Page 1

Smart Wearable ECG Monitoring Device User
Manual V 1.0
Page 2

TABLE OF CONTENTS
Safety Notes ............................................................... 5
Product Overview ...................................................... 8
Indication for Use ....................................................................... 8
Package Contents ...................................................................... 9
Product Configurations ....................................................................................... 10
Components ............................................................................. 10
Sensor ................................................................................................................... 10
Charger ................................................................................................................. 11
Body Strap Patch ................................................................................................. 12
Direct Wear Patch ................................................................................................ 13
Product Requirements ............................................................ 14
Before You Start ......................................................15
Charging the Battery ............................................................... 15
Installing the App ..................................................................... 15
Enabling Bluetooth .................................................................. 15
Getting Started.........................................................16
Creating a Profile ..................................................................... 16
Pairing........................................................................................ 17
Wearing the ECG Sensor ........................................................ 18
Wearing the ECG Sensor with the Body Strap B1............................................. 18
Wearing the ECG Sensor with the Body Strap B2............................................. 19
Direct Wear ........................................................................................................... 20
Page 3

The Q-COR App .......................................................23
Main Screen .............................................................................. 23
Menu .......................................................................................... 24
History ................................................................................................................... 24
Profile .................................................................................................................... 27
Pairing ................................................................................................................... 27
Reset ..................................................................................................................... 28
Quit ........................................................................................................................ 28
Additional Information ...........................................29
LED Indicators .......................................................................... 29
Status Indicator (Sensor)..................................................................................... 29
Charging Indicator (Charger) .............................................................................. 29
Cleaning .................................................................................... 29
ECG Electrode Information ..................................................... 29
Specifications ........................................................................... 30
Sensor Specifications .......................................................................................... 30
Charger Specifications ........................................................................................ 31
Trouble shooting ...................................................................... 31
Customer Support.................................................................... 31
EU Representative ............................................................................................... 31
Federal Communications Commission (FCC) Statement... 32
Manufacture Date ..................................................................... 33
Smart Wearable ECG Monitoring Device (CI3) .................................................. 33
Charger (CI3-C)..................................................................................................... 33
Supplier’s Declaration ............................................................. 34
Supplier's declaration and guidelines — Electromagnetic Immunity.............. 34
Recommended safety distance........................................................................... 35
Page 4

Regulatory Marks ................................................................................................. 36
Page 5

SAFETY NOTES
• The Quanta Smart Wearable ECG Monitoring Device is to be used for clinical
assessment and personal reference only. The Quanta Smart Wearable ECG
Monitoring Device is not intended for diagnostic use.
• The Quanta Smart Wearable ECG Monitoring Device consists entirely of
sophisticated medical electrical parts so maintenance can only be carried out by
professional technicians. Unauthorized disassembly of the device by the user is not
allowed.
• The Quanta Smart Wearable ECG Monitoring Device must be used with the
specified “accessories” and “electrodes”. The use of accessories and electrodes
from other brands may lead to faulty readings. For more information, see Product
Requirements.
• Do not allow the metal connectors to come into contact with any kind of power
source during use.
• Damaged or faulty accessories and electrodes should not be used.
• When the Quanta Smart Wearable ECG Monitoring Device is low on power, it will
automatically stop taking measurements and the corresponding indicator lights will
blink. Please charge the sensor as soon as it shows a low battery state.
• When the Quanta Smart Wearable ECG Monitoring Device is taking measurements,
please try and avoid using any instruments or devices that may affect the accuracy
of the readings (e.g. the use of a blood pressure meter will affect the measurement
of the pulse).
• When the Quanta Smart Wearable ECG Monitoring Device is taking measurements,
please try and avoid using other electronic devices nearby. If it must be used with
other electronic devices simultaneously, please check to make sure that the Quanta
Smart Wearable ECG Monitoring Device is continuing to take measurements
normally.
• The Quanta Smart Wearable ECG Monitoring Device-specific accessories are
defined in this user guide. The use of accessories from other brands may damage
the device.
• Do not use the Quanta Smart Wearable ECG Monitoring Device in a flammable
environment.
• When the environment temperature is 45degC, the surface temperature of Quanta
Smart Wearable ECG Monitoring Device will be 46degC.
• When ambient temperature is 40~45degC, do not use the Quanta Smart Wearable
ECG Monitoring Device for more than 4 hours to avoid low-temperature burns.
• Users have to buy ECG electrodes themselves when using the direct wear patch.
The specification for ECG electrodes can be found in ECG Electrode Information.
Page 6

