Philips REMstar SE, Respironics REMstar SE User Manual

REMstar SE
USER MANUAL
www.TheCPAPShop.com

© 2012 Koninklijke Philips Electronics N.V. All rights reserved.

1
User Manual
Table of Contents
Intended Use ............................................................................................................................................................... 2
Important ..................................................................................................................................................................... 2
Warnings ......................................................................................................................................................................2
Cautions ....................................................................................................................................................................... 3
Contraindications .......................................................................................................................................................3
Symbol Key ..................................................................................................................................................................4
System Contents ........................................................................................................................................................ 5
System Overview ....................................................................................................................................................... 5
Control Buttons ......................................................................................................................................................... 6
Available Therapies ....................................................................................................................................................6
Installing the Air Filters .............................................................................................................................................6
Connecting the Breathing Circuit .......................................................................................................................... 7
Where to Place the Device ..................................................................................................................................... 7
Supplying AC Power to the Device ....................................................................................................................... 7
Display .......................................................................................................................................................................... 8
Starting the Device .................................................................................................................................................... 9
Ramp Feature ..............................................................................................................................................................9
Humidier Preheat ..................................................................................................................................................... 9
Navigating the Patient Settings ..............................................................................................................................10
Device Messages .......................................................................................................................................................12
Enhanced Compliance Check ................................................................................................................................12
Troubleshooting .......................................................................................................................................................13
Accessories ................................................................................................................................................................15
Traveling with the System ......................................................................................................................................16
Cleaning the Device .................................................................................................................................................16
Cleaning or Replacing the Filters ..........................................................................................................................16
Cleaning the Tubing .................................................................................................................................................16
Service .........................................................................................................................................................................16
Specications .............................................................................................................................................................17
Disposal ......................................................................................................................................................................18
How to Contact Philips Respironics ....................................................................................................................18
EMC Information ......................................................................................................................................................19
Limited Warranty ....................................................................................................................................... Back Page

User Manual2
CAUTION: U. S. federal law restricts this device to sale by or on the order of a physician.
Intended Use
The Philips Respironics REMstar SE system delivers positive airway pressure therapy for the treatment of Obstructive Sleep Apnea
in spontaneously breathing patients weighing over 30kg (66 lbs). It is for use in the home or hospital/institutional environment.
Important
The device is to be used only on the instruction of a licensed physician. Your home care provider will make the correct pressure
settings according to your health care professional’s prescription.
Several accessories are available to make your OSA treatment with the REMstar SE system as convenient and comfortable as
possible. To ensure that you receive the safe, effective therapy prescribed for you, use only Philips Respironics accessories.
Warnings
A warning indicates the possibility of injury to the user or the operator.
• This manual serves as a reference. The instructions in this manual are not intended to supersede the health care professional’s
instructions regarding the use of the device.
• The operator should read and understand this entire manual before using the device.
• This device is not intended for life support.
• The device should be used only with masks and connectors recommended by Philips Respironics or with those recommended
by the health care professional or respiratory therapist. A mask should not be used unless the device is turned on and operating
properly. The exhalation port(s) associated with the mask should never be blocked. Explanation of the Warning: The device
is intended to be used with special masks or connectors that have exhalation ports to allow continuous ow of air out of the
mask. When the device is turned on and functioning properly, new air from the device ushes the exhaled air out through the
mask exhalation port. However, when the device is not operating, enough fresh air will not be provided through the mask, and
exhaled air may be rebreathed. Rebreathing of exhaled air for longer than several minutes can in some circumstances lead to
suffocation.
• If you are using a full face mask (a mask covering both your mouth and your nose), the mask must be equipped with a safety
(entrainment) valve.
• When using oxygen with this system, the oxygen supply must comply with local regulations for medical oxygen.
• Oxygen supports combustion. Oxygen should not be used while smoking or in the presence of an open ame.
• When using oxygen with this system, turn the device on before turning on the oxygen. Turn the oxygen off before turning the
device off. This will prevent oxygen accumulation in the device. Explanation of the Warning: When the device is not in
operation and the oxygen ow is left on, oxygen delivered into the tubing may accumulate within the device’s enclosure. Oxygen
accumulated in the device enclosure will create a risk of re.
• When using oxygen with this system, a Philips Respironics Pressure Valve must be placed in-line with the patient circuit between
the device and the oxygen source. The pressure valve helps prevent the backow of oxygen from the patient circuit into the
device when the unit is off. Failure to use the pressure valve could result in a re hazard.
