Qiagen GeneReader User Manual

Sample to Insight__
December 2017
QIAGEN
GeneReader
®
User Manual
For use with Advanced Process Flow (APF/HP) Instrument
Configuration and GeneReader™ Software version 1.6
For Research Use Only. Not for use in diagnostic procedures.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
2
Contents
Instrument Configuration History ...........................................................................................6
1
Introduction .............................................................................................................8
1.1 About this user manual ....................................................................................8
1.2 General information ........................................................................................9
1.2.1 Technical assistance ................................................................................9
1.2.2 Policy statement ......................................................................................9
1.3 Intended use of the GeneReader .......................................................................9
1.4 Requirements for GeneReader users ................................................................10
2
Safety Information ..................................................................................................11
2.1 Proper use ...................................................................................................12
2.2 Electrical safety ............................................................................................14
2.3 Environment .................................................................................................15
2.3.1 Operating conditions ............................................................................15
2.4 Chemicals....................................................................................................16
2.5 Waste disposal ............................................................................................16
2.6 Mechanical hazards .....................................................................................17
2.7 Maintenance safety ......................................................................................17
2.8 Symbols on the GeneReader ..........................................................................18
3
General Description ...............................................................................................20
3.1 QIAGEN GeneReader Sample to Insight® NGS workflow .................................20
3.2 GeneReader principle ...................................................................................21
3.3 External features of the GeneReader ...............................................................22
3.3.1 Status lights ..........................................................................................22
3.3.2 Flow Cell door .....................................................................................22
3.3.3 Hood ..................................................................................................23
3.3.4 Fluidic drawer ......................................................................................24
3.3.5 USB ports ............................................................................................24
3.3.6 Power switch ........................................................................................24
3.3.7 Power cord socket ................................................................................24

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
3
3.3.8 Cooling air outlet..................................................................................24
3.3.9 Workstation equipment .........................................................................25
3.4 Internal features of the GeneReader ................................................................25
3.4.1 GeneReader Flow Cell ..........................................................................25
4
Installation Procedures ............................................................................................27
4.1 System delivery and installation ......................................................................27
4.2 Site requirements ..........................................................................................27
4.3 AC Power and USB cable connections ............................................................28
4.3.1 Power requirements ..............................................................................28
4.3.2 Grounding requirements ........................................................................29
4.3.3 Installation of AC power cords, USB cables and workstation .....................29
4.3.4 Installation of AC power cords, USB cables and workstation using an
optional UPS ....................................................................................................29
4.4 Workstation requirements ..............................................................................30
4.5 Getting started .............................................................................................31
4.5.1 Powering ON the GeneReader and workstation .......................................31
4.5.2 Software upgrade .................................................................................32
5
Operating Procedures .............................................................................................34
6
GeneReader Software ............................................................................................35
6.1 Use of the GeneReader software ....................................................................35
6.1.1 Start-up ...............................................................................................35
6.1.2 Software workflow ................................................................................35
6.1.3 User interface .......................................................................................36
6.1.4 Status of Flow Cells view .......................................................................37
6.1.5 Status of Fluids view ..............................................................................38
6.1.6 Status of Configuration view ..................................................................39
6.1.7 File handling ........................................................................................41
6.2 Workflow procedures ...................................................................................41
6.2.1 Flow Cell setup .....................................................................................41
6.2.2 Loading and running the GeneReader .....................................................43
6.2.3 Staggered loading of Flow Cells ............................................................43

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
4
6.2.4 Unloading reagents and Flow Cells ........................................................43
6.2.5 Run finished .........................................................................................43
7
