Page 1
HEARTSTART FRx DEFIBRILLATOR
OWNER’S MANUAL
861304
Edition 8
Page 2
Philips Medical Systems
Page 3
HeartStart FRx Defibrillator
QUICK REFERENCE
Philips Medical Systems
Page 4

Intentionally blank.
Philips Medical Systems
Page 5
HeartStart FRx
861304
Automated External Defibrillator
OWNER’S MANUAL
Edition 8
IMPORTANT NOTE:
It is important to understand t hat survival rates for sudden cardiac arrest are
directly related to how soon victims are defibrillated. For every minute of
delay, the chance of survival declines by 7% to 10%.
Defibrillation cannot assure survival, no matter how rapid the treatment. In
some patients, the underlying problem causing the cardiac arrest is simply
not survivable despite any available care.
Philips Medical Systems
Page 6

About This Edition
The information in this guide applies to the
HeartStart FRx Defibrillator 861304. This
information is subject to change. Please
contact Philips at www.medical.philips.com/
heartstart or your local distributor for
information on revisions.
Authorized EU Representative
Philips Medizin Systeme Boeblingen GmbH
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031 463-2254
CAUTION
Edition History
Edition 8
Publication date: April 2007
Publication #: 989803138731
Assembly #: 012569-0008
Printed in the USA
Copyright
© 2007 Philips Electronics North America
Corp.
No part of this publication may be
reproduced, transmitted, transcribed,
stored in a retrieval system or translated
into any human or computer language in any
form by any means without the consent of
the copyright holder.
Unauthorized copying of this publication
may not only infringe copyright but also
reduce the ability of Philips Medical Systems
to provide accurate and up-to-date
information to users and operators alike.
FEDERAL LAW (USA) RESTRICTS THIS DEVICE TO SALE
BY OR ON THE ORDER OF A PHYSICIAN.
The Philips HeartStart is designed to be used only with
Philips-approved accessories. The HeartStart may perform
improperly if non-approved accessories are used.
Device Tracking
In the USA, this device is subject to tracking requirements
by the manufacturer and distributors. If the defibrillator has
been sold, donated, lost, stolen, exported, or destroyed,
notify Philips Medical Systems or your distributor.
Device Manufacturer
The HeartStart FRx Defibrillator is manufactured by Philips
Medical Systems, Seattle, Washington, USA.
Patents
This product is manufactured and sold under one or more
of the following United States patents: U.S. Pat. No
US6047212, US6317635, US5892046, US5891049,
US6356785, US5650750, US6553257, US5902249,
US6287328, US6662056, US5617853, US5951598,
US6272385, US6234816, US6346014, US6230054,
US6299574, US5607454, US5803927, US5735879,
US5749905, US5601612, US6441582, US5889388,
US5773961, US6016059, US6075369, US5904707,
US5868792, US5899926, US5879374, US5632280,
US5800460, US6185458, US5611815, US6556864,
and other patents pending.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 7

Philips Medical Systems
CONTENTS
1 Introduction to the HeartStart FRx
Description ........................................................................................................... 1
Sudden Cardiac Arrest ....................................................................................... 1
Indications for Use .............................................................................................. 2
Training and practice .......................................................................................... 2
State and local requirements ............................................................................ 2
For more information ......................................................................................... 3
2 Setting up the HeartStart FRx
Package contents ................................................................................................. 3
Setting up the FRx ............................................................................................... 4
Recommended accessories ............................................................................... 6
3 Using the HeartStart FRx