• Do not connect the device to an unregulated or high pressure oxygen source.
• Do not use the device in the presence of a ammable anaesthetic mixture in combination with oxygen or air, or in the presence
of nitrous oxide.
• Do not use the device near a source of toxic or harmful vapors.
• Do not use this device if the room temperature is warmer than 35° C (95° F). If the device is used at room temperatures warmer
than 35° C (95° F), the temperature of the airow may exceed 43° C (109° F). This could cause irritation or injury to your
airway.
• Do not operate the device in direct sunlight or near a heating appliance because these conditions can increase the temperature of
the air coming out of the device.
• Contact your health care professional if symptoms of sleep apnea recur.
• If you notice any unexplained changes in the performance of this device, if it is making unusual or harsh sounds, if it has been
dropped or mishandled, if water is spilled into the enclosure, or if the enclosure is broken, disconnect the power cord and
discontinue use. Contact your home care provider.
• Repairs and adjustments must be performed by Philips Respironics-authorized service personnel only. Unauthorized service could
cause injury, invalidate the warranty, or result in costly damage.
• Periodically inspect electrical cords and cables for damage or signs of wear. Discontinue use and replace if damaged.
• To avoid electrical shock, always unplug the power cord from the wall outlet before cleaning the device. DO NOT immerse the
device in any uids.
• If the device is used by multiple persons (such as rental devices), a low-resistance, main ow bacteria lter should be installed in-
line between the device and the circuit tubing to prevent contamination.

3
User Manual
• Be sure to route the power cord to the outlet in a way that will prevent the cord from being tripped over or interfered with by
chairs or other furniture.
• Using this device at an incorrect altitude setting could result in airow pressures higher or lower than the prescribed setting.
Always verify the altitude setting when travelling or relocating, and adjust the system accordingly.
• This device is activated when the power cord is connected.
• For safe operation when using a humidier, the humidier must always be positioned below the breathing circuit connection at
the mask and the air outlet on the device. The humidier must be level for proper operation.
Note: Please see the “Limited Warranty” section of this manual for information on warranty coverage.
Cautions
A Caution indicates the possibility of damage to the device.
• Medical electrical equipment needs special precautions regarding EMC and needs to be installed according to EMC information.
Contact your home care provider regarding EMC installation information.
• Mobile RF communications equipment can affect medical electrical equipment.
• Pins of connectors marked with the ESD warning symbol shall not be touched and connections shall not be made without
special precautions. Precautionary procedures include methods to prevent build-up of electrostatic charge (e.g., air conditioning,
humidification, conductive floor coverings, non-synthetic clothing), discharging one’s body to the frame of the equipment or
system or to earth. It is recommended that all individuals that will handle this device understand these precautionary procedures
at a minimum as part of their training.
• Before operating the device, ensure that the SD card cover is replaced whenever any of the accessories such as the Link Module
or Modem are not installed. Refer to the instructions that came with your accessory.
• Condensation may damage the device. If this device has been exposed to either very hot or very cold temperatures, allow it to
adjust to room temperature (operating temperature) before starting therapy. Do not operate the device outside of the operating
temperature range shown in the Specications.
• Do not use extension cords with this device.
• Do not place the device directly onto carpet, fabric, or other ammable materials.
• Do not place the device in or on any container that can collect or hold water.
• A properly installed, undamaged reusable foam inlet lter is required for proper operation.
• Tobacco smoke may cause tar build-up within the device, which may result in the device malfunctioning.
• Dirty inlet filters may cause high operating temperatures that may affect device performance. Regularly examine the inlet filters as
needed for integrity and cleanliness.
• Never install a wet filter into the device. You must ensure sufficient drying time for the cleaned filter.
• Always ensure that the DC power cord securely fits into your therapy device prior to use. Contact your home care provider or
Philips Respironics to determine if you have the appropriate DC cord for your specific therapy device.
• When DC power is obtained from a vehicle battery, the device should not be used while the vehicle’s engine is running. Damage
to the device may occur.
• Only use a Philips Respironics DC Power Cord and Battery Adapter Cable. Use of any other system may cause damage to the
device.