Maintenance .........................................................................................................48
7.1 Equipment and reagents to be supplied by user ...............................................48
7.2 Maintenance wash .......................................................................................48
7.2.1 Routine maintenance (DI water wash) ......................................................48
7.2.2 Weekly maintenance ............................................................................51
7.2.3 Preparing the GeneReader fluidics lines for long-term storage ....................58
7.2.4 Monthly cleaning procedure ..................................................................59
7.3 General cleaning procedures .........................................................................59
7.3.1 Cleaning agents ...................................................................................59
7.3.2 General instructions ..............................................................................60
7.3.3 Servicing .............................................................................................61
7.4 Cleaning the workstation hard disk .................................................................61
7.4.1 Procedure ............................................................................................61
7.5 Creating a service archive folder for troubleshooting ........................................62
7.5.1 Procedure ............................................................................................62
8
Troubleshooting .....................................................................................................64
8.1 Hardware and software errors .......................................................................64
8.1.1 Application module ..............................................................................64
8.1.2 Manage Files module ............................................................................64
8.1.3 Flow Cells ............................................................................................65
8.1.4 Run module ..........................................................................................65
8.1.5 Start Run module ..................................................................................65
9
Technical Data .......................................................................................................66
9.1 Environmental conditions – operating conditions ..............................................66
9.2 Mechanical data and hardware features .........................................................66
9.3 Workstation specifications (hardware and software) .........................................66
9.3.1 Workstation .........................................................................................66
9.3.2 Software ..............................................................................................67
Appendix A .....................................................................................................................68

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
5
Declaration of Conformity .......................................................................................68
License Terms .........................................................................................................69
Waste Electrical and Electronic Equipment (WEEE) ....................................................70
FCC Declaration ....................................................................................................71
Liability Clause ......................................................................................................72
Appendix B .....................................................................................................................73
Ordering Information ..............................................................................................73
Appendix C .....................................................................................................................74
Safety Information Translated into German and French ...............................................74
Sicherheitsinformationen ....................................................................................74
Informations de Sécurité .....................................................................................74
Index ............................................................................................................................86
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
6
Instrument Configuration History
Document title
Document
number Date
Description of changes and
specifications
Compatible software and
sequencing kits
QIAGEN
GeneReader
User Manual
for use with
APF/HP
Instrument
Configuration
HB-2503001
December 2017
Supports Advanced Process
Flow (APF) workflow and UMI
Advanced (HP) chemistry
compatible with:
• GeneRead™ QIAact
Actionable Insights Tumor
Panel, cat. no. 181910
• GeneRead QIAact BRCA 1/2
Panel, cat. no. 181920
• GeneRead QIAact Lung DNA
UMI Panel, cat. no. 181931
• GeneRead QIAact Lung
Fusion UMI Panel, cat. no.
181936
• Any future GeneRead QIAact
panel with UMI technology
Updated the following
specifications:
• Higher data output, >7%
• Enhanced maintenance wash
procedure
GeneReader instruments
released prior to the Advanced
Process Flow instrument
configuration can be upgraded
to the APF/HP instrument
configuration.
GeneReader Software
version 1.6
QIAGEN GeneRead® UMI
Advanced Sequencing Q Kit
(3), cat. no. 185251, which
consists of:
• GeneRead UMI
Advanced Sequencing Q
Add-Ons (3), cat. no.
1108488
• GeneRead UMI
Advanced Sequencing Q
Flow Cell (3), cat. no.
1108489
• GeneRead UMI
Advanced Sequencing Q
Buffers (3), cat. no.
1108487
GeneRead UMI Advanced
Sequencing Q Wash Buffers
(9), cat. no. 185905,
purchased separately
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
7
Document title
Document
number
Date
Description of changes and
specifications
Compatible software and
sequencing kits
QIAGEN
GeneReader
User Manual
for use with
APF Instrument
Configuration
HB-2325001
December 2016
Supports Advanced Process
Flow (APF) workflow compatible
with:
•
GeneRead™ QIAact
Actionable Insights Tumor
Panel, cat. no. 181910
•
GeneRead QIAact Lung
DNA Panel, cat. no.
181930
•
GeneRead QIAact BRCA
1/2 DNA Panel
Updated the following
specifications:
• Higher read length, >30%
• Higher data output, >50%
• Higher sample
multiplexing capacity per
flow cell for the GeneRead
QIAact Actionable Insights
Tumor Panel (ATP)
• Approximately 20% less
reagent consumption per
cycle
• Equal or less sequencing
time
• Parallel or staggered
processing of up to 3 flow
cells
GeneReader instruments
released prior to the Advanced
Process Flow instrument
configuration can be upgraded
to the APF instrument
configuration
GeneReader Software
version 1.4.0
QIAGEN GeneRead
Advanced Sequencing Q Kit
(3), cat. no. 185231
GeneRead Sequencing Buffer
Q Kit (16), cat. no. 185901
IMPORTANT: When used in
combination with the
QIAGEN GeneRead
Advanced Sequencing Q Kit,
the GeneRead Sequencing
Buffer Q Kit (16) will allow
the user to process 6 Flow
Cells.
