Page 1

IntelliVue MP2 Patient Monitor
Philips M8102A Technical Data Sheet
The IntelliVue MP2 portable patient monitor is
compact in size, ergonomic, and modular in design.
It provides an easy-to-use touchscreen user
interface, is highly customizable and shares a
technological platform with the Philips IntelliVue
MP5-MP90 patient monitors.
The IntelliVue series offers a complete monitoring
solution that is flexible and modular, designed to
suit a broad spectrum of monitoring needs.
Measurement Features
• Compact, rugged, lightweight monitor with built in
measurements
• ECG monitoring using any combination of three
to 10 electrodes.
• 12-lead ECG monitoring with five electrodes using
the EASI method or with 10 electrodes using the
conventional method.
• Multi-lead arrhythmia and ST segment analysis at
the bedside on all available leads.
• Mainstream or Sidestream CO
• FAST SpO2 for accurate performance even with
low perfusion.
• Invasive Pressure and Temperature measurement
• The monitor can operate using battery power for
up to 3 hours with basic monitoring configuration
to let you safely and easily monitor patients during
in-hospital transfer. AC power is provided by an
external power supply.
2
Usability Features
• Touchscreen and hardkeys as input device.
• Intuitive user interface.
PAD
Page 2

• Simple menu hierarchy gives fast access to all basic monitoring tasks.
• Patient data management with tabular and graphic trends.
• Settings “Profiles” for rapid case turnover.
• Patented automatic alarm limits help clinicians provide care more
efficiently.
• 3.5" TFT flat panel display with QVGA
(320 x 240) resolution, wide viewing angle, large numerics,
permanently visible alarm limits, and up to three real-time waves.
• Capable of functioning in a wireless infrastructure (IIT)
Intended Use
The monitor is intended to be used for monitoring and recording of,
and to generate alarms for, multiple physiological parameters of adults,
pediatrics, and neonates in a hospital environment and during patient
transport inside and outside of hospitals. The monitor is intended for
use by health care professionals.
The monitor is only for use on one patient at a time. It is not intended
for home use. Not a therapeutic device.
Rx only: U.S. Federal Law restricts this device to sale by or on the
order of a physician.
ST segment monitoring is intended for use with adult patients only and
is not clinically validated for use with neonatal and pediatric patients.
The ECG measurement is intended to be used for diagnostic recording
of rhythm and detailed morphology of complex cardiac complexes
(according to AAMI EC 11).
EMC Environment:
Main Components
Monitor
The monitor has a color LCD TFT display with a wide viewing angle,
providing high resolution waveform and data presentation.
The display, processing unit and measurements are integrated into one
device. An external power supply provides power for the monitor.
User Interface
The user interface is designed for fast and intuitive operation. The
color graphical user interface ensures that clinicians quickly feel at ease
using the monitor.
Configurable SmartKeys with intuitive icons allow monitoring tasks to
be performed quickly and easily, directly on the monitor screen.
Waves and numerics are color-coded.
The monitor displays up to three measurement waves simultaneously.
For 12-lead ECG monitoring it can display 12 real-time ECG waves,
with a rhythm strip and all ST values.
The MP2 monitor is supplied with a resistive touchscreen.
Simulated Keyboard
If alpha or numeric data entry is required, for example to enter patient
demographics, an on-screen keyboard will automatically appear on the
screen.
The monitor is suitable for use in all establishments incl. those directly
connected to the public low-voltage supply network that supplies
buildings for domestic purposes. When used with the M2741A
Sidestream CO2 sensor, the monitor can only be used in hospital
environments.
Upgradability
The MP2 monitor allows new capabilities to be added in the future as
your monitoring requirements evolve. This upgradability gives the
security of knowing that the monitors can be enhanced and updated as
practices and technologies advance, and it protects long-term
investments.
2
Mounting
The mounting options available enable flexible, space saving placement
of the monitors for an ergonomic work space. The monitor is shipped
with a low cost mounting plate if not specified otherwise.
Application Features
Critical and Cardiac Care Features
• The monitor performs multi-lead arrhythmia detection analysis
on the patient’s ECG waveform at the bedside. It analyzes for
ventricular arrhythmias, calculates heart rate, and generates alarms,
including asystole, bradycardia, and ventricular fibrillation.
• Up to 12 leads of ST segment analysis can be performed on adult
patients at the bedside, measuring ST segment elevation and
depression and generating alarms and events. The user can trend ST
changes, set high and low alarm limits, and set both ST and isoelectric
measurement points. Using ST Snippets, one-second wave segments
can be compared with a baseline segment for each measured ST lead.
• optional ST Map application shows ST changes over time in two
Page 3

multi-axis spider diagrams.
• QT/QTc interval monitoring provides the measured QT interval,
the calculated heart-rate corrected QTc value and a
'QTc value,
which tracks variation in the QT interval in relation to a baseline
value.
• optional 12-lead ECG data can be measured, using either the EASI
placement method with five standard electrodes or conventional
electrode placement with 10 electrodes.
1
• 12 realtime ECG waveforms can be displayed simultaneously.
• FAST-SpO2, using Fourier Artifact Suppression Technology,
performs accurately even in cases with low perfusion.
• Choice of sidestream or mainstream CO
monitoring for high
2
quality measurements with intubated and non-intubated patients.
- Vital Signs
- Graphic Trends
- Realtime Wave Reports
Report templates can be defined in advance, enabling print-outs
tailored to each hospital’s specific requirements to be started
quickly. Reports can be printed on centrally-connected printers or
via the IntelliVue PC Printing Solution, and they can be initiated
manually or automatically at user-defined intervals.
• The IntelliVue PC Printing Solution allows printing of reports,
waveform captures and trends from the MP2 to a standard off-the
shelf printer or to an electronic file.
Alarms
Ease of Use
• Screen layouts are easily adjustable, allowing flexible display of
measurement information.
• Temperature, height, and weight can be configured either in metric
or imperial units. Pressure measurements can be displayed in kPa or
mmHg. Gases can be displayed in kPa, mmHg.
Trends
• The trend database stores patient data from up to 30
measurement numerics. The measurement information can be
sampled every 12 seconds, one minute, or five minutes, and stored
for a period ranging from four to 48 hours.
• Horizon Trends show the deviation from a stored baseline.
Transport Features
• The monitor’s portable design means it can be used for in and out-ofhospital transport: a basic monitor weighs 1.5 kg.
• The monitor can operate using battery power for up to 3 hours, to
let you safely and easily monitor patients during procedures or inhospital transfer.
• Specially-designed mounting solutions let you quickly disconnect the
monitor for transport and reconnect to the mount after transport.
• The Universal Admit, Discharge and Transfer (ADT) feature means
that all ADT information is shared between the networked monitor
and the Information Center. Information need only be entered once.
Patient Data Documentation
• An extensive range of Patient Reports can be printed:
- 12-lead ECG Reports
- Alarm Limit Reports
The alarm system can be configured to present either the traditional
HP/Agilent/Philips alarm sounds or sounds compliant with the draft
ISO/IEC 9703-2 Standard.
Alarm limits are permanently visible on the main screen. The Alarm
Limits page provides a graphic depiction of alarm limits in relation to
the currently monitored measurement values and lets you adjust alarm
limits. It also lets you preview wide and narrow automatic alarm limits
before you apply them.
When an alarm limit is exceeded, it is signalled by the monitor in the
following ways:
• an alarm tone sounds, graded according to severity
• an alarm message is shown on the screen, color-coded according to
severity
• the numeric of the alarming measurement flashes on the screen
• alarm lamps flash for red and yellow alarms and are illuminated for
technical INOPs
If the monitor is connected via a network to a central monitoring
station, alarming is simultaneous at the monitor and at the Information
Center.
Alarms are graded and prioritized according to severity:
• Red Alarms*** identify a potentially life threatening situation for a
patient .
• Yellow Alarms** indicate conditions violating preset vital signs
limits.
• Technical Alarms (INOPS)
are triggered by signal quality
problems, equipment malfunction or equipment disconnect.
The Silence/Pause Alarms function (equivalent to Silence/Suspend
with previous monitor generations) allows the user to switch off
alarm tones with one touch.
1.EASI-derived 12-lead ECGs and their measurements are approximations to conventional
12-lead ECGs. As the 12-lead ECG derived with EASI is not exactly identical to the 12lead conventional ECG obtained from an electrocardiograph, it should not be used for
diagnostic purposes.
All alarms can be paused for a period of one, two, three, five, or 10
minutes or turned off indefinitely.
3
Page 4

