
DreamStation Go
Auto CPAP
CPAP
User manual

Table of contents
1. Safety Information .................................................................................. 1
Intended use ............................................................................................................................. 1
Warnings ....................................................................................................................................1
Cautions .................................................................................................................................... 4
Contraindications ...................................................................................................................6
Safety Symbols Glossary ...................................................................................................... 6
2. System Overview .................................................................................. 10
System Contents .................................................................................................................... 10
Accessories ..............................................................................................................................10
System Diagram ......................................................................................................................11
3. Therapy Device ..................................................................................... 12
Where to Place Your Device ............................................................................................... 12
Supplying AC Power to Your Device ................................................................................. 12
Starting the Device ................................................................................................................ 13
Navigating the Device Screens .......................................................................................... 14
Therapy On Menu Navigation Settings ........................................................................... 15
Ramp Feature ................................................................................................................. 15
Therapy O Menu Navigation Settings ........................................................................... 16
My Info .............................................................................................................................. 16
My Comfort ...................................................................................................................... 17
My Device ........................................................................................................................18
My Support ...................................................................................................................... 19
Check Mask Fit .............................................................................................................. 20
Pairing Therapy Device to Bluetooth®-enabled Mobile Device ...................... 21
Device Pop-Up Messages ...................................................................................................22
4. Tubing ..................................................................................................... 25
Tubing Types ...........................................................................................................................25
Connecting the Tubing to Your Device ............................................................................25
Changing Your Tube Type ....................................................................................................26
Cleaning the Tubing ..............................................................................................................27
Tubing Device Pop-Up Messages .....................................................................................27
5. Filter ........................................................................................................28
Filter Types ..............................................................................................................................28
Installing or Replacing the Filter ...................................................................................... 29
Filter Device Pop-Up Messages ....................................................................................... 29
6. Accessories .......................................................................................... 30
Using the USB Port and the Micro USB Port ................................................................ 30
Using the microSD Card ....................................................................................................... 31

microSD card Device Pop-Up Messages ........................................................................32
7. Battery Pack .......................................................................................... 33
Indicators and Buttons on the Battery Pack .................................................................. 33
Preparing the Battery Pack for First Use and Recharging .........................................35
Attaching the Battery Pack to the Device ......................................................................35
Disconnecting the Battery Pack ........................................................................................37
8. Care and Maintenance ........................................................................38
Caring for the Therapy Device or Battery Pack .............................................................38
Caring for the Reusable Filter ............................................................................................38
9. Troubleshooting .................................................................................. 40
Tips and Tricks ....................................................................................................................... 40
Contacting Customer Service .............................................................................................42
10. Additional Notes.................................................................................43
Traveling with the System ...................................................................................................43
Airline Travel ...........................................................................................................................43
Altitude Compensation ........................................................................................................43
Adding Supplemental Oxygen...........................................................................................43
Service ......................................................................................................................................43
Additional Notices .................................................................................................................44
Specications..........................................................................................................................45
Disposal ................................................................................................................................... 48
EMC Information ................................................................................................................... 48
Limited warranty ......................................................................................... 52
1. Safety Information
Intended use
The Philips Respironics DreamStation Go systems deliver positive airway pressure
therapy for the treatment of Obstructive Sleep Apnea in spontaneously breathing
patients weighing over 30 kg (66 lbs.). It is for use in the home or hospital/institutional
environment.
Important
This device is to be used only on the instruction of a licensed physician. Your supplier
will make the correct pressure settings according to your health care professional’s
prescription.
Several accessories are available to make your Obstructive Sleep Apnea (OSA) treatment
with the DreamStation Go system as convenient and comfortable as possible. To
ensure that you receive the safe, eective therapy prescribed for you, use only Philips
Respironics accessories.
Warnings
A warning indicates the possibility of injury to the user or operator.
Device usage This device is not intended for life support.
Personnel
qualications
Operating
and storage
temperatures
Bacteria lter If the device is used by multiple persons in a hospital environment
This manual serves as a reference. The instructions in this manual are
not intended to supersede your health care professional’s instructions
regarding the use of the device.
The operator should read and understand this entire manual before
using the device.
Do not use this device if the room temperature is warmer than 95˚ F
(35˚ C) because the temperature of the airow may exceed 109˚ F (43˚
C). This could cause thermal irritation or injury to the patient’s airway.
Do not use the device while positioned in a warm place, such as direct
sunlight or near a heating appliance. These conditions can increase
the temperature of the airow and could cause thermal irritation or
injury to the patient’s airway.
(such as rental devices), a low-resistance, main ow bacteria lter
should be installed in-line between the device and the circuit tubing
to prevent contamination.
If the device is used on multiple users, discard and replace the
bacteria lter each time the device is used on a dierent person.
1
1. Safety Information
Patient circuits
and tubing
The device should be used only with masks and connectors
recommended by Philips Respironics or with those recommended
by the health care professional or respiratory therapist. A mask
should not be used unless the device is turned on and operating
properly. The exhalation port(s) associated with the mask should
never be blocked.
Explanation of the Warning: The device is intended to be used
with special masks or connectors that have exhalation ports to
allow continuous ow of air out of the mask. When the device is
turned on and functioning properly, new air from the device ushes
the exhaled air out through the mask exhalation port. However,
when the device is not operating, enough fresh air will not be
provided through the mask, and exhaled air may be rebreathed.
If you are using a full face mask (a mask covering both your
mouth and your nose), the mask must be equipped with a safety
(entrainment) valve.
Do not pull or stretch the tubing. This could result in circuit leaks.
Inspect the tubing for damage or wear. Discard and replace the tubing
as necessary.
Improperly
functioning
device
If you notice any unexplained changes in the performance of the
device, if it is making unusual sounds, if water is spilled into the
enclosure, or if the enclosure is cracked or broken, discontinue use
and contact your supplier.
Power cord Route the power cord to the outlet in a way that will prevent the cord
from being tripped over or interfered with by chairs or other furniture.
This device is activated when the power cord is connected.
Use only power cords supplied by Philips Respironics for this device.
Use of power cords not supplied by Philips Respironics may cause
overheating or damage to the device.
To avoid strangulation hazards, ensure that all cords connected to the
device and battery pack are properly routed.
2

1. Safety Information
Accessories Do not use any accessories, detachable parts, and materials
not recommended by Philips Respironics. Incompatible parts or
accessories can result in degraded performance.
The USB charging port is designed only for use in charging a
mobile device, such as a cell phone. Ensure there are no additional
accessories attached to the mobile device while connected to this
charging port.
Use of accessories, transducers and cables other than those specied
or provided by the manufacturer of this equipment could result in
increased electromagnetic emissions or decreased electromagnetic
immunity of this equipment and result in improper operation.
Oxygen When using oxygen with this system, the oxygen supply must comply
with local regulations for medical oxygen.
Do not connect the device to an unregulated or high pressure oxygen
source.
When using oxygen with this system, a Philips Respironics pressure valve
must be placed in-line with the patient circuit between the device and
the oxygen source. The pressure valve helps to prevent the back ow
of oxygen from the patient circuit into the device when the unit is o.
Failure to use the pressure valve could result in a re hazard.
Oxygen supports combustion. Oxygen should not be used while
smoking or in the presence of an open ame.
Do not use the device in the presence of a ammable anesthetic
mixture in combination with oxygen or air, in the presence of nitrous
oxide, or in an oxygen-enriched environment.
Do not use the device near a source of toxic or harmful vapors.
When using oxygen with this system, turn the device on before
turning on the oxygen. Turn the oxygen o before turning the device
o. This will prevent oxygen accumulation in the device.
Explanation of the Warning: When the device is not in operation
and the oxygen ow is left on, oxygen delivered into the tubing may
accumulate within the device’s enclosure. Oxygen accumulated in the
device enclosure will create a risk of re.
EMC Use of this equipment adjacent to or stacked with other equipment
should be avoided because it could result in improper operation.
The Health Industry Manufacturers Association recommends that
a minimum separation of six inches be maintained between a
wireless phone and a pacemaker to avoid potential interference
with the pacemaker. The DreamStation Go on-board Bluetooth
communication should be considered a wireless phone in this regard.
3

