Powerheart AED 9200R, 9210R, 9200DR, 9210DR User manual


Powerheart AED Operation and Service Manual
CAUTION
Powerheart AED is intended for use by or on the order of a Physician or persons licensed by State law.
IMPORTANT
Read this Operation and Service Manual carefully. It contains information about your safety and the safety of others. Become
familiar with the controls and their proper use before operating the product.
The Powerheart AED Models 9200R/9210R/9200DR/9210DR are manufactured by Cardiac Science, Inc.
Corporate Headquarters:
16931 Millian Ave.
Irvine, CA 92606 USA
Internet: www.cardiacscience.com
Email: aed@cardiacscience.com
Manufacturer:
5420 Feltl Road
Minneapolis, MN 55343-7982 USA
International:
Herstedvang 8
DK - 2620 Albertslund Denmark
Trademark Information
Powerheart , MDLink, Saving Minutes Saving Lives, SmartGauge, STAR, IntelliSense, RescueReady, RescueLink and
Survivalink are trademarks and registered trademarks of Cardiac Science, Inc.
Microsoft and Windows are registered trademarks of Microsoft Corporation.
COMPACTFLASH is a trademark of SanDisk Corporation.
Limited Warranty
The Powerheart AED Operation and Service Manual and any and all information contained herein does not constitute any
warranty as to the Powerheart AED or any related products in any manner whatsoever. The “Limited Warranty” is shipped with the
Powerheart AED products and serves as the sole and exclusive warranty provided by Cardiac Science regarding the Powerheart
AED.
Customer Service
For Customer Service, call:
(800) 991-5465
(952) 939-4181
(952) 939-4191 (fax)
Technical Support
For 24-hour service, contact Technical Support at:
(888) 466-8686
(952) 939-4181
(952) 939-4191 (fax)
There is no charge to the customer for a Technical Support call. Please have the serial and model numbers available when
contacting Technical Support. (The serial and model numbers are located on the bottom of the Powerheart AED).

Notice of Rights
All rights reserved. No part of this documentation may be reproduced or transmitted in any form by any means without the
express written permission of Cardiac Science, Inc. Information in this documentation is subject to change without notice.
Names and data used in the examples are fictitious unless otherwise noted.
Defibrillator Tracking
Defibrillator manufacturers and distributors are required, under the Safe Medical Devices Act of 1990, to track the location of
defibrillators they sell. Please notify Cardiac Science Technical Support in the event that your defibrillator is sold, donated,
lost, stolen, exported, destroyed or if it was not purchased directly from Cardiac Science, Inc.

Table of Contents
Safety
Overview ........................................................................................................5
Safety Alert Definitions ...................................................................................6
Safety Alert Descriptions ................................................................................7
Symbols Descriptions ...................................................................................10
Introduction
Overview ......................................................................................................15
Powerheart AED Description .......................................................................16
Getting Started
Overview ......................................................................................................21
Unpacking and Inspecting ............................................................................22
Powerheart AED ..........................................................................................23
Powerheart AED Batteries ...........................................................................25
Electrodes ...................................................................................................27
Powerheart AED Indicators ..........................................................................28
Voice Prompt and Text Display Descriptions ...............................................32
Instructions for Use
Overview ......................................................................................................37
Step 1: Assessment and Electrode Placement ............................................38
Step 2: ECG Analysis ...................................................................................40
Step 3: Shock Delivery and CPR Mode .......................................................41
Step 4: Post Rescue .................................................................................42
Warnings .....................................................................................................43
Data Management
Overview ......................................................................................................45
Recording Rescue Data ...............................................................................46
Reviewing Rescue Data ...............................................................................47
Transfering Data to a Rescue Data Card .....................................................49
Page v © 2001 Cardiac Science, Inc. 300282-003 Rev A0

Table of Contents
Maintenance & Troubleshooting
Overview .........................................................................................51
Self-Tests........................................................................................ 52
Indicator Troubleshooting Table ......................................................53
Scheduled Maintenance ..................................................................55
Authorized Repair Service ..............................................................57
Frequently Asked Questions ...........................................................58
Technical Data
Overview .........................................................................................63
Parameters ......................................................................................64
Safety and Peformance Standards .................................................68
Summary of the RHYTHMx Clinical Study ......................................75
Accessories
Overview .........................................................................................77
List of Powerheart AED Accessories ..............................................78
300282 Rev A0 © 2001 Cardiac Science, Inc. Page vi
Section 1 Safety
Overview
This section presents safety information to guard against injury to
persons, and damage to the Powerheart AED.
Safety Alert Definitions 6
Safety Alert Descriptions 7
Symbols Descriptions 10
Topic Page
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 5

Section 1: Safety
Safety Alert Definitions
Before Operating the Powerheart AED:
Before operating the Powerheart AED, become familiar with the various
safety alerts in this section.
Safety alerts identify potential hazards using symbols and words to
explain what could potentially harm you, the patient or the Powerheart
AED.
Safety Terms and Definitions
The triangle attention symbol shown below, left, identifies the potential
hazard categories. The definition of each category is as follows:
DANGER: This alert identifies hazards that may cause serious personal
injury or death.
WARNING: This alert identifies hazards that may cause serious
personal injury or death.
CAUTION: This alert identifies hazards that may cause minor personal
injury, product damage, or property damage.
The term “Powerheart AED” refers to Models 9200R/9210R/9200DR/
9210DR..
Page 6 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0

