# of tests left
EN
x
x
Too little
Add more blood
Just right Too much
Wipe away excess
91146 A 7/2015
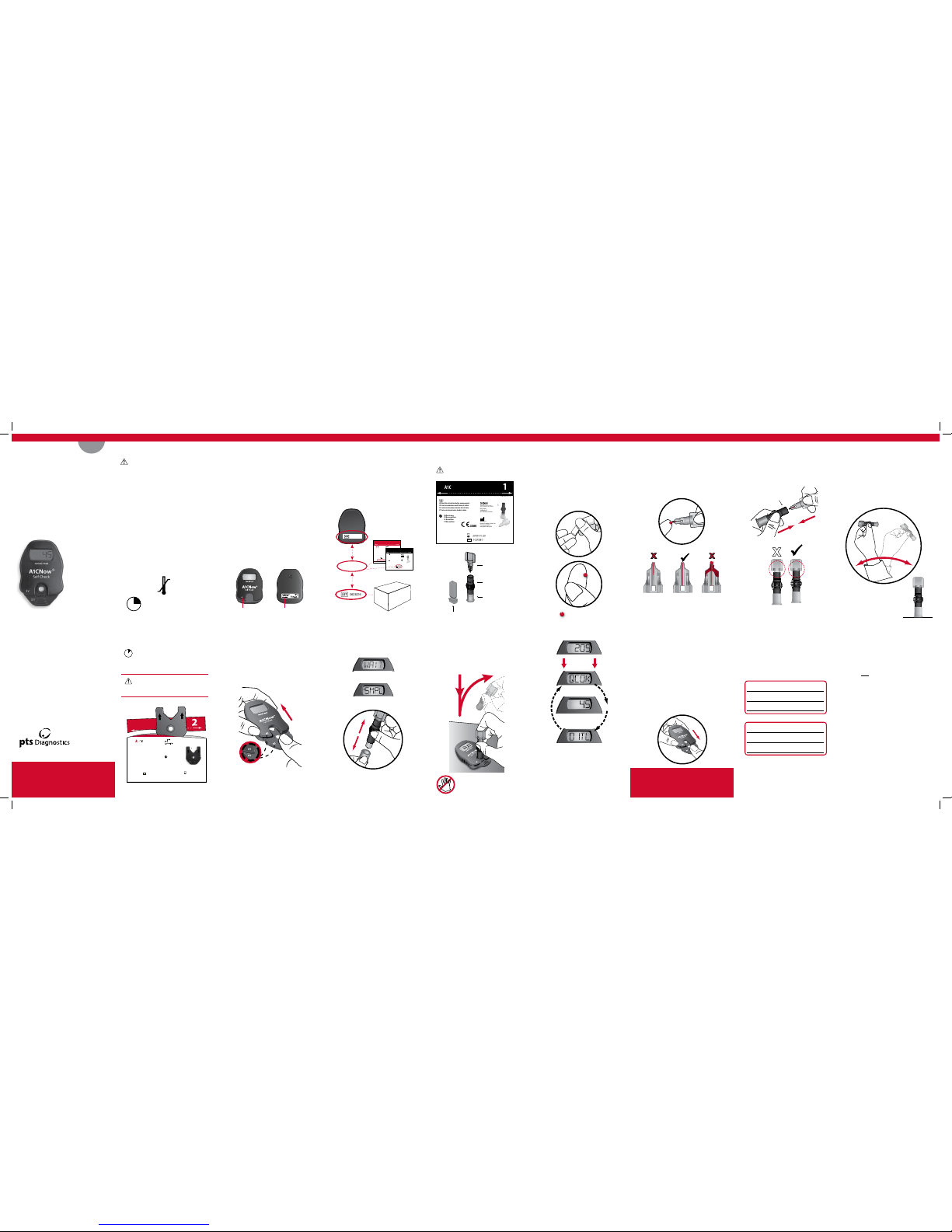
Open Plastic Shaker Pouch* Collect Blood
• Gently touch blood collector to top of
blood drop to fill.
• Make sure collector is adequately filled.
Prick Finger
• Wash hands or clean with alcohol and dry
before testing.
• Remove cap and press lancet into finger.
• Gently squeeze finger to get
adequate blood.
