Page 1
VPAP™ ST
NONINVASIVE VENTILATOR
User Guide
English
A
Respiratory Care Solutions
Making quality of care easy
Page 2

Respiratory Care Solutions
Making quality of care easy
Page 3

English
Indications for use
The VPAP ST is indicated to provide noninvasive ventilation for patients weighing more than 13 kg or
more than 30 kg in iVAPS mode with respiratory insufficiency or obstructive sleep apnoea (OSA). The
VPAP ST is intended for home and hospital use.
Contraindications
Positive airway pressure therapy may be contraindicated in some patients with the following preexisting conditions:
severe bullous lung disease
pneumothorax or pneumomediastinum
pathologically low blood pressure, particularly if associated with intravascular volume depletion
dehydration
cerebrospinal fluid leak, recent cranial surgery, or trauma.
Adverse effects
Patients should report unusual chest pain, severe headache, or increased breathlessness to their
prescribing physician. An acute upper respiratory tract infection may require temporary
discontinuation of treatment.
The following side effects may arise during the course of therapy with the device:
drying of the nose, mouth, or throat
nosebleed
bloating
ear or sinus discomfort
eye irritation
skin rashes.
Masks and humidifiers
Recommended masks and humidifiers are available on www.resmed.com on the Products page
under Service & Support. For more information on using your mask or humidifier, refer to the
manual supplied with your mask or humidifier.
English 1
Page 4

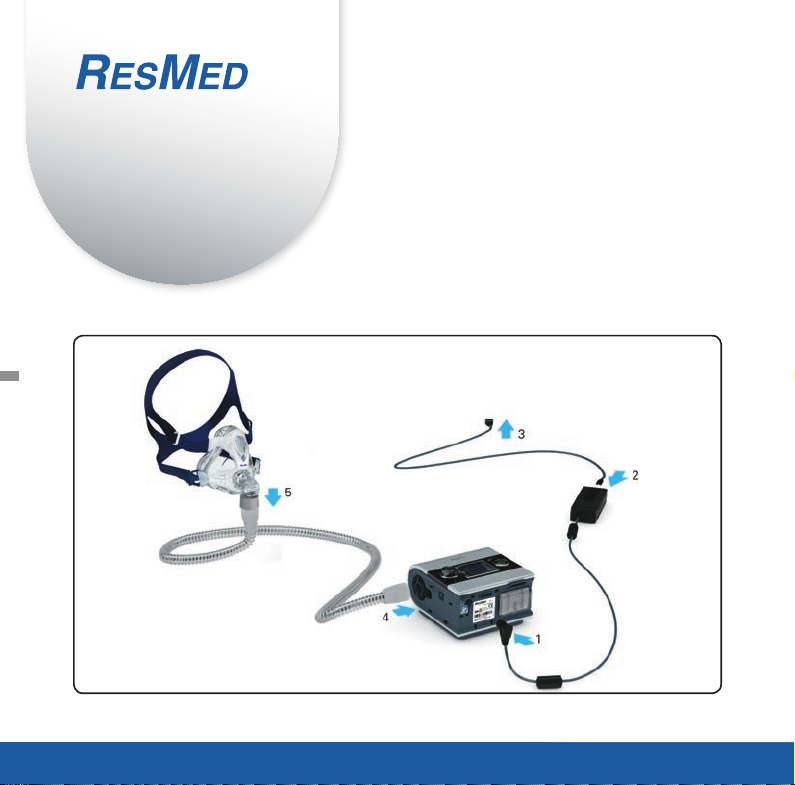
Setup
Refer to illustration A.
1. Connect the DC plug of the power supply unit to the rear of the device.
2. Connect the power cord to the power supply unit.
3. Plug the other end of the power cord into the power outlet.
4. Connect one end of the air tubing firmly onto the air outlet.
5. Connect the assembled mask system to the free end of the air tubing.
Control panel
Refer to illustration B.
The control panel of your device includes the following items:
1. Start/Stop button: Starts or stops treatment
2. LCD screen: Displays the menu, treatment and reminder screens
3. Info menu button*: Allows you to view your sleep statistics
4. Setup menu button*: Allows you to make changes to settings
5. Push dial: Turning the dial allows you to scroll through the menu and change settings. Pushing
the dial allows you to enter into a menu and confirm your choice.
*The Info and Setup menus are disabled if S9 Essentials has been enabled by your clinician.
Navigating the menus
Refer to illustration C.
In general, to navigate the menus:
1. Turn until the parameter you require is displayed in blue.
2. Press . The selection is highlighted in orange.
3. Turn until you see the setting that you require.
4. Press to confirm your choice. The screen returns to blue.
Getting started
1. Make sure the power is connected.
2. Adjust the ramp time if required.
3. Fit your mask as described in your mask user guide.
4. To start therapy, simply breathe into the mask and/or press .
5. Lie down and arrange the air tubing so that it is free to move if you turn in your sleep.
6. To stop treatment at any time, remove your mask and/or press .
2
Page 5