Section 1: Safety
Safety Alert Descriptions
The following is a list of Powerheart AED safety alerts that appear in this
section and throughout this manual. You must read, understand, and heed
these safety alerts before attempting to operate the Powerheart AED.
DANGER: Fire and Explosion Hazard
Exercise caution when operating the Powerheart AED close to flammable
gases (including concentrated oxygen) to avoid possible explosion or fire
hazard.
WARNING: Shock Hazard
Defibrillation shock current flowing through unwanted pathways is
potentially a serious electrical shock hazard. To avoid this hazard during
defibrillation abide by all of the following:
• Do not touch the patient, unless performance of CPR is indicated
• Do not touch metal objects in contact with the patient
• Keep defibrillation electrodes clear of other electrodes or metal parts in
contact with the patient
• Disconnect all non-defibrillator proof equipment from the patient before
defibrillation
WARNING: Shock and Possible Equipment Damage
Disconnect all non-defibrillator proof equipment from the patient before
defibrillation to prevent electrical shock and potential damage to the
equipment.
WARNING: Battery is Not Rechargeable
Do not attempt to recharge the battery. Any attempt to recharge the
battery may result in an explosion or fire hazard.
CAUTION: Possible Radio Frequency (RF) Susceptibility
RF susceptibility from cellular telephones, CB radios, and FM 2-way
radio may cause incorrect rhythm recognition and subsequent shock
advisory.
When attempting a rescue using the Powerheart AED, do not operate
wireless radiotelephones within 1 meter of the Powerheart AED—turn
power OFF to the radiotelephone and other like equipment near the
incident.
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 7
Section 1: Safety
CAUTION: Moving the Patient During a Rescue
During a rescue attempt, excessive jostling or moving of the patient may
cause AEDs to interrupt the ECG analysis. The prompt “Noise Detected.
Stop Patient Motion” will be issued and analysis of the ECG will restart.
It is recommended that you stop moving the patient and turn off cellular
telephones or other electronics nearby.
CAUTION: Use only Cardiac Science Approved Equipment
Using batteries, electrodes, cables, or optional equipment other than those
approved by Cardiac Science may cause the Powerheart AED to function
improperly during a rescue.
CAUTION: Serial Communication Cable
The Powerheart AED will not perform a rescue when a serial
communication cable is connected to its serial connector. The voice
prompt will say “Remove Cable to Continue Rescue.”
CAUTION: Possible Interference With Implanted Pacemaker
The Powerheart AED may not advise a defibrillation shock when the
patient has an unipolar implanted pacemaker.1 However, a defibrillation
attempt should be made with the following precautions.
• Do not place the electrodes directly over an implanted device
• Place the electrode pad at least one inch from any implanted device
CAUTION: Lithium Sulfer Dioxide Battery
Pressurized contents; never recharge, short circuit, puncture, deform, or
expose to temperatures above 65°C (149°F). Remove the battery when
discharged.
CAUTION: Battery Disposal
Recycle or dispose of the lithium battery in accordance with all federal,
state and local laws. To avoid fire and explosion hazard, do not burn or
incinerate the battery.
CAUTION: Temperature/Humidity/Pressure Extremes
Exposing the Powerheart AED with the battery installed to extremes,
outside the following operation and standby conditions, will cause the
self-tests to be disabled and could cause the Powerheart AED to function
1. Cummins, R., ed., Advanced Cardiac Life Support; AHA (1994): Ch. 4.
Page 8 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0