Insert Blood Collector
• Fully insert blood collector into shaker body.
• Push hard while twisting to fully insert.
321
4
Insert Cartridge
• “Click” test cartridge into place.
• Analyzer and test cartridge codes must match.
• If codes do not match, call
Customer Service at +1-317-870-5610.
M
% A1C
7
Overview
& Quick
Reference
Guide
PN 91171 Rev. B 02/18
Blood Collector
Shaker body - open end
Shaker base (do not remove!)
6
Open Foil Cartridge Pouch*
AFTER Blood Collection
Insert cartridge into analyzer immediately
and use within 2 minutes.
Tear foil pouch open at the notches on the sides.
A1C
2
A09
LOT 0628216
EXP 2016 01 27
LOT 0628216
EXP 2016 01 27
LOT 0628216
EXP 2016 01 27
1
Do not reuse
Ne pas réutiliser
Use at room temperature
Utiliser à la température ambiante
EN
FR
EN
FR
Usar a temperatura ambiente
Utilizar à temperatura ambiente
ES
PT
No reutilizar
Não reutilizar
ES
PT
DO NOT OPEN until shaker is prepared.
Once opened, use within 2 minutes.
Read the instructions before opening pouch.
Préparer l’agitateur AVANT D’OUVRIR le sachet.
Utiliser dans les 2 minutes suivant l’ouverture.
Lire les instructions avant d’ouvrir le sachet.
EN
FR
ES
PT
NO LA ABRA si el agitador no está preparado.
Una vez abierta la bolsa, utilice el producto antes de
que transcurran 2 minutos.
Lea las instrucciones antes de abrir la bolsa.
NÃO ABRIR até o shaker estar preparado.
Depois de aberto, usar num espaço de 2 minutos.
Leia as instruções antes de abrir a bolsa.
11 12
Dispose of Cartridge /
Save Analyzer
• Dispose of cartridge n your household
waste.
• Save analyzer for additional tests. Analyzer
will display, for example, “01TL” if there is
one test left and “00TL” if you just used your
last test.
• Record your A1C result in the result log in
Step 12.
• Analyzer will show result for 60 minutes and
will turn off automatically.
M
+1-317-870-5610
We invite you to call us and we will guide you
through the test.
5 Minutes to Results
10
mmol/mol
Display counts down
QC test result
Result
9
Prepare Shaker
Remove shaker base.
8
WAIT for SMPL to display
Ready for shaker
Mix
• Shake vigorously 6-8 times (about 5
seconds).
This will mix the blood with the solution.
• Stand shaker on table while
preparing cartridge.
5
x
Not fully inserted
Fully inserted
Self Check
Self Check
A1CNow
®
A1CNow
+
®
Sistema professionale
di autodiagnosi
A1CNow
®
A1CNow
+
®
Sistema professionale
Self Check
A1CNow
®
A1CNow
+
®
Sistema professionale
A1CNow
+
®
Sistema professionale
A1CNow
®
Self Check
di autodiagnosi
A1CNow
®
A1CNow
+
®
Sistema professionale
• FIRST, we strongly recommend you read
all instructions carefully and watch the brief
instructional video on our website at
ptsdiagnostics.com. THEN, follow all steps as you
perform the test.
• Do not open pouches until instructed.
• Make sure lot number on analyzer matches lot
numbers on pouches.
• Use indoors between 18°C - 25°C /64°F - 77°F
• DO NOT adjust your medication unless
instructed to do so by your doctor or
healthcare provider.
• If you have any questions about your A1C result,
please contact your doctor or healthcare provider.
Caution: This device contains material of animal
origin and should be handled as a potential carrier
and transmitter of disease.
For additional information, see the reverse side of
this insert.
If you are not able to access the instructional
video on our website, we encourage you to call
customer service at +1-317-870-5610 to walk
you through the test.
IMPORTANT!
Match Lot Numbers
Use analyzer only with the materials included in
the original kit. The analyzer will expire after the
programmed number of tests have been run. If
another test cartridge is inserted, the analyzer will
display “00TL.”
Open End
Analyzer Front Analyzer Back
Code Lot Number
Dispense Sample into Cartridge
• Ensure analyzer is on level surface.
