QIAGEN QIAreach Instruction Manual
Sample to Insight__
645033
QIAGEN GmbH, QIAGEN Strasse
R
L1121905EN
September 2020
QIAreach™ Anti-SARS-
CoV-2 Total Test
Instructions for Use
(Handbook)
For in vitro diagnostic use
This test has not been reviewed by the FDA.
Rx Only
Version 1
3
1, D-40724 Hilden

2
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020

QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
3
Contents
Intended Use .............................................................................................................. 5
Summary and Explanation of the Test ............................................................................ 7
Principles of the assay ....................................................................................... 7
Time required for performing the assay ............................................................... 9
Pipet use ......................................................................................................... 9
Kit Contents ............................................................................................................. 10
Materials Required but not Provided ........................................................................... 11
Storage and Handling ............................................................................................... 11
Equipment required but not provided ................................................................ 11
Kit reagents ................................................................................................... 11
Stability ......................................................................................................... 11
Warnings and Precautions ......................................................................................... 12
Limitations ..................................................................................................... 12
Precautions .................................................................................................... 12
Procedures ............................................................................................................... 15
Preparing samples .......................................................................................... 15
Detection assay .............................................................................................. 16
Results Analysis and Test Interpretation ........................................................................ 23
Quality control of test ...................................................................................... 23
Interpretation of results .................................................................................... 25
Limitations ..................................................................................................... 25
Performance Characteristics ....................................................................................... 27

4
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
Clinical performance ...................................................................................... 27
Positive and negative predictive value ............................................................... 28
Cross-reactivity ............................................................................................... 28
Interference .................................................................................................... 30
Matrix equivalency ......................................................................................... 30
Technical Information ................................................................................................ 32
Clotted plasma samples .................................................................................. 32
eHub display icons ......................................................................................... 32
Error codes .................................................................................................... 33
Troubleshooting Guide .............................................................................................. 35
Anti-CoV2 troubleshooting ............................................................................... 35
Additional user warnings................................................................................. 35
Contact Information .................................................................................................. 36
References ............................................................................................................... 37
Symbols ................................................................................................................... 40
Ordering Information ................................................................................................ 41
Document Revision History ......................................................................................... 42

QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
5
Intended Use
The QIAreach Anti-SARS-CoV-2 Total Test is a rapid, digital lateral flow serological test, using
nanoparticle fluorescence, intended for qualitative detection of total antibodies to SARS-CoV2 in human serum and plasma (heparin, EDTA). The QIAreach Anti-SARS-CoV-2 Total Test is
intended for use as an aid in identifying individuals with an adaptive immune response to
SARS-CoV-2, indicating recent or prior infection.
At this time, it is unknown for how long antibodies persist following infection and if the
presence of antibodies confers protective immunity. Testing is limited to laboratories certified
under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C 263a, to
perform high complexity tests.
Results are for the detection of SARS CoV-2 antibodies. Antibodies to SARS-CoV-2 are
generally detectable in blood several days after initial infection, although the duration of time
antibodies are present post-infection is not well characterized. Individuals may have detectable
virus present for several weeks following seroconversion.
Laboratories within the United States and its territories are required to report all positive results
to the appropriate public health authorities.
The sensitivity of the QIAreach Anti-SARS-CoV-2 Total Test early after infection is unknown.
Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected,
direct testing for SARS-CoV-2 is necessary.
False-positive results for the QIAreach Anti-SARS-CoV-2 Total Test may occur due to crossreactivity from pre-existing antibodies or other possible causes. Due to the risk of false positive
results, confirmation of positive results should be considered using a second, different blood
sample or performing a direct viral detection assay if medically indicated.

6
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
This test has not been reviewed by the FDA.

QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
7
Summary and Explanation of the Test
COVID-19 (coronavirus disease 2019) is the disease caused by SARS-CoV-2 (severe acute
1
respiratory syndrome coronavirus 2) viral infection.
2-4
symptomatic and presymptomatic
5-6
respiratory secretions.
7
of ~5 days.
The symptoms of COVID-19 are non-specific, ranging from asymptomatic to
The incubation period is estimated to be 4.6–5.8 days with a median
severe pneumonia and death.
individuals via respiratory droplets, aerosols, and upper
8
Fever and cough are the most common clinical symptoms but
also include shortness of breath, fatigue, muscle aches, headache, new loss of smell or taste,
sore throat, congestion or runny nose, diarrhea, and vomiting, which typically appear between
2–14 days following exposure to the virus.
8-10
2 will experience severe symptoms, including Acute Respiratory Distress Syndrome (ARDS)
11
that often requires mechanical ventilation.
The standard medical practice for definitive diagnosis of active SARS-CoV-2 infection relies on
the molecular detection of viral RNA.
12-14
simultaneously and shortly after viral infection with IgM ebbing rapidly in convalescence.
IgG antibody responses occur after IgM and IgA seroconversion and are longer sustained.
The QIAreach Anti-SARS-CoV-2 Total Test detects IgG, IgM, and IgA antibodies to SARS-CoV2 and is an indirect test for recent or prior infection in populations of interest. The clinical and
public health applications of serologic assays may include support to clinical assessment in
persons who present symptoms late in their illness and those suspected of having a postinfectious syndrome, understanding transmission dynamics in populations and identifying
18
asymptomatic persons previously infected.
The virus is readily transmitted from both
Roughly 20% of those infected with SARS-CoV-
Typically, IgA and IgM production occur
15-16
17
Principles of the assay
The QIAreach Anti-SARS-CoV-2 Total Test (Anti-CoV2) is a rapid serological test that detects
total antibody responses (principally IgM, IgG, and IgA) to expressed SARS-CoV-2 viral
antigens, in serum or plasma.

8
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
Antibody responses are measured on a single-use, lateral flow, digital detection cartridge
(eStick) via nanoparticle fluorescence. The eStick contains state-of-the-art optoelectronic
technology and a microprocessor that converts a fluorescent signal into a qualitative readout
for the presence of SARS-CoV-2 specific antibodies in patient test samples.
The Anti-CoV2 test is performed by inserting the Anti-CoV2 eStick into an QIAreach eHub (sold
separately). The QIAreach eHub is a connection hub that provides power to perform multiple
Anti-CoV2 tests simultaneously. The eHub acts as a power source and features a rechargeable
lithium battery to allow Anti-CoV2 tests to be performed when a continuous power supply is
not available.
To perform the test, Anti-CoV2 Diluent Buffer is first added to the Anti-CoV2 Processing Tube
and reconstitutes a SARS-CoV-2 viral S1 protein-nanoparticle conjugate that is spray dried on
an immobilized accretion pad within the tube. Patient serum or plasma is then added to the
Processing Tube and mixed with the resuspended conjugate. If anti-SARS-CoV-2 antibodies
are present in the sample, they will bind to the conjugate. The sample is then transferred from
the Processing Tube to the eStick sample port.
Once in the eStick, the test sample migrates on a nitrocellulose membrane and across the test
line. Anti-SARS-CoV-2 antibodies will bind to immobilized SARS-CoV-2 viral S1 protein at the
test line, where they will bridge the two SARS-CoV-2 S1 viral proteins on the test line and in
the conjugate. A photosensor will detect light emitted from the fluorescent nanoparticles in the
presence of excitation light filtered onto the test line. Signal is interpreted on the eStick
firmware and transmitted to the eHub, which then communicates a positive or negative test
result to the user by means of a visual display.
Anti-CoV2 test results are determined as Positive or Negative according to the assay result
algorithm on the eStick firmware.
Software is available to backup test results, generate test reports, and support online data
transfer.

QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
9
Time required for performing the assay
The time required to perform the Anti-CoV2 is estimated below. The time of testing multiple
samples when batched is also indicated.
Digital detection: Approx. 10 minutes for one test
(1 individual)
<20 minutes labor
Add up to 3 minutes for each extra eStick
Pipet use
This assay requires use of an adjustable volume pipet. Users should familiarize themselves with
pipet use prior to performing the Anti-CoV2 test.

10
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
Kit Contents
QIAreach Anti-SARS-CoV-2 Total
Test
Catalog number
Number of tests/pack
Anti-CoV2 Detection System
Components*
Anti-CoV2 eStick
Anti-CoV2 Processing Tube Packaged together with eStick in foil wrapper
Anti-CoV2 Diluent Buffer
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use (Handbook) 1
*See Warnings and Precautions for precautions and hazard statements.
Packaged together with Processing Tube in foil
wrapper
Contains recombinant SARS-CoV-2 S1 protein
and human serum albumin
Coated with recombinant SARS-CoV-2 S1 protein
and bovine serum albumin
Contains bovine serum albumin and ProClin®
300
645033
60
3 x 10 ml

QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
11
Materials Required but not Provided
Collection tubes for patient serum and plasma (lithium heparin, sodium heparin, K
EDTA, K
QIAreach eHub (including USB adapter and cable)*
EDTA)
3
Storage and Handling
Equipment required but not provided
Calibrated pipets* for delivery of 50 µl, 150 µl, and 300 µl with disposable tips
Optional: Centrifuge for isolating patient serum or plasma
Optional: Anti-CoV2 Software
Kit reagents
Store kit reagents at 2–30°C.
Stability
The test must be initiated within 60 minutes of opening the foil-wrapped eStick and
Processing Tube.
The Anti-CoV2 test should be performed in a test environment with ≤ 65% relative
humidity.
Refer to the expiration date printed on the device labeling for component shelf life.
Anti-CoV2 Diluent Buffer should be used within 3 months after opening the bottle.
2
* See Warnings and Precautions for precautions and hazard statements.
12
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
als in contact with
Warnings and Precautions
Limitations
This test has not been reviewed by the FDA.
Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is
suspected, direct testing for SARS-CoV-2 is necessary.
Results from antibody testing should not be used to diagnose or exclude acute SARS-CoV-
2 infection.
Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus
strains, such as coronavirus HKU1, NL63, OC43, or 229E.
Not for the screening of donated blood
Precautions
When working with chemicals, always wear a suitable lab coat, disposable gloves, and eye
protection goggles. For more information, please consult the appropriate safety data sheets
(SDSs).
CAUTION
The following hazards and precautionary statements apply to components of the Anti-CoV2
kit.
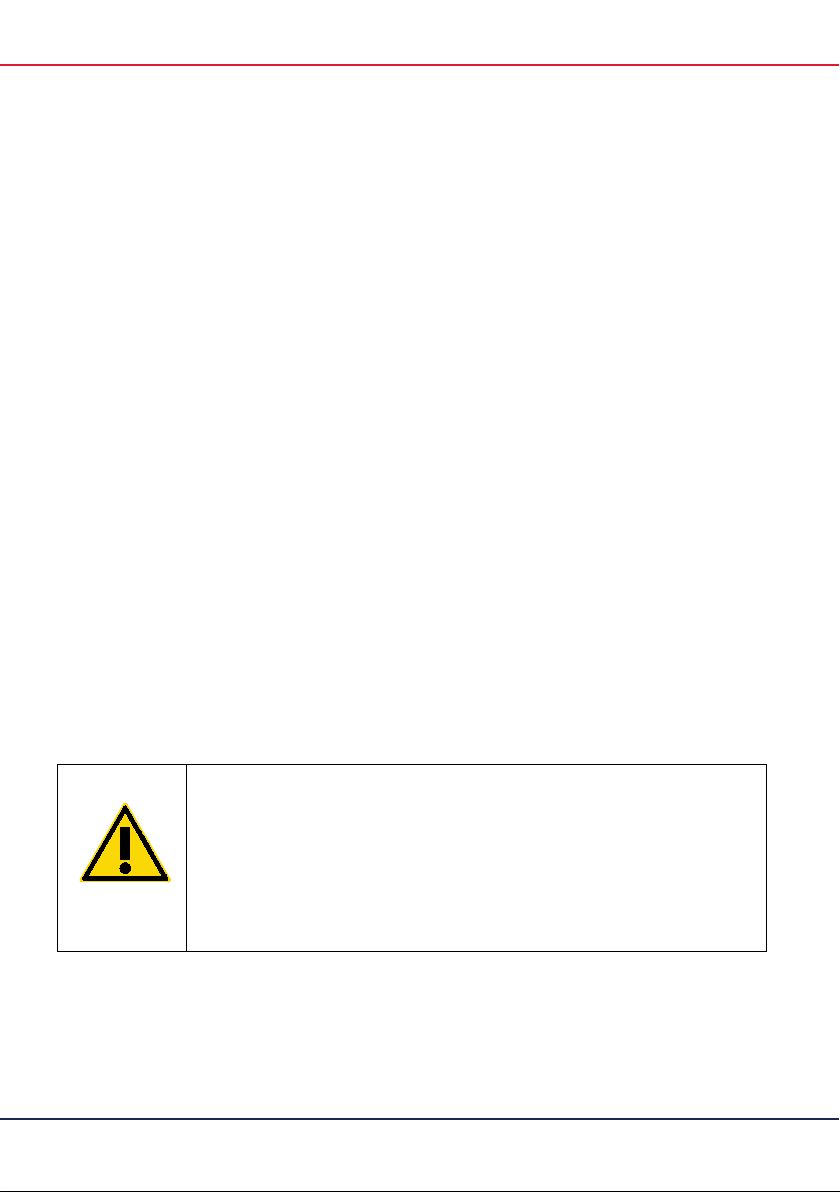
