Page 1
Conductance Data For Commonly
Used Chemicals
44-6039/rev. B
December 2010
Page 2

Page 3

1
CONDUCTANCE DATA FOR COMMONLY USED CHEMICALS
From an analytical point of view, little can be done with
a conductance reading, however accurate it may be,
unless it can be related to chemical concentration via a
table or graph. Much data has been generated in the
past 100 years in this area, but a comprehensive gathering of the information in a form useful to industry has
been lacking
1
. It is hoped that the following will provide
a ready and reasonably accurate reference of conductance values for the majority of electrolytes encountered in industrial situations.
The information is presented in graphical form to permit rapid evaluation of response patterns and to eliminate the time consuming and often impractical
(because of non-linearity) extrapolation required in
tables with widely separated concentrations. This
method of presentation sacrifices the precision gained
by listing actual values, but the accuracy retained is
more than sufficient for the great majority of industrial
uses. The intent has been to provide a useful working
tool more so than a scientific reference.
Most of the data presented is the result of extremely
careful and accurate laboratory work conducted by
various researchers over the years. It is recognized
that older sources of information, notably the extensive
International Critical Tables, have been found to be
slightly inaccurate due to changes in the definition of
basic units in recent times as well as to erroneous
alignment procedures and measurement techniques.
However, with the previously stated area of application
in mind, these minor errors are of little significance and
may safely be ignored.
A few curves are based on more casual “field condition” observations, and others are derived from data
with a low degree of resolution in the original reference. Both will be marked with a single asterisk (*) in
the index that follows.
All conductance values have been referenced to a single temperature of 25°C for convenience (isothermal
plots are given for selected chemicals of major importance). Much of the original data was presented at this
temperature, and all sources fell in the range of 15° to
25°C. Where possible, the temperature correction factor was calculated from isothermal equivalent conductances listed in the International Critical Tables. An
average was taken over as wide a range as possible
from .5N to 5N and 0-25°C. When such a calculation
was not possible, the widely accepted “average” of
2%/°C was used. Temperature factors, of course, will
vary in a non-linear manner with respect to both temperature and concentration for many chemicals, but the
small size of the adjustments render this of little importance. As the majority of corrections involved differentials of only 5° or 7°C, errors introduced from this
source will be small. With this in mind, the final effect of
temperature correction error deserves further comment.
The relative conductance values at various concentrations would not be noticeably affected. The error could
be approximately but correctly described as relating to
the reference temperature rather than conductance.
Too much or too little correction simply means that the
curve shown is really that seen at 24° or 26° rather
than the indicated 25°. It is not anticipated that errors
will exceed the +
1° examples given in any except the
most unusual cases.
No guarantees of accuracy can be given, but most of
the data should easily fall within 5% of the correct
absolute value. The choice of curve shape through
data points will be a factor in some cases, and it is for
this reason that the points were clearly indicated.
(Some were omitted in the lower portions of the scale
for the sake of clarity.)
1
An earlier and excellent series of curves was published for this purpose by Industrial Instruments Inc. It provided
very detailed data for a half-dozen or so commonly used chemicals, and has been included in the list of reference
sources.
NOTES:
1. Concentration is expressed as % by Weight of the anhydrous substance.
2. Conductance units are µmhos/cm.
3. Plotted data points are indicated by circles.
1
Page 4

2
SOURCES OF CONDUCTANCE DATA
1. International Critical Tables, Vol. Vl, pp. 230-258; McGraw Hill, 1929.
2. Handbook of Chemistry and Physics, 55th Edition: CRC Press, 1976.
3. Lange’s Handbook of Chemistry, 10th and 11 th Editions.
4. Graphs published by Industrial Instruments, Inc.; Cedar Grove, N.J.
5. Previously unpublished laboratory measurements performed at Uniloc, Inc., Irvine, Calif. 1970-1976.
6. Miscellaneous information regarding single electrolytes obtained from various reliable industrial sources.
7. Electrolyte Solutions, Robinson and Stokes: Butterworths, 1959.
8. Electrochemical Data, Dobos: Elsevier, 1975.
9. Electrolytic Conductance and the Conductances of the Halogen Acids in Water, Hamer and DeWane: National
Bureau of Standards Publication NSRDS-NBS 33, 1970.
10.Handbook of Electrochemical Constants, Parsons: Butterworths/Academic Press, 1959.
Page 5