1. Safety Information
Care and
Maintenance
Choking This device contains small parts which could result in a choking
Nebulization Nebulization or humidication can increase the resistance of
General Contact your health care professional if symptoms of sleep apnea
Periodically inspect electrical cords, cables, tubing, and accessories
for damage or signs of wear. Discontinue use and replace if damaged.
Repairs and adjustments must be performed by Philips Respironicsauthorized service personnel only. Unauthorized service could cause
injury, invalidate the warranty, or result in costly device damage.
Contact your supplier for maintenance.
Do not attempt to modify the device or battery pack in any way.
Periodically check battery pack charge status and recharge if
depleted.
To avoid electrical shock, always unplug the power cord from the wall
outlet before caring for the device. DO NOT immerse the device in any
uids.
Do not submerge the battery pack in water or any other liquid.
hazard.
breathing system lters and the operator must monitor the breathing
system lter frequently for increased resistance and blockage to
ensure the delivery of the therapeutic pressure.
recur.
Cautions
A caution indicates the possibility of damage to the device.
US Federal
Caution
EMC Medical electrical equipment needs special precautions regarding
Mobile RF
Communications
Device usage Before operating the device, ensure that both end caps are attached
4
U.S. federal law restricts this device to sale by or on the order of a
physician.
EMC and needs to be installed according to EMC information. Contact
your supplier regarding EMC installation information.
Portable RF communications equipment (including peripherals
such as antenna cables and external antennas) should be used no
closer than 30 cm (12 in.) to any part of the [ME EQUIPMENT or ME
SYSTEM], including cables specied by the manufacturer. Otherwise,
degradation of the performance of this equipment could result.
whenever any of the accessories such as the battery pack is not
installed.
Ensure that the therapy device is properly secured if it is being used
in a portable environment.
1. Safety Information
Electrostatic
Discharge (ESD)
Pins of connectors shall not be touched and connections shall not
be made without special precautions. Precautionary procedures
include methods to prevent build-up of electrostatic charge
(e.g., air conditioning, humidication, conductive oor coverings,
non-synthetic clothing), discharging one’s body to the frame
of the equipment or system or to earth. It is recommended that
all individuals that will handle this device understand these
precautionary procedures at a minimum as part of their training.
Condensation Condensation may damage the device. If the device has been
exposed to either very hot or very cold temperatures, allow it to adjust
to room temperature (operating temperature) before starting therapy.
Do not operate the device outside of the operating temperature range
shown in the Specications section later in this manual.
Filters A properly installed, undamaged Philips Respironics reusable lter or
disposable, ne lter is required for proper operation.
Clogged inlet lters may cause high operating temperatures that
may aect device performance. Regularly examine the inlet lters as
needed for integrity and to check for accumulated debris.
Never install a wet lter into the device. You must ensure sucient
drying time for the rinsed lter.
Make sure the air inlet holes on the side of the device are not blocked
by bedding, curtains, or other items. Air must ow freely around the
device for the system to work properly.
Battery Pack Do not expose the battery pack to extreme temperatures (see the
Specications section for temperature specications). If the battery
pack becomes very hot or very cold, allow it to come to room
temperature before using.
There are no user-serviceable parts inside of the battery pack;
therefore, do not attempt to disassemble or repair it.
Extension cords Do not use extension cords with this device.
Device
placement
Do not place the device in or on any container that can collect or hold
water.
Do not place the device directly onto carpet, fabric, or other
ammable materials.
Tobacco use Tobacco smoke may cause tar build-up within the device, which may
result in the device malfunctioning.
5
1. Safety Information
Contraindications
When assessing the relative risks and benets of using this equipment, the clinician
should understand that this device can deliver pressures up to 20 cm H2O. In the event
of certain fault conditions, a maximum pressure of 40 cm H2O is possible. Studies have
shown that the following pre-existing conditions may contraindicate the use of CPAP
therapy for some patients:
• Bullous Lung Disease
• Pathologically Low Blood Pressure
• Bypassed Upper Airway
• Pneumothorax
• Pneumocephalus has been reported in a patient using nasal Continuous Positive
Airway Pressure. Caution should be used when prescribing CPAP for susceptible
patients such as those with: cerebral spinal uid (CSF) leaks, abnormalities of the
cribriform plate, prior history of head trauma, and/or pneumocephalus. (Chest
1989; 96:1425-1426)
The use of positive airway pressure therapy may be temporarily contraindicated if you
exhibit signs of a sinus or middle ear infection. Not for use with patients whose upper
airways are bypassed. Contact your health care professional if you have any questions
concerning your therapy.
Safety Symbols Glossary
The following symbols may appear on the device and accessories:
Symbol Title and Meaning Reference
Operator’s manual; operating instructions
Consult instructions for use.
Approved for airline use. RTCA/DO-160G section
AC power (Alternating current)
Indicates on the rating plate that the equipment
is suitable for alternating current only; to identify
relevant terminals.
Separate collection for electrical and electronic
equipment.
6
IEC 60878
ISO 7000-1641
Symbol 5.4.3, ISO
15223-1
21, category M
IEC 60417-5032
EC Directive 2012/19/
EU
1. Safety Information
Symbol Title and Meaning Reference
Li Ion Battery -
Bluetooth® symbol
Indicates the device has Bluetooth capabilities.
IP22 Drip proof equipment
Protection against ingress of solid foreign objects ≥
12.5 mm diameter.
Protection against ingress of water with harmful
eects dripping (15° tilted).
Non-ionizing electromagnetic radiation
Indicates that the equipment includes RF
transmitters.
Caution, consult accompanying documents. IEC 60878
Prescription device
Caution: U. S. federal law restricts this device to sale
by or on the order of a physician.
Electrostatic sensitive devices (ESD warning symbol)
Attention – Observe precautions for handling
electrostatic sensitive devices.
Serial connection
Identies a connector for a serial data connection.
Class II equipment (Double Insulated)
To identify equipment meeting the safety
requirements specied for Class II equipment.
Keep away from sunlight
Indicates the medical device needs protection from
light sources.
Type BF applied part
To identify a type BF applied part complying with IEC
60601-1.
-
-
IEC 60878
IEC 60417-5140
Symbol 5.1.2, ISO
15223-1
-
IEC 60878
IEC 60417-5134
IEC 60878
IEC 60417-5850
IEC 60878
IEC 60417-5172
IEC 60878
ISO 7000-0624
Symbol 5.3.2, ISO
15223-1
IEC 60878
IEC 60417-5333
7
1. Safety Information
Symbol Title and Meaning Reference
Do not disassemble. -
For indoor use only
Equipment is designed primarily for indoor use.
Manufacturer
Indicates the medical device manufacturer.
Date of manufacture
Indicates the date when the medical device was
manufactured.
Reorder number
Indicated the manufacturer’s catalogue number so
the medical device can be identied.
Serial number
Identify the manufacturer’s serial number for the
medical device.
Keep dry
Indicates the medical device that needs to be
protected from moisture.
Fragile, handle with care
Indicates the medical device can be broken or
damaged if not handled carefully.
Humidity limitation
Indicates the range of humidity to which the medical
device can be safely exposed.
Temperature limit
Indicates the storage temperature limits to which the
medical device can be safely exposed.
IEC 60878
IEC 60417-5957
IEC 60878
ISO 7000-3082
Symbol 5.1.1, ISO 152231
IEC 60878
ISO 7000-2497
Symbol 5.1.3, ISO
15223-1
ISO 7000-2493
Symbol 5.1.6, ISO
15223-1
IEC 60878
ISO 7000-2498
Symbol 5.1.7, ISO
15223-1
IEC 60878
ISO 7000-0626
Symbol 5.3.4, ISO
15223-1
IEC 60878
ISO 7000-0621
Symbol 5.3.1, ISO
15223-1
IEC 60878
ISO 7000-2620
Symbol 5.3.8, ISO
15223-1
IEC 60878
ISO 7000-0632
Symbol 5.3.7, ISO
15223-1
8

1. Safety Information
ISO 7000:2014, Graphical symbols for use on equipment – Registered symbols
ISO 7010:2011+A1:2012+A2:2012+A3:2012+A4:2013+A5:2014+A6:2014, Graphical symbols -Safety colours and safety signs -- Registered safety signs (not currently FDA recognized)
EN 15986:2011, Symbols for medical devices containing phthalates (not currently FDA
recognized)
ISO 15223-1:2012, Medical devices—Symbols to be used with medical devices labels General requirements
IEC 60417:2002 DB, Graphical symbols for use on equipment
IEC/TR 60878:2015, Graphical symbols for electrical equipment in medical practice
9