• Pay attention to ensure that the Quanta Smart Wearable ECG Monitoring Device is
not swallowed by pets or children.
• Cardiac pacemakers or other electrical stimulators may affect the accuracy of the
measurements for the Quanta Smart Wearable ECG Monitoring Device.
• The conductive parts of the electrodes and associated connectors for type CF
applied parts, including the neutral electrode, should not make contact with other
conductive parts including the ground.
• Please read through this user guide carefully before using the Quanta Smart
Wearable ECG Monitoring Device.
• If the Quanta Smart Wearable ECG Monitoring Device must be used to take
measurements over an extended period of time, please inspect the contact point of
the electrodes at least once every 8 hours to make sure that the electrodes are in
the right position and there is no allergic skin reaction on the user.
• Do not use high-frequency instruments or electrical medical equipment such as
“defibrillators” when using the Quanta Smart Wearable ECG Monitoring Device.
• When the Quanta Smart Wearable ECG Monitoring Device is taking measurements
and a stable reading cannot be taken then stop taking measurements right away as
not everyone is suitable to sensor using the Quanta Smart Wearable ECG
Monitoring Device.
• The Quanta Smart Wearable ECG Monitoring Device can only take measurements
when the subject is stationary (e.g. while sitting or lying down) or engaging in
ordinary activity. Any activities not permitted by the attending physician may affect
the accuracy of the measurements.
• Do not use the Quanta Smart Wearable ECG Monitoring Device in an excessively
humid environment and never submerge it directly under water.
• Do not expose the Quanta Wearable ECG Monitoring Device to extremely
temperature, moist environment, dust or direct sun light.
• Do not clean or wipe the Quanta Smart Wearable ECG Monitoring Device with
corrosive and abrasive cleaning agents.
• The disposal of the Quanta Smart Wearable ECG Monitoring Device and its
accessories should comply with the relevant local regulations.
• The Quanta Smart Wearable ECG Monitoring Device has been tested and certified
to international electro-magnetic compatibility (EMC) standards for medical
equipment (EN 60601-1 and EN 60601-1-2).
• The Quanta Smart Wearable ECG Monitoring Device and its accessories should be
disposed of properly.
• Battery Caution: There is a risk of explosion if the battery for the Quanta Smart
Wearable ECG Monitoring Device is replaced by an incorrect type.
• The Quanta Smart Wearable ECG Monitoring Device’s use is not intended for
infants weighing less than 10 kg.
Page 7

• The expected service life of Quanta Smart Wearable ECG Monitoring Device is 3
years.
Page 8