3
INDEX OF ELECTROLYTES
Maximum conductance and
Chemical
point of inflection at 25°C
Mol. Wt. Graph
Substance Formula
[
µmhos/cm/% by wt.]
(Anhydrous) No.
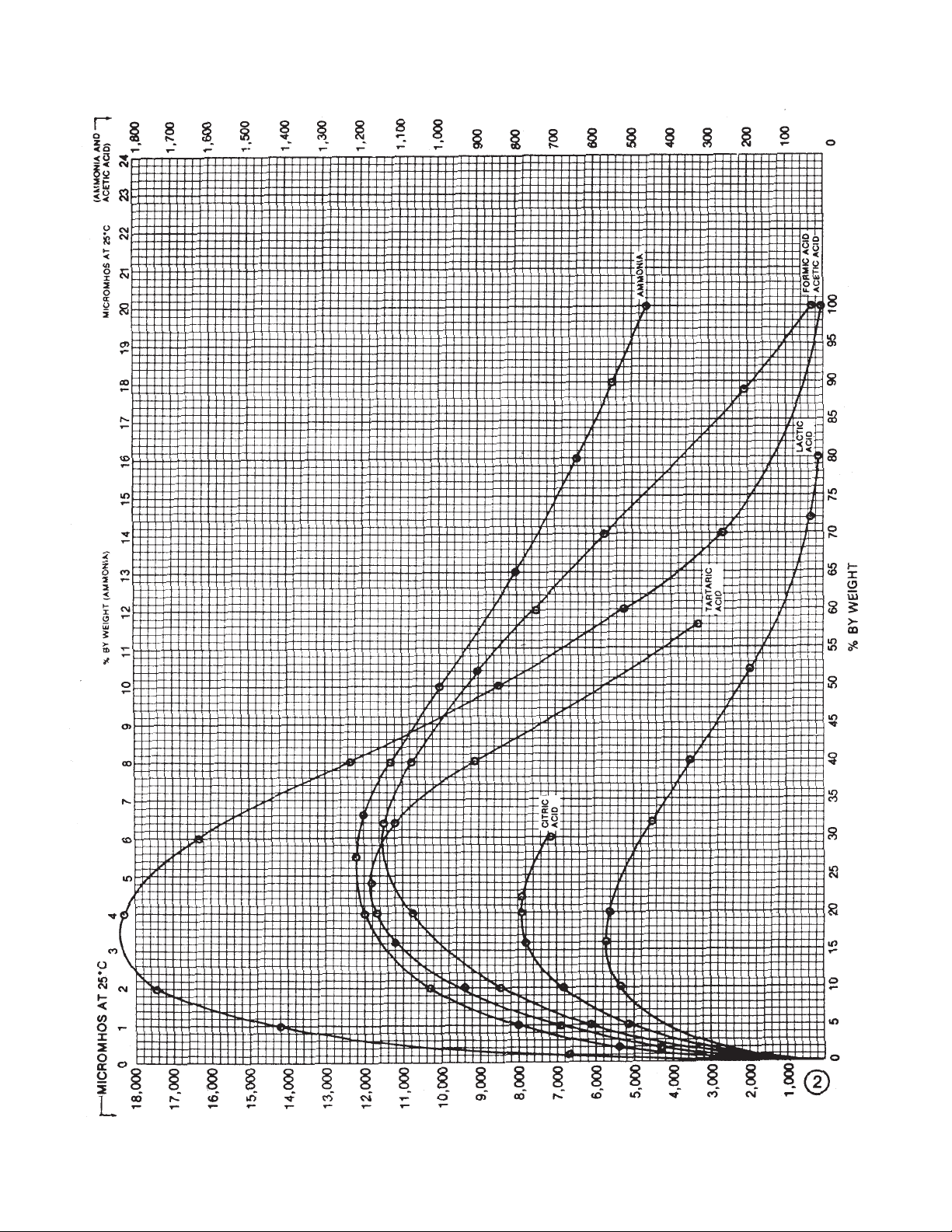
1. Acetic acid CH3COOH 1,850/17% 60.05 2
2. Aluminum chloride AlCl
3
— 133.34 6
3. *Ammonia/Ammonium Hydroxide NH3/NH4OH 1,200/5.5% 17.03/35.05 2
4. *Ammonium bifluoride NH4F•HF — 57.04 7
5. Ammonium chloride NH4Cl — 53.50 13
6. Ammonium fluoride NH4F — 37.04 7
7. Ammonium iodide NH4I — 144.94 14
8. Ammonium nitrate NH4NO
3
— 80.04 13
9. Ammonium sulfate (NH4)2SO
4
— 132.14 11
10. Ammonium thiocyanate NH4SCN — 76.12 10
11. Barium chloride BaCl
2
— 208.25 8
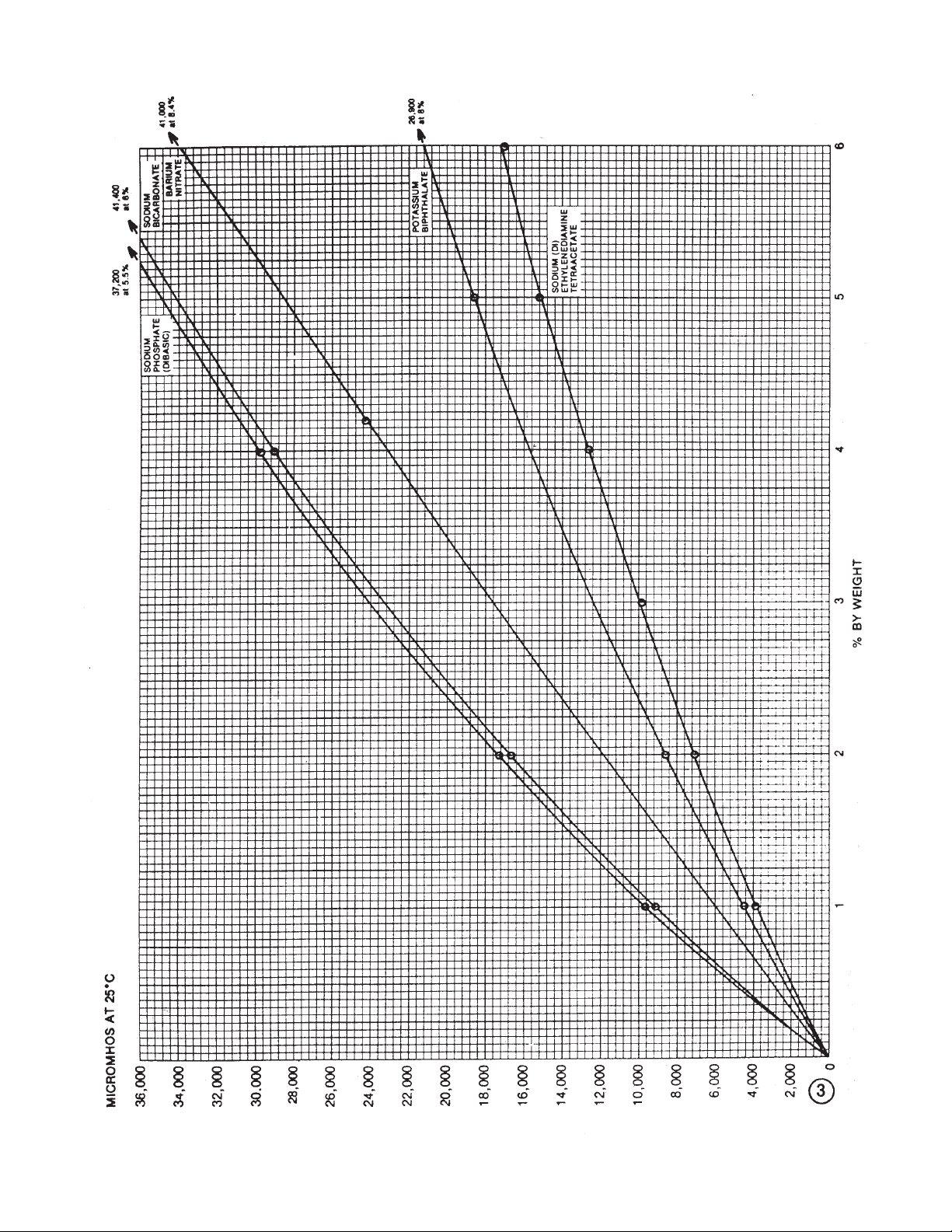
12. *Barium hydroxide Ba(OH)
2
— 171.39 4
13. *Barium nitrate Ba(NO3)
2
— 261.38 3
14. Cadmium bromide CdBr
2
30,000/32% 272.24 9
15. Cadmium chloride CdCl
2
35,000/22% 183.32 5
16. Cadmium iodide Cdl
2
366.25 9
17. Cadmium nitrate Cd(NO3)
2
108,000/32% 164.10 9
18. Cadmium sulfate CdSO
4
51,000/27% 208.48 9
19. Calcium chloride CaCl
2
204,000/24% 110.99 11
20. Calcium nitrate Ca(NO3)
2
122,000/25% 164.10 9
21. Cesium chloride CsCl — 168.37 13
22. Chromic acid CrO
3
670,000/35% 99.99 14
23. Citric acid (COOH)CH2C(OH)
(COOH)•H2O 7,900/20% 210.14 (Hyd.) 2
24. Cobaltous Chloride COCl
2
— 129.84 6
25. Cupric chloride CuCl
2
108,000/23% 134.45 11
26. Cupric nitrate Cu(NO3)
2
134,000/28% 187.55 8
27. Cupric sulfate CuSO
4
— 159.61 6
28. (Ethylenediamine) Tetraacetic acid
disodium salt, EDTA sodium Na2C10H14O8N2•2H2O — 372.24(Hyd.) 3
29. Ferric chloride FeCl
3
96,000/16% 162.22 9
30. Ferrous sulfate FeSO
4
53,000/24% 15.94 6
31. Formic acid HCOOH 11,500/30% 46.03 2
32. Hydrobromic acid HBr — 80.92 14
33. Hydrochloric acid HCl 850,000/19% 36.47 1
34. Hydrofluoric acid HF — 20.01 13
35. Hydroiodic acid HI — 127.93 14
36. Iodic acid HlO
3
— 175.93 12
37. Lactic acid CH3CHOH COOH 5,700/15% 90.08 2
38. Lanthanum nitrate La(NO3)
3
97,000/28% 324.93 8
39. Lead (plumbous) nitrate Pb(NO3)
2
— 331.23 8
40. Lithium chloride LiCl 190,000/21% 42.40 11
41. Lithium hydroxide LiOH 380,000/11% 23.95 13
42. Lithium iodide LiI — 133.86 7
43. Lithium sulfate Li2SO
4
83,000/18% 109.95 7
44. Magnesium chloride MgCl
2
160,000/18% 95.23 11
45. Magnesium nitrate Mg(NO
3)2
— 148.34 6
46. Magnesium sulfate MgSO
4
58,000/17% 120.37 7
47. Manganous chloride MnCl
2
130,000/20% 125.84 8
48. Manganous sulfate MnSO
4
51,500/22% 151.00 5
49. Nickel sulfate NiSO
4
— 154.78 7
50. Nitric acid HNO
3
865,000/29% 63.02 1
51. Oxalic acid HO
2
CCO2H — 90.04 4
52. Phosphoric acid H3PO
4
230,000/50% 98.00 12
53. Potassium acetate KCH
3CO2
150,000/32% 98.14 12
54. Potassium bicarbonate KHCO
3
— 100.12 7
55.Potassium biphthalate KHC8H4O
4
— 204.23 3
Page 6