Overview ............................................................................................................... 7
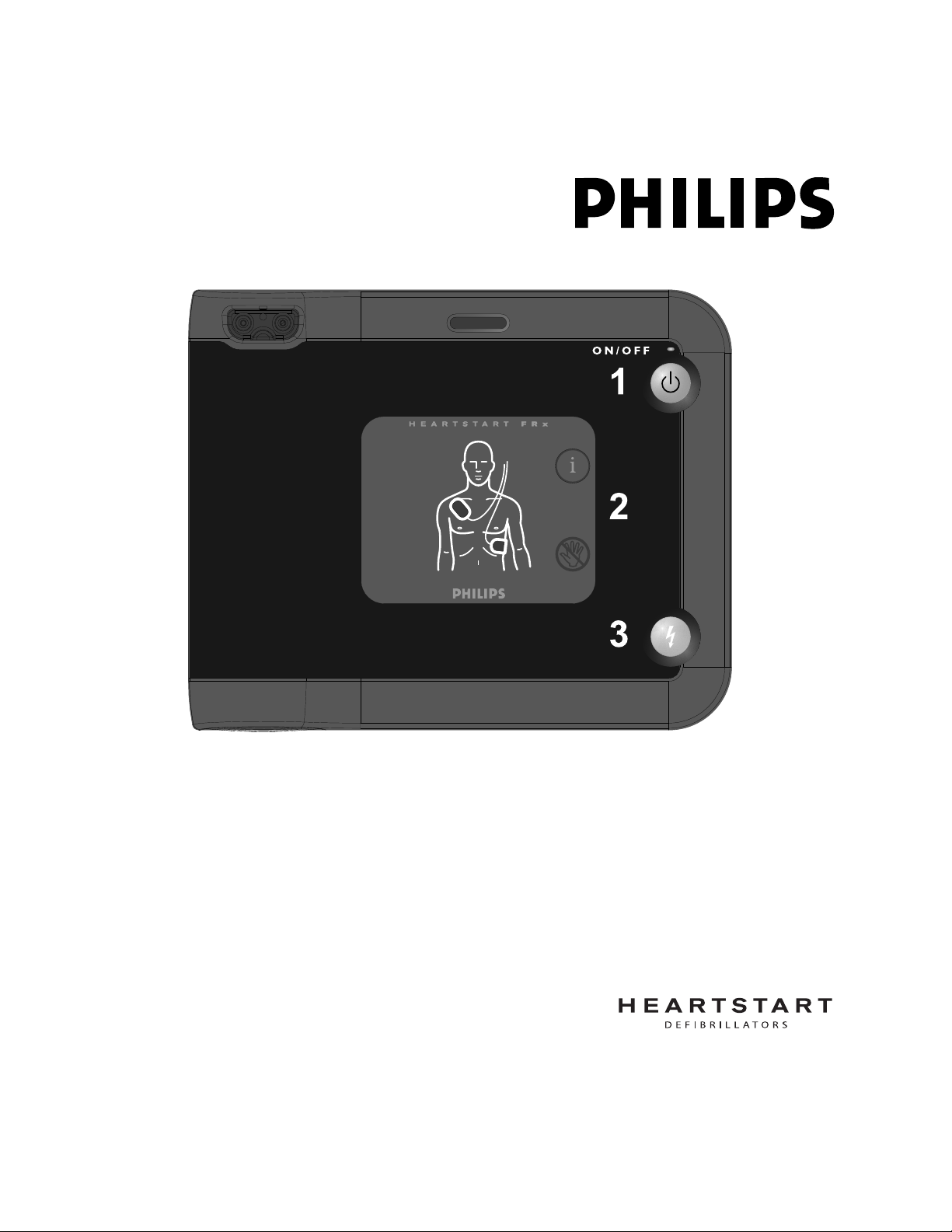
STEP 1: Press the green on/off button ........................................................... 8
STEP 2: Follow the FRx’s voice instructions ................................................. 8
STEP 3: Press the flashing orange Shock button if instructed ................... 9
Treating infants and children ............................................................................ 10
When emergency medical services arrive ..................................................... 12
4 After using the HeartStart FRx
After each use ...................................................................................................... 14
FRx data storage .................................................................................................. 15
5 Maintaining the HeartStart FRx
Routine maintenance .......................................................................................... 16
Periodic checks .................................................................................................... 17
Cleaning the FRx .................................................................................................. 17
Disposing of the FRx ........................................................................................... 17
Troubleshooting tips ........................................................................................... 18
i
Page 8

ii
APPENDICES
A Accessories ..................................................................................................................... 21
B Glossary of terms ........................................................................................................ 23
C glossary of symbols/controls ................................................................................. 27
D Warnings and Precautions ..................................................................................... 31
E Technical information ............................................................................................... 33
F Configuration .................................................................................................................41
G Testing and troubleshooting ................................................................................. 46
H Additional technical data required for European conformity ............ 50
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 9

1 Introduction to the HeartStart FRx
Description
The Philips HeartStart FRx Defibrillator 861304 (“FRx”) is an
automated external defibrillator (AED). Small, lightweight, rugged,
and battery powered, it is designed for simple and reliable operation
by minimally trained users. The FRx is highly configurable for local
protocol considerations.
Sudden Cardiac Arrest
The FRx is used to treat ventricular fibrillation (VF), the most
common cause of sudden cardiac arrest (SCA). SCA is a condition
that occurs when the heart unexpectedly stops pumping. SCA can
occur to anyone – young or old, male or female – anywhere, at any
Philips Medical Systems
time. Many victims of SCA do not have warning signs or symptoms.
Some people may have a higher risk for SCA than others. Causes
vary and may be different for infants and children than for adults.
*
VF is a chaotic quivering of the heart muscle that prevents it from
pumping blood. The only effective treatment for VF is
defibrillation.The FRx treats VF by sending a shock across the heart,
so it can start beating regularly again. Unless this is successful within
the first few minutes after the heart stops beating, the victim is not
likely to survive.
* Configurability includes timing of the “Call Emergency Medical Services”
reminder, CPR protocol variations, and other features. See Appendix F,
“Configuration,” for details.
1
Page 10

2
Indications for Use
The FRx should be used to treat someone you think may be a victim
of SCA. A person in SCA:
• does not respond when shaken, and
• is not breathing normally.
If in doubt, apply the pads. Follow the voice instructions for each
step in using the defibrillator.