PRODUCT OVERVIEW
Indication for Use
The Quanta Smart Wearable ECG Monitoring Device is intended for use in the noninvasive measurement of a functional Electrocardiography (ECG) and heart rate (HR) in
hospital, healthcare institute, and home environments by trained medical professionals
and trained-adults. The Smart Wearable ECG Monitoring Device will process ECG
signals and calculate HR. ECG and HR data will be transferred to a smartphone via
Bluetooth, and further displayed on the smartphone screen via a dedicated app called
Q-COR. The device should be worn on the human body along with a belt or a patch
with gel electrodes. The Quanta Smart Wearable ECG Monitoring Device is sold to
professional organizations such as home care givers, clinics, or hospitals rather than to
the general public. It can only be installed by trained adults or medical personnel.
Unauthorized installation by users is not allowed.
Page 9
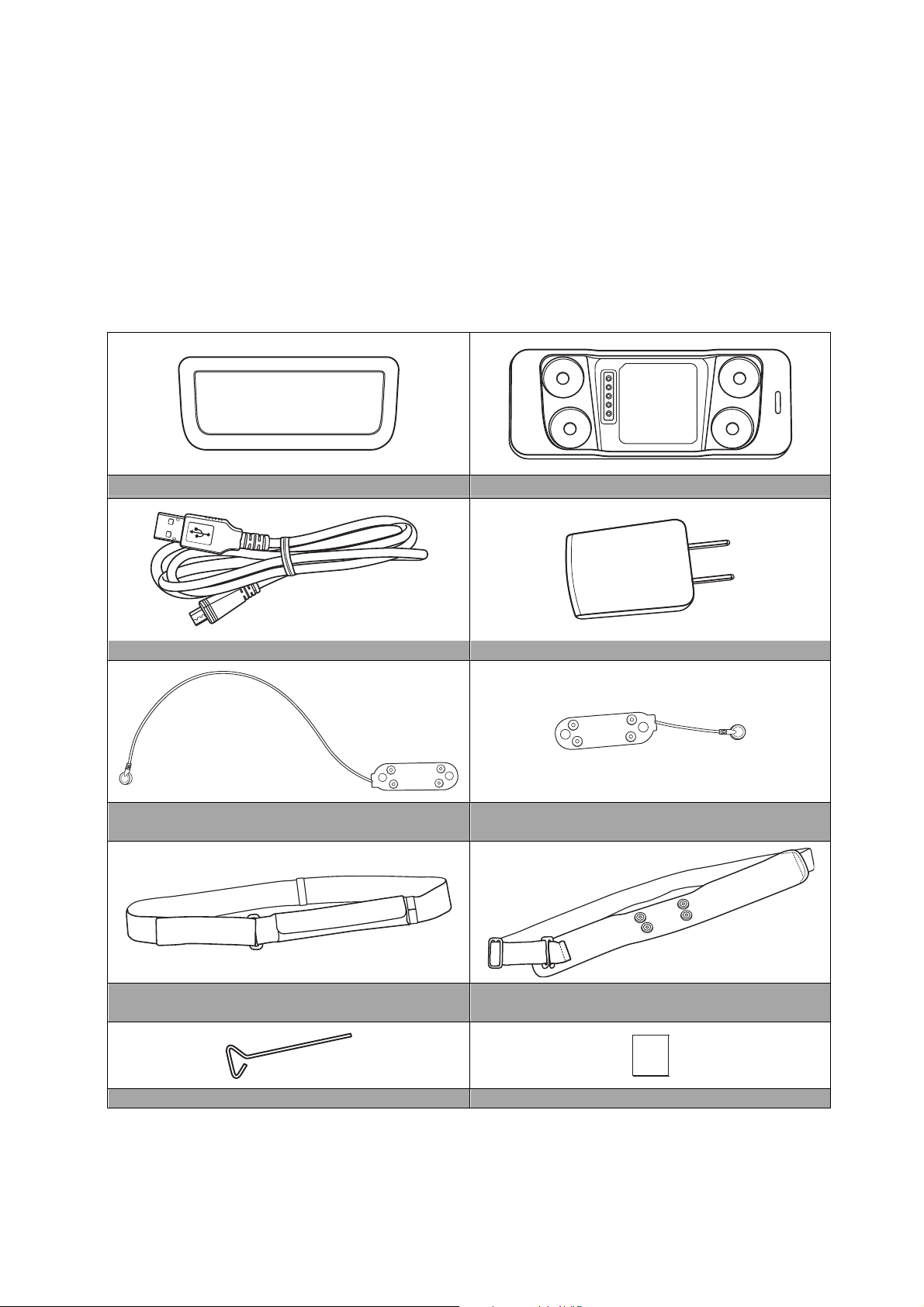
Direct Wear
Patch x 1
Body Strap
Patch x 1
Body Strap
B1
x 1
Body Strap
B2
x 1
User Manual
Pin x 1
User Manual x 1
Package Contents
After purchasing the Smart Wearable ECG Monitoring Device please check the product
package to ensure that the following items are included:
ECG Sensor x 1 Charger x 1
USB Charging Cable x 1 5V/2A Adapter x 1
(For Select Models)
(For Select Models)
(For Select Models)
(For Select Models)
Page 10
Product Configurations
Part Name
Model Number
CI3SKU1
CI3SKU2
CI3SKU3
Body Strap Patch
CI3-P2 V
V
The following is a table that lists the various product configurations for the Quanta
Smart Wearable ECG Monitoring Device and the components included for each
configuration.
Product Configuration
Smart Wearable ECG Sensor
Charger CI3-C V V V
Direct Wear Patch CI3-P1 V
Body Strap B1 CI3-B1 V
Body Strap B2 CI3-B2
CI3 V V V
Components
Sensor
Front
1
Status Indicator (see LED Indicators for more information)
Page 11
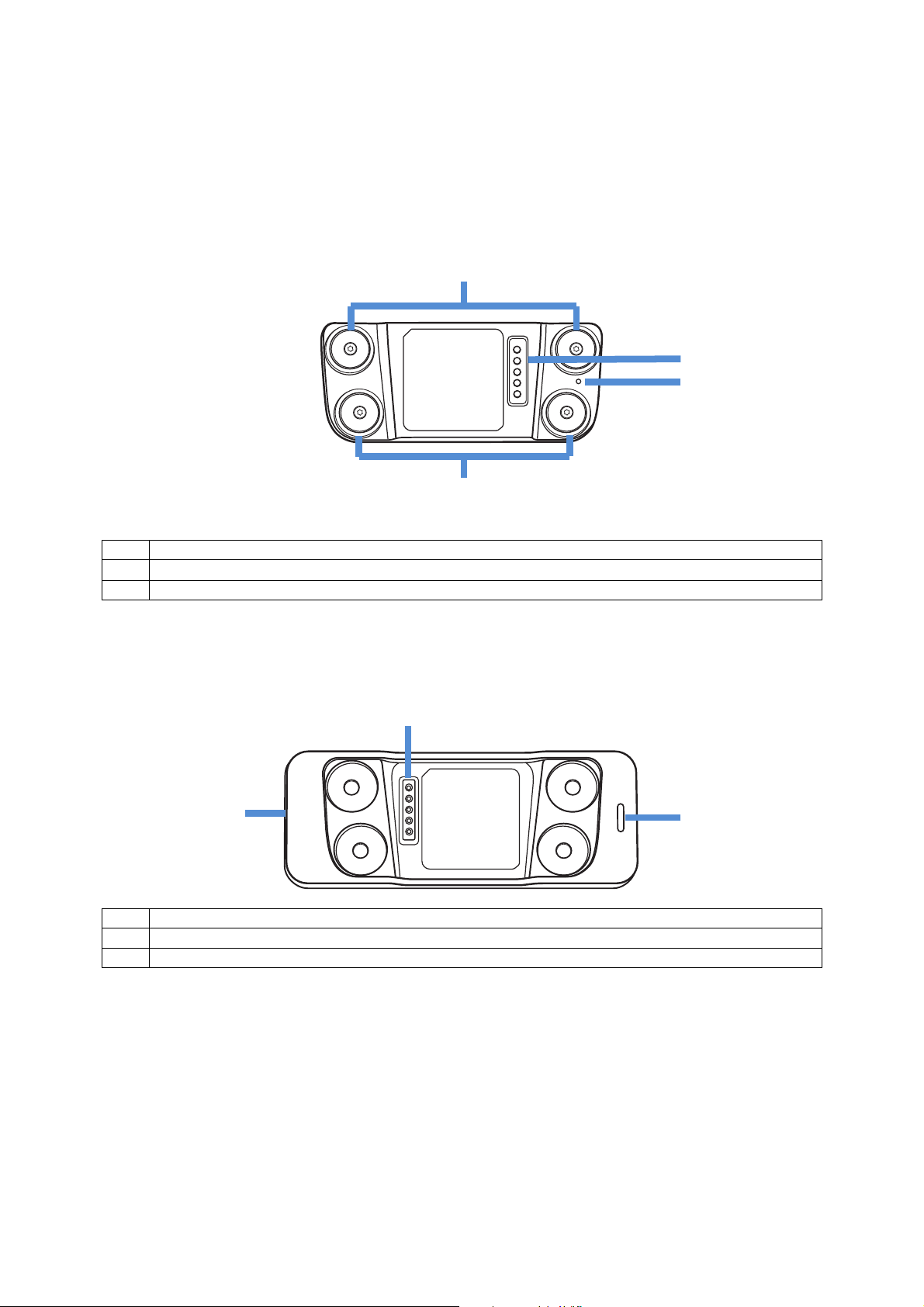
1 Patch Connectors
3
LED Indicators
3
3
Back
2 Charging Contacts
3 Bluetooth Pairing Button
Charger
1 Charging Contacts
2 USB Charging Port
Charging Indicator (see
for more information)
Page 12