Notes:
If your clinician has enabled SmartStart your device will start automatically when you breathe
into the mask and stop automatically when you remove the mask.
If power is interrupted during treatment, the device automatically restarts therapy when power
is restored.
Cleaning and maintenance
You should regularly carry out cleaning and maintenance as described in this section. Refer to your
mask and humidifier user guides for detailed instructions regarding the care of your device.
Daily:
Remove the air tubing by pulling on the finger grips on the cuff. Hang it in a clean, dry place until next
use.
Notes:
Do not hang the air tubing in direct sunlight as it may harden over time and eventually crack.
Do not wash the air tubing in a washing machine or dishwasher.
Weekly:
1. Remove the air tubing from the device and the mask.
2. Wash the air tubing in warm water using mild detergent.
3. Rinse thoroughly, hang, and allow to dry.
4. Before next use, reconnect the air tubing to the air outlet and mask.
Monthly:
1. Wipe the exterior of the device with a damp cloth and mild detergent.
2. Check the air filter for holes and blockage by dirt or dust. Replace the air filter if necessary.
Replacing the air filter:
Replace the air filter every six months (or more often if necessary).
1. Remove the air filter cover from the back of the device.
2. Remove and discard the old air filter.
3. Insert a new ResMed air filter ensuring that it is sitting flat in the air filter cover.
4. Replace the air filter cover.
Notes:
Ensure the air filter and air filter cover are fitted at all times.
Do not wash the air filter. The air filter is not washable or reusable.
English 3
Page 6

SD card
An SD card has been supplied to gather therapy data from your device and provide settings updates
from your clinician. When instructed to do so, disconnect the device from the power outlet, remove
your SD card, insert it in the protective folder and send it to your clinician. For more information on
removing and inserting your card refer to the S9 SD Card Protective Folder provided with your device.
Please retain the S9 SD Card Protective Folder for future use.
Troubleshooting
If there is a problem, try the following suggestions. If the problem cannot be solved, contact your
equipment supplier or ResMed. Do not attempt to open the device enclosure.
Problem/Possible cause Solution
No display
Power is not connected.
The DC plug is partially inserted
into the back of the device or
inserted too slowly.
Insufficient air delivered from the device
Ramp time is in use. Wait for air pressure to build up or change ramp time.
Air filter is dirty. Replace air filter.
Air tubing is not connected
properly.
Air tubing is blocked, pinched or
punctured.
Mask and headgear are not
positioned correctly.
Incorrect air tubing selected.
Non-vented mask is used. Only use a vented mask.
Mask vents might be blocked.
4
Ensure the power cord is connected and the power outlet (if
available) is on.
Fully insert the DC plug.
Check air tubing.
Unblock or free the air tubing. Check the air tubing for punctures.
Adjust position of mask and headgear.
If you are using the SlimLine, Standard or 3 m air tubing ensure
that you have the correct air tubing selected via the menu.
Check if you have sufficient venting. Unblock mask vents if
necessary.
Page 7

