
®
3125 North 126th Street, Brookfield, WI 53005
Phone: 800.669.9946 Web: www.rft.com
®®
<
Care Manager
In-Service Guide
P/N: 0510-1067-B
Release Date: 02/26/10
Users must read this Guide before using the Product.

Compliance
Federal Communication Commission (FCC) Compliance
FCC ID: KXU-PGR2CCZ24
IC: 2719A-PGR2CCZ24
This device complies with Part 15 of the FCC rules. Operation is subject to the
following to conditions: (1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that
may cause undesired operations.
Technologies could void the user’s authority to operate the equipment.
FCC and IC Radiation Exposure Statement for Portable Devices
(For the Care Manager model 9600-0500)
This equipment complies with FCC and IC radiation exposure limits set forth for an
uncontrolled environment. This equipment is in direct contact with the body of the
user under normal operating conditions. This transceiver must not be co-located or
operating in conjunction with any other antenna or transceiver.
Industry Canada Compliance
Changes or modifications not expressly approved by RF Technologies could void the user’s
authority to operate the equipment. The Term “ IC” before the radio certification number only
signifies that Industry Canada technical specifications were met.
Operation is subject to the following two conditions: (1) this device may not cause harmful
interference, and (2) this device must accept any interference received, including
interference that may cause undesired operation of the device.
This device has been designed to operate with the antennas listed below, and having a
maximum gain of 3dBi. Antennas not included in this list or having a gain greater than 3dBi
are strictly prohibited for use with this device. The required antenna impedance is 50 ohms.
Acceptable antennas are PCB antennas in all ca ses of the Router which uses a 2.4 GHz 1/2
wave RP-SMA.
To reduce potential radio interference to other users, the antenna type and its gain should be
so chosen that the equivalent isotropically radiated power (e.i.r.p.) is not more than that
permitted for successful communication.
Changes or modifications not expressly approved by RF
Copyright 2010 by RF Technologies, Inc.
All Rights Reserved. No Part of this work may be reproduced or copied in any form
or by any means without written permission from RF Technologies, Inc.

Important W arnings
It is important for your facility to implement and enforce the following W A RNINGS in order to keep
all equipment functioning properly. Disregarding the information and instructions in this document is
considered abnormal use and may result in injury or system failure.
WARNING
ACCESSORIES (SUPPLIES)—To ensure patient safety and proper operation
of equipment, use only parts and accessories manufactured or recommended
by RF Technologies, Inc. Parts and accessories not manufactured or
recommended by RF Technologies, Inc. may not meet the requirements of the
applicable safety and performance standards.
Failure to use the components and supplies specified by RF Technologies, Inc.
may result in equipment and/or system failure.
WARNING
EXPLOSION HAZARD—This device should not be used in the presence of
flammable gas mixtures. It should also not be used in oxygen enriched atmospheres.
WARNING
HIGH RISK FOR FALL—The CA 520 System may not be suitable for patients who
are at “HIGH RISK FOR FALL.” Other monitoring measures may also be required.
The Motion Alert System should not be a substitute for routine visual monitoring
protocol by caregiving personnel.
WARNING
INSTALLATION AND CONFIGURATION—It is the responsibility of the
facility to follow the installation instructions carefully, as outlined in the Series 6.0
Software Administrator Guide, and to use the components and supplies specified by
RF Technologies, Inc. for all installations.
Failure to use the components and supplies specified by RF Technologies,
Inc. may result in equipment and/or system failure.
WARNING
INSTRUCTIONS FOR SET UP AND USE—It is the responsibility of the facility to
follow the instructions for set up and use carefully, as outlined in this manual, and to
use the components and supplies specified by RF Technologies, Inc. for set up and
use. Do not attempt to use extension cords or other equipment not supplied by RF
Technologies, Inc.
Failure to use the components and supplies specified by RF Technologies, Inc.
may result in equipment and/or system failure.