Body Strap Patch
Front
1 Sensor Connectors
Back
1 Electrode Connectors
IMPORTANT: If any wear or damage to the wire shielding is found on the patch during use,
replace immediately.
Page 13

1 Electrode Connectors
Direct Wear Patch
Front
1 Sensor Connectors
Back
IMPORTANT: If any wear or damage to the wire shielding is found on the patch during use,
replace immediately.
Page 14

Product Requirements
In order to properly use the Smart Wearable ECG Monitoring Device the following items
are required:
• The ECG Sensor
• For Wear with the Body Strap B1: (A) The Body Strap Patch and (B) The Body Strap
B1
For Wear with the Body Strap B2: The Body Strap B2
For Direct Wear: (A) The Direct Wear Patch and (B) 3 ECG Electrodes*
NOTE: For more information on electrode specifications, see ECG Electrode Information.
• A Bluetooth-enabled Android Smartphone* (with Android version 5.0 or above and a
display resolution of 1920x1080 or 2560x1440)
• The ECG Sensor App Q-COR*
* Items not included in the product package.
Page 15

BEFORE YOU START
Before you start using the Smart Wearable ECG Monitoring Device you must:
1. Charge the battery on the Sensor
2. Install the Q-COR app on your Android smartphone
3. Enable Bluetooth on your smartphone
Charging the Battery
To charge the battery:
1. Plug the charger to a power source via the charging cable and adapter.
2. Place the sensor into the charger so that the charging contacts on both the sensor
and charger make contact.
3. Charge the sensor until the charging indicator on the charger lights up solid green
indicating that the battery is fully charged.
Installing the App
To install the app, search for and download “Q-COR” on Google Play Store.
NOTE: In order to install the Q-COR app your smartphone will need at least 5MB of storage capacity
available.
Enabling Bluetooth
To enable Bluetooth, enter the Settings menu on your Android smartphone and enable
Bluetooth.
Page 16

GETTING STARTED
Once you have completed the steps described in Before You Start, you can begin using
the Smart Wearable ECG Monitoring Device by following these steps:
1. Create a profile in the Q-COR app
2. Pair your smartphone to the ECG sensor via Bluetooth
3. Wear the Smart Wearable ECG Monitoring Device
Creating a Profile
The first time you launch the Q-COR app you will need to create a profile. Fill in the
following fields to create your profile:
• Name
• Gender
• Birthday
• Height
• Weight
• Resting Heart Rate
IMPORTANT: Filling in your correct resting heart rate is vital to the operation of the Smart
Wearable ECG Monitoring Device. To determine your resting heart rate measure your heart
rate after at least 5 minutes of inactivity, you may also request a trained professional to
measure your resting heart rate for a more accurate measure.
Once you have filled in your profile tap Done to continue.
Page 17

Pairing
After creating a profile, the Q-COR app will prompt you to pair the sensor to your
smartphone. To pair the sensor to your smartphone:
1. Use the pin included in the package to push the Bluetooth pairing button on the
sensor.
2. The status indicator on the sensor will alternately flash orange and green indicating
that it is in pairing mode.
3. Once the sensor is in pairing mode tap OK on your smartphone.
4. Wait until you get a message on your smartphone indicating that the pairing was
successful.
NOTE: After pushing the Bluetooth pairing button the sensor will stay in pairing mode for 1 minute. If
pairing has not been completed during that time push the pairing button and follow the steps above again.
NOTE: Not all smartphones can pair with the ECG sensor directly through the Q-COR app as Bluetooth
functionality may vary depending on smartphone model. In cases where pairing within the Q-COR app
fails (as shown in the screenshot below), enter the Bluetooth menu in the Android Settings menu
(Settings > Bluetooth > Pairing) and proceed to pair the two devices directly from there.
Page 18