2. System Overview
The DreamStation Go CPAP is a Continuous Positive Airway Pressure therapy device
designed for the treatment of Obstructive Sleep Apnea (OSA). Your supplier will choose
the appropriate pressure settings for you.
Several accessories are also available for use with your device. Contact your supplier to
purchase any accessories not included with your system.
System Contents
Your DreamStation Go system may include the following items:
• Device • microSD Card (optional)
• User Manual • Disposable Fine Filter (optional)
• Reusable Filter • Battery Pack (optional)
• 12 mm Micro-exible Tubing
(12 Typ e)
• 6 ft. (1.83 m) Power Cord
Note
If any of these items are missing, contact your supplier.
Accessories
The following accessories are available for your DreamStation Go system:
• 6 ft. (1.83 m) Serial
Communication Cable
(with ferrites)
• Small Travel Kit • Medium Travel Kit
• 15 mm Standard Tubing
(15 Type)
• Bacteria Filter • Pressure Valve (for use with
Note
Your mobile device charging cable should not be longer than 6 ft (1.83 m).
10
• 10 ft. (3.04 m) Power Cord
• 22 mm Performance Tubing (22 Type)
supplemental oxygen)
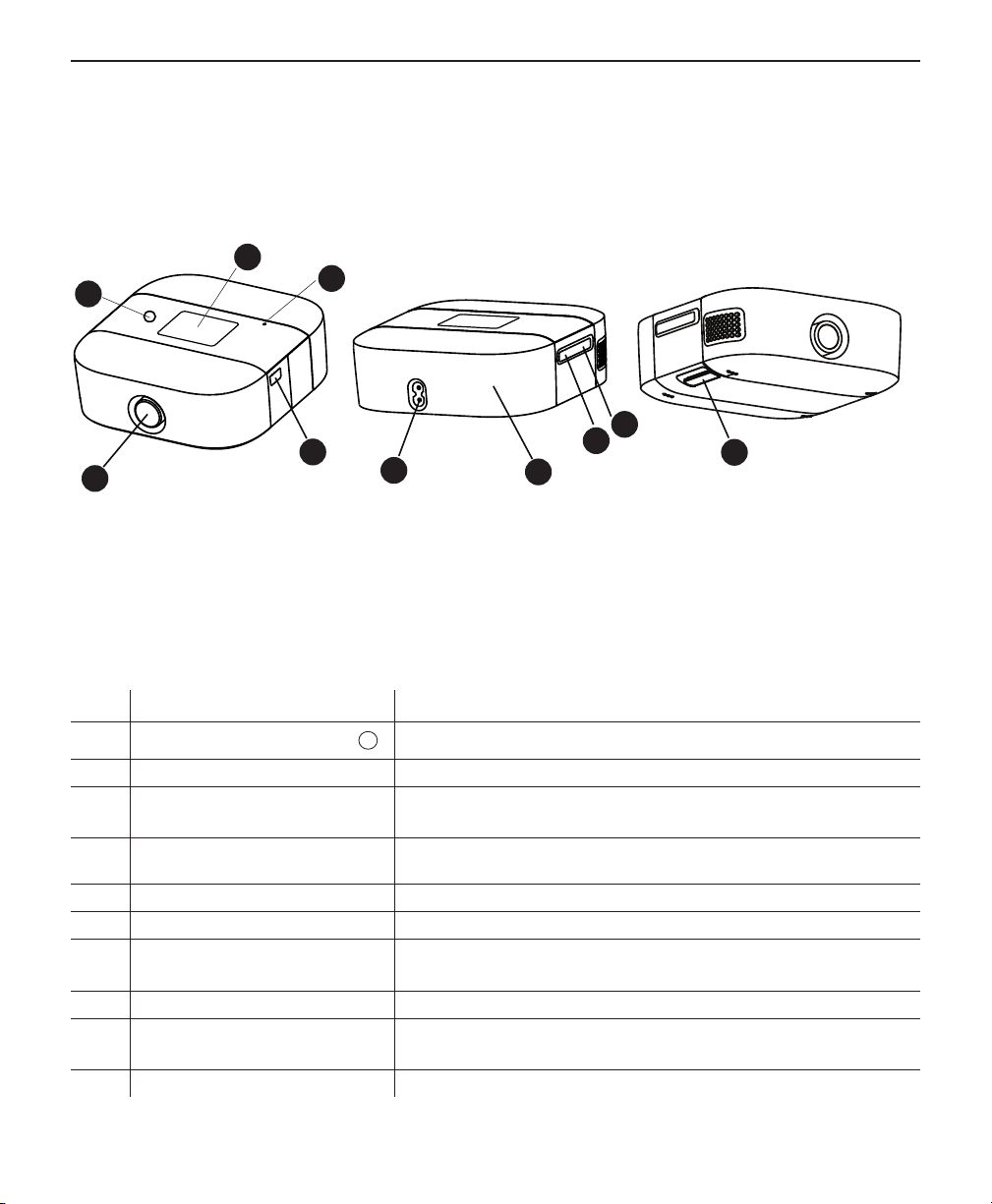
System Diagram
2
1
4
2. System Overview
3
9
5
6
8
10
7
The gure above illustrates some of the device features, described in the following table.
# Feature Description
1
2 Display Touchscreen This is the User Interface for the therapy device.
3 Ambient Light Sensor
4 Air Outlet Port Connect the tubing here.
5 Serial Connector Access the serial connector here.
6 AC Power Inlet Connect the power cord here.
7 Battery Pack Access
8 microSD Card Access the microSD card here.
9 Mobile Charging Port
10 Filter Access Access the lter here.
Therapy on/o button
11
Starts and stops the airow for therapy.
Detects room light levels and adjusts brightness of
the display screen.
This end cap slides o for access to the battery pack
connection.
Access the USB charging port here for mobile device
usage.
3. Therapy Device
Where to Place Your Device
Place the device on a rm, at surface somewhere within easy reach of where you will
use it. The device should sit at a level lower than your sleeping position. Make sure the
device is away from any heating or cooling equipment (e.g., forced air vents, radiators, air
conditioners).
Note
When positioning the device, make sure that the power cable is accessible
because removing power is the only way to turn o the device.
Supplying AC Power to Your Device
Complete the following steps to operate the device by plugging the AC power cord into
an electrical outlet.
1. Plug the power cord connector into the power inlet on the back of the device.
2. Plug the pronged end of the AC power cord into an electrical outlet that is not
controlled by a wall switch.
3. Make sure that all connections are secure.
12
3. Therapy Device
Starting the Device
1. Ensure power is supplied to the device. Tap anywhere on the display screen to
wake up the device.
1
2
# Feature
1 Therapy on/o button
2 Display Touchscreen
2. Put on your mask assembly. Refer to the instructions supplied with the mask.
3. Tap the therapy button on top of the device to turn on airow and begin therapy. The current delivered pressure will display on the screen. The therapy button is
only for therapy.
4. A small amount of mask leak is normal and acceptable. Correct large amount of
mask leaks or eye irritation by adjusting your mask headgear. See the instructions
provided with your mask for more information, or refer to the Check Mask Fit section.
5. Tap the therapy button again to turn o the therapy. To turn o therapy when the
display screen is o, place and hold a nger on the display screen for three seconds. Alternatively, tap anywhere on the display screen to wake up the display and
then tap the therapy button to turn o therapy.
Notes
• If you are using the device in a bed with a headboard, try placing the tubing over
the headboard. This may reduce tension on the mask.
• During therapy, if there is a power loss, the device will return to the home screen
once power is restored. You may resume therapy as needed.
13
3. Therapy Device
Navigating the Device Screens
The User Interface (UI) on this device allows you to adjust the device settings and view
information about your therapy. The UI consists of the display screen and the touch
panel. Swipe left or right on the touch panel to scroll through the menu options on the
display screen.
To adjust a setting:
1. Swipe the touchscreen until you nd your desired menu option.
2. Tap the desired menu option.
3. Swipe the touchscreen until you nd the sub-menu option and tap to select that
setting.
4. Swipe the touchscreen to change the setting.
5. Tap the icon or tap the up arrow in the upper left corner of the display to save
the setting, and return to the previous menu option.
Notes
• The swipe icon on any screen indicates to swipe the display left or right to
perform an action.
• The tap icon on any screen indicates to press the display to perform an action.
• Tapping the down arrow on the display when the down arrow appears on any
screen will take you to a sub-menu with more menu options. Tapping the up arrow
when the up arrow appears on any sub-menu will return you back to the main
menu.
• The screens shown throughout this manual are examples for reference only. Actual
screens may vary based upon device model and supplier settings.
14