• Push down completely to dispense diluted sample
and remove in a continuous motion.
• Remove sampler from cartridge quickly.
• “RUN” will appear.
Do not handle analyzer again
until test is complete!
* Pouch illustrations may vary.
Note: All instances of the box or pouches in this insert
are representative ONLY. Please refer to the packaging
included with your specific kit.
25°C
(77°F)
18°C
(64°F)
Complete test within 15 minutes.
Test System
Caution: Do NOT open foil pouch until this step.
Use within 2 minutes of opening.
If foil pouch is damaged, do not use.
2
DO NOT OPEN until shaker is prepared.
Once opened, use within 2 minutes.
Read the instructions before opening pouch.
Préparer l’agitateur AVANT D’OUVRIR le sachet.
Utiliser dans les 2 minutes suivant l’ouverture.
Lire les instructions avant d’ouvrir le sachet.
1
18-28°C (64-82°F)
A1C
EN
FR
ES
PT
P/N 91069 Rev. C
NO LA ABRA si el agitador no está preparado.
Una vez abierta la bolsa, utilice el producto antes de
que transcurran 2 minutos.
Lea las instrucciones antes de abrir la bolsa.
NÃO ABRIR até o shaker estar preparado.
Depois de aberto, usar num espaço de 2 minutos.
Leia as instruções antes de abrir a bolsa.
Do not reuse
Ne pas réutiliser
Use at room temperature
Utiliser à la température ambiante
EN
FR
EN
FR
Usar a temperatura ambiente
Utilizar à temperatura ambiente
ES
PT
No reutilizar
Não reutilizar
ES
PT
0628216
LOT 0628216
EXP 2016 01 27
LOT 0628216
EXP 2016 01 27
LOT 0628216
EXP 2016 01 27
P/N 91070 Rev. E
A1C
1
Polymer Technology Systems, Inc.
7736 Zionsville Road
Indianapolis, Indiana USA
Manufacturer
MDSS GmbH
Schigraben 41
30175 Hannover, Germany
Authorized representative
in the European Community
Do not reuse
Ne pas réutiliser
No reutilizar
Não reutilizar
EN
FR
ES
PT
1
Niet hergebruiken
Non riutilizzare
Nicht wiederverwenden
Nie używać ponownie
NL
IT
DE
PL
EN Read the instructions before opening pouch.
FR Lire les instructions avant d'ouvrir le sachet.
ES Lea las instrucciones antes de abrir la bolsa.
PT Leia as instruções antes de abrir a bolsa.
NL Lees de instructies alvorens de zak te openen.
IT Leggere le istruzioni prima di aprire la busta.
DE Lesen Sie die Anweisungen, bevor Sie den
Beutel önen.
PL Przed otwarciem woreczka przeczytać instrukcje.
NOTE: To run another test, use a new shaker, blood
collector, test cartridge, and lancet from the same
kit and return to Step 1.
• This result cycle remains displayed for
60 minutes or until the next test cartridge
is inserted.
• If “QCOK” is not displayed, please refer
to the troubleshooting section or
contact Customer Service.
Do NOT open foil cartridge pouch until Step 6.
A1C
1
P/N 91143 Rev. C
A1C
1
Polymer Technology Systems, Inc.
7736 Zionsville Road
Indianapolis, Indiana USA
Manufacturer
MDSS GmbH
Schigraben 41
30175 Hannover, Germany
Authorized representative
in the European Community
1
Do not reuse
Ne pas réutiliser
No reutilizar
Não reutilizar
EN
FR
ES
PT
EN Read the instructions before opening pouch.
FR Lire les instructions avant d'ouvrir le sachet.
ES Lea las instrucciones antes de abrir la bolsa.
PT Leia as instruções antes de abrir a bolsa.
Niet hergebruiken
Non riutilizzare
Nicht wiederverwenden
Nie używać ponownie
NL
IT
DE
PL
MediPurpose Pte. Ltd.
15 Hoe Chiang Road #12-02
Tower Fifteen
089316 Singapore
Manufacturer
Obelis S.A.
Bd. Général Wahis 53
1030 Brussels, Belgium
Authorized representative
in the European Community
NL Lees de instructies alvorens de zak te openen.