Section 1: Safety
improperly. Storing the Powerheart AED outside the stated temperature
conditions for 5 consecutive days will result in a “service required” alert.
• Temperature 0°C to 50°C (32°F to 122°F)
• Humidity 5% to 95% (non-condensing)
• Pressure 57kPa (+15,000 ft) to 170kPa (-15,000 ft)
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 9
Section 1: Safety
Symbols Descriptions
The following symbols appear in this manual, on the Powerheart AED, or
on its optional components. Some of the symbols represent standards and
compliances associated with the Powerheart AED and its use.
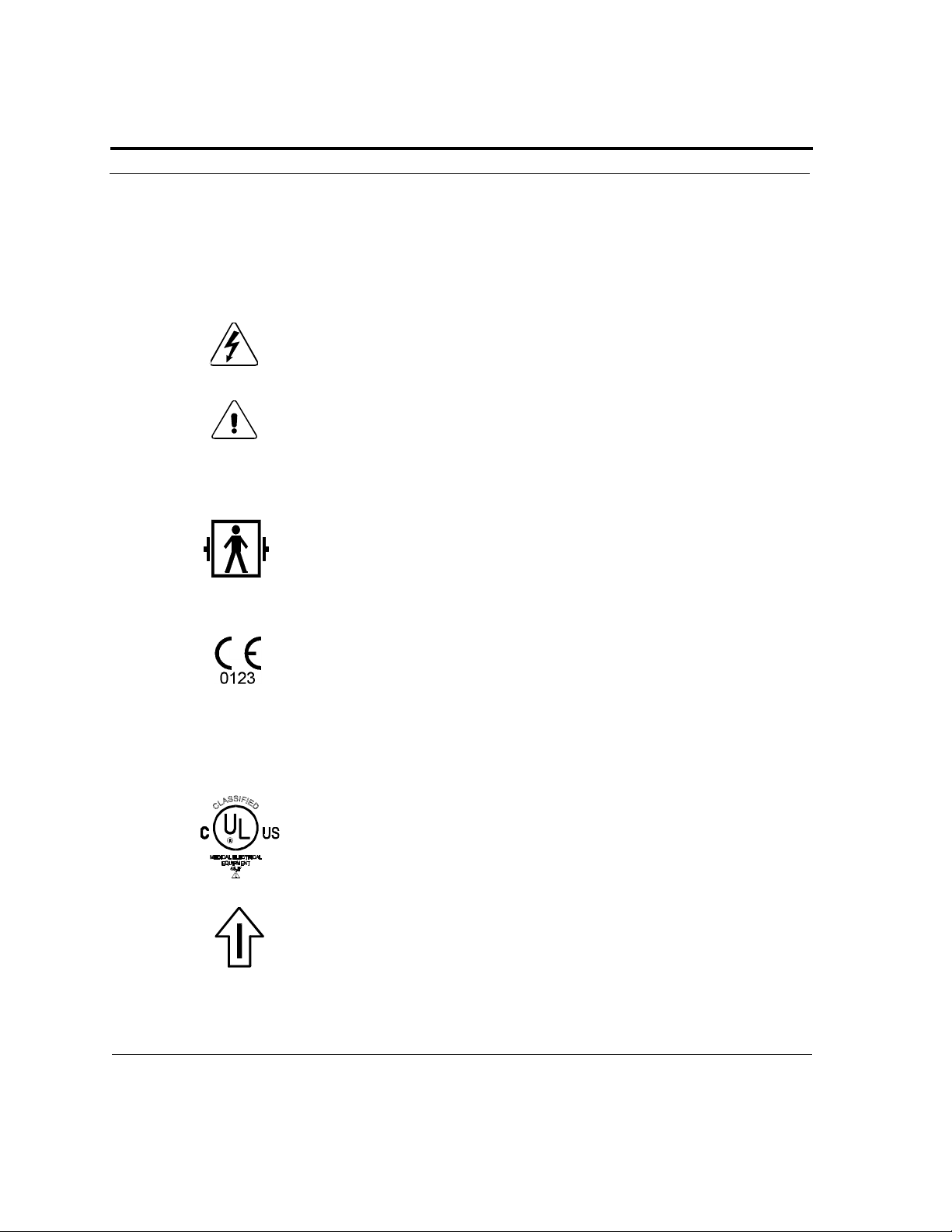
Dangerous Voltage: The defibrillator output has high voltage and can
present a shock hazard. Please read and understand all safety alerts in this
manual before attempting to operate the Powerheart AED.
Attention!: Identifies important information in this manual, on the
Powerheart AED, or on its component parts regarding the safe and proper
use of the Powerheart AED.
Defibrillator Proof Type BF Equipment: The Powerheart AED, when
connected to the patient’s chest by the electrodes, can withstand the
effects of an externally applied defibrillation shock without diverting the
shock from the patient or into the Powerheart AED.
CE Mark: This equipment conforms to essential requirements of the
Medical Device Directive 93/42/EEC.
IP23
Page 10 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0
The Powerheart AED is protected against the effects of spraying water in
accordance with IEC 529.
Classified by Underwriters Laboratories Inc. with respect to electric
shock, fire and mechanical hazards only in accordance with UL 2601-1
and IEC 601-2-4, IEC SC 62D/WG2 (O’Dowd) and CAN/CSA C22.2
No.601.1-M90.
International symbol for ON. Open the lid to turn ON the Powerheart
AED.
Section 1: Safety
TSO-C97
FAA TSO marking: This battery conforms to the FAA lithium sulfer
dioxide batteries technical standard order, TSO C97.
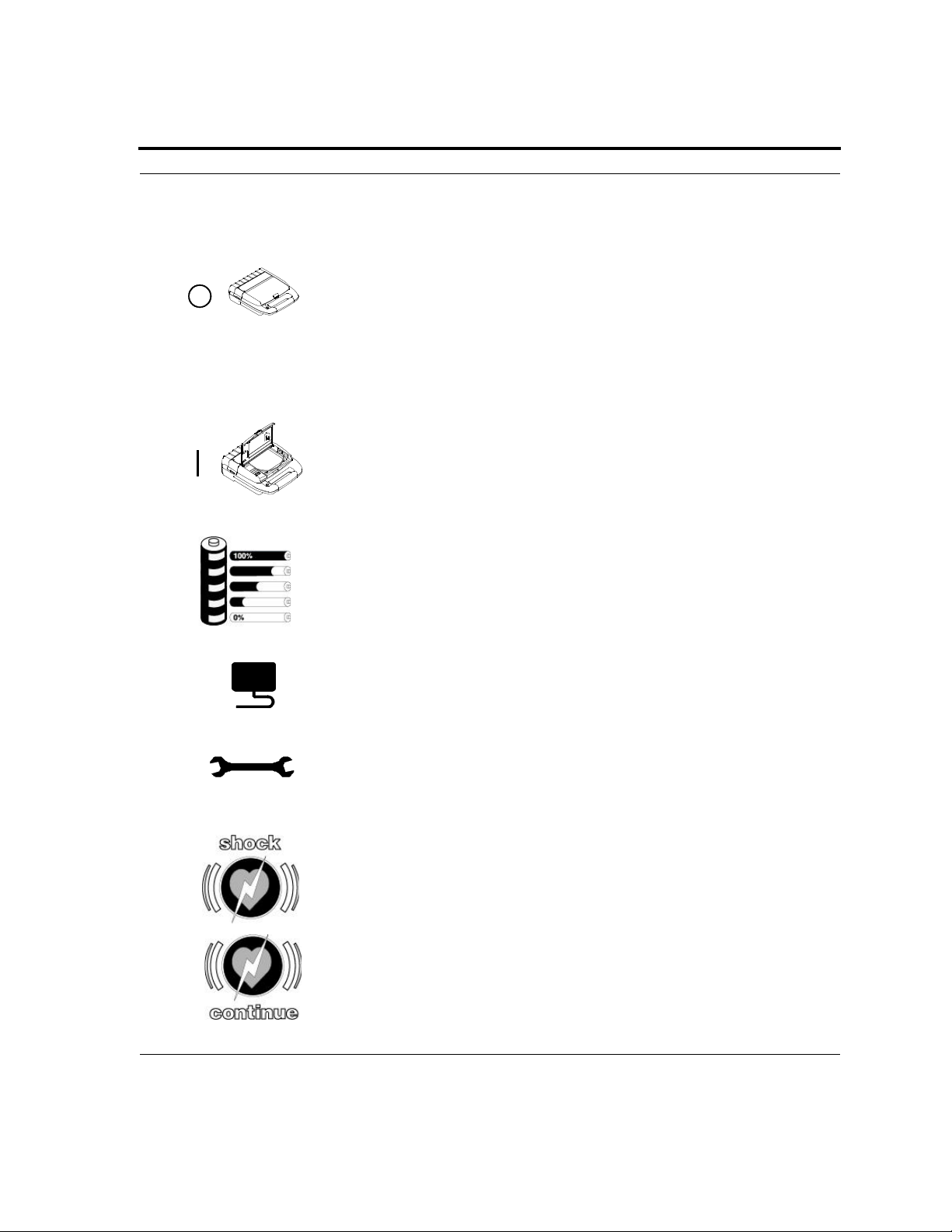
International symbol for OFF. Close the lid to turn OFF the Powerheart
AED.
Open the lid to turn ON the Powerheart AED.
Indicates the Powerheart AED battery status. The shaded areas indicate