Alarm strip recordings are available on a centrally-connected
recorder or via the IntelliVue PC Printing Solution.
Patented automatic alarm limits automatically adapt the alarm limits
to the patient’s currently measured vital signs within a safe margin
defined individually for each patient.
Visual and/or audible latching and non-latching alarm handling is
available.
Profiles
Profiles are predefined configuration settings for Screens,
measurement settings, and monitor settings. Each Profile can be
designed for a specific application area and patient category, for
example OR adult, or ICU neonatal. Profiles enable a quick reaction to
patient and care location changes: activating a Profile with a particular
patient category (Adult, Pediatric or Neonatal) automatically applies
suitable alarm and safety limits and saves time usually spent carrying
out a complete set-up procedure.
Profiles can be created directly on the monitor or remotely on a
personal computer and transferred to the monitor using the IntelliVue
Support Tool. A selection of Profiles for common monitoring
situations is provided with the monitor. These profiles can be changed,
added to, renamed, or deleted.
Network Interface
The network interface provides the system with networking capability
via a wired or wireless network connection.
Wireless Network (optional)
The monitor can function within a telemetry infrastructure compatible
with the Philips Cellular Telemetry System (CTS) in the WMTS and
ISM bands. Additional components are required to complete the
system. Please refer to the M3185A IntelliVue Clinical Network
Technical Data Sheet for further information.
Monitor Specifications
Safety Specifications
The monitor complies with the Medical Device Directive 93/42/EEC
) and with IEC 60601-1:1988 + A1:1991 + A2:1995; EN60601-
(CE
0366
1:1990 + A1:1993 + A2:1995; UL 60601-1:2003; CAN/CSA
C22.2#601.1-M90; JIS T 0601-1:1999; IEC 60601-1-1:2000; EN 606011-1:2001.
All applied parts are Type CF unless otherwise specified. They are
protected against damage from defibrillation and electrosurgery.
Optional Networking Capabilities
The monitor can operate as part of a wired or wireless hospital
network system, using the Philips IntelliVue Clinical Network interface.
Service Features
• The Support Tool helps technical personnel to
- carry out configuration, upgrades and troubleshooting via the
network, or on an individual monitor
- share configuration settings between monitors
- back up the monitor settings.
• A password-protected Service Mode ensures that only trained staff
can access service tests and tasks.
• The Configuration Mode is password-protected and allows trained
users to customize the monitor configuration.
Device Connections
The monitor can be connected to:
• an Information Center (for example M3150B)
•a PC
• MMS Extensions (M3012A, M3014A, M3015A, M3016A)
1
The possibility of hazards arising from software errors was minimized
in compliance with
and IEC 60601-1-4:1996 + A1:1999.
The monitor complies with the EMC standards
IEC 60601-1-2:2001; EN 60601-1-2:2001
This ISM device complies with Canadian ICES-001. Cet appareil ISM
est conforme a la norme NMB-001 du Canada.
The MP2 patient monitor can be used in a transport environment such
as road ambulance, airplane or helicopter, except when used with the
M2741A Sidestream CO2 sensor. For this purpose, the monitor fulfills
the following additional mechanical, EMC and environmental
requirements:
• Shock Tests according to IEC TR 60721-4-7, Class 7M3. Test
procedure according to IEC/EN 60068-2-27 (peak acceleration up to
100g).
• Random Vibration according to IEC TR 60721-4-7, Class 7M3. Test
procedure according to IEC/EN 60068-2-64 (RMS acceleration 5g).
• Sinusoidal Vibration according to IEC TR 60721-4-7, Class 7M3.
Test procedure according to IEC/EN 60068-2-6 (acceleration up to
amplitude 2g).
1.The MMS Extensions will only function when the monitor is connected to the
external power.
ISO 14971:2000, EN60601-1-4:1996 + A1:1999
4
Page 5
• Bump Test according to IEC/EN60068-2-29 (peak acceleration 15g,
1000 bumps).
• Free Fall Test according to EN1789 (covers also IEC TR 60721-4-7
and Class 7M3). Test procedure according to EN 60068-2-32 (height
0.75 m).
• Specification for degrees of protection provided by enclosures
according to IEC/EN 60529: IP 32
• EN 1789 +A1:2003 Medical vehicles and their equipment - Road
ambulances (chapter 6 - Medical Devices).
• Radiated susceptibility 20 V/m according to EN ISO 9919 (SpO2)
and EN ISO 21647 (CO2).
• Altitude Range from -500 to 3000 m operating and -500 to 4600 m
storage and transportation.
• Extended radiated susceptibility tests
The MP2 patient monitor with its out-of-hospital parameter set
provides a general immunity level of 20 V/m with only few restrictions.
Details are as listed below:
- GSM 900: Immunity at 900 MHz (uplink mobile phone), 20V/m,
duty cycle 1:8
- GSM 1800: Immunity at 1800 MHz (uplink mobile phone), 20V/m,
duty cycle 1:8.
- DECT: Immunity at 1800 MHz (digital cordless phone), 20V/m,
duty cycle 1:24
- AM: 1 kHz Immunity from 80 MHz to 2.5 GHz (any radio
communication unit, broadcasting and TV transmitter), 20V/m,
modulation factor 80%. (ECG: 20 V/m except 0.8-1.2 GHz where
it is 10V/m)
• Operating ambient temperature testing over the range from 0 to
40 °C (32 to 104 °F).
• Operating ambient humidity testing up to 95% RH at 40 °C
(104 °F), non condensing.
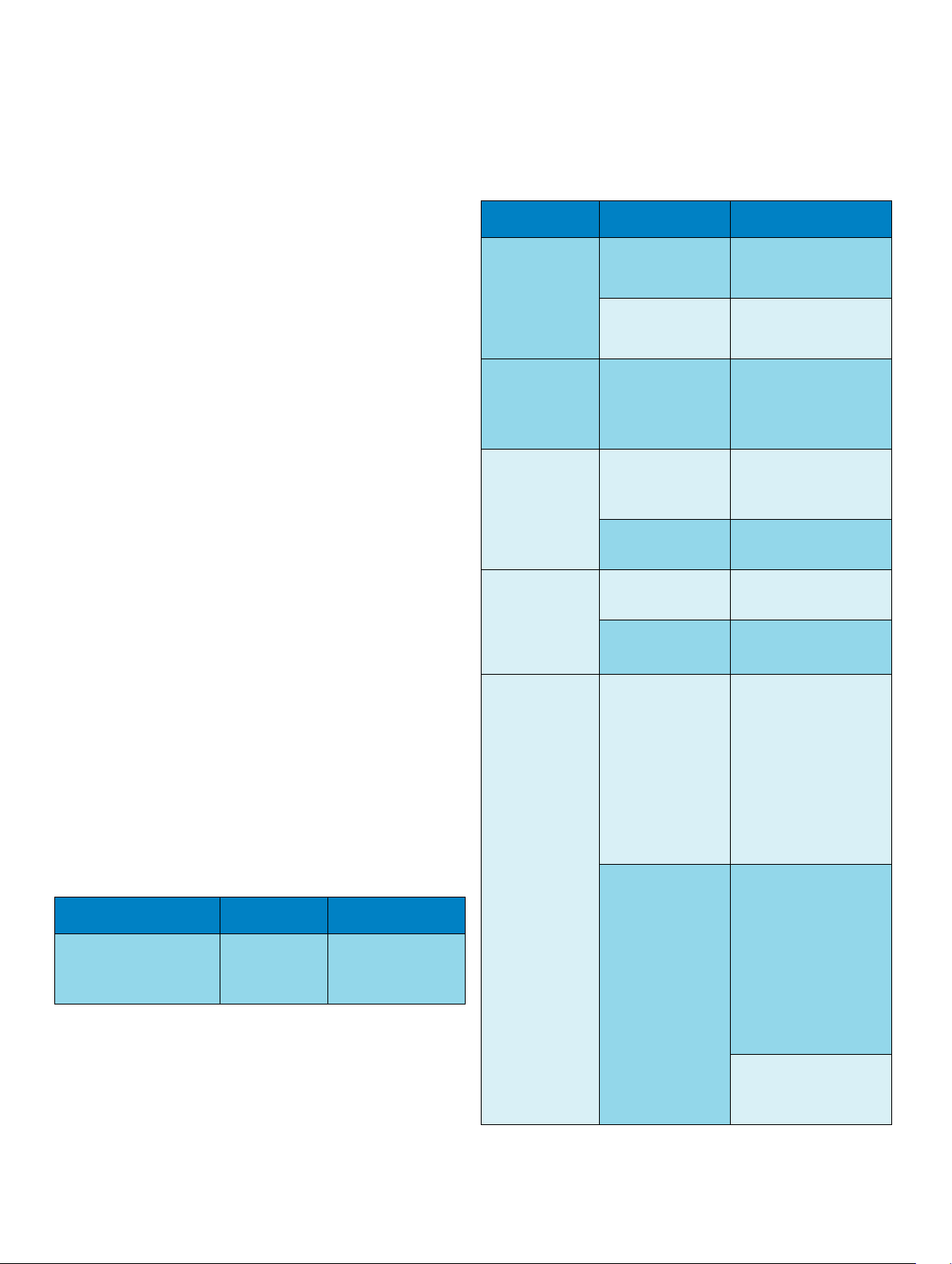
Physical Specifications
Environmental Specifications
Item Condition Range
Temperature
Range
Temperature
Range when
charging the
battery
Humidity Range Operating 15% to 95% Relative
Altitude Range Operating -500 m to 3000 m
Ingress
Protection
Operating
0 to 40oC
(32 to 104 oF)
Storage
(incl. Transport)
Operating
-20 to 60oC
(-4 to 140
0 to 35oC
(32 to 95
o
o
F)
F)
Humidity (RH) (non
condensing)
Storage and
Transport
5% to 95% Relative
Humidity (RH)
(10000 ft)
Storage and
Transport
-500 m to 4600 m
(15000 ft)
a
Monitor IP32 (protected against
the ingress of solid
foreign objects 2.5 mm
in diameter or larger,
and the ingress of water
when the water is
dripping vertically and
the monitor is tilted up
to 15°).
Product Max Weight W x H x D
M8102A
IntelliVue MP2 (without
1.5 kg
(3.3 lb)
< 188 x 99 x 86 mm
(7.4 x 3.9 x 3.4 in)
handle and options)
External Power
Supply (M8023A)
IP31(protected against
the ingress of solid
foreign objects 2.5 mm
in diameter or larger,
and the ingress of water
when the water is
dripping vertically) when
rested on its rubber feet
on a flat, level surface.
IP32 when mounted
with the connectors
facing downwards
a. sufficient for flight altitudes up to 12,000 m with pressurized cabins
5
Page 6
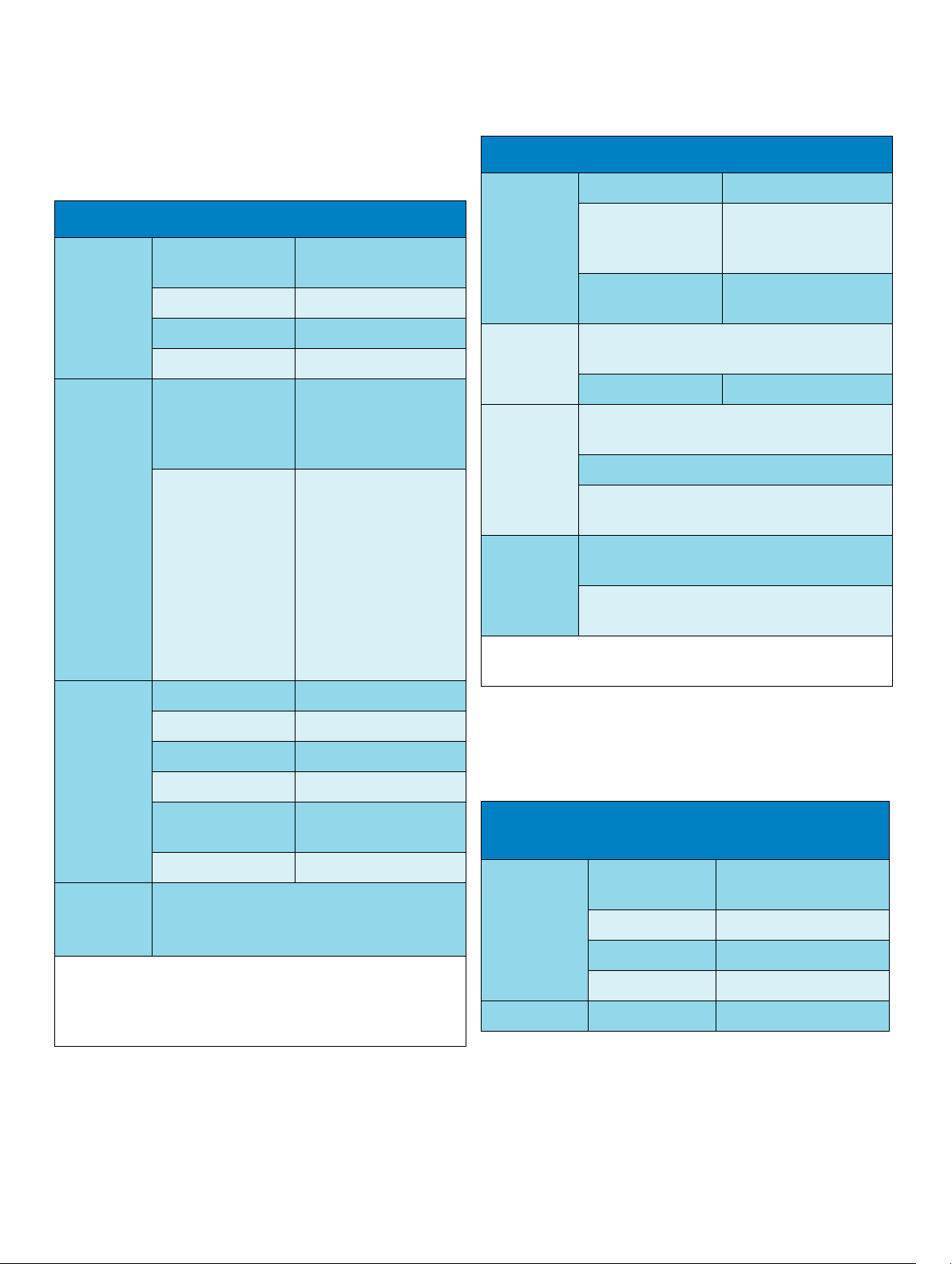
Performance Specifications
Monitor Performance Specifications
Alarm Signal System delay less than 3 seconds
Monitor Performance Specifications
Power
Specifications
Battery
Specifications
Indicators Alarms Off red LED
Power
consumption
Line Voltage 100 to 240 V ~
Current 1.3 to 0.7A
Frequency 50/60 Hz
Operating Time
(with new, fully
charged battery at
25 °C)
Charge Time When MP2 is off: 2 h
Alarms red/yellow/cyan LED
On/Standby/Error green/red LED
< 40W average, <65W
peak
Basic monitor
configuration: 3 hours
When MP2 is in use and
connected to MP20/30/
40/50/60/70/80/90
without extensions: 12 h
approx.
When MP2 is in use and
connected to the external
power
Pause duration 1,2,3 minutes or infinite,
depending on
configuration
Extended alarm
pause
Review
Alarms
Real Time
Clock
Buffered
Memory
Restart time: After power interruption, an ECG wave will be
shown on the display after 30 seconds maximum.
Information: all alarms / inops, main alarms on/off,
alarms acknowledged and time of occurrence
capacity 500 items
Range: from: January 1, 1997, 00:00 to: December
31, 2080, 23:59
Accuracy: < 4 seconds per day (typically)
Hold Time: infinite if powered by host monitor or
external power supply; otherwise at least 48 hours
Contents: Active settings, trends, patient data,
realtime reports, review alarms
Hold Time: infinite if powered by external power
supply; otherwise at least 48 hours
5 or 10 minutes
M8023A External Power Supply Performance
Specifications
AC Power green LED
Battery yellow (charging)/red
blinking (empty) LED
External Power green LED
Sounds Audible feedback for user input. Prompt tone.
QRS tones, or SpO
different alarm sounds.
Trends:
12, or 16 numerics @ 12 sec, 1 minute, 5 minute resolution.
Multiple choices of number of numerics, resolution and duration
depending on trend option and application area.
6
modulation tone. Four
2
M8023A External Power Supply Performance
Specifications
Power
Specifications
Indicators AC Power green LED
Power
Consumption
Line Voltage 100 to 240 V ~
Current 0.7 to 0.4 A
Frequency 50/60 Hz ~
< 12 W average
< 30 W peak
Page 7
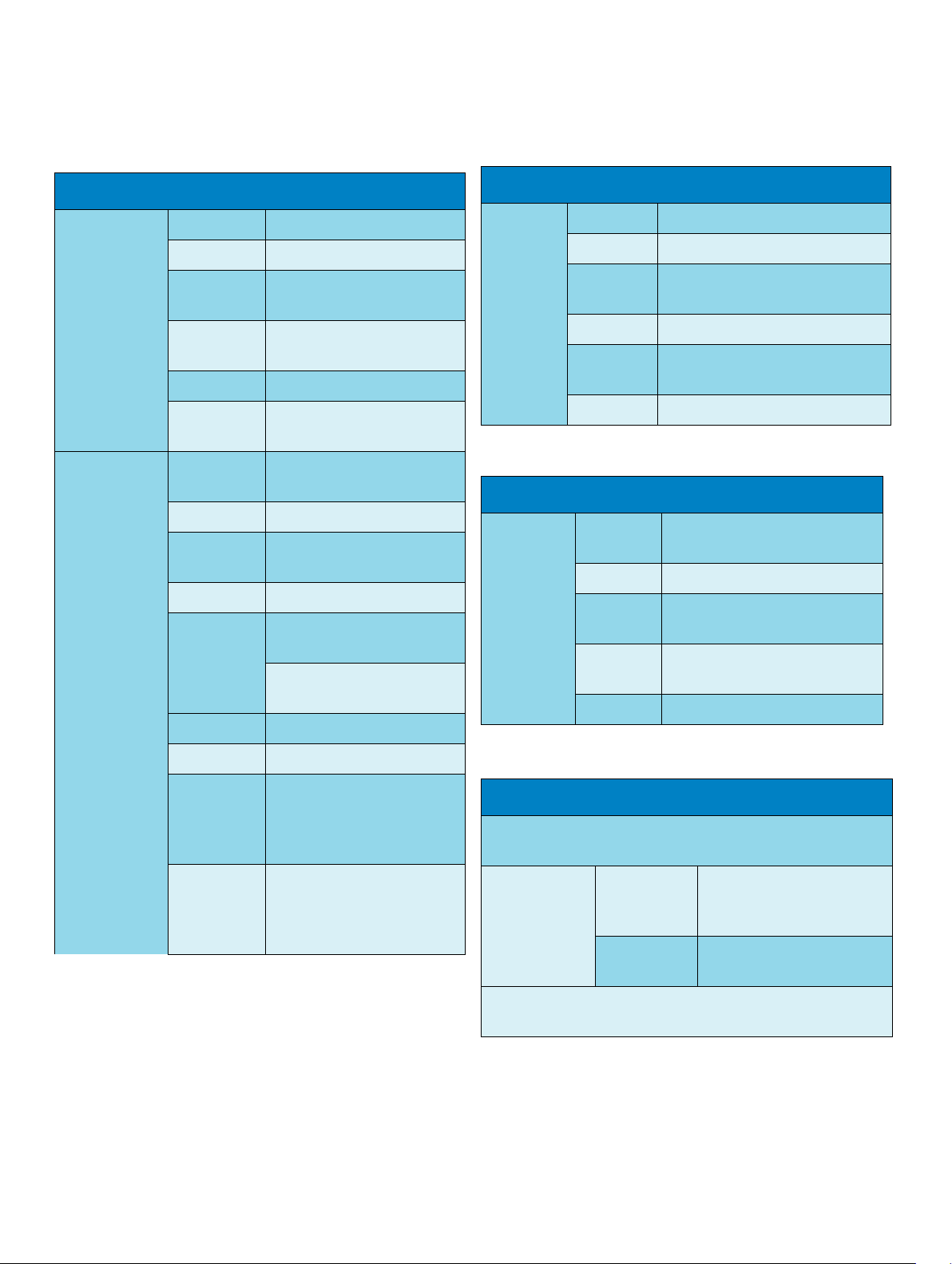
Interface Specifications
MP2 (M8102A) Interface Specifications
Measurement
Link (MSL)
Connectors Female ODU (Proprietary)
Power 30 V to 60 V input
Power Sync RS-422 compliant input
78.125 kHz (typical
LAN signals IEEE 802.3 10-Base-T
complaint
Serial signals RS-422 compliant
Local signals Provided for connecting MMS
extensions
ECG Sync Pulse
Output
Cable
Detection
Yes
Marker In No
Wave
No
Output
Connector Binder Series 709/719
Output
Levels
Output low <0.8V @ I = 4mA
Output high >2.4 V @ I=
4mA
Isolation None
M8023A External Power Supply Interface Specifications
Measurement Link
(MSL)
Connectors Male ODU (Proprietary)
Power 48 V output
Power Sync. RS-422 compliant output
78.125 kHz (typical)
LAN signals IEEE 802.3 10-Base-T complaint
Serial
signals
RS-422 compliant output
78.125 kHz (typical)
Local signals Not connected
Display Specifications
Integrated
QVGA
Display
Sweep
6.25, 12.5, 25 and 50 mm/s;
Speeds
Resolution 320 x 240
Refresh
60 Hz
frequency
Useful
72x54mm (2.8x2.1in)
screen
Pixel size 0.22 x 0.22 mm
Pulse Width 100 +/- 10 ms (high)
Delay from
R-wave peak
20 ms maximum per AAMI
EC13
to start of
pulse
Minimum
0.5 V
required Rwave
amplitude
MP2 (M8102A) Compatible Devices
IntelliVue Instrument Telemetry Wireless Network
(USA only)
Internal WMTS
Adapter
Technology compatible with Philips
Cellular Telemetry System
(CTS), cellular infrastructure
Frequency
Band
WMTS, 1395-1400 MHz and
1427-1432 MHz
IntelliVue Instrument Telemetry Wireless Network
(except USA)
7
Page 8
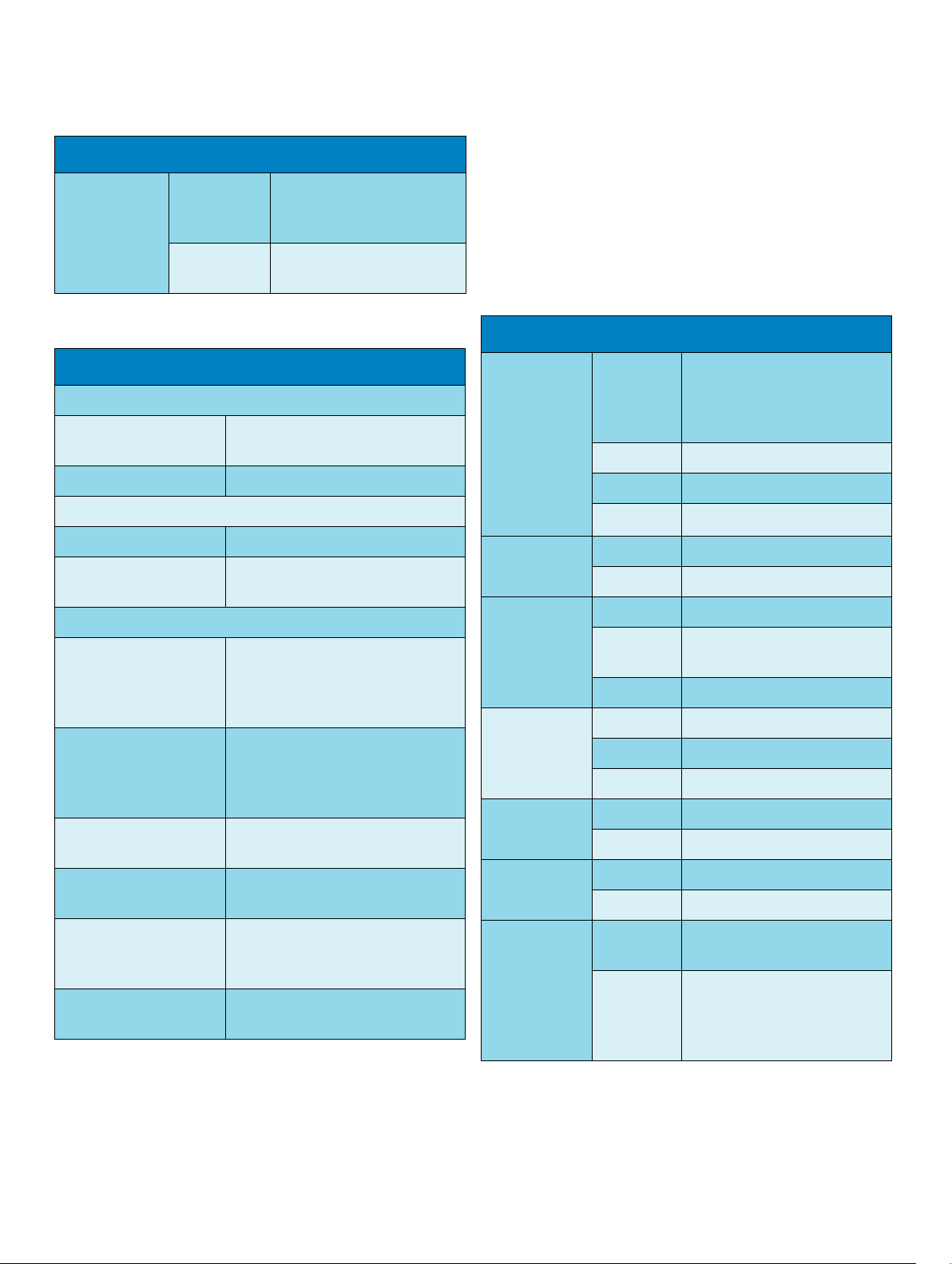
MP2 (M8102A) Compatible Devices
Measurement Specifications
Internal ISM
Adapter
M4607A Battery Specifications
Physical Specifications
W x D x H 66 mm (2.36 in) x 80 mm (3.15 in) x
Weight 160 g ±5%
Performance Specifications
Nominal Voltage 10.8 Volt
Rated Capacity at
discharge C/5
Environmental Specifications
Temperature Range Discharge 0 to 60qC (32 to 122qF)
Humidity Range Operating: 15 % to 95 % Relative
Battery Type Lithium Ion Mangan, 10.8 V,
Safety complies with UL 1642 (UL
Technology compatible with Philips
Cellular Telemetry System
(CTS), cellular infrastructure
Frequency
Band
2.4 GHz ISM
20 mm (0.79 in)
1000 mAh (typical)
Charge 0 to 60qC (32 to 122qF)
Storage and Transportation: -20 to
65qC (-4 to 140qF)
Humidity (RH)
Storage and Transportation: 5 % to
95 % Relative Humidity (RH)
1000 mAh,
Recognized)
ECG/Arrhythmia/ST/QT
Complies with IEC 60601-2-25:1993 + A1:1999 /EN60601-2-25:1995
+ A1:1999, IEC 60601-2-27:2005/EN60601-2-27:2006, IEC 60601-251:2003 /EN 60601-2-51:2003 and AAMI EC11/EC13:1991/2002.
ECG/Arrhythmia/ST Performance Specifications
Cardiotach Range Adult/pedi:
15 to 300 bpm
Neo range:
15 to 350 bpm
Accuracy ±1% of range
Resolution 1 bpm
Sensitivity t200 μV
PVC Rate Range 0 to 300 bpm
Resolution 1 bpm
ST Numeric Range -20 to +20 mm
Accuracy ±0.5 mm or 15%, whichever is
greater
Resolution 0.1 mm
QT Numeric Range 200 to 800 ms
Accuracy ±30 ms
Resolution 8ms
QTc Numeric Range 200 to 800 ms
Resolution 1ms
'QTc Numeric Range -600 to +600 ms
Resolution 1ms
peak
Electromagnetic
Compatibility (EMC)
Communication Standard complies with the SMBus specification
8
complies with the requirements for
FCC Type B computing Device, and
EN 61000-4-2 and EN 61000-3-2
v1.1
QT-HR
Numeric
Range adult
Range pediatric
and
neonatal
15 to 300 bpm
15 to 350 bpm
Page 9
ECG/Arrhythmia/ST Performance Specifications
Sinus and SV
Rhythm Ranges
Brady Adult: 15 to 60 bpm
Pedi: 15 to 80 bpm
Neo: 15 to 90 bpm
Normal Adult:60 to 100 bpm
Pedi: 80 to 160 bpm
Neo: 90 to 180 bpm
Tachy Adult: > 100 bpm
Pedi: >160 bpm
Neo: >180 bpm
Bandwidth Diagnostic
Adult/neo/pedi: 0.05 to 150Hz
Mode
Extended
Neo/pedi: 0.5 to 150Hz
Monitoring
Mode
Monitoring
Mode
Adult: 0.5 to 40Hz
Neo/pedi: 0.5 to 55Hz
Filter Mode Adult/neo/pedi: 0.5 to 20Hz
Differential Input Impedance >2M: RA-LL leads (Resp)
>5M: at all other leads (at 10Hz
including patient cable)
Common Mode Rejection
Ratio
Diagnostic mode: >86 dB (with a
51 k:/47 nF imbalance).
Filter mode: >106 dB (with a 51
k:/47 nF imbalance).
Electrode Offset Potential
±500mV
Tolerance
Auxiliary Current
(Leads off Detection)
Active electrode: <100 nA
Reference electrode: <900 nA
Input Signal Range ±5 mV
ECG/
Arrhythmia/
ST Alarm
Range Adjustment
Specifications
HR 15 to 300 bpm
maximum delay: 10
seconds according to
AAMI EC 13-1992
standard
Adult:1 bpm steps (15
to 40 bpm)
5 bpm steps (40 to 300
bpm)
Pedi/Neo:1 bpm steps
(15 to 50 bpm)
5 bpm steps (50 to 300
bpm)
Extreme Tachy Difference to high
5 bpm steps
limit 0 to 50 bpm
Clamping at 150 to
5 bpm steps
300 bpm
Extreme Brady Difference to low
5 bpm steps
limit 0 to 50 bpm
Clamping at 15 to
5 bpm steps
100 bpm
Run PVCs 2 PVCs Not adjustable by user
PVCs Rate 1 to 99 PVCs/minute 1 PVC
Vent Tach HR 20 to 300 bpm 5 bpm
Vent Tach Run 3 to 99 PVCs/minute 1 PVC
Vent Rhythm
2 to 99 PVCs/minute 1 PVC
Run
SVT HR 120 to 300 bpm 5 bpm
SVT Run 3 to 99 SV beats 1 SV beat
ST High -19.8 to +20 mm 0.2 mm
ST Low -20 to +19.8 mm 0.2 mm
QTc High 200 ms to 800 ms 10 ms steps
'QTc High 30 ms to 200 ms 10 ms steps
9
Page 10