4
INDEX OF ELECTROLYTES (Continued)
Maximum conductance and
Chemical
point of inflection at 25°C
Mol. Wt. Graph
Substance Formula
[
µmhos/cm/% by wt.]
(Anhydrous) No.
56. Potassium bromide KBr — 119.01 13
57. Potassium carbonate K
2CO3
258,000/34% 138.21 12
58. Potassium chloride KCl — 74.55 1
59. Potassium chromate K
2
CrO
4
— 194.20 10
60. Potassium cyanide KCN — 65.11 6
61. Potassium dichromate K2Cr2O
7
— 294.21 6
62. Potassium ferricyanide K
3
Fe(CN)
6
— 329.26 13
63. Potassium ferrocyanide K4Fe(CN)
6
— 368.36 6
64. Potassium fluoride KF 288,000/34% 58.10 12
65. Potassium hydroxide KOH 625,000/26% 56.11 14
66. Potassium iodide Kl — 166.03 14
67. Potassium nitrate KNO
3
— 101.10 7
68. Potassium oxalate K2C2O
4
— 166.22 7
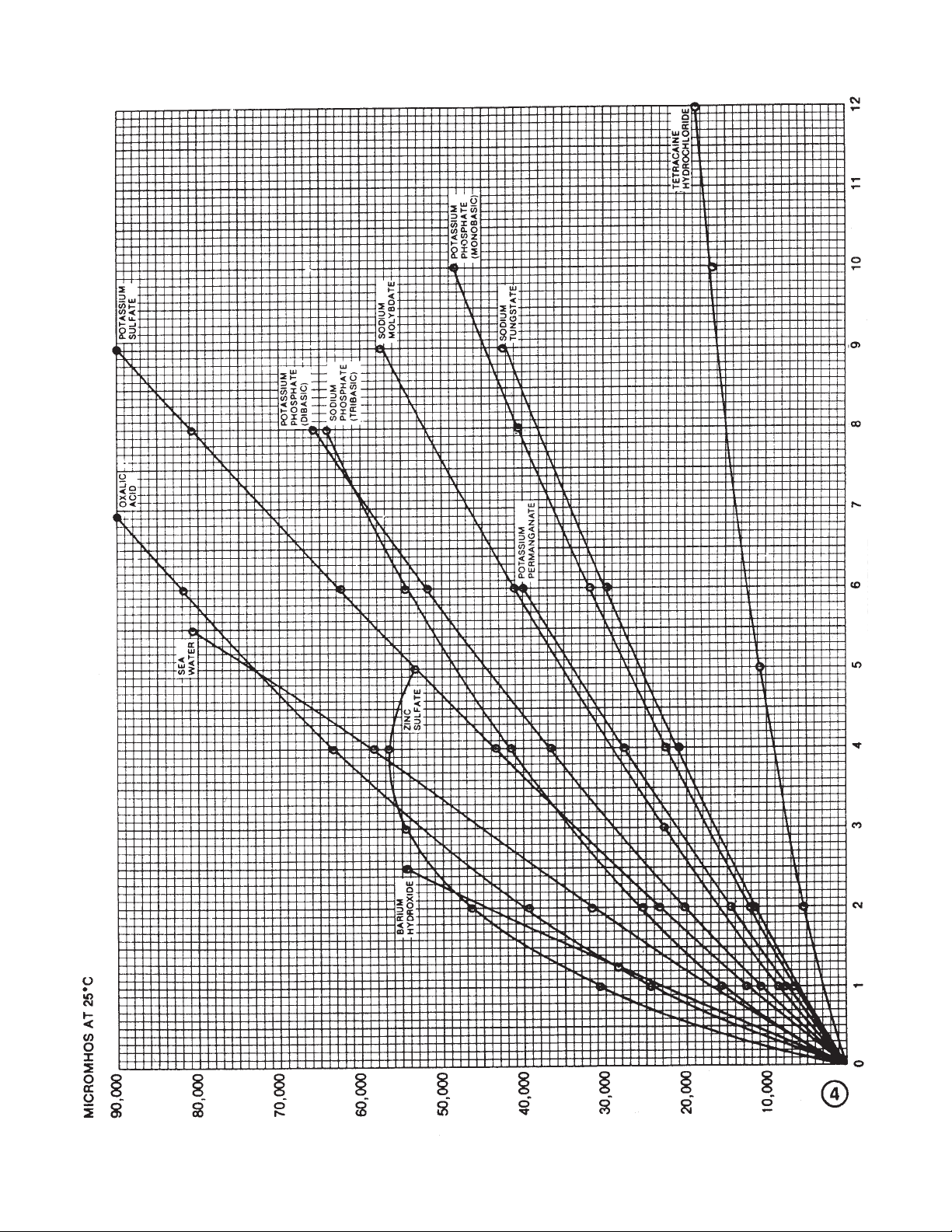
69. Potassium permanganate KMnO
4
— 158.04 4
70. Potassium phosphate (monobasic) KH2PO
4
— 136.13 4
71. Potassium phosphate (dibasic) K2HPO
4
— 174.18 4
72. Potassium sulfate K2SO
4
— 174.26 4
73. Potassium sulfide K2S 535,000/30% 110.26 14
74. Potassium thiocyanate KSCN — 97.18 10
75. Procaine hydrochloride C6H4[COOCH2CH2N
(C2H5)2] (NH2)•HCI-1,4 34,000/32% 272.78 5
76. Sea water — — — 4
77. *Silver fluoride AgF — 126.88 10
78. Silver nitrate AgNO
3
— 169.89 12
79. Sodium acetate NaCH3CO
2
78,000/22% 82.04 5
80. Sodium bicarbonate NaHCO
3
— 84.01 3
81. Sodium bromide NaBr — 102.91 10
82. Sodium carbonate Na2CO
3
103,000/19% 106.01 6
83. Sodium chloride NaCl — 58.44 1
84. Sodium citrate Na3C6H5O
7
64,500/23% 258.07 5
85. Sodium diatrizoate (Hypaque) Na(CH3CONH)2C6l3CO
2
18,500/40% 635.92 5
86. Sodium dichromate Na2Cr2O
7
165,000/40% 261.97 9
87. Sodium ferrocyanide Na
4
Fe(CN)
6
— 303.92 10
88. Sodium hydroxide NaOH 410,000/15% 40.01 1
89. Sodium molybdate Na
2
MoO
4
— 205.95 4
90. Sodium nitrate NaNO
3
— 85.01 11
91. Sodium phosphate (monobasic) NaH2PO
4
60,000/28% 119.97 8
92. Sodium phosphate (dibasic) Na
2
HPO
4
— 141.98 3
93. Sodium phosphate (tribasic) Na
3PO4
— 163.96 4
94. Sodium sulfate Na2SO
4
— 142.07 7
95. Sodium sulfide Na
2
S 262,000/15% 78.06 10
96. Sodium tartrate NaOOC(CHOH)2COONa 68,500/24% 194.07 5
97. Sodium thiocyanate NaSCN 206,000/34% 81.08 12
98. Sodium thiosulfate Na
2S2O3
152,000/29% 158.13 8
99. Sodium tungstate Na
2WO4
— 293.92 4
100. Strontium chloride SrCl
2
198,000/30% 158.55 11
101. Strontium nitrate Sr(NO3)
2
113,000/30% 211.65 8
102. Sulfuric acid H
2SO4
825,000/30% 98.08 1
103. Tartaric acid HO
2
C(CHOH)2CO2H 11,800/24% 150.09 2
104. Tetracaine hydrochloride C
l5H24N2O2
•HCI — 300.84 4
105. Trichloracetic acid CCl3COOH — 163.38 10
106. Zinc chloride ZnCl
2
104,000/27% 136.29 12
107. Zinc Sulfate ZnSO
4
56,500/4% 161.44 4
Page 7