IT Leggere le istruzioni prima di aprire la busta.
DE Lesen Sie die Anweisungen, bevor Sie den Beutel önen.
PL Przed otwarciem woreczka przeczytać instrukcje.
1008
1
Log Result
Chart your progress here.
Record your A1C results here, and bring this
with you when you see your doctor. Mark down
your A1C goal in the space provided.
Date:
A1C Result:
A1C Goal:
Date:
A1C Result:
A1C Goal:
Result Interpretation
• Do not take any decision of medical
relevance without first consulting your
healthcare practitioner.
• When this device is used for monitoring of
an existing disease, adapt the treatment
only if you have received the appropriate
training to do so.
There should be NO GAP between the
blood collector and the shaker.
Actual size required
Match codes
Lancet
LOT 0628216
INTENDED USE
The A1CNow® Self Check test system provides quantitative measurement
of glycated haemoglobin levels in capillary (ngerprick) blood samples.
The test is for home use to monitor glycemic control in people with
diabetes.
Before using this test, please read all instructions carefully.
If you need further help, call +1-317-870-5610.
We invite you to call and we will guide you through the test.
Do not take any decision of medical relevance without rst
consulting your medical practitioner.
INTRODUCTION
The concentration (mmol/mol HbA1c) of A1C in your blood today tells
you how well you have been controlling your blood sugar (glucose) levels
over the past 2-3 months. About 50% of the A1C result is from the past
30 days of blood sugar levels, about 25% is from the past 30-60 days, and
about 25% is from the past 60-90 days.
1
The American Diabetes Association (ADA) recommends that your A1C
levels should be tested at least 2 times per year if you are meeting
your diabetes treatment goals and your blood sugar is stable. If your
treatment changes or you are not meeting your treatment goals, the ADA
recommends that you test at least every 3 months.
2
The A1CNow Self Check test system is an easy-to-use at home test to
measure your A1C levels, with results in 5 minutes. In addition to blood
sugar testing, you can further participate in your diabetes care using
this test. You can have the results ready before you have your checkups
to share with your healthcare professional. Contact your healthcare
professional if you have any concerns about your A1C result. The A1CNow
Self Check test system is not a substitute for regular assessment in a
doctor’s oce or laboratory setting where a quality control program is
in eect.
MATERIALS PROVIDED
The box contains materials for multiple A1C tests. See outside box for
quantity as it may vary. Make sure all of the following parts are in the
box. DO NOT open the pouches until ready to use.
• A1CNow Self Check analyzer (see box label for quantity)
• Cartridge pouch (see box label for quantity)
• Shaker pouch (see box label for quantity), each containing:
- Shaker (1)
- Blood collector (1)
- Lancet, disposable (1)
• Product insert(s)
MATERIALS NEEDED BUT NOT PROVIDED
• Gauze pad or cotton ball
• Bandage
Contact Customer Service for a list of liquid controls.
PREPARING TO TAKE THE TEST
You may run your A1C test any time of the day. Remember to wash your
hands prior to performing the test.
No special diet is necessary (you do not have to be fasting when taking
this test). You may want to run this test at the same time as you do a
blood sugar test.
In order to help ensure an accurate result, please complete the test from
beginning to end within 15 minutes. Avoid running the test in direct
sunlight, on hot or cold surfaces or near sources of heat or cold. If the test
has recently been at high temperatures (greater than 25°C/ 77°F) or at
low temperatures (lower than 18°C/ 64°F), allow the kit parts to come to
room temperature (18-25°C/ 64-77°F) for at least one hour before you
run your test. Leave the parts in their sealed pouches while waiting.
Do not move the analyzer while test is in progress.
WHAT TO DO WITH THE RESULT
The analyzer will not store your results for more than 60
minutes, so write down the result and the test date on the result
log on the front of this insert to prevent loss of information.
WHAT THE TEST RESULT MEANS
For most people with diabetes, the American Diabetes Association (ADA)
recommends that your A1C should be under 53 mmol/mol (7%).
2
Your
healthcare professional will tell you what target level is right for you.
HOW DOES THIS TEST COMPARE WITH THE A1C TEST FROM
THE DOCTOR’S OFFICE OR THE LABORATORY?