ECG/Arrhythmia/ST Supplemental Information as
required by AAMI EC11/13
Respiration Excitation
Sinusoidal signal, 260 PA, 39 kHz
Waveform
Noise Suppression RL drive gain 44 dB max., max.
voltage 1.8 Vrms
ECG/Arrhythmia/ST Supplemental Information as
required by AAMI EC11/13
Heart Rate Meter Accuracy
and Response to Irregular
Rhythm
Ventricular bigeminy: 80 bpm
Slow alternating ventricular
bigeminy: 60 bpm
Rapid alternating ventricular
bigeminy: 120 bpm
Bidirectional systoles: 90 bpm
Time to
Alarm for
Tachycardia
Vent
Tachycardia
1mVpp,206
bpm
Gain 0.5, Range 6.5 to 8.4
seconds, Average 7.2 seconds
Gain 1.0 Range 6.1 to 6.9
seconds, Average 6.5 seconds
Gain 2.0, Range 5.9 to 6.7
seconds, Average 6.3 seconds
Vent
Tachycardia
,195b
2mV
pp
pm
Gain 0.5, Range 5.4 to 6.2
seconds, Average 5.8 seconds
Gain 1.0, Range 5.7 to 6.5
seconds, Average 6.1 seconds
Gain 2.0, Range 5.3 to 6.1
seconds, Average 5.7 seconds
Tall T-Wave Rejection
Capability
Exceeds ANSI/AAMI EC 13 Sect.
3.1.2.1(c)
minimum recommended 1.2 mV
T-Wave amplitude
Heart Rate Averaging Method Three different methods are
used:
Normally, heart rate is computed
by averaging the 12 most recent
RR intervals.
For runs of PVCs, up to 8 RR
intervals are averaged to
compute the HR.
If each of 3 consecutive RR
intervals is greater than 1200 ms
(that is, rate less than 50 bpm),
then the 4 most recent RR
intervals are averaged to
compute the HR.
Response Time of Heart Rate
Meter to Change in Heart
Rate
HR change from 80 to 120 bpm:
Range: [6.4 to 7.2 seconds]
Average: 6.8 seconds
HR change from 80 to 40 bpm:
Range: [5.6 to 6.4 sec] Average:
6.0 seconds
Accuracy of Input Signal
Reproduction
Methods A and D were used to
establish overall system error
and frequency response.
Respiration
Respiration Performance Specifications
Respiration
Rate
Bandwidth 0.3 to 2.5Hz (–6dB)
Noise Less than 25m:(rms) referred to
Respiration
Alarm Speci-
fications
High Adult/pedi:
Low Adult/pedi: 0
Range Adult/pedi: 0 to 120 rpm
Neo: 0 to 170 rpm
Accuracy at 0 to 120 rpm ±1 rpm
at 120 to 170 rpm ±2 rpm
Resolution 1 rpm
the input
Range Adjustment Delay
under 20 rpm: 1
10 to 100
rpm
Neo: 30 to
rpm steps
over 20 rpm: 5
rpm steps
150 rpm
under 20 rpm: 1
to 95 rpm
Neo:
0 to 145 rpm
rpm steps
over 20 rpm: 5
rpm steps
max. 14
seconds
for limits
from 0 to
20 rpm:
max. 4
seconds
for limits
above 20
rpm: max.
14 seconds
10
Page 11