the remaining battery capacity.
Check the electrodes when illuminated. The electrodes are either missing
or out of specification. Also, on the electrode packaging, this symbol
represents one pair.
When illuminated indicates Powerheart AED requires maintenance by
authorized service personnel.
When flashing, push this button to deliver a defibrillation shock.
When flashing: push this button to clear the internal memory to allow
storage of new rescue data in the Powerheart AED.
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 11
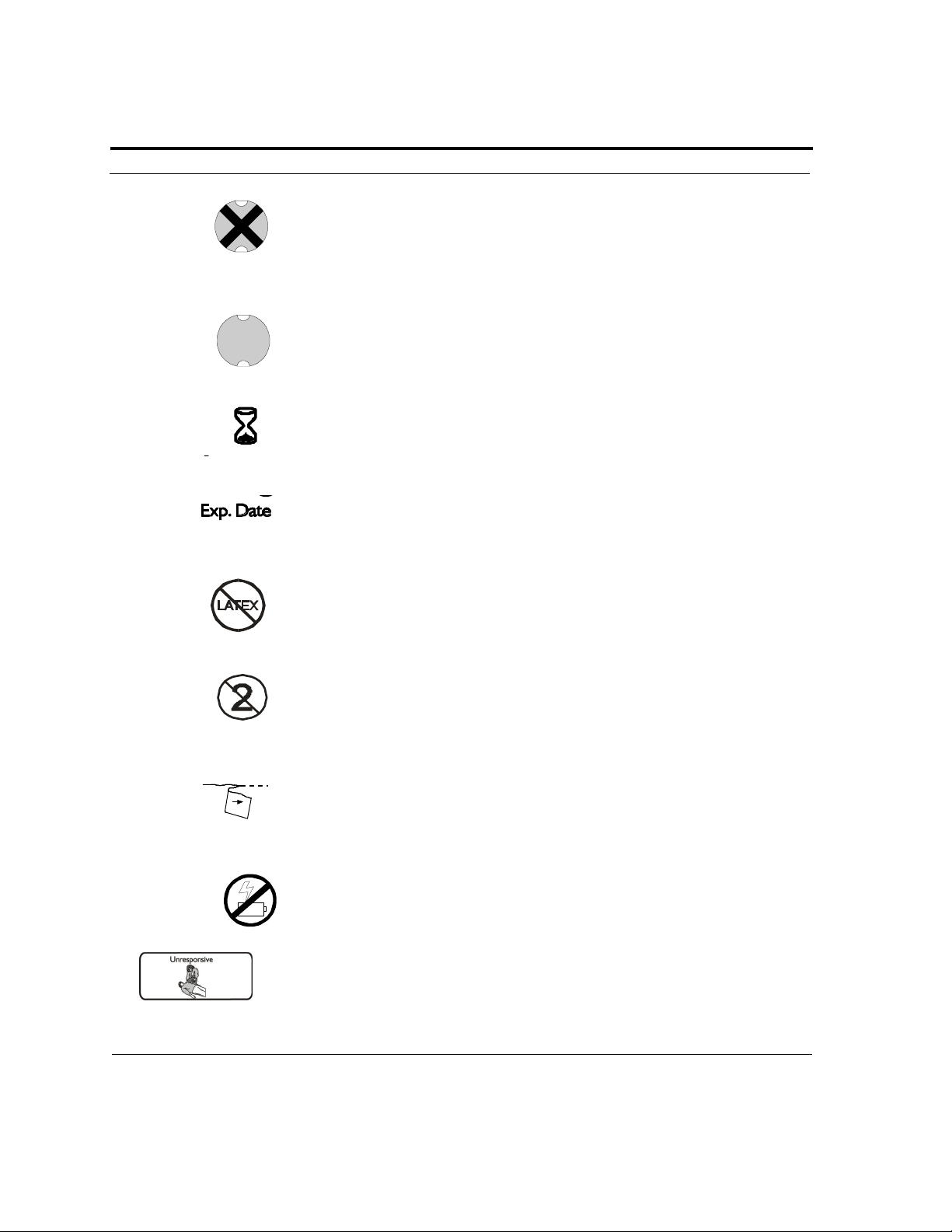
Section 1: Safety
RED with a BLACK X means the Powerheart AED requires operator
attention or maintenance, and is not RescueReady. For purposes of
retaining simple, clear instructions, this symbol will be referred to as RED
in the remainder of this manual.
GREEN without a BLACK X means the Powerheart AED is
RescueReady. For purposes of retaining simple, clear instructions, this
symbol will be referred to as GREEN in the remainder of this manual.
Use by or install by date.
Expiration Date. Replace by this date.
Latex Free.
Disposable. Single patient use only.
Tear here to open.
Do not recharge battery.
The patient is unconscious.
Page 12 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0
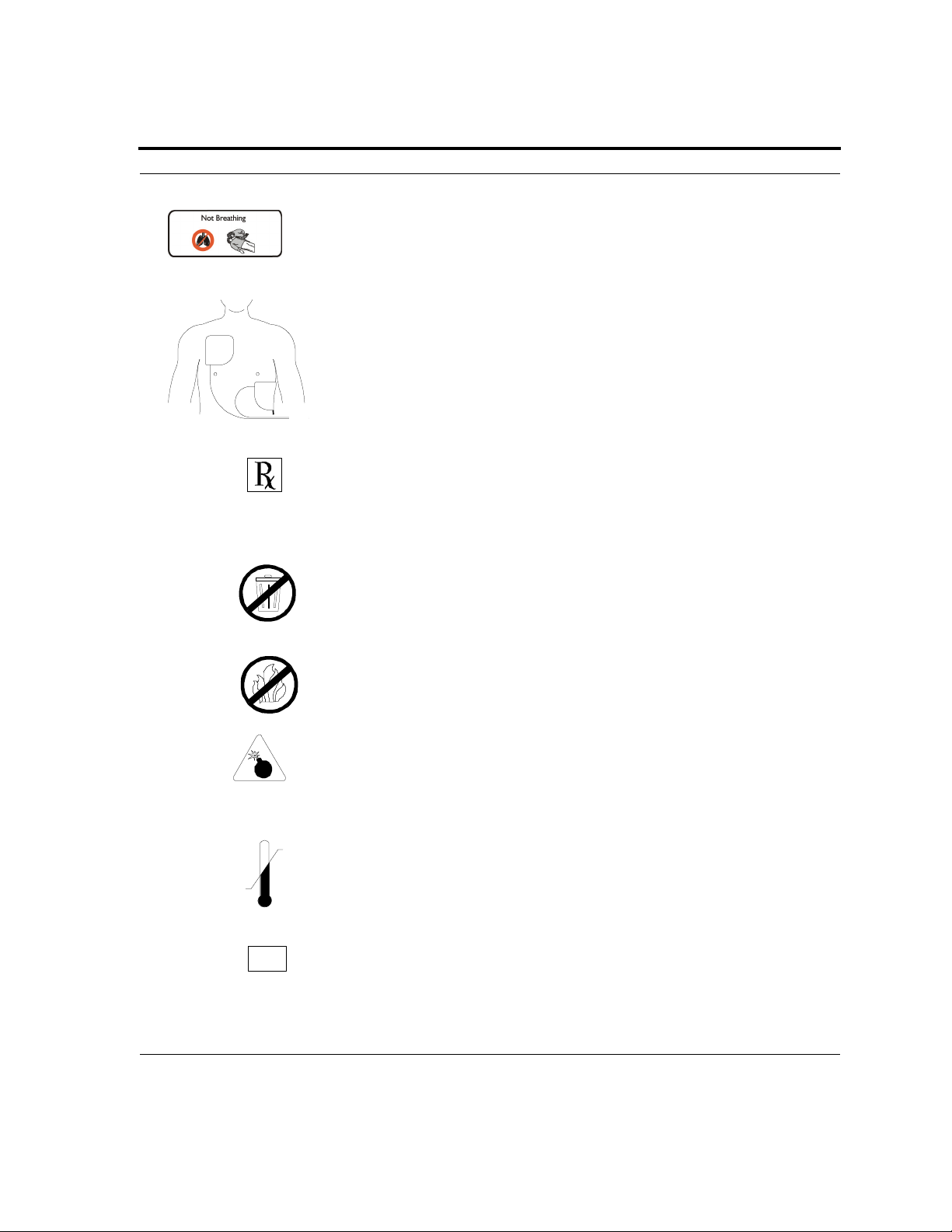
Section 1: Safety
The patient is not breathing.
Place the electrodes on the chest of the patient.
For use by or on the order of a Physician, or persons licensed by state,
province or country law.
Dispose of properly in accordance with all state, province, and country
regulations.
Do not incinerate or expose to open flame.
Explosion Hazard: Do not use in the presence of a flammable gas,
including concentrated oxygen.
Upper and lower temperature limits.
Serial Number.
SN
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 13
Section 1: Safety
Lot Number.
Date of Manufacture.
Additional information is provided in the Powerheart AED Operation and
Service Manual.
Points to important information regarding the use of the Powerheart AED.
Lift Here
Page 14 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0