Problem/Possible cause Solution
EPAP may be set too low. Talk to your clinician about your settings.
Device does not start when you breathe into the mask
Breath is not deep enough to
trigger SmartStart/Stop.
SmartStart/Stop is disabled
because Leak Alert is enabled.
SmartStart/Stop is disabled
because Confirm Stop is
enabled.
SmartStart/Stop is disabled. Talk to your clinician about enabling the SmartStart/Stop feature.
There is excessive leak. Adjust position of mask and headgear.
Device does not stop when you remove your mask
SmartStart/Stop is disabled
because Leak Alert is enabled.
SmartStart/Stop is disabled
because Confirm Stop is
enabled.
SmartStart/Stop is disabled. Talk to your clinician about enabling the SmartStart/Stop feature.
SmartStart/Stop is enabled but the device does not stop automatically when you remove your
mask
Incompatible mask system
being used.
Incorrect mask setting being
used.
The patient is using a nasal
pillows mask with a set
pressure less than 6 cm H2O.
Take a deep breath in and out through the mask.
Press Start/Stop to start therapy.
A message appears on the screen. To stop therapy, select Yes and
press the Push Dial.
Connect the air tubing firmly at both ends.
Press Start/Stop to stop therapy.
A message appears on the screen. To stop therapy, select Yes and
press the Push Dial.
Only use equipment recommended by ResMed.
Check the selected mask type in the Setup menu. Change it if
necessary.
Disable SmartStart/Stop.
English 5
Page 8

Problem/Possible cause Solution
The patient is using a paediatric
mask with a set pressure less
than 8 cm H2O.
Pressure rises inappropriately
Talking, coughing or breathing in
an unusual manner.
Mask cushion is buzzing against
the skin.
Cushion seated incorrectly
causing excessive leak.
Displays message: High temperature fault, refer to user manual
Device has been left in a hot
environment.
Air filter is blocked.
Air tubing is blocked.
Humidity level setting is too
high, resulting in accumulation
of water in the air tubing.
Displays message: Check ResMed 30/90W Power Supply Unit and fully insert the connector
The DC plug is partially inserted
into the back of the device or
inserted too slowly.
A non-ResMed power supply
unit is connected to the device.
The power supply unit is being
covered by bedding.
Disable SmartStart/Stop.
Avoid talking with a nasal mask on, and breathe as normally as
possible.
Adjust the headgear.
Adjust headgear or re-fit cushion.
Allow to cool before re-use. Disconnect the power cord and then
reconnect it to restart the device.
Replace your air filter. Disconnect the power cord and then
reconnect it to restart the device.
Check your air tubing and remove any blockages. Disconnect the
power cord and then reconnect it to restart the device.
Turn the humidity level setting down and empty the water from the
air tubing.
Fully insert the DC plug.
Remove the power supply unit and replace with a ResMed power
supply unit.
Make sure that the power supply unit is free from bedding, clothes
or other objects that could cover it.
6
Page 9

Problem/Possible cause Solution
Displays message: No tube, please check your tube is connected
Flow is high because air tubing
is not connected properly.
Note: The tube disconnection
check may not operate when an
antibacterial filter is used.
Displays message: Tube blocked, please check your tube
Air tubing is blocked.
Displays message: High leak, please check system setup and all connections
There is excessive leak.
Note: If Leak Alert is enabled,
an audible alert is activated and
a high leak message is
displayed.
The following message is displayed on the LCD after you try to update settings or copy data to
the SD card: Card error, please remove SD card and contact service provider
SD card is not inserted correctly. Ensure that the SD card is inserted correctly.
You may have removed the
SD card before settings were
copied to the device.
The following message is NOT displayed on the LCD after you try to update the settings using
the SD card: Settings updated successfully, press any key
The settings were not updated. Contact your clinician/service provider immediately.
English 7
Connect the air tubing firmly at both ends.
Check your air tubing and remove any blockages. Disconnect the
power cord and then reconnect it to restart the device.
Adjust position of mask and headgear.
Connect the air tubing firmly at both ends.
Reinsert the SD card and wait for the Home screen or the
"Settings updated successfully, press any key" message to
appear on the LCD.
Note: This message only appears once. If you re-insert the SD card
after you have updated your settings, the message will not be redisplayed.
Page 10