Training and practice
The FRx is intended to be used under the oversight of a physician as
part of a well-designed emergency response plan. Any emergency
response plan should provide for training of FRx users in
cardiopulmonary resuscitation (CPR) and defibrillator use. Philips
recommends that you train on the device you will be using.
Several national and local organizations offer combined CPR/
defibrillator training. Contact your Philips representative, or visit us
on-line at www.medical.philips.com/heartstart, for information about
training programs in your area.
Philips Medical Systems
NOTE: Training accessories are available from Philips for
practicing use of the FRx. See Appendix A for information on
ordering accessories.
State and local requirements
Check with your state health department to see if there are any local
or state requirements about owning and using a defibrillator.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 11

For more information
Contact your local Philips distributor for additional information
about the FRx. We will be happy to answer any questions you may
have and to provide you with copies of the clinical summaries of
several key studies using Philips automated external defibrillators.
You can also find the clinical summaries online at
www.medical.philips.com/heartstart. Technical information about all
Philips HeartStart automated external defibrillators is also available
online, in the Technical Reference Manual for HeartStart
Defibrillators.
2 Setting up the HeartStart FRx
3
*
Package contents
Philips Medical Systems
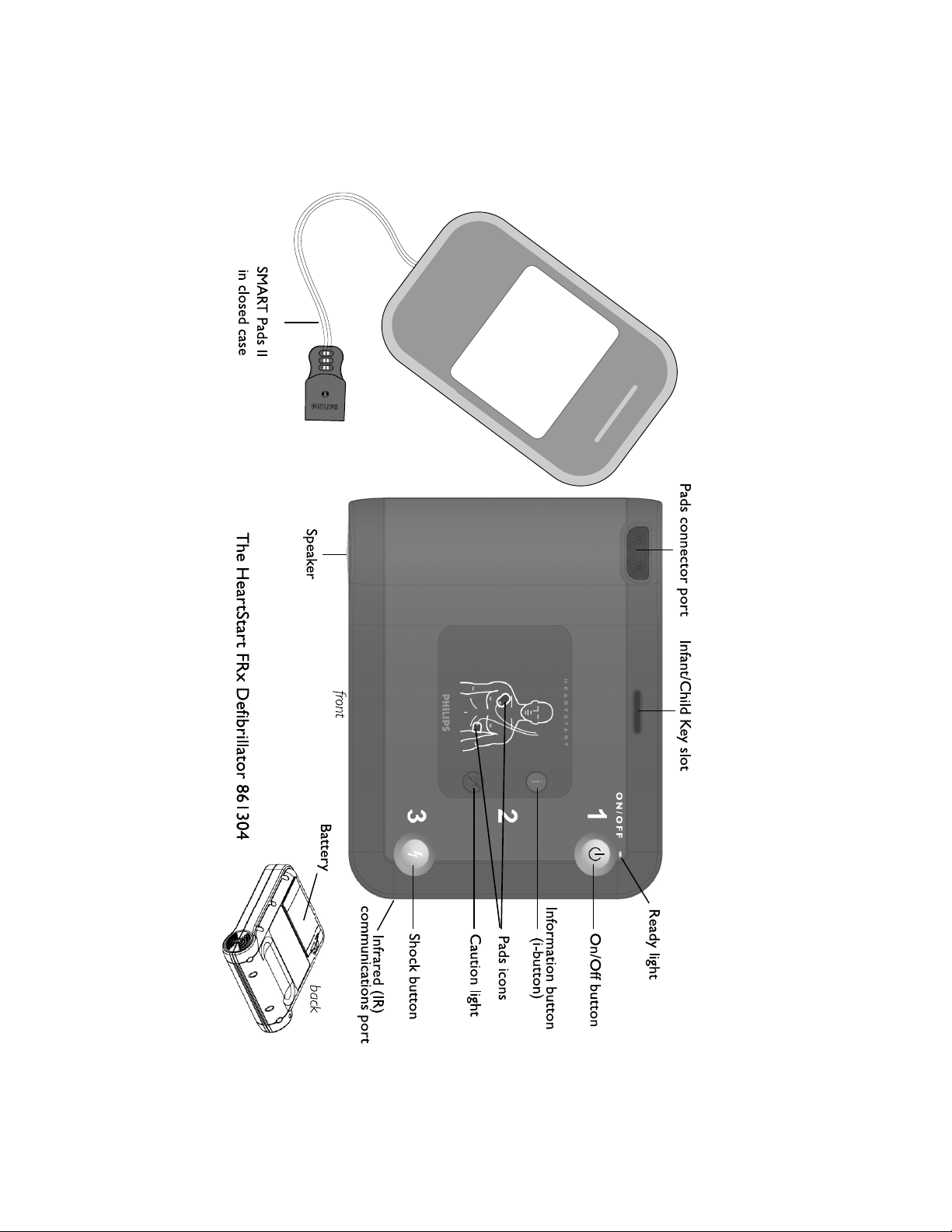
Check the contents of the FRx box to be sure it contains:
• 1 Philips HeartStart FRx Defibrillator 861304
• 1 four-year battery M5070A
• 1 package of HeartStart SMART Pads II 989803139261,
containing a pair of single-use adhesive defibrillation pads in a
disposable plastic case
• 1 Owner’s Manual
• 1 Quick Reference Guide
* Clinical summaries also include defibrillators sold as Heartstream ForeRunner
and FR2.