Handle all human blood, serum, and plasma as if potentially
infectious. Observe relevant blood and blood product handling
guidelines. Dispose of samples and materi
blood or blood products in accordance with federal, state, and
local regulations.
(C1)
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
13
clothing/ eye
Hazard statements
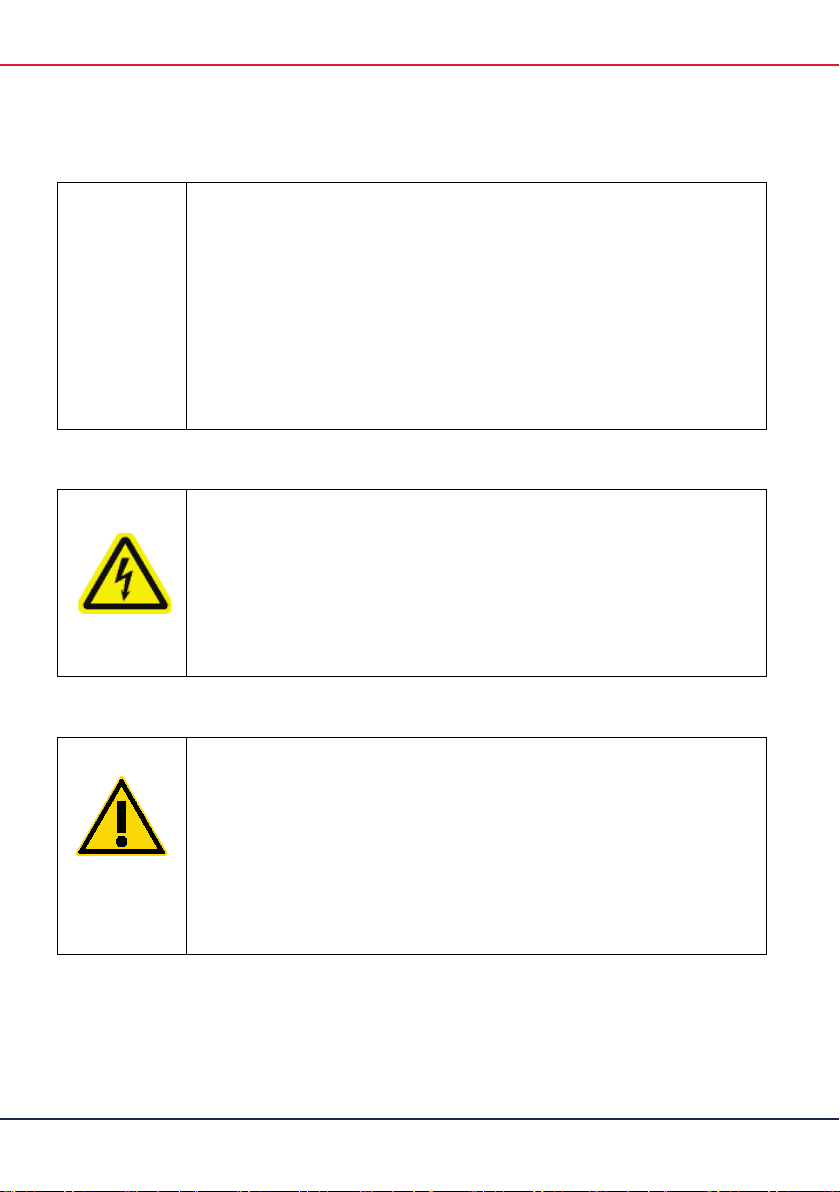
WARNING QIAreach Anti-SARS-CoV2 Total Diluent Buffer
Contains: Mixture of 2-methyl-1,2-thiazol-3(2H)-one, and 5chloro-2-methyl-1,2-thiazol-3(2H)-one. Warning! May cause an
allergic skin reaction. Harmful to aquatic life with long lasting
effects. Wear protective gloves/ protective
protection/ face protection.
WARNING QIAreach eHub
Do not open the eHub. No serviceable parts inside. Opening
of the eHub device could lead to electric shock or damage of
the device.
CAUTION QIAreach Anti-SARS-CoV-2 Total eStick
(W1)
(W2)
(C2)
Do not open the eStick. No serviceable parts inside. Opening
of the eStick could lead to user exposure of infectious patient
body fluids. Opening the eStick could also damage the eStick
device.

14
QIAreach Anti-SARS-CoV2 Total Test Instructions for Use 09/2020
Further information
Deviations from the QIAreach Anti-SARS-CoV-2 Total Test Instructions for Use may yield
erroneous results. Please read the instructions carefully before use.
Important: Inspect materials prior to use. Do not use kit if the Diluent Buffer, Processing
Tube, or eStick show signs of damage or leakage, or if the seals have been
compromised prior to use. Do not handle broken eSticks.
Discard used or unused materials and biological samples in accordance with local and
government regulations.
Do not use the Anti-CoV2 kit after the expiration date.
Do not mix consumables and reagents from multiple lots.
 Loading...
Loading...