Contraindications
When assessing the relative risks and benefits of using this equipment, the clinician should understand that this device can deliver
pressures up to 20 cm H2O. In the event of certain fault conditions, a maximum pressure of 30 cm H2O is possible. Studies have
shown that the following pre-existing conditions may contraindicate the use of CPAP therapy for some patients:
• Bullous Lung Disease
• Pathologically Low Blood Pressure
• Bypassed Upper Airway
• Pneumothorax
• Pneumocephalus has been reported in a patient using nasal Continuous Positive Airway Pressure. Caution should be used when
prescribing CPAP for susceptible patients such as those with: cerebral spinal fluid (CSF) leaks, abnormalities of the cribriform
plate, prior history of head trauma, and/or pneumocephalus. (Chest 1989; 96:1425-1426)
The use of positive airway pressure therapy may be temporarily contraindicated if you exhibit signs of a sinus or middle ear
infection. Not for use with patients whose upper airways are bypassed. Contact your health care professional if you have any
questions concerning your therapy.

User Manual4
Symbol Key
The following symbols may appear on the device and power supply:
Sy m b o l De fi ni t io n
Consult accompanying instructions for use.
AC Power
DC Power
IP22
Drip Proof Equipment
Caution, consult accompanying documents.
ESD Warning symbol
Class II (Double Insulated)
Type BF Applied Part
For Indoor Use Only.
Do not disassemble.
For Airline Use. Complies with RTCA/DO-160F section 21, category M.
Separate collection for electrical and electronic equipment per EC Directive
2002/96/EC.
Use only with the standard 60W power supply 1091398.
(not for use with Heated Tubing)
Use only with the Heated Tubing compatible 80W power supply 1091399.
(can also be used when Heated Tubing is not in use)
5
User Manual
System Contents
Your REMstar SE system may include the following items:
• Device • Side cover panel
• User manual • SD card
• Carrying case • Reusable gray foam filter
• Flexible tubing • Disposable ultra-fine filter (optional)
• Power cord • Humidifier (optional)
• Power supply (60W 1091398, or 80W 1091399)
Note: If any of these items are missing, contact your home care provider.
System Overview
The REMstar SE is a CPAP (Continuous Positive Airway Pressure) device designed for the treatment of Obstructive