General technical specifications
Power supply 90W power supply unit
Environmental
conditions
Aircraft use
Electromagnetic
compatibility
IEC 60601-1
classification
8
Input range: 100–240V, 50–60Hz, 115V, 400Hz nominal for aircraft use
Typical power consumption: 70W (80VA)
Maximum power consumption: 110W (120VA)
30W power supply unit
Input range: 100–240V, 50–60Hz, 115V, 400Hz nominal for aircraft use
Typical power consumption: 20W (40VA)
Maximum power consumption: 36W (75VA)
90W DC/DC converter
Nominal inputs: 12V, 24V
Typical power consumption: 70W
Maximum power consumption: 110W
Operating temperature: +5°C to +35°C
Note: The air flow for breathing produced by this therapy device can be higher
than the temperature of the room. Under extreme ambient temperature
conditions (40ºC) the device remains safe.
Operating humidity: 10 to 95% non-condensing
Operating altitude: Sea level to 2,591 m; air pressure range 1013 hPa to 738 hPa
Storage and transport temperature: -20°C to +60°C
Storage and transport humidity: 10 to 95% non-condensing
ResMed confirms that the device/s meets the Federal Aviation Administration
(FAA) requirements (RTCA/DO-160, section 21, category M) for all phases of air
travel.
Product complies with all applicable electromagnetic compatibility requirements
(EMC) according to IEC60601-1-2, for residential, commercial and light industry
environments.
It is recommended that mobile communication devices are kept at least 1 m
away from the device.
Information regarding the electromagnetic emissions and immunity of this
ResMed device can be found on www.resmed.com, on the Products page
under Service and Support. Click on the PDF file for your language.
Class II (double insulation), Type BF, Ingress protection IP21
Page 11

VPAP ST technical specifications
Mode pressure
ranges
Maximum single
fault pressure
Physical
Air filter
Sound
DECLARED
DUAL-NUMBER
NOISE EMISSION
VALUES in
accordance with
ISO 4871:1996
Supplemental
oxygen
English 9
CPAP mode
Set Pressure: 4–20 cm H2O
S, ST, T and PAC modes
IPAP: 4–25 cm H2O; EPAP: 2–25 cm H2O
iVAPS mode
PS: 0–23 cm H2O; EPAP: 2–25 cm H2O
Maximum single fault steady state pressure: 30 cm H2O—if pressure exceeded
for > 6 sec; 40 cm H2O—if pressure exceeded for >1 sec
Nominal dimensions (L x W x H): 153 mm x 140 mm x 86 mm
Weight: 835 g
Housing construction: Flame retardant engineering thermoplastic
Air outlet: 22 mm conical air outlet (complies with ISO 5356-1:2004)
Hypoallergenic air filter: Acrylic and polypropylene fibers in a polypropylene
carrier
Standard air filter: Polyester non-woven fiber
Pressure level (CPAP mode)
With SlimLine air
tubing:
With Standard air
tubing:
With either SlimLine
or Standard air
26 dBA with uncertainty of 2 dBA as measured according
to EN ISO 17510-1:2009
27 dBA with uncertainty of 2 dBA as measured according
to EN ISO 17510-1:2009
28 dBA with uncertainty of 2 dBA as measured according
to EN ISO 17510-1:2009
tubing and H5i:
Power level (CPAP mode)
With SlimLine air
tubing:
With Standard air
tubing:
With either SlimLine
or Standard air
34 dBA with uncertainty of 2 dBA as measured according
to EN ISO 17510-1:2009
35 dBA with uncertainty of 2 dBA as measured according
to EN ISO 17510-1:2009
36 dBA with uncertainty of 2 dBA as measured according
to EN ISO 17510-1:2009
tubing and H5i:
Recommended maximum supplemental oxygen flow: 15 L/min (CPAP, S, ST, T,
PAC); 4 L/min (iVAPS)
Page 12

Air tubing technical specifications
Air tubing Material Length Inner diameter
ClimateLine heated air tubing Flexible plastic and electrical
components
Flexible plastic and electrical
components
ClimateLine
tubing
MAX
heated air
SlimLine air tubing Flexible plastic 1.8 m 15 mm
Standard air tubing Flexible plastic 2 m 19 mm
3 m air tubing Flexible plastic 3 m 19 mm
Heated air tubing temperature cut-out: 41°C
Notes:
The manufacturer reserves the right to change these specifications without notice.
The temperature and relative humidity settings displayed for Climate Control are not measured
values.
Check with your clinician/service provider before using the SlimLine air tubing with devices other
than the S9 or H5i.
The electrical connector end of the heated air tubing is only compatible with the H5i air outlet
and should not be fitted to the device or mask.
When using the SlimLine or ClimateLine above 20 cm H2O, the device optimum performance
may not be reached if used with an antibacterial filter. The device performance must be checked
prior to prescribing the SlimLine for use with an antibacterial filter.
The ClimateLine or ClimateLine
MAX
is designed only for use with the H5i.
2 m 15 mm
1.9 m 19 mm
10
Page 13