† The FRx sold for aviation applications includes a TSO-certified battery,
REF: 989803139301, instead of the M5070A.
†
Page 12
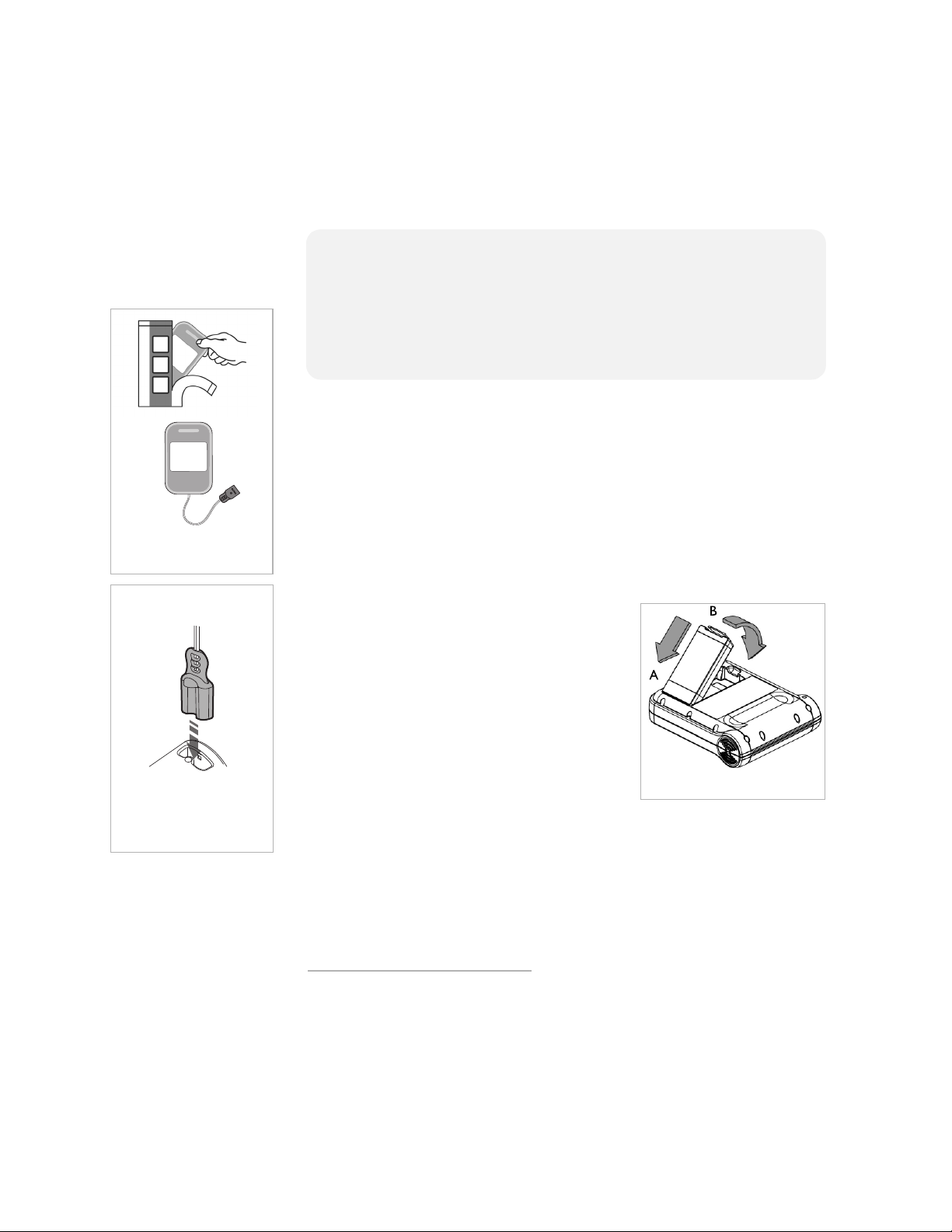
4
2. Insert battery in FRx.
1A. Remove Pads
Case from packaging.
1B. Plug in pads
cable connector.
IMPORTANT NOTE: The FRx is designed to be used with a carry
case. A number of carry cases are offered to meet the needs of
your individual defibrillation program. These include a standard
carry case and a hard-shell carry case. See Appendix A for
information about these as well as a list of training materials and
other accessories available from Philips.
Setting up the FRx
Setting up the FRx is simple and quick.