Section 2 Introduction
Overview
This section presents information about the Powerheart AED, its use, and
the training requirements for operation.
Topic Page
Powerheart AED Description 16
Indications for Use 16
Contraindications for Use 16
Powerheart AED ECG Analysis Algorithm 17
Powerheart AED Rescue Protocol 18
Powerheart AED STAR Biphasic Waveform 19
Powerheart AED Operator Training Requirements 19
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 15

Section 2: Introduction
Powerheart AED Description
The Powerheart AED is a self-testing battery-operated automated
external defibrillator (AED). After applying the Powerheart AED’s
electrodes to the patient’s chest, the Powerheart AED automatically
analyzes the patient’s electrocardiogram (ECG) and advises the user to
push the button and deliver a shock if needed. The Powerheart AED uses
one button and guides you through the rescue using a combination of
voice prompts, audible alerts, and visible indicators.
Indications for Use
The Powerheart AED with STAR Biphasic is intended to be used by
personnel who have been trained in its operation. The user should be
qualified by training in basic life support or other physician-authorized
emergency medical response. The device is indicated for emergency
treatment of victims exhibiting symptoms of sudden cardiac arrest who
are unresponsive and not breathing. Post-resuscitation, if the victim is
breathing, the AED should be left attached to allow for acquisition and
detection of the ECG rhythm. If a shockable ventricular tachyarrythmia
recurs, the device will charge automatically and advise the operator to
deliver therapy. The Powerheart AED with STAR Biphasic is intended to
be used on patients older than eight years.
Patients at risk of cardiac arrest include persons that:
• Are not responsive
• Are not conscious
• Are conscious and have just been revived by a defibrillator shock
• Are conscious and complain of dizziness, and/or chest pain
• Suffers from syncopy (fainting spells)
• Suffer shortness of breath
• Experience unexplained palpitation, coldness or sweat
• Experience radiating pain from the neck to the shoulder
• Experience unexplained chest pains.
Contraindications for Use
Do not use the Powerheart AED for emergency treatment if the patient is
under eight years of age.
Page 16 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0