Humidifier performance
The following settings have been tested at 22°C ambient temperature:
Mask pressure
cm H2O
Setting 3 Setting 6 Setting 3 Setting 6
3 90 100 10 18
10 95 100 11.5 21
20 95 100 11 18
25 100 100 12 13.5
a. AH - Absolute Humidity in mg/L.
b. BTPS - Body Temperature Pressure Saturated.
Pneumatic flow path
Flow (maximum) at set pressures
The following are measured at the end of the specified air tubing:
Pressure, cm H2O VPAP ST and
4 200 170 195 170
8 200 170 190 170
12 200 170 184 170
16 200 170 175 170
20 190 170 168 161
25 180 161 144 125
English 11
Standard, L/min
RH output % Nominal system output AHa, BTPS
1. Flow sensor
2. Blower
3. Pressure sensor
4. Mask
5. Air tubing
6. H5i
7. Device
8. Inlet filter
VPAP ST, H5i and
Standard, L/min
VPAP ST and
SlimLine, L/min
VPAP ST, H5i and
ClimateLine,
L/min
b
Page 14

Displayed values
Value Range Display resolution
Pressure sensor at air outlet
Mask pressure 4-20 cm H2O (CPAP); 2-25 cm
H2O (S, ST, T, PAC, iVAPS)
Flow derived values
Leak 0–200 L/min 1 L/min
Tidal volume 0–4000 mL 1 mL
Respiratory rate 0–50 BPM 1 BPM
Minute ventilation 0–30 L/min 0.1 L/min
Ti 0.1–4.0 sec 0.1 sec
I:E ratio 1:50–2:1 0.1
Value Accuracya
Pressure measurementa
Mask pressure
±0.5 cm H2O (+4% of measured value)
Flow measurementsa
Leakb
Tidal volume
b.c
Respiratory rate
Minute ventilation
b,c
b, c
±12 L/min or 20% of reading, whichever is greater, at 0 to
60 L/min
±20%
±1 BPM
±20%
a. Results are expressed at ATPD (Ambient Temperature and Pressure, Dry).
b. Accuracy may be reduced by the presence of leaks, supplemental oxygen, tidal volumes <100 mL
or minute ventilation <3 L/min.
c. Measurement accuracy verified as per EN ISO 10651-6:2009 for Home Care Ventilatory Support
Devices (Figure 101 and Table 101) using nominal ResMed mask vent flows.
0.1 cm H2O
12
Page 15

Pressure accuracy
Maximum static pressure variation at 10 cm H2O according to EN ISO 17510-1:2009
Without H5i 9.89 cm H2O to 9.97 cm H2O 9.76 cm H2O to 9.87 cm H2O
With H5i 9.82 cm H2O to 9.98 cm H2O 9.78 cm H2O to 9.88 cm H2O
Maximum dynamic pressure variation according to EN ISO 17510-1:2009
Pressure (cm H2O) 10 BPM 15 BPM 20 BPM
4 0.18 / 0.18 0.30 / 0.30 0.51 / 0.51
8 0.21 / 0.20 0.26 / 0.24 0.38 / 0.36
12 0.21 / 0.20 0.26 / 0.23 0.34 / 0.31
16 0.22 / 0.21 0.27 / 0.26 0.36 / 0.33
20 0.23 / 0.22 0.26 / 0.28 0.38 / 0.35
25 0.30 / 0.31 0.54 / 0.50 0.74 / 0.71
Pressure (cm H2O) 10 BPM 15 BPM 20 BPM
4 0.22 / 0.20 0.28 / 0.29 0.47 / 0.53
8 0.23 / 0.19 0.32 / 0.29 0.41 / 0.42
12 0.22 / 0.21 0.35 / 0.29 0.41 / 0.45
16 0.22 / 0.23 0.41 / 0.33 0.44 / 0.50
20 0.24 / 0.27 0.37 / 0.34 0.48 / 0.50
25 0.31 / 0.31 0.50 / 0.54 0.78 / 0.84
Symbols
The following symbols may appear on your product or packaging.
Caution; Read instructions before use; Protection against insertion of fingers and against
vertically dripping water; Type BF equipment; Class II equipment; Start/Stop;
Manufacturer; European RoHS; Batch code; Catalogue number; Serial number;
English 13
Standard air tubing SlimLine air tubing
VPAP ST and Standard air tubing without H5i / VPAP ST and Standard air
tubing with H5i
VPAP ST and SlimLine air tubing without H5i / VPAP ST and SlimLine air
tubing with H5i
Page 16