Wearing the ECG Sensor
Depending on the model you have purchased you may either wear the ECG sensor
using the provided body strap, or wear the ECG sensor directly on your body.
IMPORTANT: When wearing the ECG sensor directly you must use separately purchased
electrodes for the device to work properly. For more information on electrode specifications see
ECG Electrode Information.
IMPORTANT: When wearing the ECG sensor either directly or with the body strap, ensure that
the contacts on the electrodes or the strap are in direct contact with your skin and that the
contact area on the skin is sufficiently clean and dry. You may wipe the skin clean with a wet
towel or alcohol swab and allow it to dry to before wearing the ECG sensor.
IMPORTANT: If the skin at the contact point shows signs of rash/blisters/reddening or other
discomfort, please contact a medical professional or physician.
Wearing the ECG Sensor with the Body Strap B1
To wear the ECG sensor using the body strap B1 follow the directions below:
1. Open up the front pocket of the body strap.
2. Attach the patch to the front of the body strap so that the left sensor side of the patch
attaches to the connector on the strap next to the ring loop (see image below).
3. Attach the ECG sensor to the front of the patch so that all four patch connectors on
the sensor are properly clicked into the four sensor connectors on the patch (see
image below).
Page 19

Sensor
Side
Ring
Loop
NOTE: Once connected, the status indicator on the sensor will light green then slowly flash indicating
that the sensor is connected to the patch, but that the patch has not properly made contact with a
body.
4. Fold over and adhere the Velcro tabs to close the front pocket of the body strap.
5. Wrap the body strap around your torso and tighten it so that it fits firmly around
middle of your torso.
6. Adjust the positioning of the strap so that the pocket is slightly under your pectoral
area and the midpoint between the first and second contact on the strap aligns with
the middle of the rib cage (see image below).
7. Once the ECG sensor is properly worn on your body the Q-COR app will start
showing an ECG reading.
Wearing the ECG Sensor with the Body Strap B2
To wear the ECG sensor using the body strap B2 follow the directions below:
1. Attach the ECG sensor to the front of the body strap so that all four patch connectors
on the sensor are properly clicked into the four sensor connectors on the body strap
(see image below).
NOTE: Once connected, the status indicator on the sensor will light green then slowly flash indicating
that the sensor is connected to the patch, but that the patch has not properly made contact with a
body.
Page 20

2. Wrap the body strap around your torso and then clip the strap clip to the front of the
strap (see image below). Tighten the body strap so that it fits firmly around middle of
your torso.
3. Adjust the positioning of the strap so that the pocket is slightly under your pectoral
area and the second contact on the strap aligns with the middle of the rib cage (see
image below).
4. Once the ECG sensor is properly worn on your body the Q-COR app will start
showing an ECG reading.
Direct Wear
To directly wear the ECG sensor on your body follow the directions below:
1. Attach an ECG electrode to each of the 3 connectors on the back of the patch (see
image below).
NOTE: For more information on electrode specifications, see ECG Electrode Information.
2. Attach the ECG sensor to the front of the patch so that all four patch connectors on
the sensor are properly clicked into the four sensor connectors on the patch (see
image below).
Page 21

NOTE: Once connected, the status indicator on the sensor will light green then slowly flash indicating
that the sensor is connected to the patch but that the patch has not properly made contact with a
body.
3. Unpeel the paper strips on the contact side of each of the ECG electrodes.
4. Adhere the 2 electrodes attached to the sensor side of the patch to under your left
pectoral area (see image below).
NOTE: The electrodes feature adhesive on the contact side to ensure that they adhere to your body,
if an electrode does not properly adhere please replace the electrode with a new one.
5. Adhere the electrode attached to the cable side of the patch to the area slightly
under your right collarbone (see image below).
6. Once the ECG sensor is properly worn on your body the Q-COR app will start
showing an ECG.
IMPORTANT: When directly wearing the ECG sensor, ensure that all 3 electrodes are firmly
adhered to your body and that neither parts of the patch are left dangling, as seen in the image
below, which may lead to damage or short-circuit if contact is made with another power
Page 22