GeneReader
User Manual
HB-2023001
December 2015 Specifications:
• Compatible with
GeneRead QIAact Panels,
Powered by QCI™, cat.
no. 181910
• Approximately 45 hours
sequencing time
• Parallel or staggered
processing of up to 4 Flow
Cells
GeneReader Software up to
version 1.1.3
GeneRead Sequencing Q Kit
(1), cat. no. 185200
GeneRead Sequencing Q Kit
(4), cat. no. 185201
GeneRead Sequencing Buffer
Q Kit, (16) cat. no. 185901

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
8
1 Introduction
Thank you for choosing the GeneReader. We are confident it will become an integral part of your
laboratory.
Before using the GeneReader, it is essential that you read this user manual carefully and pay
particular attention to the safety information. The instructions and safety information in the user
manual must be followed to ensure safe operation of the instrument and to maintain the instrument
in a safe condition.
1.1 About this user manual
This user manual provides information about the GeneReader in the following sections:
Instrument Configuration History
Introduction
Safety Information
General Description
Installation Procedures
Operating Procedures
Maintenance
Troubleshooting
Technical Data
Appendices
Index
The appendices contain the following information:
Declaration of Conformity
License Terms
Waste Electrical and Electronic Equipment (WEEE)
FCC Declaration
Liability Clause
GeneReader Accessories
Safety Information Translated into German and French

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
9
1.2 General information
1.2.1 Technical assistance
At QIAGEN, we pride ourselves on the quality and availability of our technical support. Our
Technical Services Departments are staffed by experienced scientists with extensive practical and
theoretical expertise in molecular biology and the use of QIAGEN® products. If you have any
questions or experience any difficulties regarding the GeneReader or QIAGEN products in general,
do not hesitate to contact us.
QIAGEN customers are a major source of information regarding advanced or specialized uses of
our products. This information is helpful to other scientists as well as to the researchers at QIAGEN.
We therefore encourage you to contact us if you have any suggestions about product performance
or new applications and techniques.
For technical assistance, contact QIAGEN Technical Services (see back cover).
1.2.2 Policy statement
It is the policy of QIAGEN to improve products as new techniques and components become
available. QIAGEN reserves the right to change specifications at any time. In an effort to produce
useful and appropriate documentation, we appreciate your comments on this user manual. Please
contact QIAGEN Technical Services (see back cover).
1.3 Intended use of the GeneReader
The GeneReader is designed to perform next-generation sequencing (NGS) applications by
integrating highly parallel fluorescence-based sequencing chemistry with detection of the
corresponding fluorescent signals from templates that have been clonally amplified using the
GeneRead QIAcube®.
GeneReader software provides a wizard for setting up the sequencing, data storage management,
and the functionality for base calling and generation of FASTQ files.
The GeneReader is intended to be used only in combination with QIAGEN kits indicated for use
with the GeneReader for applications described in the respective QIAGEN kit product sheets or
handbooks.
The GeneReader is intended for Research Use Only. Not for use in diagnostic procedures.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
10
The GeneReader is intended for use by professional users trained in molecular biological techniques
and in the operation of the GeneReader.
1.4 Requirements for GeneReader users
Table 1covers the general level of competence and training necessary for transportation,
installation, use, maintenance and servicing of the GeneReader.
Table 1. Recommended training and skill proficiency to use, maintain and service GeneReader.
Task Personnel Training and experience
Transportation No special requirements No special requirements
Installation
QIAGEN Field Service Specialists
only
Special training required
System relocation
QIAGEN Field Service Specialists
only
Special training required
Routine use (running protocols) Laboratory technicians or equivalent
Appropriately trained and experienced
personnel familiar with use of
computers and automation in general
Regular and weekly maintenance Laboratory technicians or equivalent
Appropriately trained and experienced
personnel familiar with use of
computers and automation in general
Annual preventative maintenance and
servicing
QIAGEN Field Service Specialists
only
Special training required
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
11
2 Safety Information
Before using the GeneReader, it is essential that you read this user manual carefully and pay
particular attention to the safety information. The instructions and safety information in the user
manual must be followed to ensure safe operation of the instrument and to maintain the condition
of the instrument to ensure its continued safe operation.
IMPORTANT: safety information in German (Sicherheitsinformationen) and French (Informations
de Sécurité) is provided in Appendix C, page 74.
The following types of safety information appear throughout the QIAGEN GeneReader User Manual
for Advanced Process Flow (APF/HP) Instrument Configuration.
WARNING The term WARNING is used to inform you about situations that could result in
personal injury to you or others.
Details about these circumstances are given in a box like this one.