Direct current; Lock/unlock; China pollution control logo 1; China pollution
control logo 2; European Authorised Representative; Not drip proof; Keep dry;
Environmental information
WEEE 2002/96/EC is a European Directive that requires the proper disposal of electrical and
electronic equipment. This device should be disposed of separately, not as unsorted municipal waste.
To dispose of your device, you should use appropriate collection, reuse and recycling systems
available in your region. The use of these collection, reuse and recycling systems is designed to
reduce pressure on natural resources and prevent hazardous substances from damaging the
environment.
If you need information on these disposal systems, please contact your local waste administration.
The crossed-bin symbol invites you to use these disposal systems. If you require information on
collection and disposal of your ResMed device please contact your ResMed office, local distributor or
go to www.resmed.com/environment.
Servicing
The VPAP ST device is intended to provide safe and reliable operation when operated in accordance
with the instructions provided by ResMed. ResMed recommends that the VPAP ST device be
inspected and serviced by an authorised ResMed Service Centre if there is any sign of wear or
concern with device function. Otherwise, service and inspection of the devices generally should not
be required during the five year design life of the device.
Limited warranty
ResMed Ltd (hereafter 'ResMed') warrants that your ResMed product shall be free from defects in
material and workmanship from the date of purchase for the period specified below.
Product Warranty period
Mask systems (including mask frame, cushion, headgear and
tubing)—excluding single-use devices
Accessories—excluding single-use devices
Flex-type finger pulse sensors
Humidifier water tubs
Batteries for use in ResMed internal and external battery
systems
90 days
6 months
14
Page 17

Product Warranty period
Clip-type finger pulse sensors
CPAP and bilevel device data modules
Oximeters and CPAP and bilevel device oximeter adapters
Humidifiers and humidifier cleanable water tubs
Titration control devices
CPAP, bilevel and ventilation devices (including external
power supply units)
Battery accessories
Portable diagnostic/screening devices
This warranty is only available to the initial consumer. It is not transferable.
If the product fails under conditions of normal use, ResMed will repair or replace, at its option, the
defective product or any of its components.
This Limited Warranty does not cover: a) any damage caused as a result of improper use, abuse,
modification or alteration of the product; b) repairs carried out by any service organization that has not
been expressly authorized by ResMed to perform such repairs; c) any damage or contamination due
to cigarette, pipe, cigar or other smoke; and d) any damage caused by water being spilled on or into
an electronic device.
Warranty is void on product sold, or resold, outside the region of original purchase.
Warranty claims on defective product must be made by the initial consumer at the point of purchase.
This warranty replaces all other expressed or implied warranties, including any implied warranty of
merchantability or fitness for a particular purpose. Some regions or states do not allow limitations on
how long an implied warranty lasts, so the above limitation may not apply to you.
ResMed shall not be responsible for any incidental or consequential damages claimed to have
resulted from the sale, installation or use of any ResMed product. Some regions or states do not
allow the exclusion or limitation of incidental or consequential damages, so the above limitation may
not apply to you.
This warranty gives you specific legal rights, and you may also have other rights which vary from
region to region. For further information on your warranty rights, contact your local ResMed dealer or
ResMed office.
1 year
2 years
English 15
Page 18

