Philips Series 50 (M1351-53) User manual

Series 50 A (M1351A)
Series 50 IP-2 (M1353A)
Fetal Monitors
INSTRUCTIONS FOR USE
M1353-9001J
Printed in Germany February 2002

Philips makes no warranty of any kind with regard to this material, including, but not limited
to, the implied warranties of merchantability and fitness for a particular purpose. Philips shall
not be liable for errors contained herein or for incidental or consequential damages in
connection with the furnishing, performance or use of this material.
The information contained in this document is subject to change without notice.
Philips assumes no responsibility for the use or reliability of its software on equipment that is
not furnished by Philips.
Responsibility of the Manufacturer
Philips only considers itself responsible for any effects on safety, reliability and performance of
the equipment if:
• assembly operations, extensions, re- adjustments, modifications or repa irs are carried out by
persons authorized by Philips, and
• the electrical installation of the relevant room complies with national standards, and
• the instrument is used in accordance with the Instructions for Use or User’s Guide.
Intended Use (M1351A)
The intended use of this device is to monitor the condition of the fetus by measuring fetal
heart rate (FHR), maternal uterine activity and optionally fetal movements simultaneously.
The M1531A is a fetal monitor designed for the antepartum testing area. The fetal heartbeat
is detected by an ultrasound transmitter/receiver probe applied to the abdominal wall.
The intended use of this device is to provide information of both FHR and maternal uterine
activity on a digital display, on a strip char t recorder and on an interface for optional remote
data management.
Intended Use (M1353A)
The intended use of this device is to monitor the condition of the fetus by measuring fetal
heart rate (FHR), maternal uterine activity and optionally fetal movements simultaneously.
The M1353A is a fetal monitor designed for the antepartum and intrapartum testing area.
The fetal heartbeat may be detected by ECG electrodes connected to the fetal scalp or by an
ultrasound transmitter/receiver probe connected to the abdominal wall. The uterine pressure
may be detected by a pressure probe connected to the intrauterine pressure via a fluid filled
tube or by a pressure probe connected to the abdominal wall.

The intended use of this device is to provide information of both FHR and maternal uterine
activity on a digital display, on a strip chart recorder and on an interface f or optional remote
data management.
The monitor should only be used by, or under the direct supervision of, a licensed physician
or other health care practitioner who is trained in the use of fetal and maternal heart rate
monitors and in the interpretation of fetal and maternal heart rate traces. US law restricts this
device to sale by, or on the order of, a physician.
Conventions Used in This Guide
Warning
A warning alerts you to a potential serious outcome, adverse event or safety hazard.
Failure to observe a warning may result in death or serious injury to the use r or pati ent.
Caution
A caution alerts you to situations where specia l care is necessary for the safe and
effective use of the product. Failure to observe a caution may result in minor or
moderate personal injury or damage to the product or other property, and possi bly in a
remote risk of more serious injury.
Note—A note
calls your attention to an important point in the text.
On your monitor, this sign indicates that there is
detailed information in this book which you must read
before proceeding with your task
2002 Philips Medizinsysteme Boeblingen GmbH
All rights are reserved. Reproduction in whole or in part is prohibited without the prior
written consent of the copyright holder.


Contents
1. Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
About This Guide. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
About the Monitors. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Major Parts and Keys. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Recorder Keys. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Major Keys at a Glance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Display Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
2. General Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Fastening a Belt around the Mother . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Clipping a Transducer to the Belt . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Attaching a Patient Module to the Belt. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Connecting a Transducer or Patient Module
to the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Signal Quality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Suspected Fetal Demise. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Marking an Event . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
After Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
3. Getting Started. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Before Connecting to the Power Supply. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Connecting to the Power Supply. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Loading Paper. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Paper-Out Alert. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Choosing Paper Speed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Setting the Paper Speed. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Tearing Off the Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Switching on the Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Displaying the Time and Date . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Setting the Time and Date . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Mounting the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Fitting the Monitor to a Wall . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Fitting the Monitor to an Angle Mount. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Fitting the Monitor to a Cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Contents v