Section 2: Introduction
Powerheart AED ECG Analysis Algorithm
The Powerheart AED ECG analysis algorithm, provides superior ECG
detection capabilities allowing it to be placed on patients at risk for
sudden cardiac arrest. The features available with the Powerheart AED
include the following:
• Detection Rate
• Supraventricular Tachycardia (SVT) Rate
• SVT Discriminators
• Asystole Threshold
• Noise Detection
• Continuous Monitoring
• Non-Committed Shock
• Synchronized Shock
• Pacemaker Signal Detection
Detection Rate
All ventricular fibrillation (VF) and ventricular tachycardia (VT) rhythms
at or above this rate will be classified as shockable. All rhythms below
this rate will be classified as non shockable. The default Detection Rate is
160 bpm (beats per minute). This rate is selectable between 120 bpm and
240 bpm via MDLink Software by the Medical Director.
SVT Rate
All rhythms with rates between the Detection Rate and SVT Rate will be
screened through a number of SVT Discriminator to classify them into
VF/VT or SVT. Rhythms classified as SVT between the two set rates are
not shockable. All rhythms at or above the SVT Rates will be shockable.
The default SVT Rate is 200 bpm. The SVT rate must be greater than or
equal to the Detection Rate and is selectable between 160 and 240 bpm
via MDLink Software by the Medical Director.
SVT Discriminators
These are sophisticated filters that analyze the morphology of the ECG
waveforms and distinguish VF/VT from SVT and Normal Sinus
Rhythms (NSR). The SVT Discriminator will only be applied to rhythms
that fall between the Detection Rate and the SVT Rate.
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 17