supply.
Page 23

THE Q-COR APP
Profile
4
5
2
3
Main Screen
Once you have successfully paired and worn the ECG sensor, the Q-COR app’s main
screen will be displayed on your mobile device. The main screen displays a real-time
ECG which you can present to a medical professional for evaluation. The following
items can comprise the main screen:
1
No
1 Menu Button
2 Profile Settings
3 Real-Time ECG Displays your real-time ECG in 5 second intervals.
4 Battery Status Displays the current charge level for the battery.
5 Connection Status
NOTE: When using the Q-COR app the default Back button on your Android smartphone will be disabled,
in order to exit the Q-COR app you must use the Quit button in the Q-COR app’s menu, for more
Item Description
Tap to access the app menu. For more information see
NOTE: When using the Q-COR app the default Back button on
your Android smartphone will be disabled, in order to exit the QCOR app you must use the Quit button in the Q-COR app’s
menu, for more information see Quit.
NOTE: The Q-COR will trigger your smartphone to vibrate when
the battery on the sensor is low.
Menu.
Displays the profile settings that were entered during the
profile creation process. For more information on changing
profile settings, see
Displays the connection status for the ECG sensor. If the
ECG sensor is disconnected while in use, see Pairing for
instructions on pairing your device.
.
Page 24

information see Quit.
1
5
4
NOTE: The Q-COR will trigger your smartphone to vibrate when the battery on the sensor is low.
Menu
When you tap the menu button in the Q-COR app’s main screen, the app menu will
appear on the left side of the main screen. The menu allows you to: view either the realtime ECG or an ECG history, change profile settings, pair your ECG sensor to an
Android smartphone, reset the Q-COR app, or exit the Q-COR app.
History
Tap History in the Q-COR app menu to view a scrollable and scalable ECG history.
The following items can be found on the ECG history screen:
2
3
No
Item Description
Page 25

1 Date Button
or backward on your ECG history.
Display Options
2 Menu Button Tap to access the app menu.
3 ECG History
Tap to view an ECG from a different date, for more information,
see Date.
Displays your ECG history. Swipe left or right to move to forward
Tap to rescale the view of the ECG history, for more information,
see Date
When you tap the date button in the History screen a
calendar view will appear. The calendar view allows you to
view your ECG on days when an ECG was recorded
(indicated by a circled date) by tapping on the respective
date.
Display Options
4
Button
5 Timeline Scroll Bar
NOTE: The Q-COR app will store ECG data for up to 7 days.
Allows you to quickly scroll to a different point in time of your ECG
history. The numbers on the scroll bar represent the hour of the
day shown, and each point on the scroll bar represents 10
minutes.
Date
When you tap the date button in the History screen a calendar view will appear. The
calendar view allows you to view your ECG on days when an ECG was recorded
(indicated by a circled date) by tapping on the respective date.
NOTE: The Q-COR app will store ECG data for up to 7 days.
Page 26

Display Options
The ECG displayed on the Q-COR app is displayed on a grid where the horizontal axis
measures time (in seconds), and the vertical axis measures the electrical activity (in
millivolts). Each unit on the grid is 1mm in size. By changing the display options for the
ECG history you will be able to rescale the ECG on the grid for either a more detailed or
a more general view. The following display options are available for the ECG history:
• Speed: Using a lower speed option will shrink the ECG along the horizontal axis of
the grid, while using a higher speed option will expand the ECG along the horizontal
axis of the grid.
• Sensitivity: Using a lower sensitivity option will shrink the ECG along the vertical
axis of the grid, while using a higher sensitivity option will expand the ECG along the
vertical axis of the grid.
Page 27

Profile
Tap Profile in the Q-COR app menu to change the profile settings used in the Q-COR
app. Follow the same instructions in Creating a Profile to change the profile settings.
Pairing
Tap Pairing in the Q-COR app menu to pair/re-pair the ECG sensor to an Android
smartphone. Follow the same instructions in
Page 28

Pairing to pair the ECG sensor to a smartphone.
Reset
Tap Reset in the Q-COR app to reset the app so that all previous profile settings and
ECG history are deleted. Pairing information will still be stored on the device after
resetting.
Quit
Tap Quit in the Q-COR app to exit the app and return to the Android interface.
Page 29

ADDITIONAL INFORMATION
Indicator Color
Status
Item
Cleaning Method
CI3 Sensor
CI3 Charger
Wipe with a
dry cloth
Body Strap
B1/B2
LED Indicators
The following tables describe the indicators on both the sensor and charger:
Status Indicator (Sensor)
Indicator Color Status
Flashing Orange Battery Low
Alternate Green and Orange (1 min. intervals) Pairing in Progress
Green for 5 seconds followed by 3 flashes Connected to Sensor Connectors
Charging Indicator (Charger)
Solid Orange Charging In Progress
Solid Green Fully Charged
Cleaning
Below is a table that describes the cleaning methods for various items included with the
Smart Wearable ECG Monitoring Device:
Wipe with a dry cloth
Patch Carefully wipe with a dry cloth
Place within a wash bag for use in a washing machine;
DO NOT USE BLEACH;
DO NOT DRY IN A CLOTHES DRYER
ECG Electrode Information
When using the direct wear patch for the Smart Wearable ECG Monitoring Device you
must use separately purchased electrodes for the ECG sensor to work properly.
Suggested electrodes include Kendall Ag/AgCl electrodes or any electrodes that feature
the following specifications:
Page 30