Test results will rarely match exactly. This is true even for repeated tests
done in the same lab.
A1C results may be dierent due to: slight dierences between labs,
normal variation within each test method, and the time between
tests.
STORAGE AND HANDLING
• Store and use at room temperature at 18-25°C (64-77°F) and out of
direct sunlight.
• If you cannot condently store the kit under these recommended
conditions, you have the option of refrigerating the kit at 2-8°C
(36-46°F). DO NOT freeze. However, you must bring the kit to room
temperature for at least one hour prior to use.
• If the temperature label, placed on the outside of every kit, is exposed
to a temperature in excess of 50°C (122°F), the dot on the label will
turn red and the product should not be used.
• DO NOT use the test after the expiration date shown on the box.
• If disinfection of the analyzer is desired, Super Sani-Cloth® wipes
are recommended (EPA Reg. No. 9480-4, Professional Disposables
International (PDI), Orangeburg, New York), concentration of active
ingredients (0.25%) and with a contact time of 2 minutes. The active
ingredients in this disinfectant are n-Alkyl dimethyl ethylbenzyl
ammonium chlorides.
• Store analyzer in protective package when not in use.
WARNINGS AND PRECAUTIONS
• When this analyzer is used for the monitoring of an existing
disease, you should only adapt the treatment if you have
received the appropriate training to do so.
• For self-testing use outside of the body only (in vitro diagnostic use).
• To ensure proper test performance, carefully read and follow the
steps located on the front side of this product insert.
• DO NOT adjust your medication unless instructed to do so by
your doctor or healthcare professional.
• DO NOT substitute this test for blood sugar monitoring.
• The following conditions may aect the accuracy of your A1C result:
haemoglobin variants (HbS, HbC), elevated HbF, anemia, recent
signicant blood loss, a recent blood transfusion, or high amounts of
rheumatoid factor.
• People with hemophilia (bleeding disorder) or on anti-coagulant
therapy (blood thinning medicine) should consult their doctor or
healthcare professional before using this kit.
• DO NOT use the test kit if any parts are cracked or broken.
• DO NOT eat or drink any parts of this kit.
• Keep out of reach of children under the age of 7 years. When children
are performing the test, be sure that testing is done under adult
supervision.
• DO NOT use any other body uids or food to perform this test. Use
ONLY your ngerprick blood sample.
• DO NOT reuse the shaker or the cartridge. Throw these parts away
after using them once. Refer to Step 11 on the front side of this insert.
• If the solution from inside the shaker touches your skin or your eyes,
ush with water.
• Leave the cartridge pouch sealed until ready for use.
• DO NOT add your blood directly to the cartridge. Your blood must rst
be added to the shaker.
• DO NOT touch the white circle area of the cartridge.
• This test is to be used at room temperature between 18° and 25°C
(64° and 77°F). Using the test outside this temperature range will
give you an error code.
• The test cartridges should not be used if the foil pouch or any other
protective packaging is damaged.
• Caution: The analyzer contains material of animal origin and
should be handled as a potential carrier and transmitter of
disease.
LIMITATIONS
• This test is NOT for the screening or diagnosis of diabetes.
• If the user has high levels of Haemoglobin F, HaemoglobinS,
Haemoglobin C, or other haemoglobin variants, the A1CNow system
may report incorrect results.
• Any cause of shortened red cell survival (e.g., haemolytic anemia or
other haemolytic diseases, pregnancy, recent signicant blood loss,
etc.) will reduce exposure of red cells to glucose. This results in a
decrease in A1C concentrations (mmol/mol). Glycated haemoglobin
results are not reliable in users with chronic blood loss and
consequent variable erythrocyte life span.
• Rheumatoid Factor in high amounts will cause low results, or an
error code. It is recommended that A1C be re-checked by alternate
methodology, such as boronate anity, by a healthcare professional.
• This test is NOT a substitute for regular healthcare provider visits and
blood glucose monitoring.
• As with any laboratory procedure, a large discrepancy between
clinical impression and test results usually warrants investigation.
QUALITY CONTROLS
Each PTS Diagnostics A1CNow Self Check analyzer performs over 50
internal chemical and electronic quality control checks, including
potential hardware and software errors (e.g. cartridge alignment,
programming), and potential test strip errors (e.g. insucient sample
volume, invalid calculations). The analyzer has been programmed to
report an error code if these quality checks are not passed. Contact
Customer Service at +1-317-870-5610, if you receive any errors.