Fitting the Paper Take-Up Tray . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
4. Monitoring FHR and FMP Using Ultrasound . . . . . . . . . . . . . . . .29
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Ultrasound Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
What You Need . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Getting Started . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Fetal Movement Profile. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
Switching FMP On and Off. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
FMP Statistics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
5. Monitoring FHR Using DECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
DECG: Contraindications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
What You Need . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Getting Started . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
With DECG legplate M1357A. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
With Patient Module M1364A. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Using the DECG Adapter Cable M1362B to Monitor DECG. . . . . . . . . . . . . . . . . . . . . 44
With DECG Legplate M1357A . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
With Patient Module M1364A. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
Monitoring DECG. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
Switching Arrhythmia Logic On and Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50
Why use Arrhythmia Logic?. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Removing the Fetal Scalp Electrode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
6. Monitoring Twin FHRs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Things to Remember During Monitoring. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Monitoring Internally . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Monitoring Externally . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
Cross-Channel Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
Separating Twin FHR Traces: “Twins Offset” . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 59
Using Keys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
Using the Barcode Reader . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 60
Twins Offset: On . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
Twins Offset: Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
vi Contents

7. Monitoring Uterine Activity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
What You Need. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
For External Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
For Internal Monitoring. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
External Toco Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66
Internal Toco Monitoring (IUP Monitoring) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
External Toco . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
Internal Toco . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
8. Measurements Using External Devices . . . . . . . . . . . . . . . . . . . . 71
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 71
Supported External Devices. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
Connecting External Devices to the Monitor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
Trace Display on Obstetrical Surveillance Systems . . . . . . . . . . . . . . . . . . . . . . . . . 74
Monitoring Maternal NIBP. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
Example Maternal NIBP Trace. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
Monitoring FSpO
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Example FSpO
Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
FSpO
2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .78
External Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .77
Trace . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
2
9. Monitoring Maternal ECG. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
Monitoring Maternal ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 81
To Begin Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 82
Using MECG Transducer M1359A . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 82
Using a Patient Module M1364A. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 82
Cross-Channel Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84
Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 85
10. Fetal Heart Rate Alerting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Recognizing an Alert . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Acknowledging an Alert . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 87
Turning Alerting ON or OFF . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
Contents vii

Changing Alert Limits. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 88
11. Non Stress Test Timer. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .91
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Setting the NST Timer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
12. Recording Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .93
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 93
Recording A Note. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 93
Deleting a Barcode Note . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 94
Recording a Patient’s Name . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 94
Recording Several Barcodes as One Note. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 94
Recording Several Barcodes as Separate Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 95
13. Modem Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97
Connecting the Modem Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97
Connecting Peripheral Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 98
Connecting to the Telephone System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99
PCMCIA Modem. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100
Entering and Storing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101
Barcode Reader . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101
Getting Started. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 101
Setting Phone Numbers and Patient ID. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 102
Keeping Patient Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 102
Clearing Patient Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 103
Clearing Trace Memory. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 103
Storing Fetal Trace Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 104
Displaying Memory. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 104
Stopping Storage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 104
Transmitting Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
Trace Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
Stopping Transmission . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 105
Troubleshooting and Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 106
Error Message 77 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 6
Error Messages. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 107
Power Failure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 108
14. Upgrade Key . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .109
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 109
viii Contents

Upgrade Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 109
15. Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113
Self Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 113
Quick Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 114
Parameter Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 115
Testing Transducers. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 117
Toco. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 117
Ultrasound . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 117
IUP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 118
Testing Patient Modules and Legplates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 118
Testing a Barcode Reader . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 119
Error Messages. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 119
A. Care and Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 123
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 123
Cleaning the Monitor and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 124
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 125
Cleaning Agents. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 126
Disinfecting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 127
Sterilizing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128
Belts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128
Storing Recorder Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 129
Preventive Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130
Visual Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130
Routine Inspection. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130
Mechanical Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131
Calibration and Electrical Safety Checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131
Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131
B. Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 133
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 133
General Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 133
Electrical Safety. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 134
Series 50 A. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 134
Series 50 IP-2. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 135
ESU, MRI and Defibrillation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 135
Leakage Current. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 136
Maximum Input/Output Voltages. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 136
Service Socket for Upgrade Key . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 136
Contents ix