3. Therapy Device
Therapy On Menu Navigation Settings
While the device is delivering therapy, you can adjust or view the following settings.
# Feature Description
1 Therapy pressure Displays the current delivered pressure.
2 Ramp Feature The device is equipped with an optional ramp feature that your
supplier can enable or disable.
1
2
Ramp Feature
This feature reduces the air pressure when you are trying to fall asleep and then gradually
increases (ramps) the pressure until your prescription setting is reached, allowing you to fall
asleep more comfortably.
If ramp is enabled on your device, after you turn on the airow, tap the Ramp ( ) icon on the
display. You can use the ramp feature as often as you wish.
When you tap the Ramp icon, the therapy screen will change to reect the ramp in pressure,
and the numbers within the blue circle will reect the gradual increase in pressure.
Your device has two ramp modes. Your supplier will select the one that is most appropriate
for you. The standard ramp mode increases pressure at a steady rate. Alternately, the
SmartRamp mode maintains a constant lower pressure until the device detects that you
require more pressure.
15

3. Therapy Device
Therapy O Menu Navigation Settings
From the Home screen, you can scroll between the following four options:
Battery My Info My Comfort My Device My Support
Battery This menu is visible when the battery pack is connected. See Chapter 7,
Battery Pack for details.
My Info This menu provides summary statistics of your therapy use.
My Comfort This menu contains comfort settings that you can adjust as needed.
My Device This menu contains device settings that you can change.
My Support This menu contains information that your supplier may direct you to read
to them so they can better assist you over the phone.
My Info
When you select My Info, you will be able to view the following screens. You cannot
change settings in the My Info menu. These screens are only for reference. Your supplier
may periodically ask you for this information. If any of the below options are not visible,
your supplier did not enable those options.
Icon Text Description
AHI
16
Therapy
Hours
AHI This screen displays the nightly Apnea/Hypopnea indices (AHI)
This screen displays the amount of time the user is actually
receiving therapy on the device for the most recent 1-day time
frame.
value for the most recent 1 day time frame.

3. Therapy Device
Icon Text Description
Mask Fit Displays the value “100% minus Large Leak”. Large Leak is the
percentage of time that the mask leak was so high that it is no
longer possible for the device to identify respiratory events with
statistical accuracy. Displays the value for the most recent 1 day.
My Comfort
When you select My Comfort, you will be able to view the following screens. You
can change the settings in the setup menu. These screens will only display if they are
available and enabled on your device. If any of the below options are not visible, your
supplier did not enable those options. If a lock icon is displayed on this screen, it
indicates that your supplier has locked this setting and you cannot change it.
Icon Text Description
Ramp This displays the ramp starting pressure. You can increase or
decrease the ramp starting pressure in 0.5 cm H2O increments.
Ramp time When you set the ramp time, the device increases the pressure
from the value set on the ramp screen to the therapy pressure
setting over the length of time specied here.
Flex This allows you to adjust the level of air pressure relief that you
feel when you exhale during therapy. Your supplier can enable
or disable this feature. When your supplier enables Flex, a level
will already be set for you on the device. You can increase or
decrease the setting from 1 to 3. The setting of “1” provides a
small amount of pressure relief, with higher numbers providing
additional relief.
Mask type This setting allows you to adjust the level of air pressure relief
based on the specic Philips Respironics mask. Each Philips
Respironics mask may have a “System One” resistance control
setting. Contact your supplier if you cannot nd this resistance
setting for your mask.
17

3. Therapy Device
Icon Text Description
Tube type This setting allows you to select the correct tubing type that you
are using with the device. You can choose (12) for the Philips
Respironics 12 tubing type, (15) for the Philips Respironics 15
tubing type, or (22) for the Philips Respironics 22 tubing type.
Note: The 12 type and 15 type tubing are identied on the cu
with the tubing identier symbol: “12” or “15”. The 22 tubing
type does not have any identier on the cu.
Check
mask t
This feature allows you to check the t of your mask prior to
starting therapy. This is done by measuring the amount of leak.
My Device
When you select My Device, you will be able to view the following screens. You can
change the settings in the setup menu. These screens will only display if they are
available and enabled on your device. If any of the below options are not visible, your
supplier did not enable those options.
Icon Type Description
Therapy
Ring
Language This feature allows you to choose which language to display on
Bluetooth This feature allows you to turn Bluetooth o and on. Also, it
Time This setting allows you to adjust the time. The default setting
18
This setting controls the therapy button LED light ring during
therapy. The LED light ring will remain on during therapy if you
select Light On. The LED light ring will fade with the display
backlight if you select Light Dims.
the interface.
allows you to clear the pairing with a compatible Bluetooth
device.
is Greenwich Mean Time, but you may adjust the time in 30
minute increments to match your local time zone.
Note: This time setting should not be used as a clock function
on the device. It is to align your therapy data for your
supplier’s data reports.

3. Therapy Device
My Support
When you select My Support, you will be able to view the following screens. You cannot
change settings in the support menu. These screens are only for reference. Your supplier
may periodically ask you for this information. If any of the below options are not visible,
your supplier did not enable those options.
Icon Text Description
Device Info This screen displays your therapy device information: serial
number, model, and software version.
Performance
Check
Phone-In This screen displays the total therapy hours and total blower
Your device is equipped with a self-diagnostic tool called
“Performance Check.” This tool can evaluate your device for
certain errors. It also allows you to share key device settings
with your supplier. Use Performance Check when directed to
by your supplier.
At conclusion of the scan, the screen displays a green
checkmark if no issue is detected. If device displays a red “X,”
please contact your supplier for assistance.
hours for the device, and a compliance check number used by
your supplier to validate that data provided by you is the data
taken from this screen.
19

3. Therapy Device
Check Mask Fit
The optional Check Mask Fit feature can be enabled or disabled by your supplier. This feature
allows you to check the t of your mask prior to starting therapy.
1. Put on your mask assembly. Refer to your mask instructions if needed.
2. Navigate to the Check Mask Fit screen under My Comfort and tap the display to initiate the check.
3. The device will deliver a test pressure while the screen counts down 40 seconds.
4. After the test is complete, the screen will either display a green checkmark or a red
“X”. The green checkmark indicates that there is an appropriate amount of leak.
The red “X” indicates that the leak may aect device performance, however, the
device will remain functional and deliver therapy.
Note
If you choose to try to improve your mask fit, you can stop therapy, adjust the fit
of your mask, and rerun the Check Mask Fit feature. Refer to the instructions that
came with your mask and headgear for the proper fitting procedure.
20

3. Therapy Device
Pairing Therapy Device to Bluetooth®-enabled Mobile Device
Your device may have Bluetooth wireless technology, which is one method by which you
can transfer your therapy device’s data to DreamMapper. DreamMapper is a mobile and
web-based system designed to help you enhance your sleep therapy experience.
Notes
• You can only pair your therapy device to one mobile device at any given time.
• Pairing works best when your therapy device and mobile device are in the same
room.
• The current version of DreamMapper will guide you through these instructions.
• After initiating pairing, you will have 30 seconds to complete the setup. After this
time, it will be cancelled automatically.
Follow the steps below to manually pair to your mobile phone or tablet.
1. Install DreamMapper on your mobile device.
2. With your therapy device powered up and the blower o, initiate Bluetooth Setup
from the DreamMapper mobile app.
3. The therapy device will appear as PR BT XXXX (XXXX will be the last four digits of
the serial number listed on the bottom of your therapy device or in My Support
settings).
4. Your mobile device will require you to conrm pairing via one of these two methods:
— Enter a PIN code
The following icon will appear on your therapy device screen with Pair?:
Swipe left or right to select “yes,” and tap the display to conrm your setting. Your
therapy device will display a 6 digit PIN. Enter this PIN on your mobile device to
complete pairing.
— Confirm a PIN code
The following icon will appear on your therapy device screen with a 6-digit PIN
and Pair?:
Verify that the PIN is the same on both the therapy device and the mobile device.
If so, swipe the therapy device’s display to select “yes” and tap the display to
select. Then, accept on the mobile device to complete pairing.
21