WARNING
PATIENT GENERATED ALARMS—Do not rely exclusively on patient
generated alarms for patient care and safety. The alarm function of equipment
in the possession of patients must be verified periodically and regular patient
surveillance is recommended.
WARNING
PATIENT MONITORING—The most reliable method of patient monitoring
combines close personal surveillance with correct operation of monitoring
equipment. It is the responsibility of the facility to periodically check on
patients in possession of RF Technologies, Inc.'s equipment (i.e. Pendants,
Pull Cords, Control Units) to mitigate risk of inappropriate use of equipment
or strangulation and stumbling hazards from cables and cords
WARNING
PRODUCT WARRANTIES—Failure to follow the Warnings and Cautions in
this guide voids any and all Product Warranties
WARNING
ST ATIC DISCHARGE—Do not touch the conductor portion of any conductor
or port. Damage tot he device may result.
WARNING
STRANGULATIONS AND TRIPPING HAZARD—Due to the possibility of
strangulation, all cables and cords should be routed away from the patient’s throat.
Cables and cords must be routed in a way to prevent tripping hazards.
WARNING
SYSTEM INSPECTION—It is the responsibility of the facility to establish
and facilitate a regular inspection schedule for your system. RF Technologies,
Inc. recommend quarterly inspections of your system for safety and
performance by a qualified RF Technologies, Inc. representative.
To arrange for a quarterly inspection by RF Technologies, Inc., call our
Technical Support Department at (800)-669-9946 or (262) 790-1771.
Failure to provide regular inspection of these products may result in equipment
and/or system failure.
WARNING
SYSTEM MAINTENANCE AND TESTING—It is the responsibility of the
facility to establish and facilitate a regular maintenance schedule for your
system, as outlined in the Series 6.0 Software Administrator Guide . This includes
regular inspection, testing, and cleaning. RF Technologies, Inc. recommend
monthly maintenance and testing of your system. It is also recommended that
your facility keep records of maintenance and test completions.
Failure to provide regular maintenance and testing of these pr oducts may result
in equipment and/or system failure.
WARNING
SYSTEM WIRING—All permanent supply connections must be done in
accordance with National Electric Code, NFP A 70.
WARNING
USER TRAINING—Only users who have received adequate training on the use of
the system, as outlined in this manual, should use the system. It is the responsibility of
the facility to ensure all users have been trained.
Failure to adequately train employees may cause system failure due to us er error.
In addition, incorrect use of the equipment may also result in system failure.
WARNING
WORN OR DAMAGED PARTS—If the control unit pads or cables are worn or
damaged, you must have the product serviced. For more information, see the section
entitled “Service and Return.”
WARNING
All RF Technologies transmitters, pendants and banding material
“PRODUCT” have been determined to be MR Unsafe as defined by ASTM F
2503-05. Use of “PRODUCT” in a Magnetic Resonance Imaging system will
cause injury to patients and staff, MR system malfunction or “PRODUCT”
malfunction. Do not bring “PRODUCT” into the MR system area and follow
your facilities policies to classify and label “PRODUCT” as MR Unsafe.
CAUTION
DISPOSAL—At the end of their service life the products described in this
manual, as well as accessories (i.e. lithium batteries, banding material,
disposable pads, etc.), must be disposed of in compliance with all applicable
federal, state and local guidelines regulating the disposal of products
containing potential environmental contaminants. Dispose of the packaging
material by observing the applicable waste control regulations.

This page intentionally left blank.

Contents
Preface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
About this Guide. . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Additional Detailed Documentation . . . . . . . . . . . . 2
Contact Information . . . . . . . . . . . . . . . . . . . . . . . . .2
Product Warranty. . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Chapter 1—Operations . . . . . . . . . . . . . . . . . . 3
Main Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Alarms Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
Silence Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
No Signal (option not shown). . . . . . . . . . . . . . . . . . 6
Help Call/Clear . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Charging the Care Manager . . . . . . . . . . . . . . . . . . 6
Chapter 2—Configuration . . . . . . . . . . . . . . . . 7
Chapter 3—Specifications. . . . . . . . . . . . . . . . 13
Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Code Alert Care Manager (0510-1067-B) - In-Service Manual i

Contents
This page intentionally left blank.
ii Code Alert Care Manager (0510-1067-B) - In-Service Manual