Combined Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 137
Modem Interface Module. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 138
Protective Earth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 138
Environment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 139
Spillage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 140
Electromagnetic Compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 140
EMC Testing. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 140
System Characteristics. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 141
Avoiding Interference. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 141
C. Replacing Fuses and Batteri es . . . . . . . . . . . . . . . . . . . . . . 14 3
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 143
Replacing the Batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 143
Replacing the Fuses. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 145
D. Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 147
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 147
Standard Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 147
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 149
Optional Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 150
Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 151
Gels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 151
Heart Rate Transducers and Patient Modules. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 152
Electrodes and Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 152
Disposable Scalp Electrodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 153
IUP Transducers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 153
IUP Catheters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 154
Domes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155
IUP Transducer Holder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155
Belts and Buttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155
Barcode Booklets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 157
Modem Interface Module Barcode Sheet. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 157
Digital Interface Protocol Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 157
E. Manufacturer’s Information . . . . . . . . . . . . . . . . . . . . . . . . 159
Manufacturer’s Responsibility. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 159
Warranty. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 159
USA Law. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 162
Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163
Patient Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 163
Operating and Environmental. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164
x Contents

Ultrasound, External and Internal Toco . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 166
Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 166
Scales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 167
Testing Facilities. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 167
Declaration. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 168
Contents xi

xii Contents

Introduction
This chapter contains general information about your Instructions for
Use and about your fetal monitor. You will learn about:
• The most important functions of your monitor
• The major parts and keys of your monitor.
About This Guide
This book tells mi dwiv es, nurses, and oth er hea lth c are pr ofes siona ls ho w
to use the Philips Series 5 0A fetal monitor and the Philips S eries 5 0IP-2
fetal monitor.
1
Overview
It discusses and illustrates all the parameters and features of both
monitors. Your monitor may not have every one of these features and
may look slightly different to the monitor shown in the illustrations in
this guide. A la bel in the margin of the book will indicate whether the
text refers to:
Both the Series 50 A and the Series 50 IP-2 fetal monitors
•
• The
• The Series 50 IP-2 fetal monitor only.
Chapter 1 - Overview 1
Series 50 A fetal monitor only
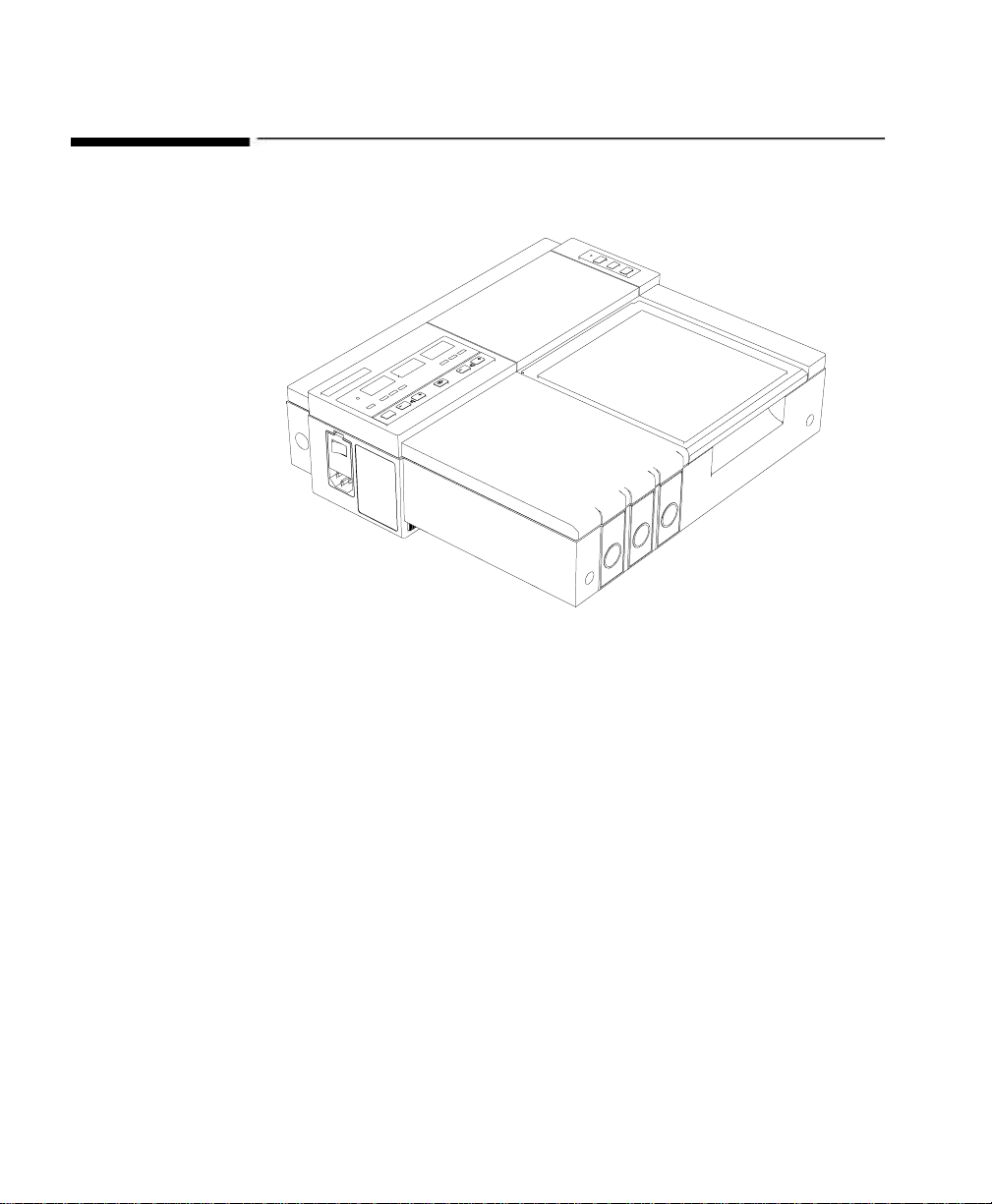
About the Monitors
About the Monitors
Series 50 A Dual Ultrasound Model
Series 50 IP-2
Series 50 A The Series 50 A Single Ultrasound model monitors a single fetal heart
rate. The Series 50 A Dual Ultrasound model can monitor one or twin
fetal heart rates.
The Single Ultrasound model looks slightly different from the Dual
Ultrasound model. For the illustrations, we have used the Dual
Ultrasound model.
Series 50 IP-2 The Series 50 IP-2 can monitor one or twin fetal h eart rates, one using
ultrasound and t he seco nd usi ng ECG or ultraso und. I t looks very si milar
to the Series 50 A Dual Ultrasound model.
Note—Not all parameters and features detailed in this manual are
available on all monitors
2 Chapter 1 - Overview