Respiration
Alarm Speci-
Range Adjustment Delay
fications
Apnea Alarm 10 to 40
5 second steps
seconds
SpO
2
Complies with EN ISO 9919:2005 (except alarm system; alarm system
complies with IEC 60601-2-49:2001).
Measurement Validation: The SpO
accuracy has been validated in
2
human studies against arterial blood sample reference measured with a
CO-oximeter. Pulse oximeter measurements are statistically
distributed, only about two-thirds of the measurements can be
expected to fall within the specified accuracy compared to COoximeter measurements. Display Update Period: Typical: 2 seconds,
Maximum: 30 seconds. Max. with NBP INOP suppression on60
seconds.
SpO2 Performance Specifications
SpO2* Range 0 to 100%
Accuracy Philips Reusable Sensors:
M1191A, M1191AL,
M1191ANL, M1191B,
M1191BL, M1192A, M1192AN:
2% (70% to 100%)
M1193A, M1193AN, M1194A,
M1194AN, M1195A,
M1195AN, M1196A:
3% (70% to 100%)
Philips Reusable Sensors
with M1943A(L):
M1191T, M1192T, M1193T
(Adult), M1196T:
3% (70% to 100%)
M1193T (Neonate):
4% (70% to 100%)
Philips Disposable Sensors
with M1943A(L):
M1132A, M1133A (adult/
infant): 2%
M1131A, M1133A (neonate),
M1901B, M1902B, M1903B,
M1904B: 3% (70% to 100%)
SpO2 Performance Specifications
SpO2* Accuracy
NellcorPB® Sensors with
M1943A(L):
MAX-A, MAX-AL, MAX-P,
MAX-I, MAX-N, D-25, D-20, I20, N-25, OxiCliq A, P, I, N:
3% (70% to 100%)
Masimo Reusable Sensors
®
with LNOP MP12 or LNC
MP10:
LNOP DC-I, LNOP DC-IP,
LNOP YI, LNCS DC-I, LNCS
DC-IP, LNCS TF-I:
2% (70% to 100%)
LNOP TC-I, LNCS TC-I:
3.5% (70% to 100%)
Masimo Disposable
Sensors
®
with LNOP MP12
or LNC MP10:
LNOP Adt, LNOP Adtx, LNOP
Pdt, LNOP Pdtx, LNOP Inf-L,
LNCS Adtx, LNCS Pdtx, LNCS
Inf-L: 2% (70% to 100%)
LNOP Neo-L, LNOP NeoPt-L,
LNCS Neo-L, LNCS NeoPt-L:
3% (70% to 100%)
Resolution 1%
Pulse Range 30 to 300 bpm
Accuracy ±2% or 1 bpm, whichever is
greater
Resolution 1 bpm
Sensors Wavelength range: 500 to 1000
nm
Emitted Light Energy: d 15mW
Information about the
wavelength range can be
especially useful to clinicians
(for instance, when
photodynamic therapy is
performed)
Pulse Oximeter Calibration
70 - 100%
Range
*The specified accuracy is the root-mean-square (RMS) difference between the measured
values and the reference values
11
Page 12