• Adapter: Stud
USB
Battery
Battery
L
ife
Working
T
emp
erature
/
Altitude
Enclosure Rating
Weight
Dimension
• Biocompatibility:
ISO 10993 approved
Latex free
• CE Mark according to MDD93/42/EEC CE Marked
Specifications
Sensor Specifications
Continuous ECG data acquisition and calculation
Measuring Lead: Lead 1 (Lead 2 with direct wear patch)
Frequency Response: Monitor 0.1 to 40 Hz (-3db)
Heart rate measurement range: 30 – 240 bpm
ECG Sensor
Activity Detection
Energy Expenditure Calculated from HR and activity data
*HR is calculated based on R-R interval of ECG
**If HR calculated falls out of 30-240bpm range, Q-COR app will
display “--“.
Heart rate accuracy: ± 3 bpm or ±3% whichever is greater
Differential Input Impedance: > 10MΩ
Common Mode Rejection Ratio: > 70 dB
Sampling rate: 256Hz
Activity Status detection by G-sensor
G-sensor (3 axis): ±8g
G-sensor sampling rate: 52Hz
G-sensor accuracy: ±0.0156g
Lead-off Detection
Network
Humidity
Storage Temperature /
Humidity
Atmospheric Pressure
Range
Detect when the sensor gets loosened from patch or belts
Bluetooth 4.0 Dual mode (BT3.0+BLE)
Transmit distance: 10 meters (open space)
USB2.0
3.7V/140mAh
9 – 10 hours
5 – 45⁰C, 10% – 95% non-condensing
-20 – 60⁰C, 10% – 95% non-condensing
800 hPa to 1013 hPA
2000m
IP22
20g
69.89mm (L) x 29.6mm(W) x 10.7mm(H)
Page 31

Charger Specifications
Input
Working Temperature
Storage Temperature
-
20 – 60°
C
Dimension
90mm (L) x 34.4mm(W) x 13.66mm(H)
Charging indicator
Weight 25g
Green LED: Fully charge
Orange LED: Charging in process
5V/0.5A
0 – 40°C
Trouble shooting
1. Cannot pair CI3 sensor with Q-COR app: refer to the notes listed in the Pairing
chapter.
2. LED on cradle does not light up when Smart Wearable ECG Monitoring Device is
placed in: check the AC adaptor input or try to put Smart Wearable ECG
Monitoring Device in again.
Customer Support
For additional technical information, contact Quanta Customer Support Department:
Quanta Computer Inc.(QCI)
Address:
No. 188, Wenhua 2nd Rd., Guishan Dist., Taoyuan City 333, Taiwan
TEL: +886-3-327-2345
FAX: +886-3-318-4207
Email: MedicalSales@quantatw.com
EU Representative
EU Representative: MedNet GmbH
Address: Borkstrasse 10, 48163 Münster, Germany
Page 32

Federal Communications Commission (FCC) Statement
15.21
You are cautioned that changes or modifications not expressly approved by the part responsible for
compliance could void the user’s authority to operate the equipment.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
1) This device may not cause interference and.
2) This device must accept any interference, including interference that may cause undesired operation of
the device.
15.105(b)
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant
to part 15 of the FCC rules. These limits are designed to provide reasonable protection against harmful
interference in a residential installation.
This equipment generates uses and can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful interference to radio communications. However,
there is no guarantee that interference will not occur in a particular installation. If this equipment does
cause harmful interference to radio or television reception, which can be determined by turning the
equipment off and on, the user is encouraged to try to correct the interference by one or more of the
following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the receiver is
connected.
• Consult the dealer or an experienced radio/TV technician for help.
FCC RF Radiation Exposure Statement:
This equipment complies with FCC radiation exposure limits set forth for an uncontrolled environment.
End users must follow the specific operating instructions for satisfying RF exposure compliance. This
transmitter must not be co-located or operating in conjunction with any other antenna or transmitter.
Page 33

IP22
QUANTA COMPUTER INC.
MODEL : CI3
Use dedicated charger to charge.
MADE IN TAIWAN
SN: CI3S5451AF
SN: CI3S5451AF
Manufacture Date
The year of manufacture label and format of the wireless home health management system's body sensor,
charger and ECG electrodes are shown below:
Smart Wearable ECG Monitoring
Device (CI3)
Charger (CI3-C)
In the serial number on the product label, the
5th character starting from the left represents
the sensor and charger's year of manufacture,
while the 6th and 7th character represents the
week of manufacture. In the example shown
the “5” represents a manufacture year of 2015
and the “45” represents the 45th week of
2015.
Page 34