About the Monitors
Series 50 A Using the Series 50 A antepartum feta l monitor you can monitor
• Fetal heart rate (FHR) , externally using ultrasound
• Uterine activity
• Fetal pul se o x imetry, by connecting a n exte rnal fet al pul se o ximeter
to your fetal monitor (optional)
• Maternal blood pressure, externally, by connecting an NIBP
monitor to your fetal monitor (optional)
• Fetal movement (optional)
Using the Series 50A Single Ultrasound model you can monitor a single
fetal heart rate; t he D ual U lt ra s ound m od el l e ts yo u m onit or on e o r t win
fetal heart rates.
With the Series 50 A fetal monitor you can also use an optional Modem
Interface Module to send fetal trace information to a Philips obstetrical
surveillance system, such as OB TraceVue.
Series 50 IP-2 Usi ng the Series 50 IP-2 intrapartum fetal monitor you can monitor
Fetal heart rate, including twins
– externally using ultrasound or
– directly using ECG
Series 50 A and
Series 50 IP-2
Chapter 1 - Overview
• Uterine activity
– externally using an Toco transducer or
– internally using an IUP catheter
• Maternal heart rate (MHR) using DECG
• Fetal pul se o x imetry, by connecting a n exte rnal fet al pul se o ximeter
to your fetal monitor (optional)
• Maternal blood pressure, externally (NIBP), by connecting a NIBP
monitor to your fetal monitor (optional)
• Fetal movement (optional)
Both monitors offer you the following features:
• Automatic printing of maternal and fetal parameters on the trace
paper
3