NBP
SpO2 Alarm
Specifica-
Range Adjustment Delay
tions
SpO
2
Adult: 50 to 100%
1% steps (0, 1, 2,
Pedi/Neo: 30 to
100%
Desat Adult: 50 to Low
1% steps
alarm limit
Pedi/Neo: 30 to
Low alarm limit
Pulse 30 to 300 bpm Adult:
1 bpm steps
(30 to 40 bpm)
5 bpm steps
(40 to 300
bpm)
Pedi/Neo:
1 bpm steps
(30 to 50 bpm)
5 bpm steps
(50 to 300
bpm)
Tachycardia Difference to high
5 bpm steps max. 14
limit 0 to 50 bpm
Clamping at 150 to
5 bpm steps
300 bpm
Bradycardia Difference to low
5 bpm steps max. 14
limit 0 to 50 bpm
Clamping at 30 to
5 bpm steps
100 bpm
3,... 30)
+ 4
seconds
max. 14
seconds
seconds
seconds
Complies with IEC 60601-2-30:1999/EN60601-2-30:2000.
NBP Performance Specifications
Measurement
Ranges
Systolic Adult: 30 to 270 mmHg (4 to 36
kPa)
Pedi: 30 to 180 mmHg (4 to 24 kPa)
Neo: 30 to 130 mmHg (4 to 17 kPa)
Diastolic Adult: 10 to 245 mmHg (1.5 to 32
kPa)
Pedi: 10 to 150 mmHg (1.5 to 20
kPa)
Neo: 10 to 100 mmHg (1.5 to 13
kPa)
Mean Adult: 20 to 255 mmHg (2.5 to 34
kPa)
Pedi: 20 to 160 mmHg (2.5 to 21
kPa)
Neo: 20 to 120 mmHg (2.5 to 16
kPa)
Pulse Rate Adult:40 to 300
Pedi: 40 to 300
Neo: 40 to 300
Accuracy Max. Std. Deviation: 8 mmHg (1.1
kPa)
Max. Mean Error: ±5 mmHg (±0.7
kPa)
Pulse Rate Measurement
Accuracy
40 to 100 bpm: ± 5 bpm
101 to 200 bpm: ± 5% of reading
201 to 300 bpm: ± 10% of reading
(average over NBP measurement
cycle)
Heart Rate Range 40 to 300 bpm
Measurement Time Typical at HR > 60bpm
Auto/manual: 30 seconds (adult)
25 seconds (neonatal)
Stat: 20 seconds
Maximum time: 180 seconds (adult/
pediatric)
90 seconds (neonates)
12
Page 13

NBP Performance Specifications
Cuff Inflation Time Typical for normal adult cuff: Less
than 10 seconds
Typical for neonatal cuff: Less than 2
seconds
Initial Cuff Inflation
Pressure
Adult: 165 ±15 mmHg
Pedi: 130 ±15 mmHg
Neo: 100 ±15 mmHg
Auto Mode Repetition
Times
1, 2, 2.5, 3, 5, 10, 15, 20, 30, 45, 60
or 120 minutes
STAT Mode Cycle Time 5 minutes
Venipuncture Mode Inflation
Inflation
Pressure
Adult 20 to 120 mmHg (3 to 16 kPa)
Pediatric 20 to 80 mmHg (3 to 11 kPa)
Neonatal 20 to 50 mmHg (3 to 7 kPa)
Automatic
deflation
after
Adult/
170 seconds
pediatric
Neonatal 85 seconds
Measurement Validation: In adult and pediatric mode, the blood
pressure measurements determined with this device comply with the
American National Standard for Electronic or Automated
Sphygmomanometers (ANSI/AAMI SP10 - 1992) in relation to mean
error and standard deviation, when compared to intra-arterial or
auscultatory measurements (depending on the configuration) in a
representative patient population. For the auscultatory reference the
5th Korotkoff sound was used to determine the diastolic pressure.
In neonatal mode, the blood pressure measurements determined with
this device comply with the American National Standard for Electronic
or Automated Sphygmomanometers (ANSI/AAMI SP10 - 1992 and
AAMI/ANSI SP10A -1996) in relation to mean error and standard
deviation, when compared to intra-arterial measurements in a
representative patient population.
NBP Alarm
Specifications
Range Adjustment
Systolic Adult: 30 to 270 mmHg
(4 to 36 kPa)
Pedi: 30 to 180 mmHg (4
to 24 kPa)
Neo: 30 to 130 mmHg
(4 to 17 kPa)
Diastolic Adult: 10 to 245 mmHg
(1.5 to 32 kPa)
Pedi: 10 to 150 mmHg
(1.5 to 20 kPa)
Neo: 10 to 100 mmHg
(1.5 to 13 kPa)
Mean Adult: 20 to 255 mmHg
(2.5 to 34 kPa)
Pedi: 20 to 160 mmHg
(2.5 to 21 kPa)
Neo: 20 to 120 mmHg
(2.5 to 16 kPa)
NBP Overpressure Settings
Adult > 300 mmHg (40 kPa)
> 2 sec
Pedi > 300 mmHg (40 kPa)
> 2 sec
Neo > 150 mmHg (20 kPa)
> 2 sec
10 to 30 mmHg: 2
mmHg (0.5 kPa)
> 30 mmHg: 5
mmHg (1kPa)
not user adjustable
13
Page 14