Preface
Overview
This guide provides detailed information about the Code Alert Care Manager, a
components 9600 Wireless Call System. It provides instructions about using and
configuring the Care Manager as well as Care Manager specification.
The Care Manager is a pocket-size battery-powered device carried by a care-provider. The
Care Manager allows a care-provider to receive patient calls for help and other alarms while
roaming anywhere within the facility coverage area. Each text page indicates the type of
alarm, patient name, and location. The Care Manager two-way communications allows a
care-provider to assign a Joint Commission reason from the Care Manager, saving time by
eliminating frequent trips to a PC. Two-way communications also allows the care-provider
to call other care-providers for help.
The 9600 Series Wireless Call System fully supervises the Care Manager and will alert staff
if a problem develops or if a signal is not received by the system. The Care Manager is
battery operated, and the battery can be replaced by the facility. When the battery is low,
staff is continuously notified until the battery is changed.
About this Guide
This Guide is intended for users who use the Care Manager, in conjunction with the 9600
Series Wireless Call System. It includes detailed information about Menus, Configuration
and Charging the Care Manager.
Care Manager (0510-1067-B) - In-Service Manual 1

: Preface
Additional Detailed Document ation
Documentation for your system is available in Portable Document Format (PDF) on the
System Documentation CD-ROM. Please contact your RF Technologies sales
representative for replacement CD-ROMs.
Contact Information
For more information about RF Technologies, Inc. products, go to www.rft.com. For
technical support, contact the Technical Support Team at (800) 669-9946 or (262) 79 0-
1771. For questions or comments about the Care Manager documentation, contact the RF
T echnologies T echnical Publications team at techpubs@rft.com.
Product Warranty
Product Warranty information can be found on the System Documentation CD-ROM or
with your original system proposal and invoice.
2 Care Manager (0510-1067-B) - In-Service Manual
Chapter 1
SILENCE
MENU
Carol Johnson
Alarm 2
Help Call
Conguration
>
Integrated Care Management
<
T
Thumb Wheel
Select Button
Operations
Main Menu
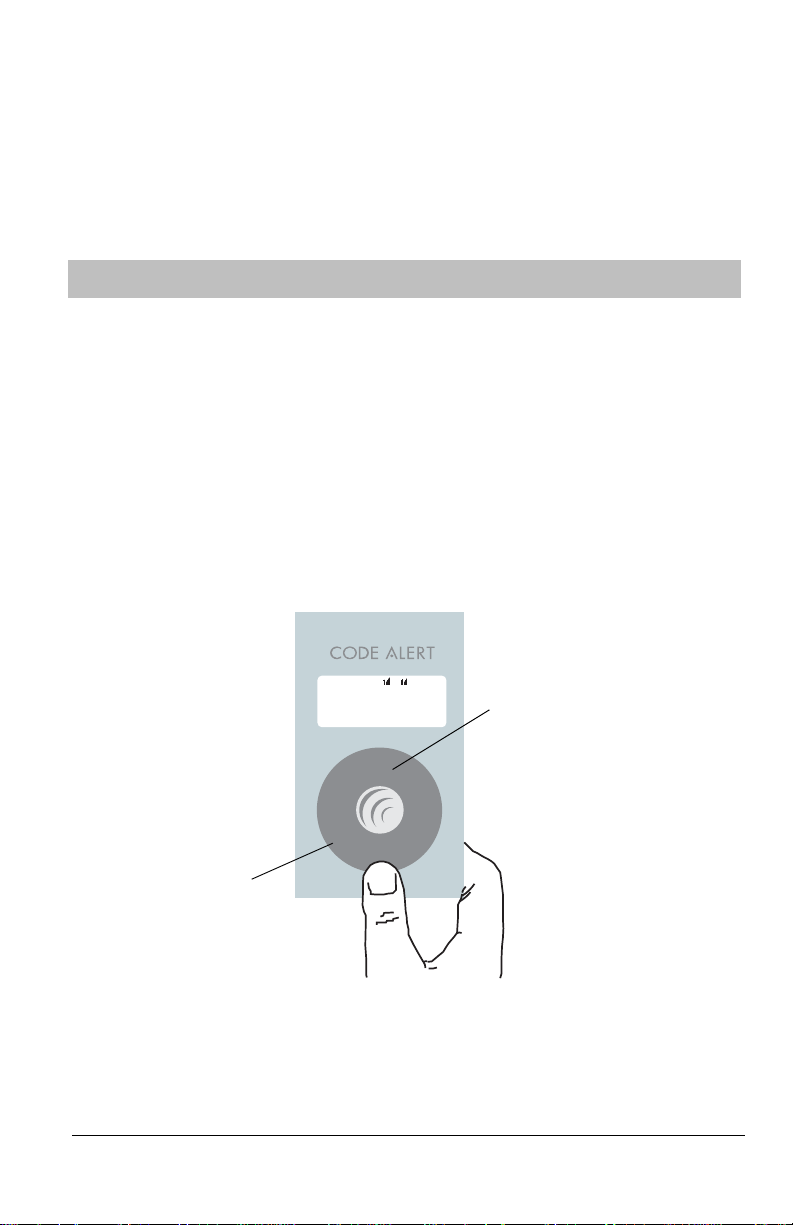
The Main Menu appears when the Code Alert Care Manager is powered.
• It displays the name of the caregiver login assigned to the Care Manager.
• It provide access to the Alarm menu and displays the number of active alarms (0-100).
• It provides access to the HELP CALL/CLEAR actions or displays No Signal when
applicable.
• It provides access to the Configuration menu.
From any menu, you can access the Main Menu by pressing MENU.
Code Alert Care Manager (0510-1067-B) - In-Service Manual 3