CAUTION
The term CAUTION is used to inform you about situations that could result in
damage to an instrument or other equipment.
Details about these circumstances are given in a box like this one.
The guidance provided in this manual is intended to supplement, not supersede, the normal safety
requirements prevailing in the user’s country.
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
12
2.1 Proper use
WARNING Risk of personal injury and material damage
(W1)
Improper use of the GeneReader may cause personal injuries or damage to the
instrument.
The GeneReader must only be operated by qualified personnel who have been
appropriately trained.
Servicing of the GeneReader instrument must only be performed by a QIAGEN
Field Service Specialist.
Perform the maintenance as described in Section 7. QIAGEN charges for repairs that are required
due to incorrect maintenance.
WARNING Risk of personal injury and material damage (W2)
The GeneReader is too heavy to be lifted by one person. To avoid personal
injury or damage to the instrument, do not lift the instrument alone.
Contact QIAGEN Technical Services to relocate the instrument.
WARNING Risk of personal injury and material damage (W3)
Do not attempt to move the GeneReader during operation.
WARNING Risk of personal injury and material damage (W4)
Load flow cell only in accordance with step-by-step instructions provided by
GeneReader software. Beware of moveable parts.
WARNING Risk of personal injury and material damage
(W5)
Do not stare into the beam of the flow cell bar code reader.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
13
CAUTION
Risk of material damage (C1)
Avoid moving the workbench and causing vibrations to the GeneReader during
operation to prevent disturbing sensitive optical measurements.
CAUTION
Damage to the instrument (C2)
Avoid spilling water or chemicals onto the GeneReader. Damage caused by
water or chemical spillage will void your warranty.
CAUTION
Risk of material damage (C3)
Do not place any items on top of the instrument.
In case of emergency, power OFF the GeneReader using the power switch at the right, rear panel
of the instrument and unplug the power cord from the power outlet.
CAUTION
Damage to the instrument (C4)
Only use QIAGEN consumables with the GeneReader. Damage caused by use
of other types of consumables will void your warranty.
CAUTION
Damage to the instrument (C5)
Make sure that the flow cell is inserted in the correct position. Incorrect insertion
of the flow cell can damage the instrument.
WARNING Fire hazard (W6)
Empty the liquid waste bottle before each run and make sure to place it in the
correct orientation back in the GeneReader instrument. Spilling of liquid-waste
may cause an electrical short-circuit and fire.
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
14
2.2 Electrical safety
Disconnect the line power cord from the power outlet before servicing.
WARNING Electrical hazard (W7)
Any interruption of the protective conductor (earth/ground lead) inside or
outside the instrument or disconnection of the protective conductor terminal is
likely to make the instrument dangerous.
Intentional interruption is prohibited.
Lethal voltages inside the instrument
When the instrument is connected to line power, terminals may be live and
opening covers or removing parts is strongly discouraged as doing so may
result in electric shock or death.
WARNING Damage to electronics (W8)
Before powering ON the instrument make sure that the correct supply voltage
is used.
Incorrect use of supply voltage may cause damage to electronics.
See specifications indicated on the type plate of the instrument.
WARNING Risk of electric shock (W9)
Do not open any panels on the GeneReader.
Risk of personal injury and material damage
Only perform maintenance that is specifically described in this user manual.
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
15
To ensure satisfactory and safe operation of the GeneReader, follow the advice below:
The line power cord must be connected to a line power outlet that has a protective conductor
(earth/ground).
Place instrument in a location so that the power cord is accessible and can be
connected/disconnected.
Use only the power cord delivered by QIAGEN.
Do not adjust or replace internal parts of the instrument.
Do not operate the instrument with any covers or parts removed.
If liquid has spilled inside the instrument, switch off the instrument, disconnect it from the
power outlet and contact QIAGEN Technical Services.
If the instrument becomes electrically unsafe, prevent other personnel from operating it and contact
QIAGEN Technical Services (see back cover).
The instrument may be electrically unsafe when:
It or the line power cord appears to be damaged.
It has been stored under unfavorable conditions for a prolonged period.
It has been subjected to severe transport stresses.
2.3 Environment
2.3.1 Operating conditions
WARNING Explosive atmosphere (W10)
The GeneReader is not designed for use in an explosive atmosphere.
WARNING Risk of explosion (W11)
The GeneReader is intended for use with reagents and substances supplied with
QIAGEN kits. Use of other reagents and substances may lead to fire or
explosion.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
16
CAUTION
Damage to the instrument (C6)
Direct sunlight may bleach parts of the instrument and cause damage to plastic
parts.