*
1. Open the SMART Pads II
(A). Do not open the pads case until you need to use the pads in an
emergency. Plug the pads cable connector into the connector
port on the FRx (B). Store the unopened Pads Case in the
pocket provided in the defibrillator carry case.
2. Open the battery package and remove
the battery. Place the bottom end (A)
of the battery into the bottom of the
compartment on the back of the FRx,
then firmly press down the top (latch)
end of the battery into the
compartment, until it clicks into place
(B).
package and take out the Pads Case
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
3. Set the FRx and the Pads Case on a flat surface and let the FRx
run its automatic battery insertion self-test. Testing the Shock
button and the On/Off button is part of the self-test. The FRx
will say “shock button test” and then instruct you to push the
Shock button. It will then say “On/Off button test” and instruct
you to push the On/Off button. Push the buttons when
* Unless otherwise noted, references to pads in this document are to HeartStart
SMART Pads II.
Page 13
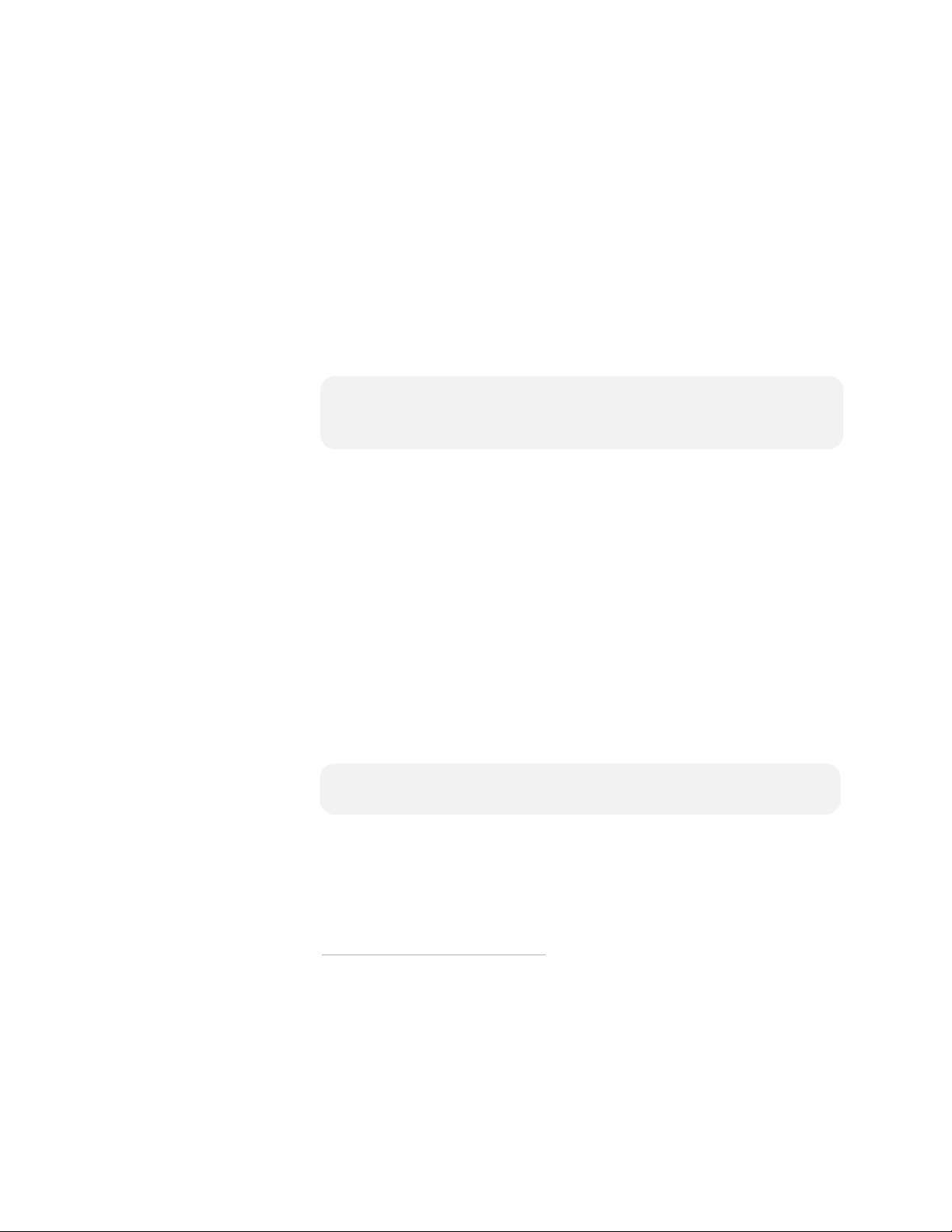
5
instructed. When the self-test is over, the FRx will report the
results, then turn off and go to standby mode. The blinking green
Ready light shows the defibrillator is ready for use.
*
4. Place the Quick Reference Guide, a brief illustrated guide for
using the FRx to treat a victim of sudden cardiac arrest, in the
defibrillator carry case.