Invasive Pressure and Pulse
Complies with IEC 60601-2-34:2000/EN60601-2-34:2000.
Invasive Pressure Performance Specifications
Measurement Range –40 to 360 mmHg
Pulse Rate Range 25 to 350 bpm
Accuracy ±1% Full Range
Resolution 1 bpm
Input Sensitivity Sensitivity:5μV/V/mmHg (37.5μV/V/
kPa)
Adjustment range:±10%
Transducer Load Impedance:200 to 2000 :
(resistive)
Output Impedance:d3000 :
(resistive)
Frequency Response dc to 12.5 Hz or 40 Hz
Zero
Adjustment
Range ±200 mmHg (±26 kPa)
Accuracy ±1 mmHg (±0.1 kPa)
Drift Less than 0.1mmHg/°C (0.013 kPa/
°C)
Gain
Accuracy
Accuracy ±1%
Drift Less than 0.05%/°C
Non
linearity
Error of d0.4% FS (@CAL
200 mmHg)
and
Hysteresis
Overall
Accuracy
Volume displacement
(including
transducer)
± 4% of reading or ± 4 mmHg (± 0.5
kPa), whichever is greater
0.1 mm3 /100 mmHg
of CPJ840J6
Invasive
Pressure
Alarm
Range Adjustment Delay
Specifications
Pressure –40 to 360
mmHg
(–5.0 to 48
kPa)
-40 to 30
mmHg
2 mmHg (0.5
kPa)
> 30 mmHg
5 mmHg (1
kPa)
Extreme High Difference to
high limit 0 to
5 mmHg steps
(0.5 kPa)
25 mmHg
Clamping at 40 to 360
5 mmHg steps
(1.0 kPa)
mmHg
Extreme Low Difference to
low limit 0 to
5 mmHg steps
(0.5 kPa)
25 mmHg
Clamping at
40 to 360
5 mmHg steps
(1.0 kPa)
mmHg
Pulse 25 to 300 bpm Adult:
1 bpm steps (25
to 40 bpm)
5 bpm steps (40
to 300 bpm)
Pedi/Neo:
1 bpm steps (25
to 50 bpm)
5 bpm steps (50
to 300 bpm)
max. 12
seconds
Tachycardia Difference to
high limit 0 to
5 bpm steps max. 14
seconds
50 bpm
Clamping at
5 bpm steps
150 to 300
bpm
14
Page 15

Invasive
Pressure
Alarm
Specifications
Range Adjustment Delay
CO
2
The CO2 measurement in the monitor, M3014A and M3015A
complies with EN ISO 21647:2004 + Cor.1:2005 (except alarm system;
alarm system complies with IEC 60601-2-49:2001).
Bradycardia Difference to
5 bpm steps max. 14
low limit 0 to
50 bpm
Clamping at 25
5 bpm steps
to 100 bpm
Temp
Complies with EN 12470-4:2000
Temp Performance Specifications
Temp Range –1 to 45 qC
(30 to 113 qF)
Resolution 0.1 qC (0.2 qF)
Accuracy ±0.1 qC (±0.2 qF)
Average Time Constant Less than 10 seconds
Alarms Range –1 to 45 qC (30 to 113 qF)
Adjustment -1 to 35 qC (30 to 95 qF): 0.5 qC
(1.0 qF) steps
35 to 45 qC (95 to 113 qF): 0.1 qC
(0.2 qF) steps
seconds
M3015A Microstream CO2 Performance Specifications
CO
2
Range 0 to 98 mmHg (0 to 13 kPa), or
13 % CO
, whichever is lower
2
Accuracy Up to 5 minutes during warmup:
±4 mmHg or 12 %, whichever is
greater
After 5 minutes warmup:
0 to 40 mmHg (0 to 5.3 kPa):
±2.2 mmHg (±0.3 kPa)
Above 40 mmHg (5.3 kPa):±(5.5 % +
(0.08 %/mmHg above 40 mmHg)) of
reading
These specifications are valid for
21 % O
and N2 balance, up to 35qC
2
ambient temperature, up to 60 rpm
in adult mode and 100 rpm in
neonatal mode.
Outside of these
conditions the accuracy reaches at a
minimum ±4 mmHg or ±12 % of the
reading, whichever is greater.
Resolution Numeric: 1.0 mmHg (0.1 kPa)
Wave: 0.1 mmHg (0.01 kPa)
Stability Included in Accuracy specifications
Temp Alarm
Specifications
Temp High/
Low Alarms
Range Adjustment
–1 to 45 ºC (30
to 113 ºF)
-1 to 35 qC (30 to 95 qF), 0.5
qC (1.0 qF) steps
35 to 45 qC (95 to 113 qF),
0.1 qC (0.2 qF) steps
awRR Range 0 to 150 rpm
Accuracy 0 to 40 rpm: ±1 rpm
41 to 70 rpm: ±2 rpm
71 to 100 rpm: ±3 rpm
>100 rpm: ±5 % of reading
Warm-up Time 5 minutes for full accuracy
specification
Rise Time 190 ms for neonatal mode
(measured with FilterLine H for
neonatal)
240 ms for adult mode
(measured with FilterLine H for
adult)
Sample Flow Rate 50 + 15/-7.5 ml/minute
15
Page 16

M3015A Microstream CO2 Performance Specifications
Gas Sampling Delay Time Typical:2.3 seconds
Maximum:3 seconds
Sound Pressure Acoustic noise: <45 dBA
Total System Response
Time
The total system response time is
the sum of the delay time and the
rise time.
M3014A Mainstream CO2 Performance Specifications
CO
2
Range 0 to 150 mmHg (0 to 20.0 kPa)
Accuracy after 2 minutes warmup:
For values between 0 and 40 mmHg:
±2.0 mmHg (±0.29 kPa)
For values from 41 to 70 mmHg: ±5 %
of reading
For values from 71 to 100 mmHg:
±8 % of reading
The specifications are valid for
standard gas mixtures, balance air, fully
hydrated at 35°C, P
= 760 mmHg,
abs
flow rate = 2 l/min.
Resolution Numeric: 1.0 mmHg (0.1 kPa)
Wave: 0.1 mmHg (0.01 kPa)
Stability:
Short term
drift
Long term
±0.8 mmHg over four hours
Accuracy specification will be
maintained over a 120 hour period
drift
awRR Range 2 to 150 rpm
Accuracy ±1 rpm
M3014A Sidestream CO2 Performance Specifications
CO
2
Range 0 to 150 mmHg (0 to 20.0 kPa)
Accuracy after 2 minutes warmup:
For values between 0 and 40 mmHg:
±2.0 mmHg (±0.29 kPa)
For values from 41 to 70 mmHg: ±5 %
of reading
For values from 71 to 100 mmHg:
±8 % of reading
For values from 101 to 150 mmHg:
±10 % of reading
At respiration rates above 80 rpm, all
ranges are ±12 % of actual. The
specifications are valid for gas mixtures
, balance N2, dry gas at
of CO
2
760 mmHg within specified operating
temperature range.
Resolution Numeric: 1.0 mmHg (0.1 kPa)
Wave: 0.1 mmHg (0.01 kPa)
Stability:
Short term
drift
Long term
±0.8 mmHg over four hours
Accuracy specification will be
maintained over a 120 hour period
drift
awRR Range 2 to 150 rpm
Accuracy ±1 rpm
Warm-up Time 2 minutes with CO2 sensor attached
for full accuracy specification
Sample Flow Rate 50 ±10 ml/minute
Total System Response
3seconds
Time
Warm-up Time 2 minutes with CO2 transducer
attached for full accuracy specification
Response Time Less than 60 ms (with adult or infant
reusable or disposable adapter)
16
Operating Temperature 0 to 40°C (32 to 104°F)
M3014A Mainstream and Sidestream CO2 Humidity Correction
Factor
Either BTPS or STPD can be selected as the humidity correction factor
for the CO
Where p = partial pressure, P
readings. The formula for the correction calculation is:
2
P
abs
---------------------------
P
STPDPBTPS
=
P
–
absPH2 O
= absolute pressure, and P
abs
H2O
= 42
mmHg @35°C and 100 % RH.
Page 17