TROUBLESHOOTING
See the table below for a description of A1CNow Self Check test system
operating and error codes (“OR”= Out of Range, “QC”= Quality Control,
“E”= Analyzer Error).
MESSAGE DESCRIPTION AND RESOLUTION
OR 1 The blood sample may have too little haemoglobin for
the test to work properly, or you added too little blood.
Call Customer Service.
OR 2 The blood sample may have too much haemoglobin for
the test to work properly, or you added to much blood.
Call Customer Service.
OR 3 The blood sample may have too little haemoglobin
A1C for the test to work properly, or you added too
little blood. Call Customer Service.
OR 4 The blood sample may have too much haemoglobin
A1C for the test to work properly, or you added too
much blood. Call Customer Service.
OR 5 The analyzer temperature is below 18°C (64°F). The
test must be repeated with a new cartridge at room
temperature (18-25°C).
OR 6 The analyzer temperature is above 25°C (77°F). The
test must be repeated with a new cartridge at room
temperature (18-25°C).
<20 The A1C is less than 20 mmol/mol. Call Customer
Service.
>120 The A1C is greater than 120 mmol/mol. Call your
healthcare professional.
QC 2 Occurs when you insert a cartridge that already has
sample added to it. Do not remove and reinsert a
cartridge after adding sample.
QC 6 Sample was added to cartridge before “SMPL” display.
This counts down one test on the analyzer. Remove
and discard cartridge. To avoid this error, do not add
sample until the “WAIT” prompt clears and “SMPL”
appears.
QC 7 The cartridge remained in the analyzer without sample
addition for 2 minutes after “SMPL” prompt. This
counts down one test on the analyzer. Discard the test
cartridge and insert a fresh one when you are ready to
dispense the shaker.
QC 30
to 33
The analyzer was unable to obtain a valid initial
reading. Be sure to remove the shaker within one
second after dispensing it into the sample port, and
do not disturb the analyzer while the test is running.
QC 50
to 51
QC 55
to 56
Insucient sample was delivered to the test cartridge.
To avoid this error be sure to fully insert the blood
collector into the shaker and shake immediately.
All other
QC Codes
The quality control checks inside the analyzer did not
pass. The test will need to be repeated with another
kit. Call Customer Service.
E Codes The analyzer is not working. Call Customer Service.
Customer Service: +1-317-870-5610
DISPOSAL OF MATERIALS
Keep the analyzer to run the other test(s) and dispose of it after the last
test has been performed. Dispose of the other used components (except
the lancet) in household waste. Each lancet, shaker, blood collector and
cartridge can be used only once.
Since the lancet has a sharp point, it should be disposed of in an
appropriate sharps container in the same way you dispose of your blood
sugar testing lancets.
The analyzer could have residual biological material and in this case it
should be regarded as contaminated waste and be disposed of in an
appropriate biohazardous waste container.
PERFORMANCE CHARACTERISTICS
Expected Values (non-diabetic population)
The expected normal range for mmol HbA1c/mol Hb (or % A1C) using
the A1CNow+ system was determined by testing blood samples from
118 presumptively non-diabetic individuals (fasting glucose levels <127
mg/dL or <7 mmol/L) across three US sites. The population included 33
males and 85 females, and an age range from 19 to 76 with a mean age
of 43. The mean HbA1c result was 33± 7.8 mmol/mol (or 5.2% ±0.71%)
(1 SD). The 95% confidence limits were 19-48 mmol/mol (or 3.9% to
6.5%). These values are similar to those reported in the literature.
Linearity
Studies were performed to evaluate the linearity of the A1CNow+
system across its dynamic range. Clinical samples representing low and
high HbA1c levels were identified, and were mixed in various proportions
into nine preparations. These samples were tested in replicates of at
least five (n = 5). The observed results were compared to the expected
results and analyzed in terms of percent recovery. The test is linear for
A1C levels between 20 and 120 mmol/mol (4% and 13%), and produces
reliable results with hematocrits between 20% and 60% packed cell
volume (PCV).