Section 2: Introduction
Asystole Threshold
The asystole peak to peak threshold is set at 0.08 mV. ECG rhythms at or
below 0.08mV will be classified as Asystole and will not be shockable.
Noise Detection
The Powerheart AED will detect noise artifact in the ECG. Noise could
be introduced by excessive moving of the patient or electronic noise from
external sources like cellular and radio telephones. When noise is
detected, the Powerheart AED will issue the prompt “Analysis
interrupted. Stop Patient Motion” to warn the user. The Powerheart AED
will then proceed to reanalyze the rhythm and continue with the rescue.
Continuous Monitoring
Powerheart AED monitors the ECG rhythms continuously throughout the
rescue including during Charge and CPR mode. Continuous Monitoring
will interrupt CPR if a shockable rhythm is detected. When CPR is
interrupted, the prompt “Do not touch patient. Analyzing rhythm” will be
issued. Only one interruption will be allowed during a single CPR mode.
CPR mode will not be interrupted if preceded by three consecutive
shocks.
Non-Committed Shock
After the Powerheart AED advises a shock, it continues to monitor the
patient ECG rhythm. If the patient’s rhythm changes to a non-shockable
rhythm before the actual shock is delivered, the Powerheart AED will
advise that the rhythm has changed and issue the prompt “Rhythm
changed. Shock cancelled.” The Powerheart AED will then be disarmed
and the ECG reanalyzed.
Synchronized Shock
Powerheart AED is designed to synchronize shock delivery on the Rwave. If delivery cannot be synchronized within one second of the button
being pressed, a non-synchronized shock will be delivered.
Powerheart AED Rescue Protocol
The Powerheart AED rescue protocol is consistent with the guidelines
recommended by the American Heart Association (AHA)1 and the
International Liaison Committee on Resuscitation (ILCOR).
Page 18 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0

Section 2: Introduction
Upon detecting a shockable cardiac rhythm, the Powerheart AED advises
you to press the “Shock” button to deliver a series of up to 3 defibrillation
shocks followed by performing 1 minute of CPR.
The 3 defibrillation shocks are delivered in a pre-programmed sequence
of escalating biphasic energies in the default configuration.
Note: The CPR protocol can be modified, such that from 1 to 3 minutes, in
increments of 5 seconds, of CPR may be administered if the first
analysis is non-shockable or following two consecutive nonshockable analysis decisions.
Powerheart AED STAR Biphasic Waveform
The Powerheart AED STAR Biphasic Waveform is designed to measure
the patient’s impedance and deliver a customized shock. This allows the
delivery of an optimized energy level to each patient. The energy levels
for the Powerheart AED are available in three different defibrillation
shock2 configurations. See table below.
9200R/9210R/
Defibrillation Shock
2
9200DR/9210DR
AED
Configurations
Standard Energy Low
Low Energy Ultra-Low
1st
Shock
Current
Current
2nd
Shock
Low Current or
High Current
Ultra-Low
Current or Low
3rd
Shock
High
Current
Low
Current
Current
Non-escalating
Energy
Low
Current
Low Current Low
Current
Powerheart AED Operator Training Requirements
Persons authorized to operate the Powerheart AED must have all of the
following minimum training and experience:
• Defibrillation training and other training as required by state, province,
or country regulations
1. “Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care” American Heart Association; 2000. Suppl. Circulation 102(8). August 22, 2000
2. The ultra-low current, low current and high current shocks are variable energy. The actual energy
is determined by the patient’s impedance.
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 19

Section 2: Introduction
• Training on operation and use of Powerheart AED
• Additional training as required by the physician or Medical Director
• A thorough understanding of the procedures in this manual
Keep certificates of training and certification as required by state,
province, or country regulations.
Page 20 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0

Section 3 Getting Started
Overview
This section presents information on unpacking and setting up the
Powerheart AED.
Topic Page
Unpacking and Inspecting 22
Powerheart AED 23
Batteries 25
Electrodes 27
Powerheart AED Indicators 28
Setting Clock 30
Voice Prompts and Text Display 32
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 21