5678910111213141516171819
Page 8
Page 9
Page 10
Page 11
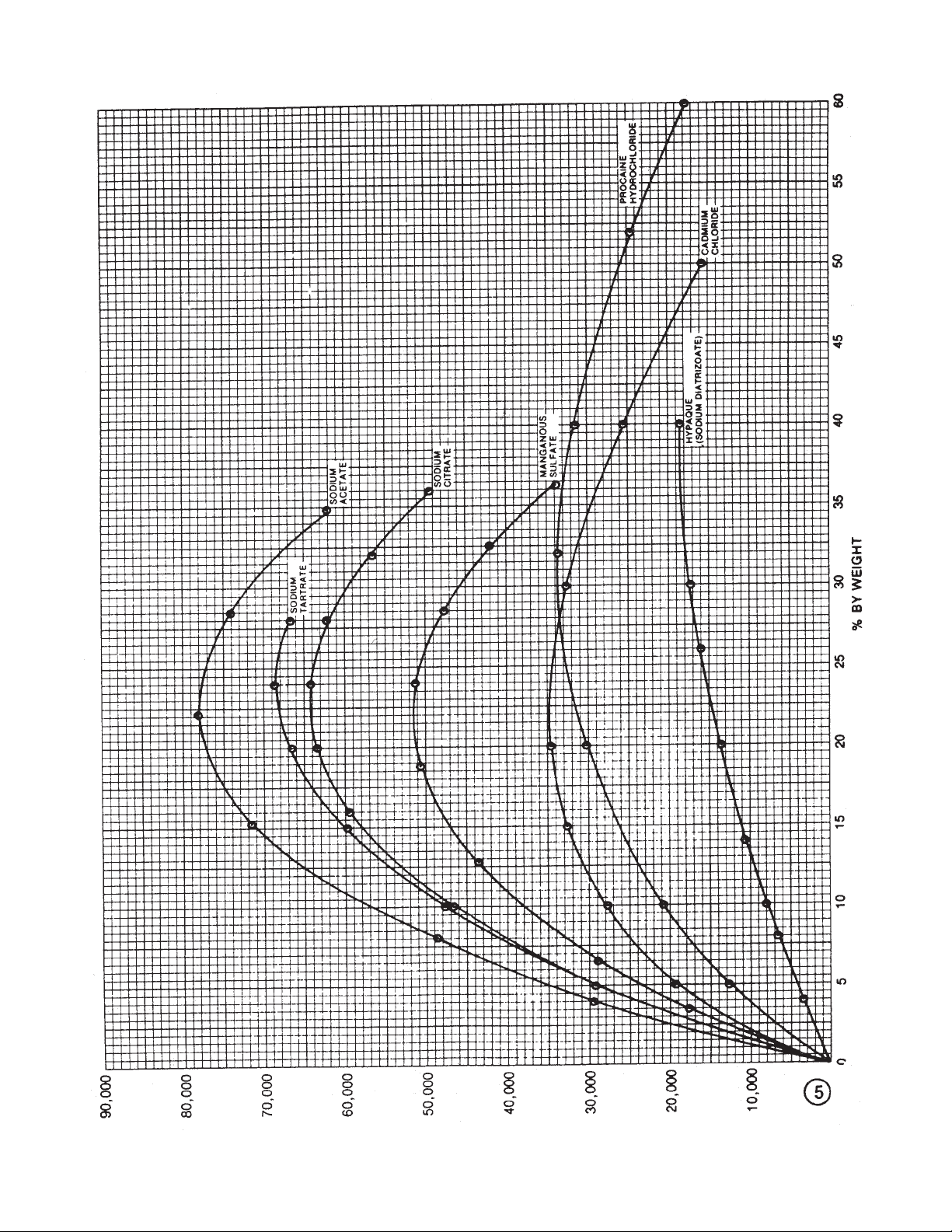
Page 12

Page 13

Page 14

Page 15

Page 16

Page 17

Page 18

Page 19

Page 20

Page 21

ELECTRICAL CONDUCTANCE OF SATURATED SOLUTIONS OF SLIGHTLY SOLUBLE
ELECTROLYTES (SALTS ARE CORRECTED FOR CONDUCTANCE OF WATER)
(Data from International Critical Tables, Vol. VI)
AgBr 21.1 0.075
AgBrO
3
19.9 663.24
AgCH3COC(CN)CO2C2H525.0 251.5
AgCI 25.0 1.794
AgCN 20.0 19.0
Ag2C2O
4
25.0 35.97
Ag2CrO
4
25.0 26.61
AgI 20.8 0.002
AgIO
3
25.0 101.27
AgONC(CN)CO2C2H
5
25.0 234.6
AgONC(CN)CO2CH(CH3)225.0 131.8
AgOOCCH
3
25.0 4,975
AgOH 24 9 35.944
Ag3PO
4
19.5 6.1
AgSCN 20.0 0.096
Agn-Propionate 25.0 1,924
Agdl-Tartratc 25.0 583.4
Ag d-Tartrate 25.0 989
Ag l-Tartrate 25.0 1009
Ag p-Toluate 25.0 251.7
Aragonite 25.0 41.0
Antipyrine Salicylate 18.0 1,000
BaCO
3
18.0 25.475
BaCrO
4
25.0 4.345
BaC2O4•?H2O 25.0 91.62
BaC2O4•2H2O 25.0 108.6
BaC2O4•3.5H2O 18.0 94.91
BaSO
4
25.0 2.923 (avg.)
Barite 25.0 3.517
CaCO
3
25.0 35.97
CaC2O4•H2O 25.0 12.37
CaF
2
26.1 50.55
CaF2(Fluorite) 25.0 45.81
CaSO
4
•2H2O 25 0 2,210
CdC2O4•3H2O 25.0 36.22
Ce(IO3)3•2H2O 25.0 636.8
Ce2(C2O4)3•10H2O 25.0 0.651
Ce2(C4H4O6)3•4.5H2O 25.0 51.66
CuI 24.6 2.128
CuSCN 18.0 0.4
Calcite 25.0 33.8
Celestite 25.0 147.4
HgCl 24.6 2.13
HgI
2
18.0 0.2 (?)
La(IO3)3•3H2O 25.0 692
La2(C2O4)3•10H2O 25.0 953
La2(C4H4O6)3•3H2O 25.0 58.5
Li2PO3•3.5H2O 25.0 274
Li3PO4•0.5H2O 25.0 937
MgC2O
4
18.0 199.3
MgCO
3
25.9 885.1
MgF
2
27.0 270.7
Mn(OH)
2
18.0 9.49
Nd2(C2O4)3•10H2O 25.0 0.764
PbBr
2
20.0 3,692
Pb(BrO3)
2
19.9 4,630.4
PbCO
3
20.0 1.39
PbC2O
4
22.0 1.54
PbCl
2
20.0 53,540
PbF
2
25.0 514
PbI
2
20.1 338.4
Pb(lO3)
2
25.0 8.75
Pb3(PO4)
2
20.0 0.14
Pb(SCN)
2
20.0 5,346
PbSO
4
25.0 40.3
Pr2(C2O4)3•10H2O 25.0 1.164
Sa2(C2O4)
3
25.0 0.82
SrF
2
25.0 204.6
SrC2O
4
25.0 70.79
SrSO
4
25.0 147.4
TlBr 25.0 293.8
TlBrO
3
19.9 1,079.
TlCl 25.0 2,160.
TlC2O
4
20.0 5,341.
TlI 25.0 36.64
TlIO
3
20.0 154.1
TISCN 20.0 1,399
TI2S 20.0 216
Y2(C2O4)•9H2O 25.0 1.74
Yb2(C2O4)3•10H2O 25.0 4.849
ZnC2O4•2H2O 25.0 10.21
SUBSTANCE TEMP.°C SOLUTION
µMHOS/CM
SUBSTANCE TEMP.°C SOLUTION
µMHOS/CM
Page 22

20
ELECTRICAL CONDUCTANCE OF VARIOUS PURE LIQUIDS
(Data from Lange's Handbook of Chemistry, 12th edition)
LIQUID TEMP.°C µMHOS/CM LIQUID TEMP.°C µMHOS/CM
ACETALDEHYDE 15 1.7
ACETAMIDE 100 <43
ACETIC ACID 25 .0112
ACETIC ANHYDRIDE 25 .48
ACETONE 25 .06
ACETONITRILE 20 7
ACETOPHENONE 25 .006
ACETYL BROMIDE 25 2.4
ACETYL CHLORIDE 25 .4
ALIZARIN 233 1.45 (?)
ALLYL ALCOHOL 25 7
AMMONIA -79 .13
ANILINE 25 .024
ANTHRACENE 230 .0003
ARSENIC TRIBROMIDE 35 1.5
ARSENIC TRICHLORIDE 25 1.2
BENZALDEHYDE 25 .15
BENZENE — .076
BENZOIC ACID 125 .003
BENZONITRILE 25 .05
BENZYL ALCOHOL 25 1.8
BENZYL BENZOATE 25 <.001
BENZYLAMINE 25 <.017
BROMINE 17.2 1.3 x 10
–7
BROMOBENZENE 25 <.00002
BROMOFORM 25 <.02
CAPRONITRILE 25 3.7
CARBON DISULFIDE 1 7.8 x 10
–12
CARBON TETRACHLORIDE 18 4 x 10
–12
CHLORINE -70 <1 x 10
–10
CHLOROACETIC ACID 60 1.4
CHLOROFORM 25 <.02
CHLOROHYDRIN 25 .5
CYANOGEN - <.007
CYMENE 25 <.02
DICHLOROACETIC ACID 25 .07
DICHLOROHYDRIN 25 12
DIETHYL CARBONATE 25 .017
DIETHYL OXALATE 25 .76
DIETHYL SULFATE 25 .26
DIETHYLAMINE -33.5 .0022
DIMETHYL SULFATE 0 .16
EPICHLOROHYDRIN 25 .034
ETHYL ACETATE 25 < .001
ETHYL ACETOACETATE 25 .04
ETHYL ALCOHOL 25 .00135
ETHYL BENZOATE 25 <.001
ETHYL BROMIDE 25 < .02
ETHYL ETHER 25 < 4x10
–7
ETHYL IODIDE 25 .02
ETHYL ISOTHIOCYANATE 25 .126
ETHYL NITRATE 25 .53
ETHYL THIOCYANATE 25 1.2
ETHYLAMINE 0 .4
ETHYLENE BROMIDE 19 <.0002
ETHYLENE CHLORIDE 25 .03
ETHYLIDENE CHLORIDE 25 <.17
EUGENOL 25 .17
FORMAMIDE 25 4
FORMIC ACID 25 64
FURFURAL 25 1.5
GALLIUM 30 36,800
mhos
GERMANIUM TETRABROMIDE
30 78
GLYCEROL 25 .064
GLYCOL 25 .3
GUAIACOL 25 .28
HEPTANE 20 <1x10
–7
HEXANE 18 <1x10
–12
HYDROGEN BROMIDE -80 .008
HYDROGEN CHLORIDE -96 .01
HYDROGEN CYANIDE 0 3.3
HYDROGEN IODIDE -35 .2
HYDROGEN SULFIDE -60 .00001
IODINE 110 .00013
iso-BUTYLALCOHOL 25 .08
Page 23