Precision
The precision analysis was performed with 110 diabetic (n=93) and nondiabetic (n=17) subjects across two US sites. Each subject performed 2
self-tests using the A1CNow Self Check test system, with blood samples
taken from two separate ngersticks. The analysis was performed on all
subjects who received a numeric result for both self-tests. The data are
provided below.
N
AVERAGE WITHIN
SUBJECT SD
AVERAGE WITHIN
SUBJECT CV
74 4.5 (0.41) 6.0% (4.57%)
Accuracy
Accuracy studies were conducted with 110 diabetic (n=93) and
non-diabetic (n=17) subjects across two US sites. Each subject
performed 2 self-tests using the A1CNow Self Check test system, with
blood samples taken from two separate ngersticks. Venous blood
was also collected from each subject for comparative testing using an
NGSP-certied laboratory method. All subject self-tests which resulted
in evaluable numeric readings were included in the analysis. Accuracy
was based on the regression of the two subject self-tests compared to the
laboratory method result, and bias calculations were conducted. The data
are provided below.
A1CNow Self Check Fingerstick Comparative Testing
(NGSP-certied method is the TOSOH A1C 2.2 Plus)
N R2 ADJ. SLOPE YINTERCEPT
178 0.924 1.010 0.135
(Analytical Bias at 42, 64 and 86 (6, 8 and 10%))
A1C VALUE BIAS STANDARD ERROR
42 (6%) 2 (0.20) 0.5 (0.05)
64 (8%) 2 (0.22) 0.4 (0.04)
86 (10%) 3 (0.24) 0.7 (0.07)
Interference Testing/Specicity
Studies were performed to assess the eect of common test interferents,
various common over-the-counter therapeutic agents, and oral
antihyperglycemic agents commonly used to treat Type Il diabetes.
Two levels of A1C (low and high, approximately 20 and 86 mmol/mol,
respectively) were tested. See table below.
INTERFERENT TEST CONCENTRATION
Bilirubin (unconjugated) 20 mg/dL 0.34 mmol/L
Triglycerides 3000 mg/dL 49 mmol/L
Haemoglobin 500 mg/dL 78E-3 mmol/L
Acetaminophen/Paracetamol 8 mg/dL 0.53 mmol/L
Ascorbic acid 5 mg/dL 0.28 mmol/L
Ibuprofen 12 mg/dL 0.53 mmol/L
Acetylsalicylic acid 1 mg/dL 56E-3 mmol/L
Glyburide (glibenclamide) 24E-3 mg/dL 490E-6 mmol/L
Metformin
(1.1-dimenthylbiguanide HCI)
2.5 mg/dL 0.15 mmol/L
The studies showed no eect from any of these potential interferents
at concentrations up to approximately 5-times their normal levels
or therapeutic doses. Studies showed no interference from modied
hemoglobins, including labile glycated hemoglobin when tested at two
levels of % A1C (low and high, approximately 31 and 97 mmol/mol
respectively). The modied hemoglobins, and the levels evaluated, were:
labile hemoglobin with 78 mmol/L glucose, carbamylated hemoglobin
at a nal concentration of 5 mmol/L potassium cyanate, and acetylated
hemoglobin at a nal concentration of 14 mmol/L acetylsalicylic acid.
There were mixed results from the testing of high levels of Hemoglobin
F, Hemoglobin S, and Hemoglobin C. Unreliable results may be obtained
from patients with elevated levels of variant hemoglobins.
FREQUENTLY ASKED QUESTIONS
When should I do the A1CNow Self Check system test?
The A1CNow Self Check system test can be performed at any time of day.
No fasting is required. You may wish to run the test at the same time you
do your blood sugar test.
Sometimes I have trouble getting a blood drop that is large
enough. What can I do?
Try washing your hands in warm water. Warm water will help increase
blood ow for a better ngerprick. You may also massage the nger
before the ngerprick.
What is the best way to ll the blood collector?
Hold the blood collector horizontally or at a 45° angle relative to the
blood drop. Touch the tip gently to the drop of blood and allow the tube
to ll. It will stop automatically when it is lled completely.
My blood collector is not lled completely. What should I do?