M3016A Mainstream CO2 Performance Specifications
CO
2
Range –4 to 150 mmHg (-0.5 to 20.0 kPa)
Accuracy after 20 minutes warmup and
calibration:
For values between 0 and 40 mmHg:
±2.2 mmHg (±0.29 kPa)
For values between 40 and 76 mmHg:
±5.5 % of reading
The specifications are valid for 45 %
and N2 or N2O balance. Outside
O
2
these conditions the accuracy reaches
at a minimum the requirements of
EN864/ISO9918.
Resolution Numeric: 1.0 mmHg (0.1 kPa)
Wave: 0.1 mmHg (0.01 kPa)
Stability ±1.0 mmHg over a 7 day period
awRR Range 0 to 150 rpm
Accuracy ±2 rpm
Warm-up Time 20 minutes with CO2 transducer
attached for full accuracy specification
Response Time Less than 125 ms (for step from 10 %
to 90 %)
Mainstream CO2 Humidity Correction Factor
Either BTPS or STPD can be selected as the humidity correction factor
for the Mainstream CO
readings. The formula for the correction
2
calculation is:
P
abs
P
STPDPBTPS
Where p = partial pressure, P
=
---------------------------
P
–
absPH2 O
= absolute pressure, and P
abs
H2O
=
47 mmHg @37qC and 100 % RH.
CO2 Alarm
Specifications
Range
etCO2 High 20 to
95 mmHg (2
to 13 kPa)
etCO2 Low 10 to
90 mmHg (1
to 12 kPa)
imCO2 High 2 to
20 mmHg
(0.3 to
3.0 kPa)
awRR High Adult/pedi:
10 to
100 rpm
Neo: 30 to
150 rpm
awRR Low Adult/pedi:
0 to 95 rpm
Neo: 0 to
145 rpm
Apnea delay 10 to
40 seconds
Adjust-
ment
1mmHg
(0.1 kPa)
steps of
1mmHg
(0.1 kPa)
under
20 rpm: 1
rpm steps
over
20 rpm:5
rpm steps
5 second
steps
Delay
M3014A/
M3016A: less
than 14 seconds
M3015A: less
than18 seconds.
M3014A/
M3016A: less
than 14 seconds
M3015A: less
than18 seconds.
M3014A/
M3016A: less
than 14 seconds
M3015A: less
than18 seconds.
M3015A:
settings
<20 rpm: less
than 8 seconds
>20 rpm: less
than 18 seconds
M3014A/
M3016A
settings
<20 rpm: less
than 4 seconds
>20 rpm: less
than 14 seconds
set apnea delay
time + 4 seconds
(M3014A/
M3016A) or
8seconds
(M3015A)
17
Page 18

Ordering Information
Ordering information for the M81052A patient monitor is given here.
Sensors and Disposables
Parameters M8102A
Order one Bxx option
ECG, Resp, NBP, SpO
ECG, Resp, NBP, SpO2, Press/Temp
ECG, Resp, NBP, SpO2, CO
2
2
B20
B22
B23
Application Options
Application Options M8102A
Full Arrhythmia Capability C01
12-Lead ECG Application (conventional) C12
ST Map C13
Full Networking C15
Hardware Options
Accessory M8102A
3-lead Accessories Bundle ICU-AAMI
Tyco low cost cable
3-lead Accessories Bundle ICU-IEC
Tyco low cost cable
5-lead Accessories Bundle ICU-AAMI
Tyco low cost cable
5-lead Accessories Bundle ICU-IEC
Tyco low cost cable
5-lead Accessories Bundle ICU-AAMI H06
5-lead Accessories Bundle ICU-IEC H07
5-lead Accessories Bundle OR-AAMI H08
5-lead Accessories Bundle OR-IEC H09
Accessories Bundle Neonatal-AAMI H14
Accessories Bundle Neonatal-IEC H15
3-lead Accessories Bundle ICU-AAMI H16
3-lead Accessories Bundle ICU-IEC H17
G06
G07
G08
G09
Hardware Add-Ons M8102A
Anti-slip pad E18
MMS Mount E20
Add 1X Lithium-Ion battery E24
Add 2X Lithium-Ion battery E26
SN3 ECG Sync Cable SN3
Interface Options
Interfaces M8102A
Instrument Telemetry 1.4 GHz J45
Instrument Telemetry 2.4 GHz J47
3-lead Accessories Bundle OR-AAMI H18
3-lead Accessories Bundle OR-IEC H19
CO2 Mainstream Sensor N01
Reusable Adult Airway Adapter (msCO2) N02
Reusable Infant Airway Adapter (msCO2) N03
Single Use Adult Airway Adapter (msCO2) N04
Single USe Infant Airway Adapter (msCO2) N05
CO2 Sidestream Sensor N11
Non-intubated Adult Airway Adapter (ssCO2) N12
Non-intubated pediatric Airway Adapter (ssCO2) N13
Intubated Adult Airway Adapter (ssCO2) N14
Intubated Pediatric Airway Adapter (ssCO2) N15
18
Page 19

Related Products
M3086A Support Tool
Mounting Information
The Intellivue MP5 Roll Stand Mounting Kit (Order No.
989803002531) is compatible with the table top mount and the
standard mounting plate. For information on other mounting
hardware, contact your local Philips sales representative. For GCX
mounting hardware information, see www.gcx.com/philips.
Documentation
Trunk Cables
Part
No.
M1669A M1668A M1667A M1663A M1665A
3-Electrode Cable Set
5-Electrode Cable Set
6-Electrode Cable Set
10-Electrode Cable set
(5+5)
10-Electrode Cable set
(6+4)
All documentation is available in .pdf format on documentation CDROM. Additionally, a printed copy of the Instructions for Use and
Quick Guide ships with each monitor.
• Instructions for Use (printed)
• Quick Guide (printed)
• Installation and Service Guide
• Configuration Guide
• Documentation CD-ROM
• Training Guide (printed)
• Computer Based Training (optional)
ECG Accessories
This symbol indicates that the cables and accessories are
designed to have special protection against electric shocks
(particularly regarding allowable leakage currents), and
are defibrillator proof.
Length 2.7m 2.7m 2.7m 2.0m 2.7m
3-Electrode Cable Sets
Description Length
OR Grabber
shielded
ICU Grabber
shielded
ICU snap
shielded
ICU Clip nonshielded
ICU Clip nonshielded
1.0m M1675A M1678A
1.0m M1671A M1672A
1.0m M1673A M1674A
0.45m M1622A --
0.7m M1624A M1626A
AAMI Part
No.
IEC Part No.
5-Electrode Cable Sets
Description Length
OR Grabber
shielded
ICU Grabber
shielded
ICU Snap shielded 1.0m/1.6m M1644A M1645A
ICU Miniclip nonshielded
1.0m/1.6m M1973A M1974A
1.0m/1.6m M1968A M1971A
0.7m/1.3m M1647A M1648A
AAMI
Part No.
IEC Part
No.
19
Page 20

6-Electrode Cable Sets
One-piece Cables
Description Length
OR Grabber 1.0m/1.6m M1684A M1685A
ICU Grabber 1.0m/1.6m M1680A M1681A
ICU Snap 1.0m/1.6m M1682A M1683A
AAMI Part
No.
IEC Part
No.
10-Electrode (5+5)Cable Sets
Description Length
ICU Grabber,
chest, shielded
ICU Snap, chest,
shielded
OR Grabber,
chest, shielded
For Limb Leads see 5-electrode cable sets
1.0m M1976A M1978A
1.0m M1602A M1604A
1.0m M1979A M1984A
AAMI Part
No.
IEC Part
No.
Description Length
3-lead Grabber,
ICU
5-lead Grabber,
ICU
1.0m 989803143181 989803143171
1.0m 989803143201 989803143191
AAMI Part
No.
IEC Part No.
Radio-translucent Cables
Pack of five single wires, radio-translucent, 0.9m, M1649A
Set Combiners and Organizers
Set combiners and organizers Part No.
Set combiner 3-electrode M1501A
5-electrode M1502A
Set organizer for
shielded leadsets grabber and snap
3-electrode M1503A
4-electrode M1664A
5-electrode M1504A
10-Electrode (6+4)Cable Sets
Description Length
ICU Grabber,
chest, shielded
ICU Snap, chest,
shielded
OR Grabber,
chest, shielded
For Limb Leads see 6-electrode cable sets
1.0m M1532A M1533A
1.0m M1537A M1538A
1.0m M1557A M1558A
AAMI Part
No.
IEC Part
No.
6-electrode M1679A
Set organizer for nonshielded lead sets miniclip
Bedsheet clip M1509A
Replacement red cover for trunk cable (for 5electrode cable sets)
3-electrode M1636A
5-electrode M1638A
989808148861
20
Page 21

Philips FAST SpO2Accessories
Part Number Description Connector Type
Philips Reusable Sensors
Part Number Description Connector Type
M1191A/B Adult Sensor
(2m cable)
M1191AL/
BL
Adult Sensor
(3m cable)
M1191ANL Adult Sensor
(3m cable)
Nellcor OxiMax-
compatible
a
M1191T Adult Sensor
(requires M1943A
(1.1m) or M1943AL
(3m) adapter cable)
M1192A Small Adult/Pediatric
sensor (1.5m cable)
M1192AN Small Adult/Pediatric
sensor (1.5m cable)
Nellcor OxiMax-
compatible
a
M1192T Small Adult Pediatric
sensor (requires
M1943A (1.1m) or
M1943AL (3m)
adapter cable)
Philips 8-pin
Generic D-Sub
Philips 8-pin
Generic D-Sub
M1194A Adult/Pediatric Clip
Philips 8-pin
Sensor (ear) (1.5m
cable)
M1194AN Adult/Pediatric Clip
Sensor (ear) (1.5m
cable)
Nellcor OxiMax-
compatible
M1195A Infant Sensor (1.5m
a
Philips 8-pin
cable)
M1195AN Infant Sensor (1.5m
cable)
Nellcor OxiMax-
compatible
M1196A Adult Clip Sensor
a
Philips 8-pin
(3m cable)
M1196T Adult Clip Sensor
Generic D-Sub
(requires M1943A
(1.1m) or M1943AL
(3m) adapter cable)
a. only in combination with Philips FAST-SpO2 and Philips OxiMax-compatible patient
monitors.
Philips Disposable Sensors
M1193A Neonatal Hand/Foot
Sensor (1.5m cable)
M1193AN Neonatal Hand/Foot
Sensor (1.5m cable)
Nellcor OxiMax-
compatible
a
M1193T Neonatal Sensor
(requires M1943A
(1.1m) or M1943AL
(3m) adapter cable)
Philips 8-pin
Generic D-Sub
Part Number Description Connector Type
M1131A Adult/Pediatric
Generic D-Sub
Sensor (requires
M1943A (1.1m) or
M1943AL (3m)
adapter cable)
M1132A Infant Sensor
Generic D-Sub
(requires M1943A
(1.1m) or M1943AL
(3m) adapter cable)
21
Page 22