Chapter 1: Operations
T o Select an Item
Selecting an item is a simple two step process.
1. Rotate the thumb wheel to position the arrow (>) on an option
2. Press the select button to access the selected options.
Integrated Care Management
T
Carol Johnson
Alarm 2
Help Call
Conguration
>
SILENCE
MENU
Rotate Thumb Wheel
<
Integrated Care Management
T
Carol Johnson
Alarm 2
>
Help Call
Conguration
SILENCE
MENU
Press Select Button
<
Alarms Menu
The ALARMS menu displays the number of active alarms (0-100). If the number is
greater than 0 you can access the ALARMS menu by positioning the arrow (>) next
to ALARMS and pushing the select button.
When you access the Alarms Menu, the most recent, highest priority alarm will be
displayed first; rotate the wheel to select a different alarm. The information in the
Alarm window includes the name and location of the person generating the alarm,
the event type, the number of active alarm messages and the number of minutes this
alarm has been active. From the ALARMS menu you can select a reason for the
alarm or delete the alarm.
Selecting a reason records the reason, saves it and returns you to the next highest
priority alarm on the MAIN MENU if no alarms are active. The Select Reasons
listed are: Fall, Water. Food. Talk, Bathroom, Test, Other, Responding, Escalate
and Delete Alarm.
4 Code Alert Care Manager (0510-1067-B) - In-Service Manual

Alarms Menu
Integrated Care Management
<
SILENCE
MENU
Betty Smith
Room 221
Assistance Required
Msg 2 of 2 15min
Press select button to classify
the alarm shown
Î
SILENCE
MENU
Î
Rotate thumb wheel to scroll
next/previous alarms
Integrated Care Management
<
James Doe
East Hallway
Assistance Required
Msg 1 of 2 30min
T
T
Integrated Care Management
<
Integrated Care Management
<
Press select button to record
and save reason
SILENCE
MENU
Rotate thumb wheel to
scroll reasons
<
SILENCE
MENU
Select reason
Fall
Water
Food
>
Reason recorded
saved
Î
T
T
Silence Alarms
Alarms can be silenced by pressing SILENCE on the thumb wheel. Alarms must be
SILENCE before the next alarm can be displayed.
Code Alert Care Manager (0510-1067-B) - In-Service Manual 5