3. Therapy Device
Device Pop-Up Messages
Device pop-ups are messages that show up on the user interface screen. Additional popup messages are contained in each chapter.
The following summary table summarizes the messages:
Condition Icon Description Possible
Cause
Time Prompts to set the
time.
Sleep
Progress
Change
Accepted
Pair?: 123456
Yes/No
n/a Shows a three night
summary of therapy.
Conrms acceptance of
prescription change or
device upgrade.
Prompts to accept or
decline pairing to a
Bluetooth compatible
device. This device can
be identied by the
digits displayed.
n/a Set the time on the
n/a Tap the display to
n/a Tap the display to
n/a Swipe the display to
Action
device.
acknowledge and clear
the message.
acknowledge and clear
the message.
accept pairing (Yes),
or decline (No), then
tap the display to
conrm selection. The
pop-up will timeout
after 30 seconds and
the pairing will be
cancelled if you do not
select Yes.
22

3. Therapy Device
Condition Icon Description Possible
Cause
Bluetooth LE
Passkey
Prompts to accept or
decline pairing to a
n/a If you selected Yes
Bluetooth compatible
device before
displaying the pairing
passkey.
Patient
Message
Change
Rejected
Message from your
supplier.
A prescription or
settings change was
rejected.
n/a Tap the display to
Change
missing or
incorrect.
Action
to accept pairing,
the Bluetooth LE
Passkey will display a
passkey on the screen.
Enter the passkey on
your mobile device
to pair. The pop-up
will timeout after
30 seconds and
the pairing will be
cancelled if you do not
use the passkey.
acknowledge and clear
the message.
Contact your supplier.
Service
Required
Indicates an error
which enters device
into “Safe State.”
This allows power to
remain on but airow is
disabled.
Automatic O Displayed when
therapy ends due to
automatic o function.
Loading
Language
and
Displayed when a new
language is selected
from the menu.
Rebooting
Device error Disconnect device
from power. Reattach
power cord to restore
power. If the alert
continues to occur,
contact your supplier.
The mask
has been
removed.
Put your mask back
on, conrm good t,
and turn airow on to
resume therapy.
n/a No action needed.
Times out when
complete.
23

3. Therapy Device
Condition Icon Description Possible
Cause
Busy
Displayed when the
n/a No action needed.
device is temporarily
inaccessible due to
data communication.
Software
Upgrade
Prompts to update the
device for software
n/a Choose between
changes.
Action
“Yes”/”No” when
asked to upgrade the
software. If “yes” is
selected, the upgrade
will be made. Do not
remove from power.
If you select “no”,
the message will be
cleared.
24

4. Tubing
Tubing Types
There are three dierent types of tubing that you may use with your DreamStation Go
therapy device. You must select the tube type on your device.
15
12
12 tubing type 15 tubing type 22 tubing type
The 12 tubing type will have a “12” identied on the tubing cu (as shown in the image
above). The 15 tubing type will have a “15” identied on the tubing cu (as shown in the
image above). The 22 tubing type does not have any number or symbol on the tubing cu
(as shown in the image above).
Connecting the Tubing to Your Device
To connect the tubing to your device, you will need the following Philips Respironics
accessories:
• Nasal or full face mask (interface) with built-in exhalation, or a nasal or full face
mask (interface) with a separate exhalation device attached (such as the Whisper
Swivel II)
• Flexible tubing, 6 ft. (1.83 m)
• Mask headgear
25

4. Tubing
Follow these steps to connect tubing to your device:
1. Insert the 12, 15 or 22 tubing type cu into the air outlet port on your therapy
device.
2. Connect the tubing to your mask. For proper placement and positioning, refer to
the instructions that came with your mask.
Note
You may use a standard tube with a bacteria lter. If required, connect a bacteria
lter to the device air outlet, and then connect the exible tubing to the outlet of
the bacteria lter. When using the bacteria lter, the device performance may be
aected. However, the device will remain functional and deliver therapy.
Changing Your Tube Type
Change your tube type by navigating to My Comfort -> Tube Type. Swipe left or right to
switch between tube types.
For more information on navigation or selection, please refer to Chapter 3, Navigating
the Device Screens or My Comfort.
26

Cleaning the Tubing
Clean the tubing before rst use and daily.
1. Disconnect the exible tubing from the device.
2. Gently wash the tubing in a solution of warm water and a mild detergent.
3. Rinse thoroughly.
4. Air dry. Inspect the tubing for damage or wear. Discard and replace as necessary.
Tubing Device Pop-Up Messages
Device pop-ups are messages that show up on the user interface screen.
4. Tubing
Condition Icon Description Possible
Cause
Low leak:
Check mask
and tube
Blocked
airway
Blockage at
tube or mask
Action
Check tube is not crushed or folded,
such that airow is restricted. Check
mask is attached properly and
without any obstruction.
27

5. Filter
Filter Types
You may use either a reusable lter that is washable, or a disposable, ne lter.
Reusable Filter Disposable Fine Filter
The reusable lter screens out normal household dust and pollen. The reusable lter is
supplied with your device.
The disposable, ne lter provides more complete ltration of ne particles. The
disposable, ne lter is recommended for people who are sensitive to tobacco smoke or
other small particles. The disposable, ne lter is sold separately. The disposable, ne
lter contains Philips Respironics branding in the media (shown in the image above).
DO NOT rinse the disposable, ne lter.
When using the disposable, ne lter, the device performance may be aected. However,
the device will remain functional and deliver therapy.
28

5. Filter
Installing or Replacing the Filter
One of the lters must be in place at all times to operate the device. If a lter is not
already installed in the device, you must at least install the reusable lter before using
your device.
The device has an automatic air lter reminder. Every 30 days, your device will display a
message reminding you to check your lters and replace them as needed.
Note
The lter reminder is a message only. The device does not detect the performance
of the lters, nor does it recognize when a lter has been replaced.
Follow these steps to install/replace a lter into your device:
1. If replacing an existing lter, pull out the old lter assembly.
2. Insert a dry, reusable lter or a new, disposable, ne lter into the lter access on
the device.
Filter Device Pop-Up Messages
Device pop-ups are messages that show up on the user interface screen.
Condition Icon Description Possible
Cause
Inlet blocked.
Check lter.
29
Blocked
airway
Blockage at
device inlet.
Check device air inlet is not
obstructed. Check air lter(s) are
installed properly; replace if needed.
Action

6. Accessories
There are several accessories available for your DreamStation Go system, such as a
microSD card, a travel kit or a battery pack. The device also comes with a USB port and a
micro USB port. The travel kit is available for convenient portability while traveling with
your device. Contact your supplier for additional information on the available accessories.
When using optional accessories, always follow any instructions enclosed with the
accessories.
Using the USB Port and the Micro USB Port
The DreamStation Go device comes with a USB port and a micro USB port. The USB port
may be used to charge your mobile devices. The micro USB port may be used by your
supplier to extract therapy data. Remove the cover over each port to access.
30
USB Port Micro USB Port

6. Accessories
Using the microSD Card
The DreamStation Go system may come with a microSD card inserted in the microSD
card slot on the side of the device to record information for your supplier. Your supplier
may ask you to periodically remove the microSD card and send it to them for evaluation.
You will use the lter to remove the microSD card.
Turn o therapy and follow these steps to remove the microSD card:
1. Remove the lter from the device. Refer to the Installing or Replacing the Filter
section in Chapter 5 of this manual.
2. Use the end of the lter to push in on the microSD card. This will push the microSD
card out of the device.
31

6. Accessories
microSD Card Device Pop-Up Messages
Device pop-ups are messages that show up on the user interface screen.
Condition Icon Description Possible Cause Action
Data Activity:
Do not
remove
microSD card
microSD card
removed
microSD card
error: Remove
and reinsert
microSD card
full
microSD card read/
write underway.
Indicates microSD card
has been removed from
therapy device and
not reinserted before
the start of the current
therapy session.
microSD card error
detected.
microSD card is full. microSD card is
n/a No action needed.
microSD card was
not reinserted
into device.
Device cannot
read the microSD
card. A problem
may exist with
the microSD
card or it was
ejected during a
writing activity,
or it was inserted
incorrectly.
full.
Message will clear
when the microSD
card activity is
nished.
Reinsert microSD
card, or click to
clear alert.
Remove microSD
card and reinsert.
If message
reappears,
contact your
supplier for a
replacement card.
Remove microSD
card and replace
with a new
card from your
supplier.
32