21
ELECTRICAL CONDUCTANCE OF VARIOUS PURE LIQUIDS
(Data from Lange's Handbook of Chemistry, 12th edition)
LIQUID TEMP.°C µMHOS/CM LIQUID TEMP.°C µMHOS/CM
KEROSENE 25 <.017
m-CHLOROANlLlNE 25 .05
m-CRESOL 25 <.017
MERCURY 0 10,629.6
mhos
METHYL ACETATE 25 3.4
METHYL ALCOHOL 18 .44
METHYL IODIDE 25 <.02
METHYL NITRATE 25 4.5
METHYL THIOCYANATE 25 1.5
METHYLETHYL KETONE 25 .1
NAPTHALENE 82 .0004
NITROBENZENE 0 .005
NITROMETHANE 18 .6
NONANE 25 <.017
o- or m- NlTROTOLUENE 25‘ <.2
OLEIC ACID 15 <.0002
PENTANE 19.5 <.0002
PETROLEUM — 3 x 10
–7
PHENETOLE 25 <.017
PHENOL 25 <.017
PHENYL ISOTHIOCYANATE 25 1.4
PHOSGENE 25 .007
PHOSPHORUS 25 .4
PHOSPHORUS OXYCHLORIDE 25 2.2
PINENE 23 <.0002
PIPERIDENE 25 < .2
PROPIONALDEHYDE 25 .85
PROPIONIC ACID 25 <.001
PROPIONITRILE 25 < .1
n-PROPYL ALCOHOL 25 .02
iso-PROPYL ALCOHOL 25 3.5
n-PROPYL BROMIDE 25 < .02
PYRIDINE 18 .053
QUINOLINE 25 .022
SALICYLALDEHYDE 25 .16
STEARIC ACID 80 < 4x10
–7
SULFONYL CHLORIDE 25 2
SULFUR 115 1x10
–6
SULFUR 130 5x10
–5
SULFUR 440 .12
SULFUR DIOXIDE 35 .015
SULFURIC ACID 25 10000
SULFURYL CHLORIDE 25 .03
TOLUENE — < 1x10
–8
o-TOLUIDINE 25 < 2
p-TOLUIDINE 100 .062
TRICHLOROACETIC ACID 25 .003
TRIMETHYLAMINE -33.5 .00022
TURPENTINE — 2 x 10
–7
iso-VALERlC ACID 80 <4 x 10
–7
WATER 18 .044
XYLENE — 1.43 x 10
–11
Page 24

22
SUMMARY OF FORMULAE,
CONVERSION DATA AND MISCELLANEOUS INFORMATION
1. To obtain the conductance at temperatures other
than reference when the temperature coefficient is
known:
CT= CR[1 + tc (T – TR)] for the general case.
CT= CR[1 + tc (T – 25)] for 25°C reference.
T = temperature of TR= reference temperature
interest
tc = temperature CT= conductivity at tem-
coefficient perature of interest
CR= conductivity at C25= conductivity at 25°C
reference
2. To obtain the conductance at 25°C when conductance values at two other temperatures are
known:
C25= [CT2(T1– 25) – CT1(T2– 25)]/(T1– T2)
3. To obtain the temperature coefficent, referenced
to 25°C, when the conductance at any two temperatures is known:
tc = (CT1– CT2)/[CT2(T1– 25) – CT1(T2– 25)]
4. Centigrade - Fahrenheit conversion:
°C = 5/9(°F – 32) °F = 9/5(°C) + 32
A rule-of-thumb method for making the conversion is to
recall the easily remembered values at freezing and
boiling, and that a change of 5°C is matched by a
change of 9°F. Thus, 35°C is seven “increments”
above freezing. Multiply the seven by the Fahrenheit
“increments” of 9 to obtain a Fahrenheit reading that is
63 degrees above freezing, or 95°F.
5. Concentration conversions:
Multiply the original concentration value by the
conversion factor shown.
6. Resistance values to simulate contacting (electrode) sensor:
Resistance(ohms) = Cell Constant x 10
6
_______________________
µmhos of solution at 25°C
7. For a 1.0 cell constant:
Conductance, µmhos Resistance, ohms
1 1,000,000
10 100,000
100 10,000
1,000 1,000
10,000 100
100,000 10
1,000,000 1
Here is an easy way to remember it — there are
always a total of six zeros in the conductance/ resistance combination for the even decade values shown.
So, 100 umhos, having two, will require a resistance
with four, which is 10,000 ohms.
FROM Weight Conc. Molarity Molality Weight%
TO
Weight Conc. Grams Solute Wm 103p Wm 10p
______________ _____________
Liter solution 103+ C Wm
Molarity 1 Moles solute 103p 10p
______ _____________ _____________ _____
Wm Liter Solution 103+ CWm Wm
Molality 10
3
10
3
Moles Solute 10
3
______________ _____________ _____________ _______________
Wm(103p – Wc) 103p – MWm 1000 Grams Wm(102– W%)
Solvent
Weight % 1 Wm 102Wm Grams Solute
_____ _____ ___________ __________________
10p 10p 103+ CWm 100 Grams Solution
ppm = W% x 10
4
Normality = Molarity x Equivalents per mole
Wm = Molecular weight of solute (g) p = Solution density (g/cm3)
Wc = Weight concentration W%= Weight %
M = Molality C = Molarity
Page 25

23
Actinium Ac (227)
Aluminum Al 26.9815
Americium Am (243)
Antimony Sb 121.75
Argon Ar 39.948
Arsenic As 74.9216
Astatine At (210)
Barium Ba 137.34
Berkelium Bk (245)
Beryllium Be 9.01218
Bismuth Bi 208.9806
Boron B 10.81
Bromine Br 79.904
Cadmium Cd 112.40
Calcium Ca 40.08
Californium Cf (248)
Carbon C 12.011
Cerium Ce 140.12
Cesium Cs 132.9055
Chlorine Cl 35.453
Chromium Cr 51.996
Cobalt Co 58.9332
Copper Cu 63.546
Curium Cm (247)
Dysprosium Dy 162.50
Einsteinium Es (254)
Erbium Er 167.26
Europium Eu 151.96
Fermium Fm (253)
Fluorine F 18.9984
Francium Fr (223)
Gadolinium Gd 157.25
Gallium Ga 69.72
Germanium Ge 72.59
Gold Au 196.9665
Hafnium Hf 178.49
Helium He 4.00260
Holmium Ho 164.9303
Hydrogen H 1.0080
Indium In 114.82
Iodine I 126.9045
Iridium Ir 192.22
Iron Fe 55.847
Krypton Kr 83.80
Lanthanum La 138.9055
Lawrencium Lr (257)
Lead Pb 207.2
Lithium Li 6.941
Lutetium Lu 174.97
Magnesium Mg 24.305
Manganese Mn 54.9380
Mendelevium Md (256)
Mercury Hg 200.59
Molybdenum Mo 95.94
Neodymium Nd 144.24
Neon Ne 20.179
Neptunium Np 237.0482
Nickel Ni 58.71
Niobium Nb 92.9064
Nitrogen N 14.0067
Nobelium No (254)
Osmi um Os 190.2
Oxygen O 1 5.9994
Palladium Po 106.4
Phosphorus P 30.9738
Platinum Pt 195.09
Plutonium Pu 239.05
Polonium Po 210
Potassium K 39.102
Praseodymium Pr 140.9077
Promethium Pm (147)
Protactinium Pa 231.0359
Radium Ra 226.0254
Radon Rn (222)
Rhenium Re 186.2
Rhodium Rh 102.9055
Rubidium Rb 85.4678
Ruthenium Ru 101.07
Samarium Sm 150.4
Scandium Sc 44.9559
Selenium Se 78.96
Silicon Si 28.086
Silver Ag 107.868
Sodium Na 22.9898
Strontium Sr 87.62
Sulfur S 32.06
Tantalum Ta 180.9479
Technetium Tc 98.9062
Tellurium Te 127.60
Terbium Tb 158.9254
Thallium Tl 204.37
Thorium Th 232.0381
Thulium Tm 168.9342
Tin Sn 118.69
Titanium Ti 47.90
Tungsten (Wolfram) W 183.85
Uranium U 238.029
Vanadium V 50.9414
Xenon Xe 131.30
Ytterbium Yb 173.04
Yttrium Y 88.9059
Zinc Zn 65.37
Zirconium Zr 91.22
ATOMIC
NAME SYMBOL WEIGHT
ATOMIC
NAME SYMBOL WEIGHT
TABLE OF THE ELEMENTS
Page 26