The GeneReader must be located out of direct sunlight.
2.4 Chemicals
WARNING Hazardous chemicals (W12)
Some chemicals used with this instrument may be hazardous or may become
hazardous after completion of the protocol run. Always wear safety glasses,
gloves, and a lab coat. The responsible body (e.g., laboratory manager) must
take the necessary precautions to ensure that the surrounding workplace is safe
and that the instrument operators are not exposed to hazardous levels of toxic
substances (chemical or biological) as defined in the applicable Safety Data
Sheets (SDSs) or OSHA,* ACGIH† or COSHH‡ documents.
Venting for fumes and disposal of wastes must be in accordance with all
national, state, and local health and safety regulations and laws.
* OSHA: Occupational Safety and Health Administration (United States of America).
†
ACGIH: American Conference of Government Industrial Hygienists (United States of America).
‡
COSHH: Control of Substances Hazardous to Health (United Kingdom).
2.5 Waste disposal
Used labware may contain hazardous chemicals. Waste must be collected and disposed of
properly according to local safety regulations.
For more information about how to dispose of the GeneReader instrument, see “Waste Electrical
and Electronic Equipment (WEEE)” in Appendix A, page 70.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
17
WARNING Hazardous chemicals and infectious agents
(W13)
The waste contains samples and reagents. This waste may contain toxic or
infectious material and must be disposed of properly. Refer to your local safety
regulations for proper disposal procedures.
2.6 Mechanical hazards
The door of the GeneReader must remain closed during operation of the instrument. Only handle
the flow cell loading station when the flow cell door has been released by the software.
Note: Only power OFF the instrument if the process has been properly terminated by the
software and the flow cell door is closed.
WARNING Moving parts (W14)
To avoid contact with moving parts during operation of the GeneReader, the
instrument must be operated with the flow cell door closed.
If the door sensor is not functioning correctly, contact QIAGEN Technical
Services.
WARNING Risk of overheating (W15)
To ensure proper ventilation, maintain a minimum clearance of 10 cm at the
sides and rear of the GeneReader.
Slits and openings that ensure the ventilation of the GeneReader must not be
covered.
2.7 Maintenance safety
WARNING Risk of personal injury and material damage
(W16)
Only perform maintenance that is specifically described in this user manual.
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
18
CAUTION
Damage to the instrument (C7)
Do not use bleach, solvents, or reagents containing acids, alkalis, or abrasives
to clean the GeneReader.
2.8 Symbols on the GeneReader
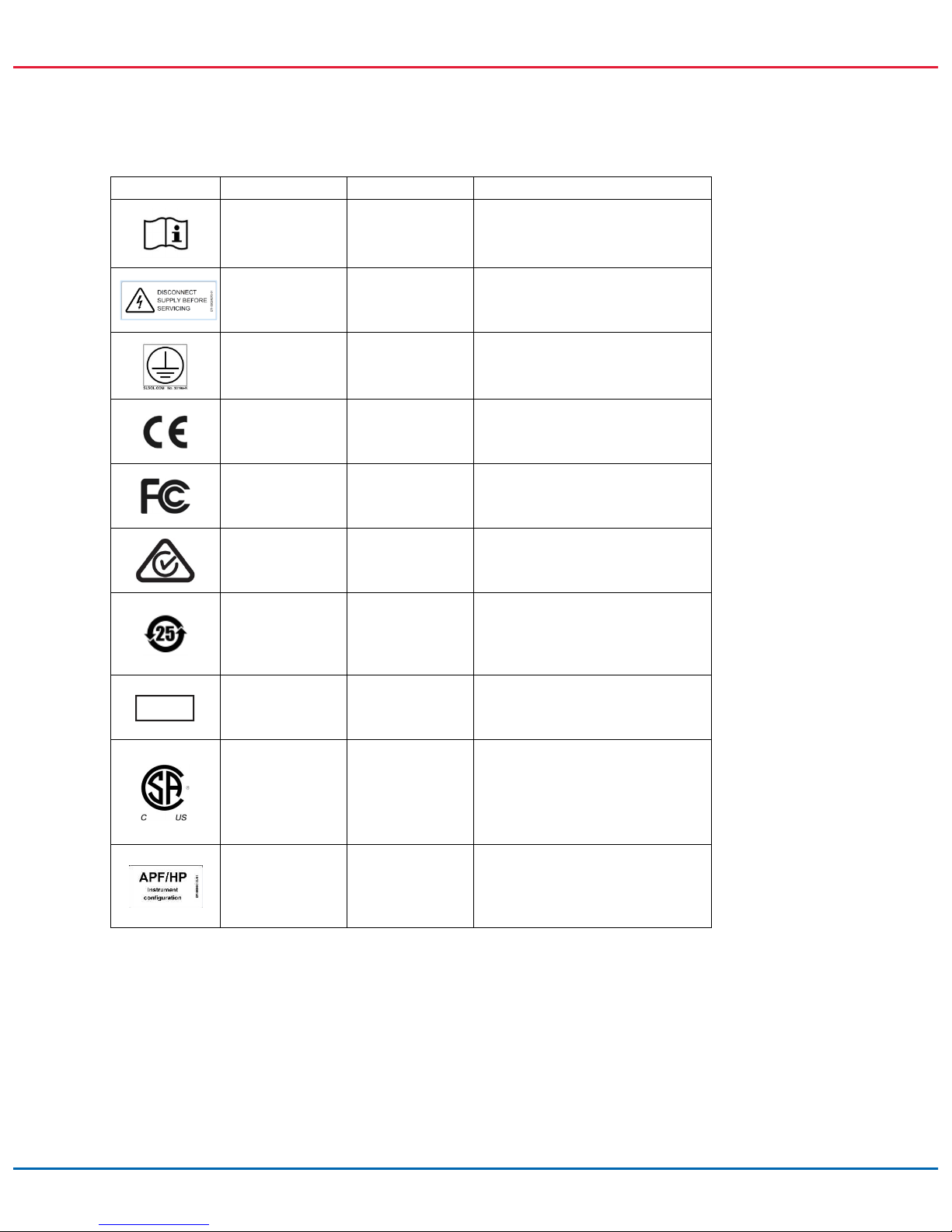
Symbol Location Language Description