Part Number Description Connector Type
MASIMO LNOP®2 Reusable Sensors:
M1133A Adult/Infant/
Generic D-Sub
Neonatal Sensor
(requires M1943A
(1.1m) or M1943AL
(3m) adapter cable)
NELLCOR® Disposable Sensors1:
Purchase Nellcor OxiCliq sensors and adapter cables directly from
Tyco Healthcare.
Philips
Product Number Description
OxiMax MAX-A
OxiMax MAX-AL
a
a
Adult Sensor
Adult Sensor
(long cable)
OxiMax MAX-P
OxiMax MAX-I
OxiMax MAX-N
a
a
a
Pediatric Sensor
Infant Sensor
Neonatal Sensor
Part
Number
M1904B
n/a
M1903B
M1902B
M1901B
Product
Number
LNOP DC-I Adult Sensor 989803140321
LNOP DC-IP Pediatric Sensor 989803140331
LNOP-YI Reusable Multi-Site
LNOP TC-I Tip Clip reusable
MASIMO LNCS®
Product
b
b
b
b
Number
LNCS DC-I Adult Sensor 989803148281
LNCS DC-IP Pediatric Sensor 989803148291
LNCS-TC-I Reusable Ear
LNCS TF-I Reusable Forehead
Description
Sensor
Sensor
1
Reusable Sensors:
Description
Sensor
Sensor
Philips
Part Number
n/a
989803140341
Philips
Part Number
989803148301
989803148311
Oxisensor II D-25
Oxisensor II D-20
Oxisensor II I-20
Oxisensor II N-25
OxiCliq A
Oxicliq P
OxiCliq I
OxiCliq N
a. Requires M1943 A(L) adapter cable
b.not available from Philips in the U.S.A.
c. Requires M1943 A(L) and OC3 adapter cables
1.Nellcor, OxiMax and OxiCliq are trademarks of Nellcor Puritan Bennett Inc., a part
of Tyco Healthcare.
c
c
c
c
a
a
a
a
Adult Sensor n/a
Pediatric Sensor n/a
Infant Sensor n/a
Neonatal Sensor n/a
Adult Sensor n/a
Pediatric Sensor n/a
Infant Sensor n/a
Neonatal Sensor n/a
2.LNOP and LNCS are federally registered trademarks of Masimo Corporation
22
Page 23

MASIMO LNOP® Disposable Adhesive Sensors:
Extension/Adapter Cables:
Product
Number
Description
Philips
Part Number
LNOP Adt Adult Sensor 989803140231
LNOP Adtx Adult Sensor n/a
LNOP Pdt Pediatric Adhesive
989803140261
Sensor
LNOP Pdtx Pediatric Sensor n/a
LNOP INF-L Neo/Infant
989803140311
Adhesive Sensor
LNOP NEO-L Neo Adhesive
989803140291
Sensor
LNOP NEOPT-LNeo Pre-Term
989803140301
Sensitive Skin
Adhesive Sensors
MASIMO LNCS® Disposable Adhesive Sensors:
Product
Number
Description
LNCS Adtx Adult Finger
Sensor
Philips
Part Number
989803148231
Part Number Description
M1941A Extension Cable (2m) (8-pin to 8-pin)
M1943A Adapter Cable (1.1m) for Philips and
Nellcor disposable sensors
(8-pin to 9-pin D-Sub)
M1943AL Adapter Cable (3m) for Philips and
Nellcor disposable sensors
(8-pin to 9-pin D-Sub)
OC3 Adapter cable for OxiCliq Sensors
(available from Nellcor only)
LNOP MP12
(451261000761)
LNOP MP Series Patient Cable (3.6 m)
Adapter Cable for Masimo LNOP
Sensors
LNC MP10
(989803148221)
LNCS MP Series Patient CAble (3.0 m)
Adapter Cable for Masimo LNCS
Sensors
Non Invasive Blood Pressure Accessories
These cuffs and tubings are designed to have special
protection against electric shocks (particularly regarding
allowable leakage currents), and are defibrillator proof.
LNCS Pdtx Pediatric Finger
989803148241
Sensor
LNCS INF-L Infant Toe Sensor 989803148251
LNCS NEO-L Neo Foot Sensor
989803148271
or Adult Finger
Sensor
LNCS NEOPT-L Neo Pre-Term
989803148261
Sensitive Skin
Adhesive Sensors
The Philips M8102A uses Masimo certified pulse
oximetry for reduced noise and low perfusion
performance with Masimo Sensors under the
Masimo NR&LP protocol available from Masimo.
Multi-Patient Comfort Cuffs and Disposable Cuffs
Patient Category Disposable cuff Reusable cuff
Adult (Thigh) M1879A M1576A
Large Adult M1878A M1575A
Adult M1877A M1574A
Small Adult M1876A M1573A
Pediatric M1875A M1572A
Infant M1874A M1571A
Tubing: Use M1598B or M1599B
Reusable Cuff Kits Part No.
Infant, pediatric, small adult, adult M1577A
23
Page 24

Reusable Cuff Kits Part No.
Small adult, adult, large adult, thigh M1578A
Infant, pediatric, small adult, adult, large
M1579A
adult, thigh
Adult/Pediatric Soft Single Patient Single-Hose Disposable
Cuffs
Patient
Category
Limb
Circumference
Bladder
Width
Disposable
cuff
Part No.
Adult/Pediatric Antimicrobial Coated
Reusable cuffs
Cuff Size
(color)
Infant
(orange)
Pediatric
(green)
Small Adult
(royal blue)
Adult
(navy blue)
Adult
X-long
Circumference
(cm)
Bladder
Width
9.0 - 14.8 5.4 cm
2.1 inches
13.8 - 21.5 8.0 cm
3.1 inches
20.5 - 28.5 10.6 cm
4.2 inches
27.5 - 36.5 13.5 cm
5.3 inches
27.5 - 36.5 13.5 cm
5.3 inches
(navy blue)
Large Adult
(burgundy)
Large Adult
X-long
35.5 - 46.0 17.0 cm
6.7 inches
35.5 - 46.0 17.0 cm
6.7 inches
(burgundy)
Thigh
(grey)
45 - 56.5 21.0 cm
8.3 inches
Single-
Hose Part
No.
M4552A
M4553A
M4554A
M4555A
M4556A
M4557A
M4558A
M4559A
Large Adult
35.5-46.0 cm 16.4 cm M4578A
X-long
Large Adult 35.5-46.0 cm 16.4 cm M4577A
Adult
27.5 to 36.5 cm 16.4 cm M4576A
X-long
Adult 27.5-36.5 cm 13.1 cm M4575A
Small Adult 20.5-28.5 cm 10.4 cm M4574A
Pediatric 15.0-21.5 cm 8.0 cm M4573A
Infant 9.0-15.0 cm 5.6 cm M4572A
Tubing: Use M1598B or M1599B
Neonatal/Infant Cuffs (Disposable, non-sterile)
Cuffs
Limb
Circumference
Bladder
Width
Part No.
Size 1 3.1 to 5.7 cm 2.2 cm M1866A
Size 2 4.3 to 8.0 cm 2.8 cm M1868A
Size 3 5.8 to 10.9 cm 3.9 cm M1870A
Size 4 7.1 to 13.1 cm 4.7 cm M1872A
Tubing: Use M1596B or M1597B
Tubing: Use M1598B or M1599B
Adult/Pediatric Soft Single Patient Single-Hose Disposable
Cuffs
Patient
Category
Limb
Circumference
Bladder
Width
Disposable
cuff
Part No.
Adult (Thigh) 45.0-56.5 cm 20.4 cm M4579A
24
Cuff Tubing
Adult 1.5 m /4.9’ M1598B
3.0 m/9.8’ M1599B
Neonatal 1.5 m /4.9’ M1596B
3.0 m/9.8’ M1597B
Page 25

Temperature Accessories
Pressure Transducers and Accessories Part No.
Temperature Probes Part No.
Reusable
General purpose probe 21075A
Small flexible vinyl probe
(Infant/Pediatric)
Attachable surface probe 21078A
Disposable
General purpose probe M1837A
Skin probe 21091A
Esophageal/Stethoscope Probe
(12 French)
Esophageal/Stethoscope Probe
(18 French)
Esophageal/Stethoscope Probe
(24 French)
Foley Catheter Probe
(12 French)
21076A
21093A
21094A
21095A
M2255A
Transducer holder for CPJ840J6
(pack of 4)
IV pole mount for CPJ840J6 CPJ84447
Disposable (EU/EFTA only. Not available in USA)
Single channel disposable sensor kit (20) M1567A
Dual channel disposable sensor kit (20) M1568A
Transducer holder for M1567/8A M2271A
IV pole mount for M1567/8A M2272C
Adapter cable for disposable sensor kit, 3.0m,
for M1567/8A
CPJ84046
M1634A
Foley Catheter Probe
(16 French)
Foley Catheter Probe
(18 French)
Adapter cable 1.5m/4.9’ 21082B
Adapter cable 3.0m/9.8’ 21082A
21096A
21097A
PRESS Accessories
These transducers and accessories are designed to have
special protection against electric shocks (particularly
regarding allowable leakage currents), and are defibrillator proof.
Pressure Transducers and Accessories Part No.
Reusable
Reusable pressure transducer
5 PV/V/mmHg sensitivity
Sterile disposable pressure domes for
CPJ840J6 (pack of 50)
CPJ840J6
CPJ84022
25
Page 26

Page 27

Page 28

Philips Medical Systems is part of
Royal Philips Electronics
Interested?
Would you like to know more about our
imaginative products? Please do not hesitate to
contact us. We would be glad to hear from you.
On the web
www.medical.philips.com
Via e-mail
medical@philips.com
By fax
+31 40 27 64 887
By mail
Philips Medical Systems
Global Information Center
P.O Box 1286
5602 BG Eindhoven
The Netherlands
By phone
Asia
Tel: +852 2821 5888
Europe, Middle East, Africa
Tel: +49 7031 463 2254
Latin America
Tel: +55 11 2125 0764
North America
Tel: +1 800 229 6417
© 2007 Koninklijke Philips Electronics N.V.
All rights are reserved.
Philips Medical Systems Nederland B.V. reserves the right to make changes in specifications and/or to discontinue any product at any time without notice
or obligation and will not be liable for any consequences resulting from the use of this publication.
Printed in The Netherlands.
4522 962 26701/862 * DEC 2007
0366
M8102A complies with the requirements of the
Council Directive 93/42/EEC of 14 June 1993
(Medical Device Directive).
 Loading...
Loading...