24
CONDUCTANCE IN DILUTE AQUEOUS SOLUTIONS
(Data obtained from Handbook of Electrochemical, Constants,
Parsons; Academic Press/Butterworths, 1959)
Conductance values at lower concentrations can be
approximately determined by use of the following formula:
Specific Conductance at 25°C
~
_
1000 C Ao (1-a
3
—
C + bC )
Ao, a and b are obtained from the tables that follow.
Parts Per million concentration
C = Normality
~
_
———————————————
1000 X Equivalent weight
The equivalent weight may be obtained from the tables
also. The formula is useful for values of C between
0.0001 and 0.1 only. No allowance was made for solution density as it will be near that of pure water at lower
concentrations. However, if it is known and greater
accuracy is desired, simply multiply the value already
calculated for C times the density to obtain a more precise answer. If normality is known, rather than ppm or
wt. %, use it directly for C.
Example 1: What is the specific conductance of a
10,000 ppm solution of silver nitrate at 25°C?
C
~
_
10,000/
(1000 x 169.89)
= .05886
Specific Conductance
———
~
_
1000 (.05886) (133.3) [
(1-.68
3
.05886 + .35(.05886)]
~
_
6,713 mhos/cm
How much would density correction affect the reading?
The relative density at 10,000 ppm is 1.007 (at 20 C).
C = 10,000 (1.007)/
(1000 x 169 89)
=
.05927 Corrected for density
Specific conductance
———
~
_
1000 (.05927) (133.3) [
(1-.68
3
.05927 +
.35(.05886)]
~
_
6,757 mhos
The error due to no density correction was less than 1%.
Example 2: What is the specific conductance of a 0.01 N
solution of KCI at 25°C?
Specific Conductance
~
_
———
1000 (.01) (149.8) [
1-.63 3.01 + .64 (0.1)]
~
_
1,413 umhos/cm
Because C was given as normality, no density correction
is necessary. Referring to the table of conductance values
for standard KCI solutions, the measured value at 0.01N
is 1411 umhos/cm. The calculated value of 1413 is, thus,
in error by less than two-tenths of one percent. Not all calculations will be this close, but this approximation will be
more than adequate for most industrial applications.
Page 27

25
Equivalent Min./Max. ppm for
Substance
Weight
Ao(25°C) a b
use of Formula
AgMnO
4
226.81 122 0.72 2.0 20 - 23,000
AgNO
3
169.87 133.3 0.68 0.35 16 - 17,000
Ag2SO
4
155.90 t42 1.30 -3.5 15 - 16,000
AlBr
3
88.90 139 1.64 2.2 8 - 9,000
AICI
3
44.45 137.6 1.65 2.0 4 - 5,000
AII
3
135.90 137.6 1.66 3.1 13 - 14,000
Al(NO3)
3
71.00 129.5 1.72 2.2 7 - 8,000
BaAc
2
127.72 104.2 1.59 1.7 12 - 13,000
BaBr
2
148.58 141.1 1.28 1.78 14 - 15,000
Ba(BrO3)
2
196.57 118 1.44 1.4 19 - 20,000
BaCl
2
104.13 139.5 1.28 1.74 10 - 11,000
Bal
2
195.58 141 1.28 2.7 19 - 20,000
Ba(MnO4)
2
187.61 119 1.42 1.4 18 - 19,000
Ba(NO3)
2
130.68 132 1.34 1.2 13 - 14,000
Ba(OH)
2
85.68 256 0.88 0.0 8 - 9,000
CaBr
2
99.95 133.0 1.32 2.1 9 - 10,000
CaCl
2
55.50 135.6 1.3 1.8 5 - 6,000
Ca2Fe(CN)
6
73.03 118 5.47 11.0 7 - 8,000
Ca3[Fe(CN)6]
2
90.71 138 3.87 7.2 9 - 10,000
Ca(NO3)
2
82.05 130.0 1.35 2.0 8 - 9,000
CaSO
4
68.07 104 2.9 3.6 6 - 7,000
CdBr
2
136.11 97 1.73 0.95 13 - 14,000
CdCl
2
91.66 104 1.65 0.9 9 - 10,000
Cdl
2
183.11 77 2.02 1.38 18 - 19,000
CdSO
4
104.23 105 2.89 3.7 10 - 11,000
CoAc
2
118.04 90.1 1.74 1.4 11 - 12,000
CoBr
2
109.38 126 1.35 1.9 10 - 11,000
CoCl
2
64.92 124.5 1.37 1.2 6 - 7,000
Co(NO3)
2
91.47 122.4 1.39 2 0 9 - 10,000
CoSO
4
77.50 100 2.07 1.65 7 - 8,000
CsCI 168.40 154.6 0.62 -0.7 16 - 17,000
CsOH 74.96 271 0.45 0.5 7 - 8,000
CuAc
2
90.82 60 2.36 2.2 9 - 10,000
CuBr
2
71.73 134 1.31 1.6 7 - 8,000
CuCl
2
67.22 131 1.33 1.5 6 - 7,000
Cu(NO
3)2
93.78 128.8 1.38 1 7 9 - 10,000
CuSO
4
79.80 113 2.79 3.3 7 - 8,000
FeCl
2
63.38 137 1.34 1.05 6 - 7,000
FeSO
4
75.97 99 2.08 1.7 7 - 8,000
GdBr
3
132.33 139.9 1.63 3.2 13 - 14,000
GdCl
3
87.87 140 1.63 2.5 8 - 9,000
Gdl
3
179.32 139 1.64 4.0 17 - 18,000
HBr 80.92 429.4 0.37 0.35 8 - 9,000
HBrO
3
128.92 408 0.37 -5.0 12 - 13,000
HCNS 59.09 404 0.38 0.37 5 - 6,000
HCl 36.46 426.0 0.37 0.38 3 - 4,000
HClO
3
84.46 408 0.36 0.4 8 - 9,000
HClO
4
100.46 417 0.37 0.4 10 - 11,000
H2CrO
4
59.01 207 0.97 2.2 5 - 6,000
Hl 127.91 428 0.37 0.42 12 - 13,000
HlO
3
175.91 391.2 0.38 -4.7 17 - 18,000
HMnO
4
119.95 410 0.38 0.2 11 - 12,000
HNO
3
63.01 420 0.37 0.36 6 - 7,000
KAc 98.15 115.4 0.75 1.3 9 - 10,000
Specific Conductance (25°C)
~
_
1000 CAo
(1-a
3
–
C + bC) where .0001 C .1
C = Normality = ppm concentration/
(1000 x equivalent weight)
(Multiply ppm x density for greater accuracy.)
Page 28