About the Monitors
• Transmission of maternal and fetal parameters to an obstetrical
review system
• Audible and visual alarms
• Twin heart rate separation for ease of interpretation
• Non stress test (NST) timer
• Paper-e nd alarm
• Significant event marking on the trace paper.
4 Chapter 1 - Overview

Major Parts and Keys
Recorder Keys
1. Recorder on/off key switches the recorder on and off. It also starts
the NST timer (switch off the recorder and press the recorder on/
off key for two seconds).
2. Recorder on/off light is on when the recorder is working. It flashes
when the monitor detects five pages or less remaining in the pack,
and if the paper runs out.
3. Event marker key records events on the paper trace.
4. Paper advance key advances pa per automatically to the next fold.
Tear the paper along the perforations and never pull it to advance
it.
Major Parts and Keys
3
4
Chapter 1 - Overview
5

Major Parts and Keys
Major Keys at a Glance
6 Chapter 1 - Overview

Major Parts and Keys
1. Mains socket
2. Monitor on/off switch
3. Equipotential grounding point
4. Monitor on/off light
5. Display panel
6. Time and date key
7. Paper speed key
8. Test key
9. Recorder on/off light
10. Recorder on/off key
11. Event marker key (Alert acknowledge key)
12. Paper advance key
13. Loudspeaker
14. Battery compartment
15. Paper table
16. Service socket
17. Series 50 A: US2 Socket (not present on Single Ultrasound
model)
Series 50 IP-2: US2/ECG Socket
18. Toco socket
19. Series 50 A: Single Ultrasound Model: US Socket
Double Ultrasound Model: US1 socket
Series 50 IP-2:US1 socket
20. Socket for remote event marker
21. Lock-release button
22. Combined interface module
23. Integrated carrying handle
24. Cable clamp
Chapter 1 - Overview
7

Major Parts and Keys
Display Panel
1. Monitor on/off light
2. Telemetry indicator is on when the fetal u ltrasound telemetry
receiver is connected and switched on
3. Function key: selects menus for twins offset, FMP and logic
4. US/US1 display. Shows the FHR detected by the US transducer
5. US/US1 signal quality indicator shows the quality of the heart
rate signal detected by the US transducer:
– Green (good)
– Yellow (fair to potentially poor)
– Red (unacceptable)
6. US/US1 speaker light is on when the US/ US1 heartbeat is the
one you hear
7. US/ US1 volume keys set the volume and select the US channel to
which you are listening. Use them also to change current setting of
FMP, twins offset, logic, and FHR alert.
8. Toco display shows uterine activity
9. Toco baseline key zeros the Toco display and trace to 20 units
when you are monitoring uterine activity externally or 0 units
when you are monitoring uterine activity internally
8 Chapter 1 - Overview

Major Parts and Keys
10. US2/ECG display shows the FHR detected by the US2 or DECG
transducer.
11. US2/ECG signal quality indicator shows the quality of the signal
detected by the US2 or DECG transducer.
12. US2/ECG speaker light is on when the US2 or DECG heart b eat
is heard.
13. US2/ECG volume keys set the volume and select the US2 or
DECG heartbeat.
Chapter 1 - Overview
9

Major Parts and Keys
10 Chapter 1 - Overview

Introduction
2
General Information
This section contains information about tasks you will need to perform
regularly. You will learn how to:
• Fasten a belt around the mother
• Attach a transducer or patient module to the belt
• Connect a transduc er or patient module to the monitor
• Mark a significant event on the trace paper
• Be aware of possible fetal demise
• Care for the monitor and accessories after monitoring.
Fastening a Belt around the Mother
1. Place the transducer belt across the bed, ensuring that the fixing
button will face away from the mother when it i s fa stened. Us e t wo
belts if you are monitoring uterine activity and FHR at the same
time.
2. Lie the mother on the bed and arrange the belt around her until it
is tight but still comfortable.
3. Fasten the belt by pushing the fixing button through the
overlapping section of the belt.
Chapter 2 - General Information 11