Supplier’s Declaration
The Quanta Smart Wearable ECG Monitoring Device conforms to the international EN 60601-1 and EN
60601-1-2 standards for electromagnetic compatibility with medical electrical devices and systems.
Emission Testing Compliant Standard Electromagnetic Environment Guidelines
The recommended electromagnetic environment for this instrument is shown in the table below. The
customer or user should ensure that this instrument is used in the described environment.
Radio-frequency is only used by the internal functions of
RF Emissions
CISPR 11
Group 1
this instrument. The emission energy is also very low
and does not interfere with nearby electronic
instruments.
RF Emissions
CISPR 11
Harmonic Emissions IEC 61000-3-2
Voltage
Fluctuations/
Flicker Emissions
Class B
This instrument is suitable for home use (including
residential or other mixed residential/public low-voltage
power grids)
IEC 61000-3-3
Testing IEC 60601-1-2 testing
The recommended electromagnetic environment for this instrument is shown in the table below. The
customer or user should ensure that it is used in the described environment.
Electrostatic
Discharge
(ESD)
IEC 61000-4-2
Power Frequency
(50/60Hz)
Magnetic Field
IEC 61000-4-8
±6 kV contact
±8 kV air
3 A/m 3 A/m Power frequency and magnetic
Compliant
Standard
±6 kV contact
±8 kV air
Electromagnetic Environment
Guidelines
The floor should be timber,
concrete or ceramic tile. If the
floor is covered in a synthetic
material then it should have a
relative humidity of at least
30%.
field in representative
commercial or hospital
environments.
Supplier's declaration and guidelines — Electromagnetic Immunity
Testing IEC 60601-1-2 testing
The recommended electromagnetic environment for this instrument is shown in the table below. The
customer or user should ensure that it is used in the described environment.
Compliant
Standard
Electromagnetic Environment Guidelines
Page 35

No part of the Quanta Smart Wearable ECG Monitoring Device should be placed near portable and
mobile radio-frequency communications equipment. The recommended safety distance can be
calculated using the radio frequency and the following formula.
Radio-frequency
IEC 61000-4-6
Radio-frequency
IEC 61000-4-3
The field intensity of fixed RF transmitters (e.g. radio, mobile phone, ham radio, FM and AM radio
broadcasts and TV stations) cannot be predicted from the frequency alone. To evaluate the EM field
intensity of the environment, an EM site survey may be necessary. If the measured field intensity for a
region exceeds the above recommended range, the device should be observed or checked to make sure
it operates normally. If abnormal operation is observed, further action such as adjusting the direction or
re-positioning of the equipment should be taken.
3 V/m
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
[3] V
[3] V/m
Recommended safety distance
d=1.17
d=1.17
d=2.33
P is the maximum output of the
transmitter (Unit: Watt)
d is the recommended safety distance
(Unit: Meter)
The field intensity of the fixed RF
transmitter (after an EM site survey)
should be lower than the recommended
values for the above frequency ranges.
Interference may be produced near
instruments that have the following
symbol:
P
P
80 MHz to 800MHz
P
800MHz to 2.5 GHz
Attention:
For frequencies between 80 MHz and 800 MHz, use the higher frequency range.
Attention:
These guidelines may not be applicable to all situations. Electromagnetic waves can be absorbed or
reflected by buildings, objects and people.
Recommended safety distance
The following table lists the recommended safety distance between the device and mobile RF
communications equipment.
The recommended EM environment for the Quanta Smart Wearable ECG Monitoring Device is where
RF interference is controlled. The user should maintain the recommended safety distance with the
devices and portable/mobile RF communications equipment listed in the following table (corresponding
to the maximum output of the RF transmitter).
Recommended Safety Distance (corresponding to RF transmitter output)
Maximum output of
RF transmitter (Watt)
150 kHz to 80 MHz
d=1.17
P
80 MHz to 800 MHz
d=1.17
P
800 MHz to 2.5 GHz
d=2.33
P
Page 36

0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.2 1.2 2.3
10 3.7 3.7 7.4
100 12 12 23
If the maximum output of the transmitter is not shown in the above table, use the formula to derive the
recommended safety distance d (Meter) with P (Watt) being the maximum output of the transmitter.
Attention:
For frequencies between 80 MHz and 800 MHz, use the higher frequency range.
Attention:
These guidelines may not be applicable to all situations. Electromagnetic waves can be absorbed or
reflected by buildings, objects and people.
Regulatory Marks
The Quanta Smart Wearable ECG Monitoring Device conforms to the following regulatory requirements.
Administrative Regulations on Low Power Radio Waves Radiated Devices (930322)
Article 12
Without permission granted by the NCC, any company, enterprise, or user is not allowed to change
frequency, enhance transmitting power or alter original characteristic as well as performance to an
approved low power radio-frequency devices.
Article 14
The low power radio-frequency devices shall not influence aircraft security and interfere with legal
communications. If found, the user shall cease operation immediately until no interference is achieved.
The said legal communications means radio communications is operated in compliance with the
Telecommunications Act. The low power radio-frequency devices must be susceptible with the
interference from legal communications or ISM radio wave radiated devices.
CE Mark: Indicates that the body sensor has been certified and conforms to
EC Directive 93/42/EEC on medical devices.
Type CF applied part
Indicates that the body sensor is classified as electrical or electronic
equipment requiring proper disposal (WEEE Directive)
Indicates the manufacturer's catalogue number
Attention: Catalogue number may also be referred to as the reference
number or reorder number.
Indicates the manufacture's serial number.
Page 37

Indicates the manufacturer's name and address
To indicate on the rating plate that the equipment is suitable for alternating
current only; to identify relevant terminals.
Indicates the need for the user to consult the instructions for use for important
cautionary information such as warnings and precautions that cannot, for a
variety of reasons, be presented on the medical device itself
Indicates the need for the user to consult the instructions for use.
 Loading...
Loading...