26
Equivalent Min./Max. ppm for
Substance
Weight
Ao(25°C) a b
use of Formula
KBr 119.01 151.7 0.62 0.62 11 - 12,000
KBrO
3
167.01 129.4 0.69 0.48 16 - 17,000
KCNS 97.18 140.0 0.65 0.63 9 - 10,000
KCl 74.56 149.8 0.63 0.64 7 - 8,000
KCLO
3
122.55 138.7 0.66 0.4 12 - 13,000
K2CrO
4
97.10 156 1.22 1.3 9 - 10,000
KF 58.10 128 0.70 0.5 5 - 6,000
K4Fe(CN)
6
92.09 169 2.48 3.6 9 - 10,000
K3Fe(CN)
6
109.75 167.8 1.56 1.8 10 - 11,000
K2Fe(CN)5NO 147.07 136.4 1.32 1.9 14 - 15,000
Kl 166.01 150.8 0.63 0.62 16 - 17,000
KlO
3
214.00 115 0.53 0.4 20 - 22,000
KMnO
4
158.04 136 0.67 0.5 15 - 16,000
KNO
3
101.11 144.5 o.64 0.36 10 - 11,000
KOH 56.11 271 0.45 0.4 5 - 6,000
K2SO
4
87.14 151.4 1.24 1.14 8 - 9,000
LiBr 86.85 121.4 0.72 0.5 8 - 9,000
LiCl 42.39 115 0.75 0.78 4 - 5,000
LiClO
3
90.39 104.1 0.81 0.3 9 - 10,000
Li2CrO
4
64.93 123.6 1.46 1.5 6 - 7,000
Lil 133.84 117.7 0.74 0.8 13 - 14,000
LiNO
3
68.94 111 0.77 0.45 6 - 7,000
LiOH 23.95 236.5 0.48 0.5 2 - 3,000
Li2SO
4
54.97 119.2 1.48 1.4 5 - 6,000
MgBr
2
92.07 129 1.34 2.2 9 - 10,000
MgCrO
4
70.15 125 2.64 3.2 7 - 8,000
Mg2Fe(CN)
6
65.14 172 4.75 13 6 - 7,000
Mg(NO3)
2
74.16 129.0 1.35 1.8 7 - 8,000
Mg(OH)
2
29.17 257 0.87 2.1 2 - 3,000
MgSO
4
60.19 116 2.75 3.7 6 - 6,000
MnBr
2
107.38 128 1.34 1.7 10 - 11,000
MnCl
2
62.92 126 1.36 1.6 6 - 7,000
MnSO
4
75.50 109 2.84 3.8 7 - 8,000
NH4Br 97.95 155 0.62 0.60 9 - 10,000
NH4CNS 76.12 140.8 0.65 0.5 7 - 8,000
NH4Cl 53.49 150.5 0.63 0.49 5 - 6,000
NH
4IO3
192.94 117 0.74 0 19 - 20,000
NH4Pic 246.14 104.4 0.80 0.9 24 - 25,000
(NH
4)2SO4
66.07 149.9 1.25 1.1 6 - 7,000
NaAc 82.03 91.1 0.89 0.34 8 - 9,000
NaBr 102.90 126.0 0.70 0.5 10 - 11,000
NaBrO
3
150.90 106.1 0.79 0.60 15 - 16,000
NaCNS 81.07 110.5 0.77 0.75 8 - 9,000
Na2CO
3
53.00 124.1 1.47 1.6 5 - 6,000
NaCl 58.44 126.5 0.70 0.74 5 - 6,000
NaClO
3
106.44 115 0.75 0.6 10 - 11,000
NaClO
4
122.44 110 0.77 0.6 12 - 13,000
NaCrO
4
161.97 132 1.38 1.5 16 - 17,000
NaF 41.99 106 0.79 0.6 4 - 5,000
Na4Fe(CN)
6
75.98 155 2.74 4.7 7 - 8,000
NaHCO
3
84.01 96.0 0.85 0.6 8 - 9,000
Nal 149.89 127.0 0.70 0.80 14 - 15,000
NaNO
3
84.99 123 0.72 0.36 8 - 9,000
NaOH 40.01 246.5 0.47 0.3 4 - 4,000
Page 29

27
Equivalent Min./Max. ppm for
Substance
Weight
Ao(25°C) a b
use of Formula
NaPic 251.09 81. 0.97 0.7 25 - 26,000
Na2SO
4
71.02 129.0 1.39 1.50 7 - 8,000
Na2S2O
3
79.06 135.0 1.36 1.60 7 - 8,000
NiAc
2
88.40 89.5 1.75 1.3 8 - 9,000
NiBr
2
109.27 127 1.34 1.6 10 - 11,000
NiCl
2
64.81 123.3 1.37 1.7 6 - 7,000
Ni(NO3)
2
91.36 124.5 1.37 1.8 9 - 10,000
NiSO
4
77.39 100 2.7 1.6 7 - 8,000
PbCl
2
139.05 145.0 1.26 -7.0 13 - 14,000
Pb(NO3)
2
165.60 135.7 1.32 0.89 16 - 17,000
RbBr 165.37 148 0.63 0.2 16 - 17,000
RbCl 120.92 153 0.62 0.7 12 - 13,000
Rbl 212.37 145.3 0.64 0.65 21 - 22,000
RbOH 102.48 272 0.45 0.5 10 - 11,000
SnlBr
3
130.02 140.2 1.63 2.9 12 - 14,000
SmCl
3
85.57 139.8 1.64 3.0 8 - 9,000
Sml
3
177.02 138.5 1.64 3.4 17 - 18,000
SrAC
2
102.86 101 1.63 2.0 10 - 11,000
SrBr
2
123.72 136.0 1.30 1.8 12 - 13,000
SrCl
2
79.27 136.0 1.30 1.74 7 - 9,000
Sr(NO3)
2
105.82 131.8 1.34 1.5 10 - 11,000
TlCl 239.82 150.3 0.63 -1.3 23 - 25,000
TlCIO
3
~87.82 137.6 0.65 0.45 28 - 30,000
TlOH 221.38 276.1 0.45 0.45 22 - 23,000
YBr
3
137.59 141 1.63 2.8 13 - 14,000
YCl
3
65.09 136 1.67 3.5 6 - 7,000
Yl
3
156.54 143.8 1.60 2.6 15 - 16,000
ZnAc
2
91.73 88 1.77 1.2 9 - 10,000
ZnBr
2
112.60 159 1.23 0.7 11 - 12,000
ZnCl
2
68.14 130 1.48 2.3 6 - 7,000
Zn(NO3)
2
94.69 125 1.37 2.2 9 - 10,000
ZnSO
4
80.72 105 2.90 4.2 8 - 8,000
Me3HNCl 95.56 123.6 0.71 0.76 9 - 10,000
Me4Nl 201.03 118.6 0.73 0.35 20 - 21,000
Me4NPic 290.22 76 1.02 0.5 29 - 30,000
Et4Nl 257.15 108 0.78 –.– 25 - 26,000
Et
4
NPic 346.34 63 1.18 –.– 34 - 35,000
Pr
4
Nl 313.27 100 0.83 –.– 31 - 32,000
Ac = Acetate
Et = Ethyl
Me = Methyl
Pic = Picrate
Pr = Propyl
Page 30

28
All comments, formulae, etc. regarding aqueous solutions will apply here as well (except as noted regarding limits for C and temperature).
ACETONITRIDE AT 25°C
Solute Ao a b
AgNO
3
150.0 2.28 1.4
Kl 181.4 2.02 1.5
1/3TlBr
3
140.5 2.39 5.9
1/3TICl
3
170.4 2.09 2.1
Pr4NClO
4
172.3 2.08 2.4
Pr4Nl 169.6 2.10 10.0
Pr4NPic 146.3 2.32 14.0
Am4Nl 152.0 2.26 1.0
CPh2(p-C6H4OMe)CIO
4
160.9 2.18 4.0
C(p-C6H4OMe)3ClO
4
156.7 2.22 5.0
METHANOL AT 25°C
Solute Ao a b
HBr 192.0 1.78 2.0
HCI 188.0 1.79 2.0
Hl 197.0 1.76 2.5
KCH3(CH2)COO 89.0 2.73 4.1
Kl 113.3 2.34 5.1
KOH 105.8 2.45 5.5
KOCH
3
106.8 2.42 1.0
LiCHS 101.5 2.51 5.5
LiCI 94.2 2.53 3.0
LiNO
3
100.7 2.52 5.0
NaBr 101.8 2.50 4.1
NaCNS 106.9 2.43 6.0
NaCH3(CH2)3COO 82.0 2.88 4.1
NaC6HCH3(NO2)3O 91.0 2.67 3.9
NaCI 98.4 2.56 4.0
Nal 107.8 2.42 4.8
NaOH 95.7 2.60 5.6
NaOH
3
98.4 2.55 5.0
NaPic 91.4 2.68 4.6
Et4Nl 117.6 2.30 2.0
Me3NCH2Phl 96.8 2-58 5.0
(C5H11)Nl 86.9 2.77 4.0
C3H5H2Pic 102.4 2.49 2.0
i-C4H9H3NCl 97.4 2.57 6.0
C5H12NC6HMc(NO2)3O 94.4 2.63 2.8
PhH3NC6HMc(NO2)3O 82.0 2.88 3.8
CONDUCTANCE IN DILUTE NON-AQUEOUS SOLUTIONS
(Data obtained from Handbook of Electrochemical Constants,
Parsons; Academic Press/Butterworths, 1959)
Page 31