Apply pressure to your nger to get more blood. Again, touch the tip
gently to the drop of blood and allow the tube to ll. You may have to
re-prick your nger to get the necessary blood. If the blood collector does
not ll, call Customer Service: +1-317-870-5610.
There is extra blood on the tip of the blood collector. What
should I do?
Carefully wipe the tip of the blood collector with a piece of gauze or
tissue. If some of the blood comes out while doing this, touch the tip
gently to the blood drop to re-ll the blood collector.
The shaker seemed to leak when I pushed the blood collector
into it. What should I do?
Call Customer Service.
The cartridge will not insert into the analyzer.
What should I do?
Make sure you are inserting the cartridge facing correctly. The code
should be on top as you insert the cartridge into the analyzer.
I accidentally opened the cartridge pouch too early. What should
I do?
Throw away the cartridge that has been opened for more than 2 minutes.
Inaccurate results can be obtained. Use another cartridge in the kit
instead.
The codes on the cartridge and the analyzer are not the same.
Do not use the cartridge. Save the packaging materials and call Customer
Service.
The analyzer did not turn on after I inserted the cartridge. What
should I do?
Take the cartridge out. Re-insert in until it ‘clicks’. If the analyzer still does
not turn on, this means that it may have a problem and can’t be used. Call
Customer Service.
I did not see ‘RUN’ and a countdown after I added the sample
using the shaker. What should I do?
Call Customer Service.
My result says ‘QCOK’ and a number. What should I do?
‘QCOK’ means the analyzer is working correctly. The number you see is
your A1C result. Write your result down in the result log on the front of
this insert. The analyzer will show the result for 60 minutes and will turn
o automatically.
Review your result with your healthcare professional.
My result is not ‘QCOK’ and a number. What should I do?
If “QCOK” is not displayed, refer to troubleshooting section or contact
Customer Service.
What should I do with the test after I am done with it?
After you write down your result, you can throw away the used blood
collector, shaker, and cartridge appropriately. These items can be used
only once. Note that the lancet is also a single-use item.
Save the analyzer for additional tests (see outside box for quantity as it
may vary). The analyzer will display, for example, “01TL” showing that
there is one test left. When analyzer is displaying “00TL”, it indicates that
you have used all tests. Once you have used all tests, you can discard the
analyzer appropriately.
Self Check
A1CNow
®
A1CNow
+
®
Sistema professionale
di autodiagnosi
A1CNow
®
A1CNow
+
®
Sistema professionale
A1CNow
®
Self Check
Sistema di analisi
QUESTIONS OR COMMENTS
Call Customer Service: +1-317-870-5610.
REFERENCES
1. Burtis, C.A., Ashwood, E.R., Tietz Textbook of Clinical Chemistry, 3rd Edition, W.B.
Saunders Co., 1999.
2. American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes
Care, 34 (S1) 2011, pp. S11-S61.
CUSTOMER SERVICE
For assistance with PTS Diagnostics products, please contact
PTS Diagnostics Customer Service (M-F, 6 a.m. - 9 p.m. US EST) or your
local authorized dealer.
Toll-free inside the USA and Canada: 1-877-870-5610
Direct: +1-317-870-5610
Fax: +1-317-870-5608
E-mail: customerservice@ptsdiagnostics.com
The A1CNow system is manu fac tured in the United States by Polymer
Technology Systems, Inc., Indianapolis, IN 46268 USA.
© 2018 Polymer Technology Systems, Inc.
A1CNow is a trademark of Polymer Technology Systems, Inc.
All other trademarks and product names are the property of their
respective owners.
EXPLANATION OF SYMBOLS
Use by
Catalog number
Lot number
Consult instructions for use
Caution
Contains sucient for <n> tests
Authorized representative in the European Community
Keep away from sunlight
Keep dry
In vitro diagnostic medical device
Manufacturer
This product fullls the requirements of European Directive
98/79/EC on in vitro diagnostic medical devices
Temperature limitation
MDSS GmbH
Schigraben 41
30175 Hannover, Germany
Polymer Technology Systems, Inc.
7736 Zionsville Road,
Indianapolis, IN 46268 USA
+1-317-870-5610
Product requires separate collection for electrical and electronic equipment
per the WEEE Directive
 Loading...
Loading...