7. Battery Pack
Indicators and Buttons on the Battery Pack
1
2
3
# Feature
1 Push Button
2 LED Display
3 AC Power Inlet
• Push Button - The push button is located on the LED display of the battery pack.
• LED Display - The battery pack uses one green LED light to indicate the battery
pack charge status when the battery pack is charging while not connected to the
therapy device (standalone charging). The LED will be in one of the three modes:
* Steady when the battery pack is fully charged
* Blinking when charging
* O when connected to the therapy device
33

7. Battery Pack
• Battery Pack Charge Indicator During Therapy - The display touchscreen shows
the current battery pack charge status in the lower right hand corner when the
pack is connected and therapy is active (shown below). A fully charged battery
pack is indicated by four charge bars. The white charge bars disappear as the
battery pack charge decreases. Depending on your settings, mask leak and
environmental conditions, a fully charged battery pack typically lasts 8 hours. For
further information, speak with your supplier.
• Battery Pack Charge Indicator When Therapy Is Not Active - The display
touchscreen will show the battery charge percentage when the battery is
connected to the therapy device but not in use (shown below). This screen will
appear in your main menu selections.
• Battery Pack State of Charge Alert - The display touchscreen will display a battery
with a question mark in the center (shown below) when the charge level cannot be
determined.
34

7. Battery Pack
• Battery Pack Fault Alert - The display touchscreen will show a battery with an X
inside (shown below) when a battery fault is detected.
Preparing the Battery Pack for First Use and Recharging
1. Remove the battery pack from the packaging.
2. Plug the end of the AC power cord into the battery pack.
3. Plug the AC power cord into an AC outlet. The battery pack will begin to charge automatically.
4. Once the battery pack is fully charged, it is ready for use with the DreamStation Go
therapy device.
Notes
• Periodically charge the battery pack if not used regularly.
• Retain your packaging in case you ever need to return your battery pack to Philips
Respironics.
• Before using the battery pack for the rst time, you must plug it in until it is fully
charged. This may take up to 5 hours.
Attaching the Battery Pack to the Device
After charging, your battery pack is ready to use. It can either be disconnected from the
AC outlet and used as an external battery pack (standalone mode), or remain plugged
into the AC outlet for a continuous, fully charged battery pack (uninterruptible power
supply (UPS) mode).
To use the battery pack in UPS mode, follow the below steps:
1. Keep the battery pack plugged into the power source and connected to an AC
outlet. This will allow you to use the battery pack continuously without losing any
charge.
35

7. Battery Pack
2. Remove the battery pack end cap on the therapy device.
3. Slide the battery pack onto the device where the end cap was. Make sure the battery pack latches onto the therapy device.
4. Attach the AC power cord to the battery pack and then to the AC outlet.
To use the battery pack in standalone mode, follow these steps:
1. Make sure the battery pack is fully charged. Disconnect the power cord from the
AC outlet and disconnect the power cord from the therapy device. It can now be
used with your therapy device as an external battery pack.
2. Remove the battery pack end cap on the therapy device.
3. Slide the battery pack onto the device where the end cap was. Make sure the battery pack latches onto the therapy device.
4. Momentarily push the battery pack push button to wake up the battery pack.
36

7. Battery Pack
Notes
• The rst time you charge your battery pack, it must be fully charged in accordance
with the Preparing the Battery Pack for First Use and Recharging section. After
the rst charge is complete, the battery pack will charge while connected in UPS
mode.
• To preserve battery life in UPS mode, the battery pack stops charging when it
reaches full charge. The battery will start charging again when it depletes to 90%
charge status.
• When the battery pack is used in standalone mode and the therapy device enters
standby mode, the therapy device automatically shuts down the battery pack to
preserve battery charge.
Disconnecting the Battery Pack
1. Disconnect the power cord.
2. Hold the battery pack push button down for 5 seconds, or the battery pack will
shut o within 30 minutes when not in use.
3. The power down pop-up message will appear and the therapy device will power
down and go dark.
4. You can now disconnect the battery pack. Disengage the battery pack by sliding
the latch on the back of the pack, and pulling the battery pack away from the
therapy device.
5. Replace the battery pack end cap on the therapy device.
37

8. Care and Maintenance
Caring for the Therapy Device or Battery Pack
Every two weeks of use, inspect your device or battery pack to see if it needs care.
1. To avoid electrical shock, make sure that the device and battery pack are disconnected from all outlets and power sources. Remove any cables attached to the
device or battery pack.
2. Wipe the outside of the device or battery pack with a cloth slightly dampened with
water.
3. Allow the device or battery pack to dry completely before reconnecting it to a
power source, battery pack, device or cable.
Note
Inspect the device, battery pack, and all circuit parts (lter, tube and mask) for
damage, such as cracks, tears or broken pieces. Replace any damaged parts.
Caring for the Reusable Filter
Under normal usage, you should rinse the reusable lter at least once every two weeks
and replace it with a new one every six months.
The disposable, ne lter should be replaced after 30 nights of use, or sooner if it appears
clogged. DO NOT rinse the ne lter.
Follow these steps to rinse the reusable lter:
1. If the device is operating, stop the airow. Disconnect the device from the power
source.
2. Remove the lter from the device. Refer to the Installing or Replacing the Filter
section in Chapter 5.
3. Take the reusable lter to a sink, turn it upside down (tabs down), and run warm tap
water through the white middle portion of the lter to rinse away any debris.
4. Shake the lter to remove as much water as possible.
5. Allow the lter to air dry completely before reinstalling it. If the lter is damaged,
replace it.
6. Reinstall the lter into the lter access area on the device. Refer to the Installing or
Replacing the Filter section in Chapter 5.
38

8. Care and Maintenance
Notes
• Only Philips Respironics supplied lters should be used as replacement lters.
• Replace the disposable, ne lter if it is damaged or has accumulated debris.
39

9. Troubleshooting
Tips and Tricks
Your device is equipped with a self-diagnostic tool called Performance Check. This
tool can evaluate your device for certain errors. It also allows you to share diagnostic
information with your supplier. Use Performance Check when directed by your supplier.
The table below lists some of the problems you may experience with your device and
possible solutions to those problems.
Contact customer service for assistance if none of the below troubleshooting tips work
for you.
Problem Why it happened What to do
Nothing happens
when you apply
power to the
device. The
backlights on the
buttons do not
light.
The airow does
not turn on.
There’s no power
at the outlet or the
device is unplugged.
There may be a
problem with the
blower.
• If you are using AC power: Check
the outlet and verify that the
device is properly plugged in, and
that there is power available at the
outlet. Make sure the AC power
cord is connected correctly to the
device’s power inlet.
• If you are using the battery pack:
Make sure your battery pack is
securely connected to your device.
If the battery pack has been
exposed to extreme temperatures,
allow the battery back to cool or
warm to room temperature. Check
to see if your battery pack needs
charged or replaced.
• Make sure the device is powered
correctly.
• Make sure the home screen
appears on the user interface.
• Press the therapy button on top
of the device to start airow. If the
airow does not turn on, there may
be a problem with your device.
40

Problem Why it happened What to do
The device’s
display is erratic.
The Ramp feature
does not work
when you press the
Ramp button.
The device has
been dropped or
mishandled, or the
device is in an area
with several electronic
devices.
Your supplier did
not enable Ramp for
you, or your therapy
pressure is already
set to the minimum
setting.
Unplug the device. Reapply power to
the device. If the problem continues,
relocate the device to an area away from
electronic equipment (such as cellular
phones, cordless phones, computers, TVs,
electronic games, hair dryers, etc.).
• If ramp has not been enabled for
you, discuss this feature with your
supplier.
• If your supplier has enabled ramp,
but the feature still does not
work, check the current pressure
setting on the therapy screen. If
the therapy pressure is set to the
minimum setting (4.0 cm H2O), or
the ramp starting pressure is the
same as the therapy pressure, the
ramp feature will not work. Make
sure that the ramp time setting is
>0.
The airow is much
warmer than usual.
The air lters may be
dirty. The device may
be operating in direct
sunlight or near a
heater.
• Rinse or replace the air lter.
• The temperature of the air may
vary somewhat based on your
room temperature.
• Make sure that the device is
properly ventilated. Keep the
device away from bedding or
curtains that could block the ow
of air around the device.
• Make sure the device is away
from direct sunlight and heating
equipment.
The airow
pressure feels too
high or too low.
The tubing type
setting may be
incorrect.
Make sure the tubing type setting (12, 15
or 22) matches the tubing that you are
using (Philips Respironics 12, 15 or 22
tubing type).
9. Troubleshooting
41