Inside instrument – Heat hazard – do not perform
maintenance before the system has
cooled down.
On the instrument – Mechanical hazard – avoid contact
with moving parts.
On front of the
instrument, open
door
– Mechanical hazard – avoid contact
with moving parts.
Inside instrument – Electric shock hazard
On the
instrument, right
side panel
EN This product contains a class 2
laser. Do not stare into the beam.
On the
instrument, right
side panel
FR This product contains a class 2
laser. Do not stare into the beam.
On front of the
instrument, open
door
– This product contains a class 2
laser. Do not stare into the beam.
Type plate on the
right side panel
– WEEE about the disposal of waste
electrical and electronic equipment
for Europe and rest of the world.
Type plate on the
right side panel
– Legal manufacturer.
QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
19
Symbol Location Language Description
On the
instrument, right
side panel
– Consult instructions for use.
On the
instrument, right
side panel
EN Disconnect power supply before
servicing.
Inside instrument – Earth (Ground)
Type plate on the
back of the
instrument
– CE mark for Europe
Type plate on the
back of the
instrument
– FCC mark of the United States
Federal Communications
Commission
Type plate on the
back of the
instrument
– RCM (former C-Tick) for Australia
(supplier identification N17965)
Type plate on the
back of the
instrument
– RoHS mark for China (the restriction
of the use of certain hazardous
substances in electrical and
electronic equipment)
Type plate on the
back of the
instrument
– Instrument serial number
Type plate on the
back of the
instrument
– Certification mark
Indicates that the product was tested
and has met the certification
requirements for electrical, plumbing
and/or mechanical products
Sticker on the
instrument, right
side panel
– Sticker indicating GeneReader with
Advanced Process Flow (APF) and
UMI Advanced (HP) instrument
configuration
SN

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
20
3 General Description
The QIAGEN GeneReader performs fully automated next-generation sequencing (NGS) by
integrating highly parallel fluorescence-based sequencing chemistry with detection of the
corresponding fluorescent signals on templates that have been clonally amplified using the
GeneRead QIAcube.
The GeneReader sequencer consists of the GeneReader, the workstation, the GeneReader software
and a handheld bar code scanner that connects to the workstation for scanning bar codes of kits
and buffers, which are then automatically entered into the GeneReader software. The initial software
installation is performed by a QIAGEN Field Service Specialist. There are two USB connections
between the GeneReader instrument and workstation. The GeneReader sequencer includes several
additional components, which are listed in Section 4.1.