†
NOTE: Do not store anything in the defibrillator carry case that it
is not designed to accommodate. Store all objects in their
intended location in the case.
5. Store the FRx in accordance with your site’s emergency
response protocol. Typically, this will be in a high-traffic area that
is easy to access, convenient for checking the Ready light
periodically, and easy to hear the alarm chirp if the battery
power gets low or the defibrillator needs attention. Ideally, the
HeartStart should be stored near a telephone, so the Emergency
Response Team or Emergency Medical Services can be alerted as
fast as possible in the event of a possible SCA. Keep a spare
Philips Medical Systems
SMART Pads II cartridge and other accessories with the
defibrillator – in the carry case – for quick access when needed.
Be sure to store the defibrillator according to its specifications.
See Appendix E for details.
NOTE: Always store the FRx with a set of SMART Pads II
connected and a battery installed, so it will be ready to use.
* As long as a battery is installed and a set of SMART Pads II is connected, turning
the FRx “off” puts it into standby mode, which means that it is ready for use.
† Any of the carry case options has room for storing the Quick Reference Guide.
Page 14
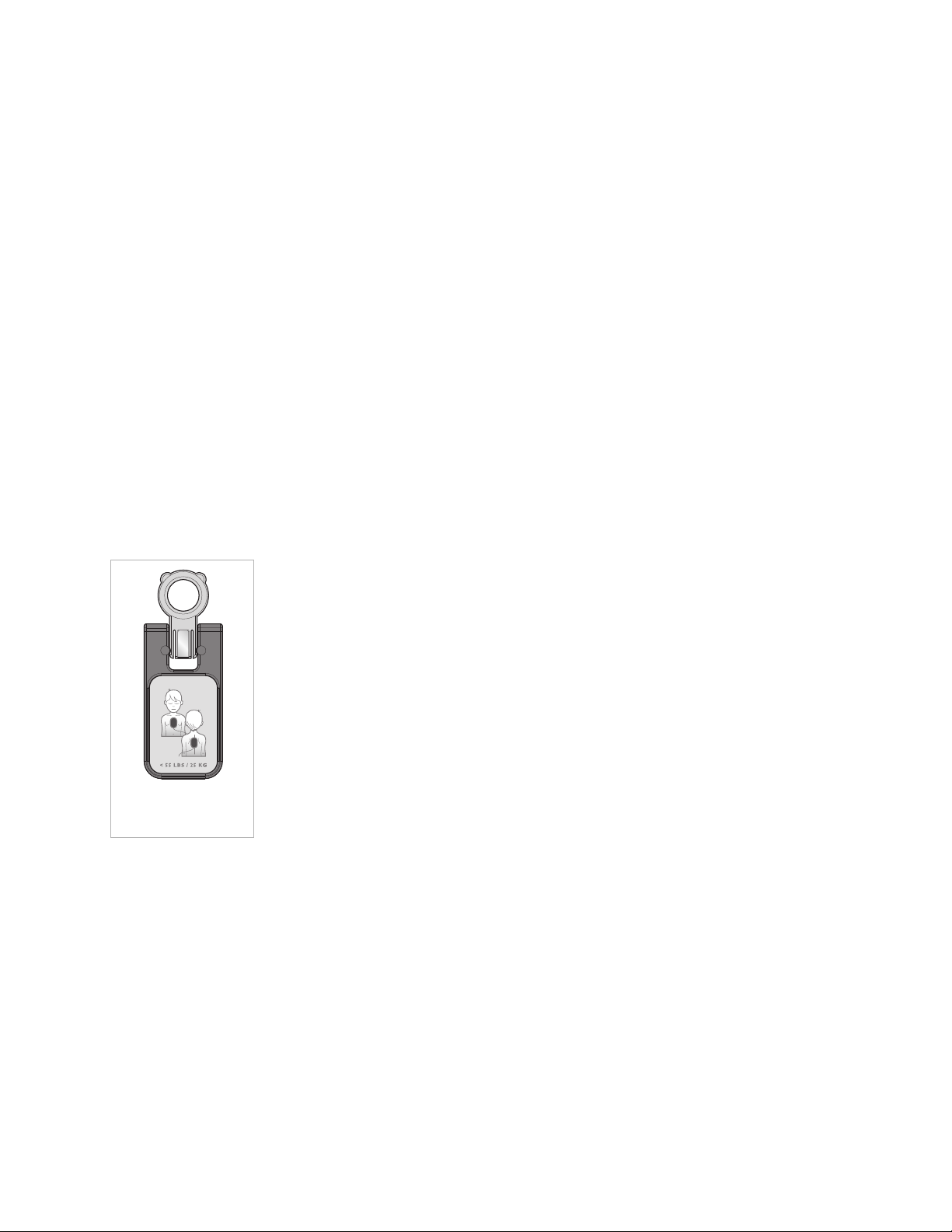
6
Infant/Child Key
accessory.
Recommended accessories
It is always a good idea to have a spare battery and a spare pads set.