9. Troubleshooting
Problem Why it happened What to do
I hear a leak or
whistling sound
coming from my
therapy device (not
related to mask
leak).
The battery pack
LEDs will not light
up while charging.
The battery pack
LED is rapidly
ashing.
“Service Required”
shown on display.
The therapy device
air inlet may be
obstructed.
Your battery pack may
have been damaged.
Your battery pack may
have been damaged.
A device error has
occurred and placed
the device into safe
state.
• Check therapy device air inlet is
not obstructed, and lter has not
accumulated excessive debris and
is properly inserted.
• Conrm that the device and tube
are connected properly and not
leaking.
If the battery pack is completely depleted
of charge, wait a few minutes for the LEDs
to light up. If the LEDs still do not light up,
replace your battery pack. If the battery
pack has been exposed to extreme
temperatures, allow the battery pack to
cool or warm to room temperature.
If the battery pack has been exposed
to extreme temperatures, allow the
battery pack to cool or warm to room
temperature. Unplug the battery pack
from the power cord, then plug the power
cord back into the battery pack. If the LED
continues to rapidly ash, replace your
battery pack.
Disconnect power cord. Reattach the
power cord to restore power. If the alert
continues, contact your supplier.
Contacting Customer Service
Should you experience trouble with this equipment or require assistance setting up,
using, or maintaining the device or accessories, please contact your supplier. If you need
to contact Philips Respironics directly, call the Philips Respironics Customer Service
department at 1-724-387-4000 or 1-800-345-6443. You can also use the following
address:
Respironics, Inc.
1001 Murry Ridge Lane
Murrysville, PA 15668
42

10. Additional Notes
Traveling with the System
When traveling, the optional case is for carry-on luggage only. The optional case will not
protect the system if it is put through checked baggage.
For your convenience at security stations, there is a note on the bottom of the device
stating that it is medical equipment and is suitable for airline use. It may be helpful to
bring this manual along with you to help security personnel understand the DreamStation
Go device.
If you are traveling to a country with a line voltage dierent than the one you are
currently using, a dierent power cord or an international plug adaptor may be required
to make your power cord compatible with the power outlets of the country to which you
are traveling. Contact your supplier for additional information.
Airline Travel
The device is suitable for use on airlines when the device is operating from an AC power
source or battery pack.
Altitude Compensation
This device automatically compensates for altitude up to 7,500 feet. No manual
adjustment is necessary.
Adding Supplemental Oxygen
Oxygen can be added to the patient circuit.
Notes
• Refer to the pressure valve’s instructions for complete setup information.
• When using oxygen with this system, turn the device on before turning on the
oxygen. Turn the oxygen o before turning the device o. This will prevent oxygen
accumulation in the device.
• Do not connect the device to an unregulated or high pressure oxygen source.
Service
The device does not require routine servicing.
43

10. Additional Notes
Additional Notices
Notices:
• The Bluetooth® word mark and logos are registered trademarks owned by
Bluetooth SIG, Inc. and any use of such marks by Philips Respironics is under
license. Other trademarks and trade names are those of their respective owners.
• The DreamStation Go Therapy Device transmits data between the therapy
device and a mobile device, but it does not store any of your personal data. This
connection between the therapy device and a mobile device is encrypted.
• This device contains a FCC certied Bluetooth radio module (located on the main
board). FCC ID: THO1116426
• Use of non-original manufacturer-approved accessories may violate your local RF
exposure guidelines and should be avoided.
• This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that
may cause undesired operation. This equipment has been tested and found to
comply with the limits for a Class B digital device, pursuant to Part 15 of the FCC
Rules. These limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates, uses, and
can radiate radio frequency energy and, if not installed and used in accordance
with the instructions, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference to radio, TV
reception, or other devices which can be determined by turning the equipment on
and o, the user is encouraged to try to correct the interference by one or more of
the following measures:
Reorient or relocate the receiving antenna (on the radio, TV, or other device).
Increase the separation between the equipment and receiver.
Connect the equipment into an outlet on a circuit dierent from that to which the
receiver is connected.
Consult the dealer of the device for help.
• Any changes or modications made to the device that are not expressly approved
by Respironics may void the user’s authority to operate the equipment.
44

Specications
Environmental
10. Additional Notes
Operating
Temperature
Storage
Temperature
Relative Humidity
(operating &
storage)
Atmospheric
Pressure:
Device: 5° to 35° C (41° to 95° F)
Battery Pack: 5° to 35° C (41° to 104° F)
-20° to 60° C (-4° to 140° F)
15 to 95% (non-condensing)
Device: 101 to 77 kPa (0 - 2286 m / 0 - 7500 ft)
Battery Pack: 101 to 77 kPa (0 - 2286 m / 0 - 7500 ft)
Physical
Dimensions Device: 150.8 x 150.8 x 58.8 mm (5.937” L x 5.937” W x 2.315” H)
Battery Pack: 122 x 150.8 x 58.8 mm (4.803” L x 5.937” W x 2.315” H)
Weight Device: Approximately 854 g (1.88 lb.)
Battery Pack: Approximately 696 g (1.53 lb.)
Service life
The expected service life of the DreamStation Go therapy device is 5 years.
The expected service life of the battery pack is 3 years.
Standards compliance
This device is designed to conform to the following standards:
• IEC 60601-1 General Requirements for Basic Safety and Essential Performance of
Medical Electrical Equipment
• ISO 80601-2-70 Sleep Apnea Breathing Therapy Equipment
• EN 60601-1-2 Electromagnetic Compatibility
• RTCA/DO-160G section 21, category M; Emission of Radio Frequency Energy
45

10. Additional Notes
IEC 60601-1 classication
Type of Protection Against Electric
Shock
Degree of Protection Against
Electric Shock
Degree of Protection against
Ingress of Water
Mode of Operation Continuous
Class II Equipment/Internally Powered
Type BF Applied Part
Device: Drip Proof, IP22
First characteristic numeral - 2 - Protection against
ingress of solid foreign objects ≥ 12.5 mm diameter.
Explanation: Protected against access to hazardous
parts with a nger and protected against solid
foreign objects of 12.5 mm diameter and greater.
Second characteristic numeral - 2- Protection
against ingress of water with harmful eects
dripping (15° tilted).
Explanation: Protected against vertically falling
water drops when enclosure tilted up to 15°.
Electrical
AC Power Consumption 100–240 VAC, 50/60 Hz, 2.0-1.0 A
Fuses There are no user-replaceable fuses.
USB Charging Port Output 5 VDC, 7.5 W (1.5 A)
Electrical for the Battery Pack
Run Time > 8 hours
Output voltage range 18-24.6 VDC
Battery technology Lithium Ion
Capacity 62 Wh
Input voltage range 100-240 VAC, 50/60 Hz, 2.0-1.0 A
Output power (max continuous) 50W
Minimum life cycle ≥ 70% of rated capacity after 500 cycles
Recharge time < 5 hours
46