Sleep Apnea (OSA). CPAP maintains a constant level of pressure throughout the breathing cycle.
When prescribed for you, the device provides several special features to help make your therapy more comfortable.
The ramp function allows you to lower the pressure when you are trying to fall asleep. The air pressure will gradually
increase until your prescription pressure is reached. You also have the option of not using the ramp feature at all.
Additionally, the Flex comfort feature provides you with pressure relief when you exhale during therapy.
Several accessories are also available for use with your device. Contact your home care provider to purchase any
accessories not included with your system.
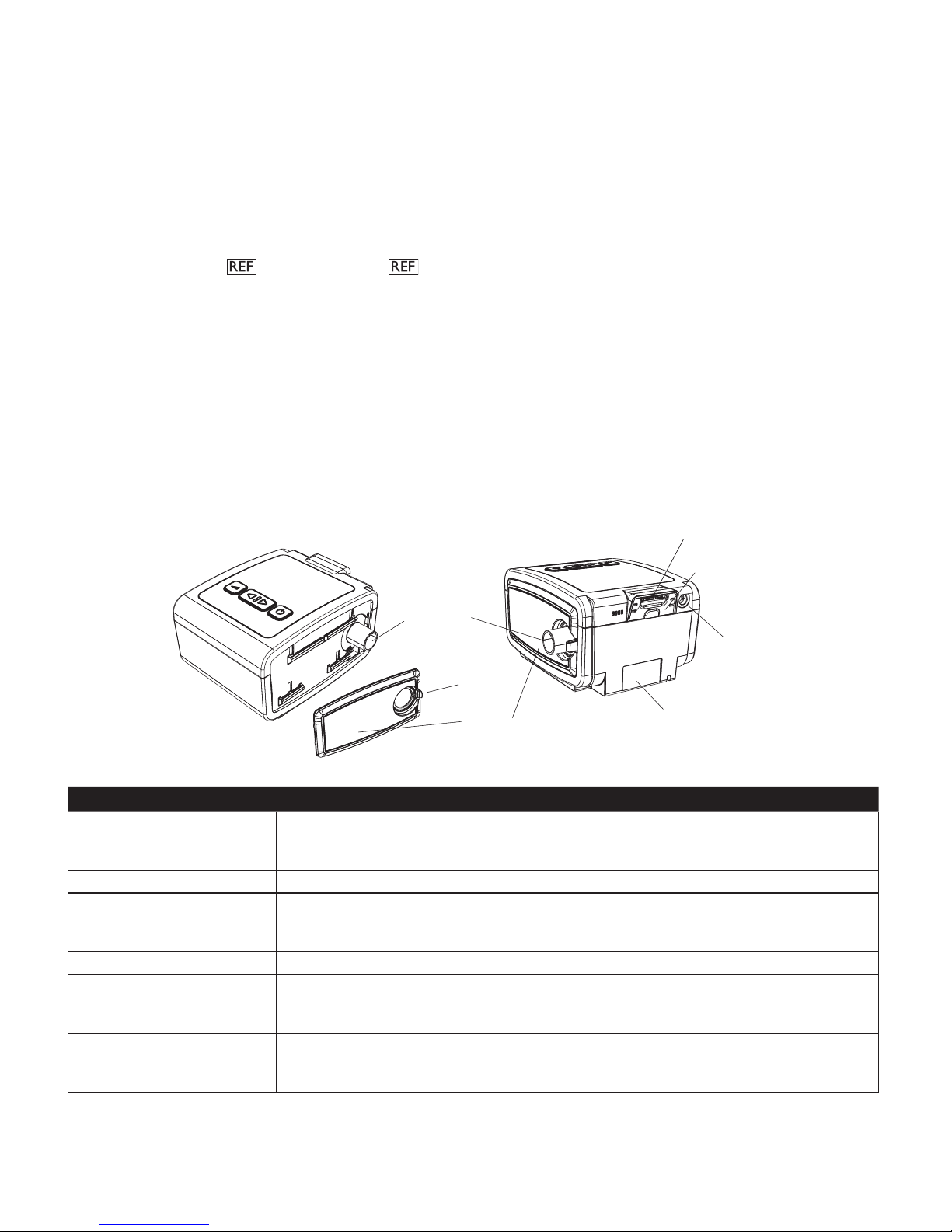
Air Outlet Port
Power Inlet
Filter Area
SD Card (Accessory) Slot
Side Cover
T a b
SD Card Cover
This gure illustrates some of the device features, described in the following table.
De v i c e fe a t u r e De S c r i p t i o n
Air Outlet Port
(conical, 22 mm)
Connect the 15 or 22 mm Philips Respironics exible tubing here.
Note: Heated Tubing should only be connected to the Air Outlet Port of the compatible
System One Heated Humidier and not to the Air Outlet Port of the therapy device.
SD Card (Accessory) Slot If applicable, insert the optional accessory SD card here.
SD Card Cover If applicable, the optional accessories such as a Link Module or Modem can be installed here.
Refer to the instructions supplied with the accessory. When not using an accessory, this
cover must be in place on the device.
Power Inlet Connect the power cord here.
Filter Area A reusable, gray foam lter must be placed in the lter area to screen out normal household
dust and pollens. A white ultra-ne lter can also be used for more complete ltration of very
ne particles.
Side Cover If using a humidier with the device, this side cover can be easily removed with the release
tab before attaching the humidier. Refer to the humidier manual. When not using a
humidier, this cover must be in place on the device.
User Manual6
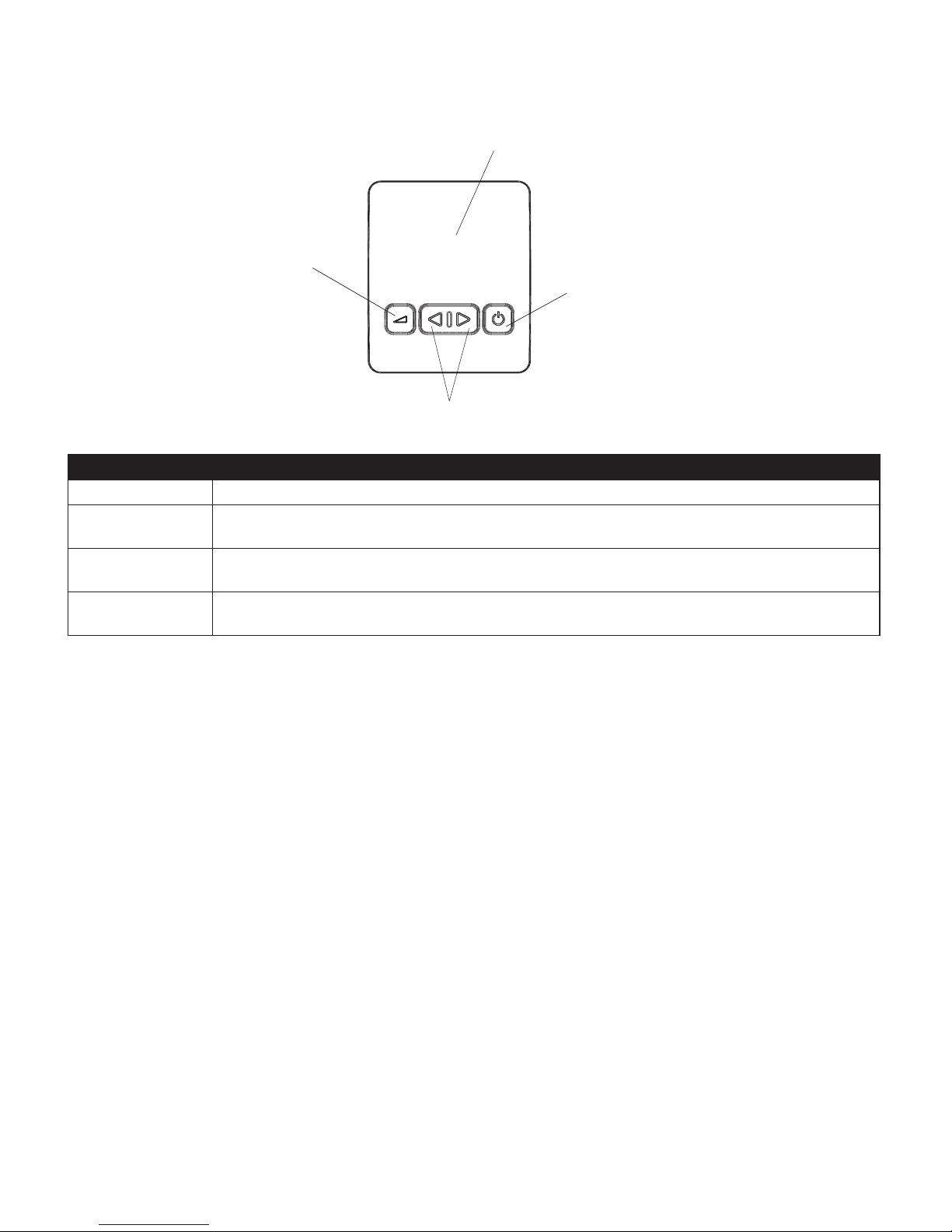
Control Buttons
Display Area
Left/Right Navigation Button
Start/Stop or Select Button
Ramp Button
These features are described below.