29
FORMAMIDE AT 25°C
Solute Ao a b
1/2 Ba (NO3)
2
30.3 1.33 1.33
1/2 Ca (NO3)
2
31.6 1.29 1.00
CsCI 29.0 0.74 0.75
CsNO
3
29.4 0.74 0.61
KCNS 28.7 0.75 1.20
KCI 28.0 0.76 0.90
Kl 27.7 0.77 1.04
LiNO
3
25.0 0.83 1.05
NH4Cl 30.4 0.72 1.60
NH4l 30.5 0.72 1.10
NH4NO
3
33.6 0.67 0.60
NaBr 25.7 0.81 0.80
1/2Na2CrO
4
26.0 1.56 1.80
Nal 25.0 0.83 1.18
NaNO
3
28.3 0.76 0.63
NaHCOO 25.1 o.83 0.65
NaPhCOO 20.0 0.99 0.78
NaSalicylate 20.6 0.97 0.60
NaPhSO
3
20.7 0.96 0.75
RbBr 28.3 0.76 1.10
RbCI 28.2 0.76 0.60
Rbl 28.0 0.76 1.00
RbNO
3
28.6 0.75 1.00
1/2Sr(NO3)
2
32.0 1.28 1.00
Me4NCl 28.7 0.75 0.65
Me4Nl 25.0 0.83 1.10
Et4NCl 28.7 0.75 0.65
Et4Nl 25.0 0.83 1.10
HYDROGEN CYANIDE AT 18°C b = 0
Valid to C = 10–5N.
Solute Ao A
CsCI 368.2 200
KBr 363.2 248
KCNS 358.0 243
KCI 363.4 280
KClO
4
353-3 275
Kl 363.3 235
KNO
3
353.9 253
LiBr 356.9 270
LiCNS 340.6 400
LiCI 345.4 335
LilCO
4
336.9 230
Lil 348.0 258
LiNO
3
336.6 402
NaBr 343.8 243
NaCNS 337.7 230
NaClO
4
335.5 235
Nal 344.9 238
NaNO
3
333.8 250
NaPic 266.9 195
RbCI 363.2 195
Et4NPic 282.3 215
Page 32

30
SULPHUR DIOXIDE AT 0°C
Solute Ao a b
Ph3CCl0
4
153.6 5.03 17.0
Ph2C(C6H4Me)ClO
4
149.8 4.93 15.0
PhC(C6H4Me)2ClO
4
141.3 5.06 16.0
C(C6H4Me)3Cl 168.5 4.69 15.0
C(C6H4Me)3ClO
4
150.0 4.92 15.0
Ph2C(C6H4Ph)Cl 78.3 6.83 16.0
C(C6H4Ph)3Cl 5.0 5.00 12.0
Ph2C(p-C6H4OMe)Cl 169.1 4.68 13.0
Ph2C(p-C6H4OMe)ClO
4
148.0 4.95 17.0
C(p-C6H4OMe)3ClO
4
144.4 5.03 16.0
PhC(p-C6H4NO2)(p-C6H4OMe)Cl 90.0 5.97 20.0
C(p-C6H4NO2)2(p-C6H4OMe)ClO
4
103.5 5.86 17.0
C(p-C6H4NO2)(p-C6H4OMe)2ClO
4
123.0 5.39 20.0
Me4NCl 160.5 4.80 11.8
Me4NBr 160.8 4.79 11.8
Me4Nl 166.0 4.72 12.0
Me3Sl 150.2 4.93 12.0
ACETONE AT 25°C
Solute Ao a b
Nal 161.0 3.71 6.0
Pr4Nl 152.0 3.83 6.0
C5Hl2NPic 89.1 5.34 9.0
CPh2(p-C6H4OMe)ClO
4
160.0 3.72 5.0
C(p-C6H4OMe)3ClO
4
160.2 3.72 8.0
Page 33

31
HYDRAZINE AT 0°C
Solute Ao a b
1/2 Cdl
2
76.0 1.97 2.2
HCI 103.0 0.858 0.7
HPhCOO 85.9 0.950 0.0
HPh3CCOO 74.8 1.03 –4.0
HCH2NO
3
87.0 0.94 –3.0
Hm–C6H4(NO2)O 86.4 0.95 –5.0
KCI 85.0 0.96 1.0
Nam–C6H4(NO2)O 58.1 1.21 4.0
Et4Nl 66.6 0.87 –1.0
HCI 153.9 0.90 0.6
KClO
4
128.2 0.90 0.6
25°C
Kl 130.0 0.99 1.3
NaClO
4
110.0 1.09 1 4
Nal 112.5 1.07 0.8
Et4NCl 99.7 1.16 1.6
AMMONIA AT –33°C
Solute Ao a b
AgNO
3
241.7 3.92 8.0
HCI 183.8 4.48 9.0
HNO
3
245.5 3.89 7.3
Kl 295.7 3.59 6.0
KNH
2
108.7 6.55 13.0
KPh2N 230.0 4.01 8.0
KPh3BNH
2
155.0 5.14 10.5
LiNO
3
225.7 4.05 8.5
NaBr 240.2 3.92 7.0
NaCI 206.9 4.22 8.0
Nal 265.1 3.76 7.2
NaNO
3
224.8 4.05 8.0
NaEtS 201.8 4.27 9.0
NaPhS 219.6 4.32 10.0
NaPh2N 198.7 4.3 10.0
NaMe3Sn 249.0 3.86 10.0
NaPh3BNH
2
202.5 4.26 9 8
Et2HNCl 183.0 4.43 8.0
Me3Sl 210.0 4.19 6.9
{
Page 34

32
ETHANOL AT 25°C
Solute Ao a b
HBr 77.3 2.62 4.1
HCI 70.5 2.74 3.6
Hl 81.4 2.57 4.5
Kl 46.5 3.42 6.4
KOH 42.0 3.63 6.0
LiCI 37.0 3.90 7.0
LiNO
3
40.7 3.70 6.8
NH4CCl3COO 37.0 3.93 6.2
NH4Cl 39.7 3.75 6.2
NH4Pic 40.8 3.70 6.0
NaBr 39.0 3.80 10.0
NaCCl3COO 34.3 4.12 6.6
Nal 46.0 3.44 7.0
NaOH 38.0 3.86 6.8
Me3NCH2Phl 43.4 3.56 5.8
C5Hl2NCl 37.0 3.92 6.0
C5Hl2NPic 37.5 3.90 5.6
CPh2(p-C6H4OMe)ClO
4
61.4 3.02 6.0
CPh(p-C6H4OMe)2ClO
4
60.3 2.97 7.0
FORMIC ACID AT 25°C
Solute Ao a b
CsHCOO 75.2 1.06 1.4
KHCOO 79.6 1.03 1.5
LiHCOO 75.7 1.06 0.7
NH4HCOO 82.4 1.01 1.2
NaHCOO 75.7 1.06 1.4
RbHCOO 81.1 1.02 1.0
PhNH3HCOO 75.8 1.06 1.2
8.50°C
KCl 35.82 1.12 0.94
Me4NCl 35.70 1.12 0.94
DIMETHYL-FORMAMIDE
AT 25°C b = o
Solute Ao A
KBr 84.1 154
KCNS 90.2 151
KClO
4
82.7 137
Kl 82.6 137
KNO
3
88.5 214
NaBr 83.4 165
NaCNS 89.5 171
Nal 81.9 138
NaNO
3
87.9 263
{
Page 35

33
SPECIFIC CONDUCTANCE OF STANDARD KCl SOLUTIONS
(Data obtained from Handbook of Electrochemical Constants,
Parsons; Academic Press/Butterworths, 1959)
Concentration Conductance, µmhos/cm
0°C 18°C 20°C 25°C
1N KCl
71.3828 g KCl 65,430 98,201 102,024 111,733
per kg solution
0.1N KCl
7.43344 g KCl 7,154.3 11,191.9 11,667.6 12,886.2
per kg solution
0.01N KCl
0.746558 g KCl 775.12 1,222.69 1,275.72 1,411.45
per kg solution
Page 36

ON-LINE ORDERING NOW AVAILABLE ON OUR WEB SITE
http://www.raihome.com
Credit Cards for U.S. Purchases Only.
The right people,
the right answers,
right now.
Emerson Process Management
2400 Barranca Parkway
Irvine, CA 92606 USA
Tel: (949) 757-8500
Fax: (949) 474-7250
http://www.raihome.com
© Rosemount Analytical Inc. 2010
8
 Loading...
Loading...