WARNINGS
Read the entire manual before using the device.
Use the device only as directed by your physician or healthcare provider.
Use the device only for the intended use as described in this manual. Advice contained in this
manual should not supersede instructions given by the prescribing physician.
If you notice any unexplained changes in the performance of the device, if it is making unusual
or harsh sounds, if the device or the power supply are dropped or mishandled, if water is spilled
into the enclosure, or if the enclosure is broken, discontinue use and contact your ResMed
Service Center.
Beware of electrocution. Do not immerse the device, humidifier, power supply or power cord in
water. In the event of a spill, disconnect the device from the power supply and let the parts dry.
Always unplug the device before cleaning and make sure that all parts are dry before plugging in
the device.
Explosion hazard—do not use in the vicinity of flammable anesthetics.
Make sure the power cord and plug are in good condition and the equipment is not damaged.
Keep the power cord away from hot surfaces.
The device should only be used with masks (and connectors1) recommended by ResMed, or by
a physician or respiratory therapist. A mask should not be used unless the device is turned on.
Once the mask is fitted, ensure that the device is blowing air. The vent hole or holes associated
with the mask should never be blocked.
Explanation: The device is intended to be used with special masks (or connectors) which have
vent holes to allow continuous flow of air out of the mask. When the device is turned on and
functioning properly, new air from the device flushes the exhaled air out through the mask vent
holes. However, when the device is not operating, insufficient fresh air will be provided through
the mask, and the exhaled air may be rebreathed. Rebreathing of exhaled air for longer than
several minutes can, in some circumstances, lead to suffocation. This applies to most models of
CPAP or bilevel devices.
Oxygen supports combustion. Oxygen must not be used while smoking or in the presence of an
open flame.
Always ensure that the device is turned on and airflow generated before the oxygen supply is
turned on. Always turn the oxygen supply off before the device is turned off, so that unused
oxygen does not accumulate within the device enclosure and create a risk of fire.
1
Ports may be incorporated into the mask or in connectors that are near the mask.
16
Page 19

Do not leave long lengths of air tubing around the top of your bed. It could twist around your
head or neck while you are sleeping.
Do not use electrically conductive or antistatic air tubings.
Do not use the air tubing if there are any visible signs of damage.
Only ResMed air tubing and accessories should be used with the device. A different type of air
tubing or accessory may alter the pressure you actually receive, reducing the effectiveness of
the treatment.
Only use the ResMed 90W or 30W power supply unit. Use the 90W power supply unit to power
the system comprising the device, H5i, air tubing, DC/DC converter and battery pack. The 30W
power supply unit is designed to power the device only and recommended for travelling.
Only ResMed products are designed to be connected to the module connector port. Connecting
other devices could damage the device.
Blocking the air tubing and/or air inlet of the device while in operation could lead to overheating
of the device.
CAUTIONS
Do not open the device enclosure. There are no user serviceable parts inside. Repairs and
servicing should only be performed by an authorised ResMed service agent.
Do not use bleach, chlorine, alcohol, or aromatic-based solutions, moisturizing or antibacterial
soaps or scented oils to clean the device, humidifier or air tubing. These solutions may cause
damage and reduce the life of these products.
Incorrect system setup may result in incorrect mask pressure reading. Ensure the system is
correctly set up.
Be careful not to place the device where it can be bumped or where someone is likely to trip
over the power cord.
Make sure that the area around the device is dry and clean and clear of bedding, clothes or other
objects that could block the air inlet or cover the power supply unit.
Ensure that the device is protected against water if used outdoors. Enclose the device in the S9
travel bag for transport.
English 17
Page 20

Respiratory Care Solutions
Making quality of care easy
Page 21

368551/2 2013-02
VPAP ST
User
EUR1 EUR3
B
1 2 3
4 5
C
1 42 3
Manufacturer: ResMed Ltd 1 Elizabeth Macarthur Drive Bella Vista NSW 2153 Australia. Distributed by: ResMed
Corp 9001 Spectrum Center Boulevard San Diego CA 92123 USA.
Oxfordshire OX144RY UK. See www.resmed.com for other ResMed locations worldwide.
For patent information, see www.resmed.com/ip. S9, H5i, ClimateLine, SlimLine, SmartStart and VPAP are trademarks of
ResMed Ltd and S9, ClimateLine, SlimLine, SmartStart and VPAP are registered in U.S. Patent and Trademark Office.
© 2013 ResMed Ltd.
Global leaders in sleep and respiratory medicine www.resmed.com
ResMed (UK) Ltd 96 Milton Park Abingdon
 Loading...
Loading...