Chapter 1: Operations
No Signal (option not shown)
If the Care Manager is taken out of range and loses its signal, NO SIGNAL is displayed and
menu items disappear. Selecting this field has no action. Bringing the Care Manager back in
range brings back menu items.
Help Call/Clear
HELP CALL and CLEAR are direct action options that toggles between the two. When
HELP CALL is displayed, selecting it initiates an alarm for help. Once selected, the option
changes to CLEAR. The Care Manager continues to send out the alarm message until you
select CLEAR.
Charging the Care Manager
To charge the Care Manager, plug the AC end of the charge adapter into a working
electrical outlet. Connect the other end to the power jack on the side of the Care
Manager. When the Care Manager is plugged into the charge adapter, the RF
Technologies Code Alert information window is displayed indicating OFF DUTY
and the charge status, CHARGING or FULL CHARGED.
The Care Manager can be manually configured to function “ON DUTY with the
scroll menu and will link up with a Gateway on the same channel.
1. Press MENU then select Configuration
2. Select On/Off.
3. Select ON DUTY and Save.
The Care Manager window goes blank once the Care Manager is disconnected
from the charge adapter. The blank screen indicates that the Care Manager is in
Sleep Mode. When the Care Manager is in Sleep Mode, any activity on the thum b
wheel “wakes up” the Care Manager and activates the Main Menu.
6 Code Alert Care Manager (0510-1067-B) - In-Service Manual
Chapter 2
Integrated Care Management
<
SILENCE
MENU
SILENCE
MENU
Volume
On/O
Melody
Vibrate
>
Carol Johnson
Alarm 2
Help Call
Conguration
>
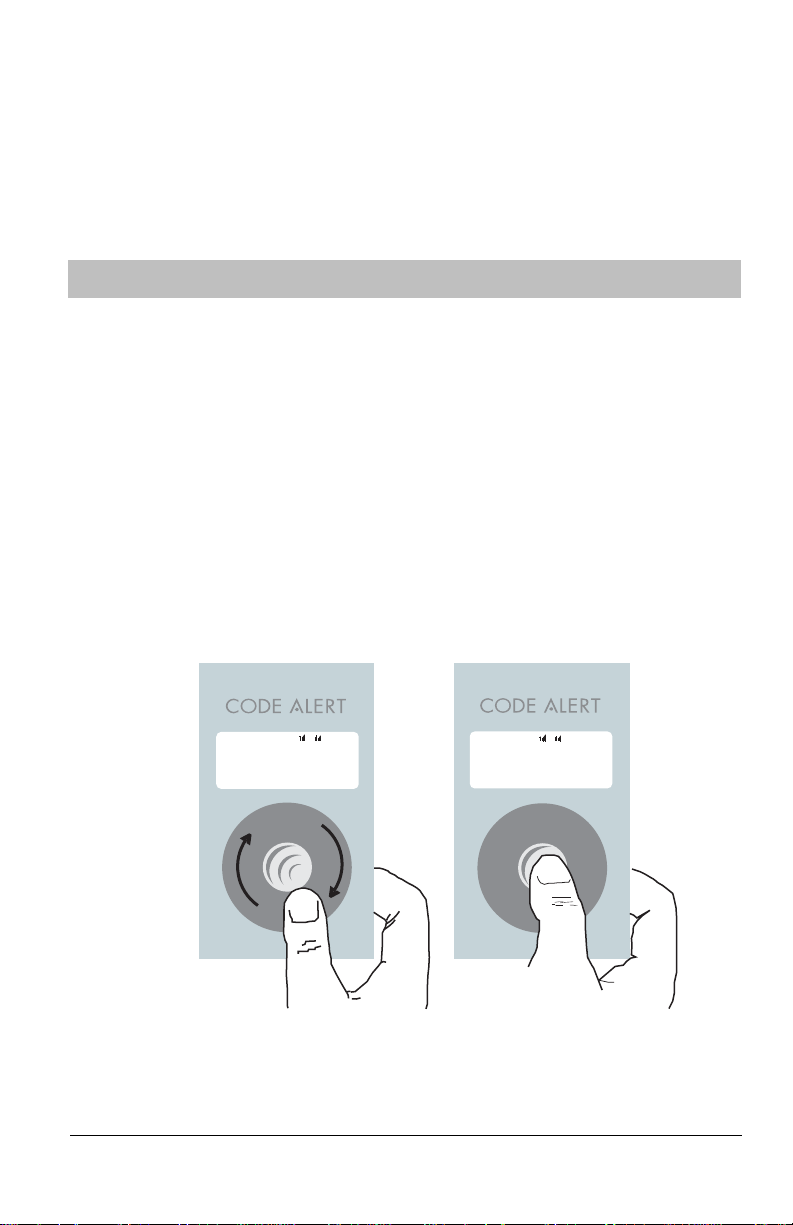