The GeneReader software provides a FASTQ file of sequence information for each analyzed sample
that is ready for QCI Analyze or GeneRead Link. QCI Analyze automatically runs an optimized
workflow for GeneReader panels and generates a VCF result file that is ready for upload to QCI
Interpret.
3.1 QIAGEN GeneReader Sample to Insight® NGS workflow
The QIAGEN GeneReader Sample to Insight workflow provides a streamlined and standardized
approach to next-generation sequencing (NGS), from sample preparation to the biological
interpretation of sequencing data.
The majority of the workflow is automated, ensuring greater standardization and more accurate
results.
QIAGEN Clinical Insight™ combines analytical tools and integrated human disease content,
providing access to current and advanced interpretations of genomic data. QCI Analyze or
GeneRead Link automatically sends samples through predefined workflows and provides a webbased result viewer. Afterwards, QCI Interpret (another web-based viewer) provides a biological
interpretation.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
21
3.2 GeneReader principle
The workflow includes the following six (6) processes:
sequencing primer hybridization
Flow Cell preparation
reagent preparation
experiment set-up
Flow Cell loading and start of run
post-run maintenance wash
The principle behind a sequencing run on the GeneReader is described below.
The first step of the GeneReader sequencing-by-synthesis technology consists of the incorporation
of unique deoxyribonucleotide triphosphates (dNTPs) which are both fluorescently labelled and
reversibly terminated. These are known as “labeled nucleotides”. This is followed by the addition
of unlabeled reversibly terminated dNTPs or dark nucleotides”.
The GeneReader sequencing chemistry uses four dye colors for labeling each dNTP to differentiate
each base (A, C, G or T) that is incorporated onto the DNA fragment. Furthermore, the reversible
terminators facilitate the addition of only one engineered nucleotide at a time to the growing strand
of all DNA templates. Upon signal detection from each bead, the fluorescent labels as well as the
terminators are removed which then allows for a new cycle of incorporation, hence ensuring highly
accurate and cost-effective next-generation sequencing.
DNA libraries are clonally amplified on beads using the GeneRead QIAcube to serve as a
sequencing template. After hybridization of a sequencing primer, the primer-template carrying
beads are immobilized via direct bead-glass interaction to produce a high-density array on a
GeneReader Flow Cell. To read out the content of templates on each bead, the array of fragments
is first subjected to reagents containing uniquely engineered dNTPs, as described above. These
bases are incorporated by the addition of a modified DNA polymerase to the end of the growing
strand of DNA in accordance with the base on the complementary strand. The array is subsequently
scanned by a high-resolution digital camera and the fluorescent output of each of the four dye colors
at each array position is measured and recorded. Finally, the array is exposed to cleavage
chemistry to break off the fluorescent dyes and reversible terminators that will then allow additional
bases to be incorporated. This cycle is then repeated on the GeneReader.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
22
3.3 External features of the GeneReader
Front view Side view (right side panel)
1
Status lights
5
USB port (GeneReader connection)
9
Cooling air outlet (back of instrument)
2
Flow cell door
6
USB port (camera connection)
10
Main Hood (QIAGEN service only)
3
Hood
7
Power switch
11
Instrument configuration sticker (right
side panel)
4
Fluidic drawer
8
Power cord socket
Figure 1. External features and functions of GeneReader.
3.3.1 Status lights
The status lights illuminate in the following patterns:
When the instrument is not running, the status lights are off.
When a protocol is running normally, the green light is on.
When a protocol is running, but a pause has been requested, or when a protocol is paused,
the green light blinks.
When all protocols have been stopped, canceled or an error has occurred, the red light is
on.
When the protocol has finished successfully, the green light blinks.
3.3.2 Flow Cell door
Opening the Flow Cell door
Flow Cells are inserted through the flow cell door. Opening the Flow Cell door is controlled by
software.
Note: The Flow Cell door cannot be opened manually.
1
2
3
4
5
6
7
8
9
10
11

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
23
Closing the Flow Cell door
Push the Flow Cell door manually until the fastener snaps in place to lock the door.
Note: The GeneReader will not work if the Flow Cell door is not locked.
3.3.3 Hood
Opening the hood
Opening the hood is controlled by software. The hood must be opened prior to opening the fluidic
drawer.
Note: The hood can be manually released if the GeneReader loses power. Insert your hand,
palm facing upward, and feel for the hole located approximately where the Main Hood meets
the fluidic drawer. When your fingers are in the hole you will feel a lever on the right and by
pulling slightly towards the front the hood will open.