Clipping a Transducer to the Belt
Ensure that the fixing button and the loose ends of the belt are at
the patient ’s side.
Clipping a Tran s du ce r to th e Belt
When you have positioned a transducer satisfactorily, you can clip it to
the belt using the snap-shut mechanism. The clip is designed to allo w the
transducer to slide along the belt for easy repositioning.
Alternatively, you can fix a button to the transducer and use this to attach
12 Chapter 2 - General Information

the transduce r to the abdominal belt. See the Installat ion Note that
comes with the Transducer Adapter Button for assembly instructions,
Attaching a Patient Module to the Belt
You can attach a patient module to the belt by pushing the fixing knob
(1) on the patient module through one of the holes in the belt.
1
Attaching a Patient Module to the Belt
Connecting a Transducer or Patient Module
to the Monitor
To connect a transducer or patient module to the monitor, first switch on
the monitor and the recorder. When you connect a transducer or a
patient module to either the US/US1 socket, the Toco socket, or the
US2/ECG socket:
• The dashes in the corresponding digital display will go out.
• The signal quality light for the heart rate display turns red, as the
transducer or patient module is not yet receiving a good signal from
the patient.
• The monitoring mode will be printed on the trace paper, and
repeated every three to four pages. Depending on the parameter
you are monitoring, this could be US, US1, US2, DECG, TOCO
int or TOCO ext.
Chapter 2 - General Information
13

Signal Quality
Signal Quality
• You will hear the fetal heartbeat from the loudspeaker.
If the Fetal Scalp Electrode and the electrode wires are connected
correctly and a good si gnal is being pr od uced, the si gnal qualit y indica tor
should be green. If an inadequate signal is being produced, or there is a
bad contact, the signal quality indicator will be red. The message
may also be displayed. See Chapter 15, “Troubleshooting,” for more
details.
Warning
NEVER immerse a transducer in liquid when it is connected to the
fetal monitor.
During monitoring, if the signal quality indicator fluctuates between red,
yellow and green, it does not necessarily mean that the transducer needs
repositioning. The fluctuation may be caused by fetal movement. Allow
time for the signal to stabilize before deciding whether to reposition the
transducer (ultrasound) or apply a new electrode (ECG). A trace is
possible when the indicator is yellow, but for the best trace it should be
continuously green.
nop
Suspected Fetal Demise
Be very careful when interpreting a trace if you suspect fetal demise. The
maternal heartrate may be atypically high and therefore confused with
that of a live fetus. Apparent fetal movement may also be detected by the
monitor but this may be a result of maternal movement causing the fetus
to move within the amniotic fluid.
Please refer to “Cross-Channel Verification” on page 84.
14 Chapter 2 - General Information

Marking an Event
Use the event marker key or the remot e event marker to record signifi cant
events on the trace paper (for example, when pain medication is
administered or when the mother changes position). The mother can use
the remote event marker to mark events herself. To mark an event on the
trace paper you can either:
• Press the event marker key “Mark” on the monitor (1)
Marking an Event
• Or press the button on the remote event marker. The remote event
marker is connected to the monitor via the socket to the left of the
US/US1 transducer socket.
Chapter 2 - General Information
15

Marking an Event
A small arrow is printed on the heart rate scale on the trace paper. The
arrow starts with the peak t o show the exact time whe n the key or but ton
has been pressed.
If the key or button is not released, a black bar is printed on the paper.
The width of the bar c orr esponds to the length of time the key or button
is pressed.
pop63sca.tif
16 Chapter 2 - General Information

After Monitoring
1. Switch off the recorder.
2. Press and release the paper advance key to advance the paper
3. Remove the transducer from the patient and, using a soft tissue,
4. Tear off the paper at the fold.
5. Switch off the monitor.
After Monitoring
automatically to the next fold.
remove any gel from it.
Don’t pull on the paper to advance it and only tear off the paper at
the fold.
Chapter 2 - General Information
17

After Monitoring
18 Chapter 2 - General Information
 Loading...
Loading...