fe a t u r e De S c r i p t i o n
Display Area This area shows the therapy settings and patient data, and other messages.
Ramp Button
When the airow is on, this button allows you to activate or restart the ramp function. Ramp lowers the
airow pressure and then gradually increases it, allowing you to fall asleep more easily.
Start/Stop or Select
Button
Starts the airow and places the device into Active state, or stops the airow, and places the device into
Standby state. Also, when navigating the patient screens, press this button to select the menu options.
Left/Right Navigation
Button
Performs display navigation or setting adjustments.
Note: The control buttons are backlit and will be on when the device is plugged into a power outlet.
Available Therapies
The REMstar SE device delivers the following therapies:
• CPAP – Delivers Continuous Positive Airway Pressure; CPAP maintains a constant level of pressure throughout the
breathing cycle.
• CPAP with Flex – Delivers CPAP therapy with pressure relief upon exhalation to improve patient comfort based
on patient needs.
Installing the Air Filters
CAUTION: A properly installed, undamaged reusable gray foam filter is required for proper operation.
The device uses a gray foam filter that is washable and reusable, and an optional white ultra-fine filter that is
disposable. The reusable filter screens out normal household dust and pollens, while the optional ultra-fine filter
provides more complete filtration of very fine particles. The gray reusable filter must be in place at all times when
the device is operating. The ultra-fine filter is recommended for people who are sensitive to tobacco smoke or other
small particles.
A reusable gray foam filter and a disposable ultra-fine filter are supplied with the device. If your filters are not already
installed when you receive your device, you must at least install the reusable gray foam filter before using the device.
To install the filter(s):
1. If you are using the white disposable ultra-fine filter, insert it into the filter area first, mesh-side facing in, towards
the device.
2. Insert the gray foam filter into the filter area after the ultra-fine filter.
Note: If you are not using the white disposable filter, simply insert the gray foam filter into the filter area.
 Loading...
Loading...