Press select button to access
the Conguration option
Rotate thumb wheel to scroll
to the Conguration option
Î
Integrated Care Management
<
T
T
Configuration
The CONFIGURATION menu allows you to configure the Care Manager and
personalize settings. The options under Configuration are:
• V olume—allows you to adjust the volume on the Care Manager.
• On/Off—manually configure to function On/Off Duty.
• Melody —allows you to select and hear an alarm melody
• Vibrate—turns vibration on or off.
• Contrast—allows you to adjust the contrast of the display.
• Name—Edit or change the name of the person carrying the Care Manager.
• ConfigReg—The Care Manager requests the latest Joint Commission cause and the
cause text.
Code Alert Care Manager (0510-1067-B) - In-Service Manual 7
Chapter 2: Configuration
Integrated Care Management
<
Integrated Care Management
<
SILENCE
MENU
SILENCE
MENU
Vibrate on
Vibrate o
>
Vibrate on
saved
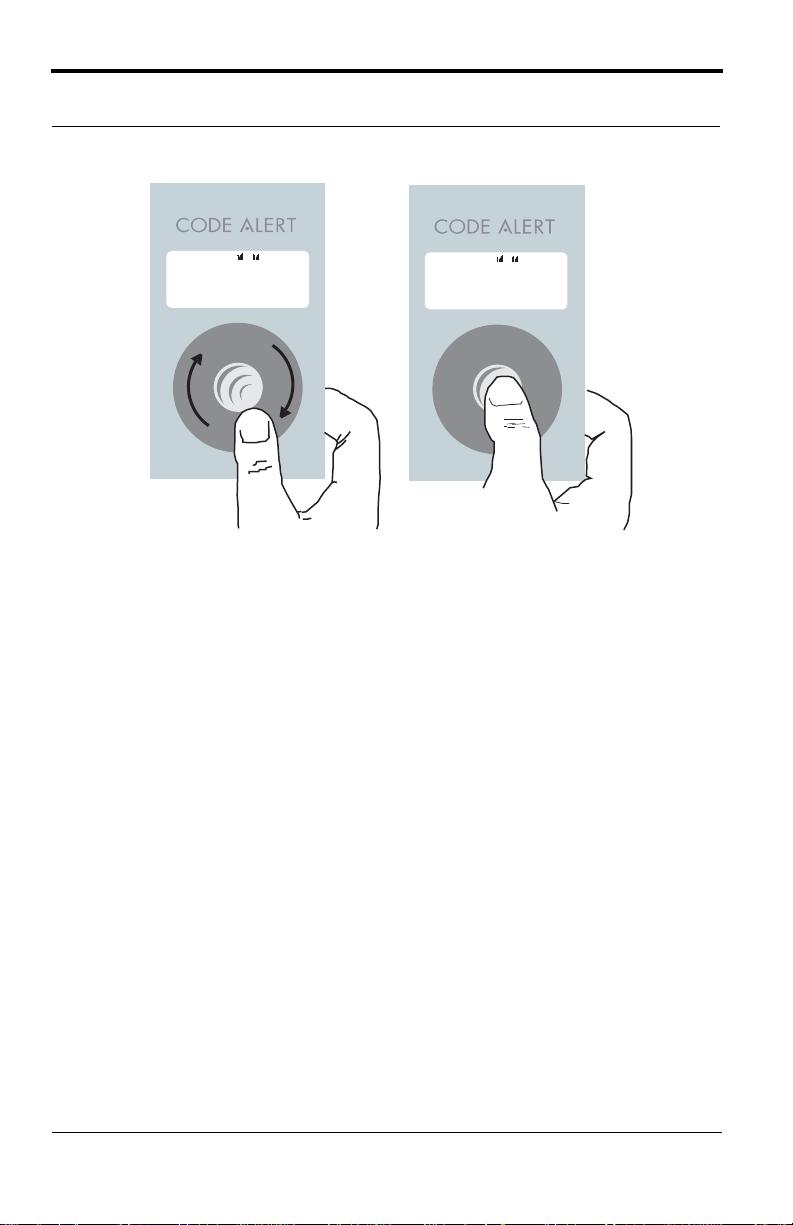
Press select button to save selection
Rotate thumb wheel to scroll
through selections
Î
Î
T
T
8 Code Alert Care Manager (0510-1067-B) - In-Service Manual

Specifications
Specifications
Power 3.7V Lithium Ion batteries
Battery Life 1 Y ear
Frequency 2.4 GHz Direct Sequence Spread Spectrum
Frequency Range 2.405 - 2.475 GHz
Transmit Power 0 dBm
Dimensions 3.88" H x 2.5" W x .69" D
W eight .22 lbs (3.5 oz.)
Color Gray
Certification FCC, Part 15
Industry Canada
Part Number 9600-0500
Chapter 3
Code Alert Care Manager (0510-1067-B) - In-Service Manual 13

Chapter 3: Specifications
This page intentionally left blank.
14 Code Alert Care Manager (0510-1067-B) - In-Service Manual


 Loading...
Loading...