10. Additional Notes
Radio Specications
Operating Frequency Range 2402 - 2480 MHz
Maximum Output Power 4.0 dBm
Modulation GFSK, P/4 DQPSK, 8DQPSK
Intake port lters
Reusable Filter 100% Polyester
88% Ecient @ 7-10 micron size
Fine Filter Blended Synthetic Fiber
95% Ecient @ 0.5-0.7 micron size
Declared dual-number noise emissions values (in accordance with ISO
4871)
Tube Size Sound Pressure
Level (L)
12 (mm) tubing type 30.4 dB(A) 2 dB(A) 38.4 dB(A) 2 dB(A)
15 (mm) tubing type 29.9 dB(A) 2 dB(A) 37.9 dB(A) 2 dB(A)
22 (mm) tubing type 29.8 dB(A) 2 dB(A) 37.8 dB(A) 2 dB(A)
Note
Values determined according to noise test code given in ISO 80601-2-70:2015,
using the basic standards ISO 3744 and ISO 4871.
Uncertainty
(K)
Sound Power
Level
Uncertainty
(K)
Pressure Accuracy
Pressure Increments: 4.0-20.0 cmH2O (in 0.5 cmH2O increments)
Maximum static pressure accuracy, according to ISO 80601-2-70:2015:
Tube Type Pressure Accuracy
12 (mm) tubing type 10 cmH2O ± 1.0 cmH2O
15 (mm) tubing type and
22 (mm) tubing type
Static pressure accuracy has a measurement uncertainty of 3.8%
47
10 cmH2O ± 0.5 cmH2O

10. Additional Notes
Maximum dynamic pressure variation, according to ISO 80601-2-70:2015:
Tube Type 10 BPM 15 BPM 20 BPM
12 (mm) tubing type ± 0.6 cmH2O ± 0.8 cmH2O ± 1.6 cmH2O
15 (mm) tubing type and 22 (mm) tubing type ± 0.7 cmH2O ± 0.7 cmH2O ± 1.0 cmH2O
Dynamic pressure accuracy has a measurement uncertainty of 3.6%.
Maximum Flow Rate (typical)
Tube Type Flow
12 (mm) tubing type Average ow at the patient
connection port (l/min)
15 (mm) tubing type Average ow at the patient
connection port (l/min)
22 (mm) tubing type Average ow at the patient
connection port (l/min)
Test pressures (cmH2O)
4.0 8.0 12.0 16.0 20.0
90 119 112 106 99
77 115 112 105 106
80 121 127 121 109
Disposal
Separate collection for electrical and electronic equipment per EC Directive 2012/19/EU.
Dispose of this device in accordance with local regulations.
EMC information
Your unit has been designed to meet EMC standards throughout its service life without
additional maintenance. There is always an opportunity to relocate your DreamStation
Go Therapy Device within an environment that contains other devices with their own
unknown EMC behavior. If you believe your unit is aected by locating it closer to another
device, simply separate the devices to remove the condition.
Pressure and Flow Accuracy
The DreamStation Go Therapy Device is designed to perform within the pressure and
owrate accuracies specied in the user manual. If you suspect that the pressure and/or
ow rate accuracy is aected by EMC interference remove power and relocate the device
to another area. If performance continues to be aected discontinue use and contact
your supplier.
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions – This device is
intended for use in the electromagnetic environment specied below. The user of this
device should make sure it is used in such an environment.
48

10. Additional Notes
Emissions TEsT ComplianCE ElECTromagnETiC EnvironmEnT - guidanCE
The device uses RF energy only for its internal
RF emissions
CISPR 11
Group 1
function. Therefore, its RF emissions are very
low and are not likely to cause any interference
in nearby electronic equipment.
RF emissions
Class B
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage uctuations/
Flicker emissions
Class A
Complies
The device is suitable for use in all
establishments, including domestic
establishments and those directly connected to
the public low-voltage power supply network.
IEC 61000-3-3
Emission of Radio
Frequency Energy
RTCA/DO-160G Section
Category M
This device is suitable for use onboard
commercial airplanes inside passenger cabin.
21
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity – This device is
intended for use in the electromagnetic environment specied below. The user of this
device should make sure it is used in such an environment.
immuniTy TEsT iEC 60601 TEsT
lEvEl
Electrostatic
±8 kV contact
Discharge (ESD)
IEC 61000-4-2
±15 kV air
ComplianCE
lEvEl
±8 kV contact
±15 kV air
ElECTromagnETiC EnvironmEnT guidanCE
Floors should be wood,
concrete or ceramic tile. If oors
are covered with synthetic
material, the relative humidity
should be at least 30%.
Electrical fast
Transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
±2 kV for power
supply lines
±1 kV for input-
output lines
±1 kV dierential
mode
±2 kV for supply
mains
±1 kV for input/
output lines
±1 kV
dierential
mode
Mains power quality should
be that of a typical home or
hospital environment.
Mains power quality should
be that of a typical home or
hospital environment.
±2 kV common
mode
±2 kV for
common mode
49

10. Additional Notes
immuniTy TEsT iEC 60601 TEsT
lEvEl
Voltage
dips, short
interruptions and
voltage variations
on power supply
<5% UT (>95% dip
in UT) for
0.5 cycle at
45 degree
increments
input lines
IEC 61000-4-11
<5% UT (>95% dip
in UT) for
1 cycle
ComplianCE
lEvEl
<5% UT (>95%
dip in UT) for
0.5 cycle at
45 degree
increments
<5% UT (>95%
dip in UT) for
1 cycle
ElECTromagnETiC EnvironmEnT -
guidanCE
Mains power quality should
be that of a typical home or
hospital environment. If the
user of the device requires
continued operation during
power mains interruptions,
it is recommended that the
device be powered from an
uninterruptible power supply or
a battery.
70% UT (30%
dip in UT) for 0.5
seconds
70% UT (30%
dip in UT) for
0.5 seconds
Power frequency
(50/60 Hz)
magnetic eld
IEC 61000-4-8
<5% UT (>95%
dip in UT) for 5
seconds
30 A/m 30 A/m Power frequency magnetic
<5% UT (>95%
dip in UT) for 5
seconds
elds should be at levels
characteristic of a typical
location in a typical hospital or
home environment.
NOTE: UT is the a.c. mains voltage prior to application of the test level.
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity – This device is
intended for use in the electromagnetic environment specied below. The user of this
device should make sure it is used in such an environment.
50

10. Additional Notes
Immunity Test IEC 60601 Test
Level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
6 Vrms
Amateur Radio
& ISM Bands
between 150 kHz
and 80 MHz
10 V/m
80 MHz to 2.7 GHz
Special
Telecommunication
Bands between
300 MHz and 5.6
GHz
Compliance Level Electromagnetic
Environment - Guidance
3 Vrms
150 kHz to 80
MHz
Portable and mobile RF
communications equipment
should be used no closer
to any part of the device,
6 Vrms
Amateur Radio
& ISM Bands
including cables, than
the recommended 30 cm
separation distance.
between 150 kHz
and 80 MHz
Interference may occur in
the vicinity of equipment
10 V/m
marked with the following
symbol:
Up to 28 V/m
51

Limited Warranty
Respironics, Inc., a Philips company, warrants that the system shall be free from
defects of workmanship and materials and will perform in accordance with the product
specications for a period of two (2) years for the therapy device and a period of one (1)
year for the battery pack from the date of sale by Respironics, Inc. to the dealer. If the
product fails to perform in accordance with the product specications, Respironics, Inc.
will repair or replace – at its option – the defective material or part. Respironics, Inc.
will pay customary freight charges from Respironics, Inc. to the dealer location only. This
warranty does not cover damage caused by accident, misuse, abuse, alteration, water
ingress, and other defects not related to material or workmanship. The Respironics, Inc.
Service department shall examine any devices returned for service, and Respironics,
Inc. reserves the right to charge an evaluation fee for any returned device as to which no
problem is found after investigation by Respironics, Inc. Service.
This warranty is non-transferable by unauthorized distributors of Respironics, Inc.
products and Respironics, Inc. reserves the right to charge dealers for warranty service of
failed product not purchased directly from Respironics, Inc. or authorized distributors.
Respironics, Inc. disclaims all liability for economic loss, loss of prots, overhead,
or consequential damages which may be claimed to arise from any sale or use of
this product. Some states do not allow the exclusion or limitation of incidental or
consequential damages, so the above limitation or exclusion may not apply to you.
This warranty is given in lieu of all other express warranties. In addition, any implied
warranties – including any warranty of merchantability or tness for the particular
purpose – are limited to two years. Some states do not allow limitations on how long
an implied warranty lasts, so the above limitation may not apply to you. This warranty
gives you specic legal rights, and you may also have other rights which vary from
state to state. To exercise your rights under this warranty, contact your local authorized
Respironics, Inc. dealer or contact Respironics, Inc. at:
52
1001 Murry Ridge Lane
Murrysville, Pennsylvania 15668-8550
1-724-387-4000

1128239
1128239 R03
AD 3/7/2017
EN-DOM
 Loading...
Loading...