Figure 2. Diagram illustrating how to manually open the hood of GeneReader.
Closing the hood
The fluidic drawer must be closed (pushed all the way in until a clicking sound is heard) prior to
manually pulling down the hood.
Note: The Main Hood on the left side of the instrument must only be opened by QIAGEN Field
Service Specialists.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
24
3.3.4 Fluidic drawer
Open the fluidic drawer to:
Load or unload 1 liter wash bottles.
Load or unload 50 ml tubes.
Insert or remove the liquid waste bottle.
Clean the cooling block, remove excessive condensation.
Clean the drawer.
Clean the dip sticks.
Clean the waste level sensor surface.
The fluidic drawer remains locked during a run.
Note: If the hood is closed, the fluidic drawer cannot be opened.
3.3.5 USB ports
The two (2) USB ports are used to connect the GeneReader with the workstation. They are located
on the right side panel of the instrument.
3.3.6 Power switch
The power switch is located on the right side panel of the GeneReader.
3.3.7 Power cord socket
The power cord socket is located on the right side panel of the GeneReader. It allows connection
of the GeneReader to a power outlet via the supplied power cord.
3.3.8 Cooling air outlet
Cooling air outlets are on the right side and back of the GeneReader. They allow cooling of the
internal components. The instrument should not be positioned in close proximity to a wall that could
block airflow.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
25
3.3.9 Workstation equipment
The GeneReader system is operated with a workstation.
The workstation specifications are listed in Section 9.3.
3.4 Internal features of the GeneReader
1
50 ml conical tubes in
cooling compartment
3
Liquid waste bottle
2 One liter bottles for
sequencing wash buffer
or Maintenance Wash
Buffer
Figure 3. Internal view of the fluidic drawer of GeneReader.
3.4.1 GeneReader Flow Cell
Sequencing beads carrying single strand DNA templates with sequencing primers annealed are
deposited into the GeneReader Flow Cell according to the current QIAGEN GeneRead UMI
Advanced Sequencing Q Handbook. The Flow Cell should be loaded into the GeneReader through
the Flow Cell door with the bar code towards the left side of the GeneReader.
2
3
1

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
26
Figure 4. Loading the Flow Cell of GeneReader.
Figure 5. View inside the Flow Cell door of GeneReader.
Flow Cell bar code reader
Upon loading of a Flow Cell, the Flow Cell bar code reader scans the bar code on the Flow Cell
and provides the information to the GeneReader software. If the Flow Fell has been inserted in the
wrong orientation an error message is displayed.

QIAGEN GeneReader User Manual for Advanced Process Flow (APF/
HP
) Instrument Configuration
12/2017
27
4 Installation Procedures
4.1 System delivery and installation
The unpacking and installation of the GeneReader is performed by a certified QIAGEN Field
Service Specialist. A person who is familiar with your laboratory and computer equipment should
be present during the installation.
The following items are delivered:
GeneReader instrument
GeneReader User Manual for Advanced Process Flow (APF/HP) Instrument Configuration
Workstation
GeneReader software (will be installed by QIAGEN Field Service during initial set up)
Additional components: 1 international power cable set, 2 USB cables, 1 handheld bar code
scanner, 1 waste container and 4 one liter bottles
Note: The computer recommended for data analysis is the QIAGEN Powerstation, however,
any hardware that meets the specifications as defined in the current version of the QIAGEN
Clinical Insight Analyze User Manual may be used.
4.2 Site requirements
The GeneReader must be located out of direct sunlight, away from heat sources, and away from
sources of vibration and electrical interference. Refer to Section 9.1 for the operating conditions
(temperature and humidity). The site of installation should be free of excessive drafts, excessive
moisture and dust, and not be subject to large temperature fluctuations.
Refer to Section 9.2 for the weight and dimensions of the instrument.
Use an appropriate workbench to accommodate the GeneReader. Ensure that the workbench is dry
and clean, and has additional space for accessories. To accommodate the GeneReader instrument
with the hood open, 125 cm (49.3 in.) minimum clearance above the workbench is required. Allow
at least 10 cm (4 in.) of free space behind the instrument for cables and cooling of the instrument.
The GeneReader must be placed within approximately 1.5 m (59 in.) of a properly grounded
(earthed) AC power outlet. The power line to the GeneReader should be voltage regulated and
surge protected, and an uninterruptable power supply (UPS) is optional.
 Loading...
Loading...