Section 3: Getting Started
Unpacking and Inspecting
Every attempt is made to ensure your order is accurate and complete.
However, to be sure that your order is correct, verify the contents of the
box against your packing slip.
If you have any question about your order, contact our Customer Service
Department at: (800) 991-5465 or (952) 939-4181 or your local
distributor. For customers from countries outside of the United States,
contact your local distributor.
Page 22 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0
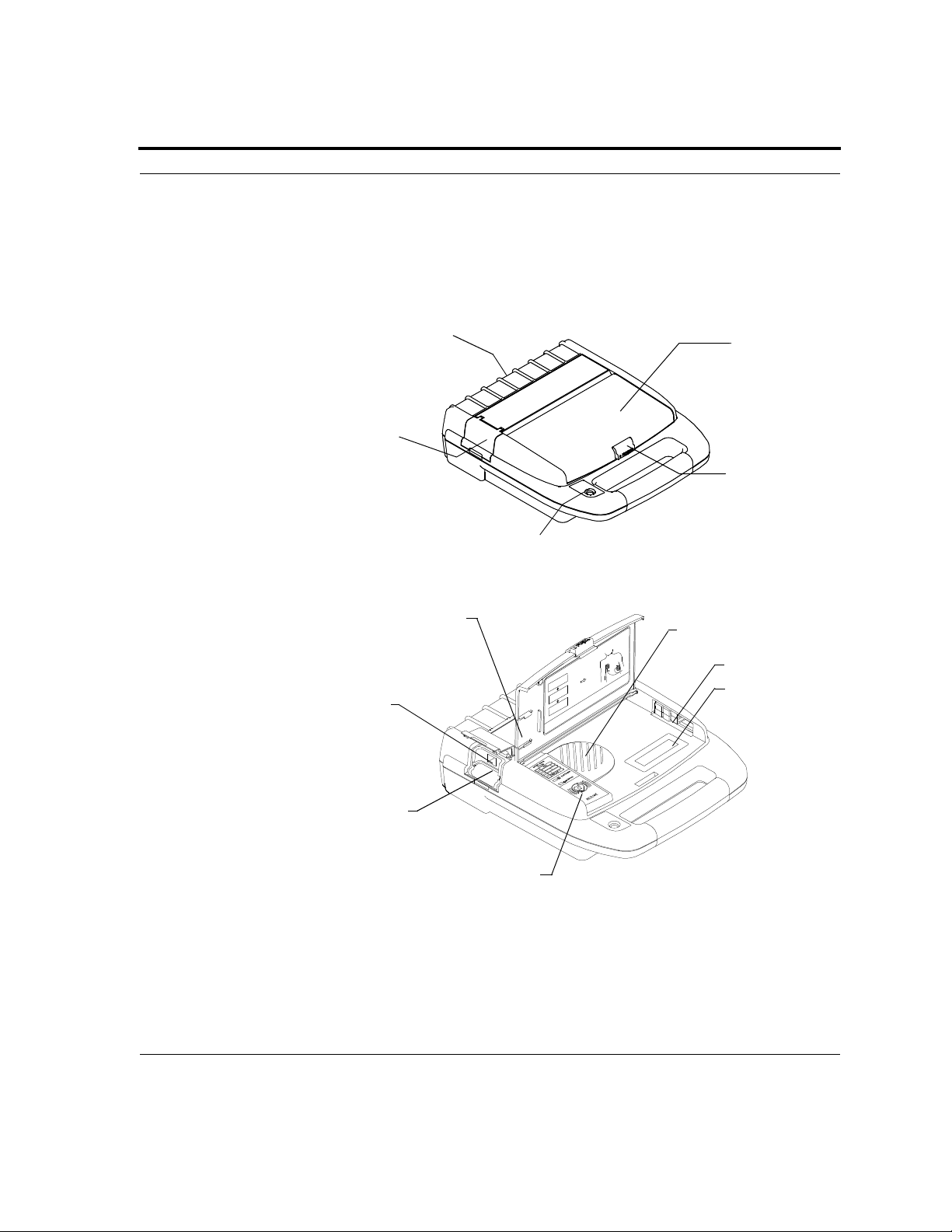
Section 3: Getting Started
Powerheart AED
The following drawings show the Powerheart AED parts and their
locations.
A = Lid
B = Latch (push in and up to
open)
C = Status indicator
D = Data access door
E = Battery compartment
F = Speaker
G = Electrode connector
H = Diagnostic panel
I = Card Slot (model 9210R and
9210DR)
J = Serial communication port
K = Spare flash card storage
L = Optional text display
E
A
D
B
C
K
F
G
L
J
I
H
The Powerheart AED has three modes of operations:
Operating Mode - is defined as having the battery installed and the lid
open. This is the mode the Powerheart AED would be in during an actual
rescue situation.
300282-003 Rev. A0 ©2001 Cardiac Science, Inc. Page 23
Section 3: Getting Started
Standby Mode - is when the battery is installed, but the lid is closed. In
this mode the Powerheart AED is not being used in a rescue, the device
will conduct its routine self-tests to ensure proper operation.
Storage Mode - is when the battery is removed, such as during shipping
or transport. With the battery removed, the Powerheart AED is unable to
perform self-tests or rescues.
Powerheart AED Operating and Standby Conditions
Temperature 0°C to 50°C (32°F to 122°F)
Humidity 5% to 95% (non-condensing)
Atmospheric Pressure 57kPa to 170kPa
CAUTION: Temperature/Humidity/Pressure Extremes
Exposing the Powerheart AED with the battery installed to extremes,
outside the operation and standby conditions, will cause the self-tests to
be disabled and could cause the Powerheart AED to function improperly.
Storing the Powerheart AED outside these conditions for 5 consecutive
days will result in a “service required” alert.
Powerheart AED Shipping and Transport Conditions
(for up to 1 week)
Temperature w/o Display -40°C to 65°C (-40°F to 149°F)
Temperature w/Display -30°C to 65°C (-22°F to 149°F)
Humidity 5% to 95% (non-condensing)
Atmospheric Pressure 57kPa to 170kPa
Page 24 ©2001 Cardiac Science, Inc. 300282-003 Rev. A0
 Loading...
Loading...