Other things that are useful to keep with the FRx include:
• scissors — for cutting the victim’s clothes if needed
• disposable gloves — to protect the user
• a disposable razor — to shave the chest if hair prevents good
pads contact
• a pocket mask or face shield — to protect the user
• a towel or absorbent wipes — to dry the victim's skin for good
pads contact
Philips has a Fast Response Kit with all these items. See Appendix A
for details.
Philips Medical Systems
If you may need to defibrillate an infant or a child under 55 pounds
(25 kg) or 8 years old, it is recommended that you order the
Infant/Child Key accessory, available separately. When the
Infant/Child Key is inserted in the FRx, the FRx automatically
reduces the defibrillation energy to 50 joules and, if optional CPR
Coaching is selected, provides coaching appropriate for infants and
children. Directions for using the Infant/Child Key are provided in
Chapter 3, “Using the HeartStart FRx.”
HEARTSTART FRx 861304 OWNER’S MANUAL
See Appendix A for a list of accessories and training products for the
FRx available from Philips.
Page 15

7
3 Using the HeartStart FRx
IMPORTANT NOTE: Be sure to read the Reminders section at the
end of this chapter as well as the warnings and precautions in
Appendix D.
Overview
If you think someone is in SCA, act quickly and calmly. If someone else
is available, ask him or her to call for emergency medical assistance
while you get the FRx. If you are alone, follow these steps:
• Call your emergency services provider.
• Quickly get the FRx and bring it to the victim’s side. If there is
any delay in getting the defibrillator, check the patient and
perform cardiopulmonary resuscitation (CPR) if needed until the
FRx is available.
Philips Medical Systems
• If the victim is an infant or child, see directions for treating
infants and children starting on page 10.
• Check the immediate environment for flammable gases. Do not
use the FRx in the presence of flammable gases, such as an
oxygen tent. However, it is safe to use the FRx on someone
wearing an oxygen mask.
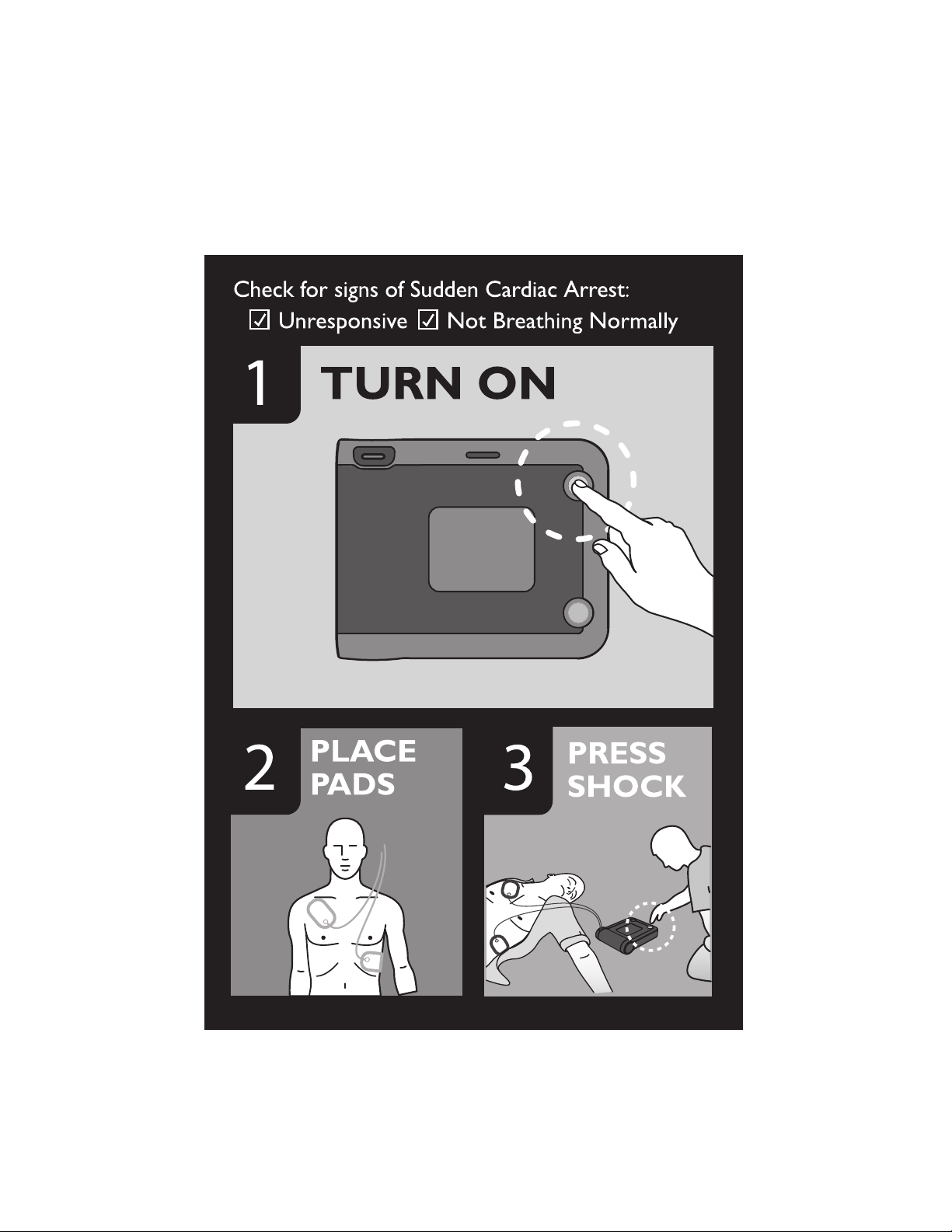
There are three basic steps to using the defibrillator to treat
someone who may be in sudden cardiac arrest:
1. Press the green On/Off button.
2. Follow the FRx’s voice instructions.
3. Press the flashing orange Shock button if instructed.
Page 16
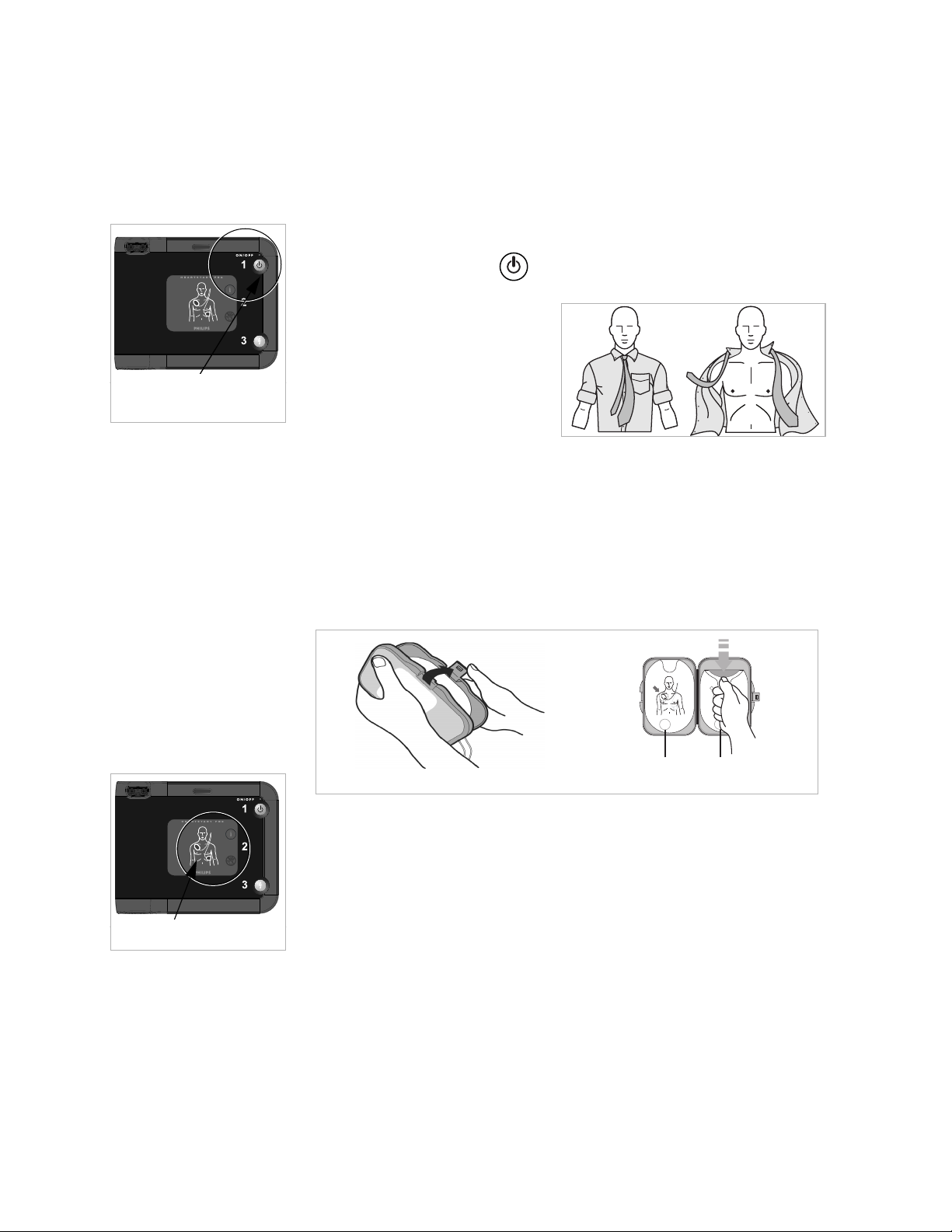
8
Press green On/Off
button.
Pads icons flash.
Open Pads Case. Peel one pad from case.
STEP 1: Press the green on/off button
Press the On/Off button to turn on the FRx.
The FRx tells you to remove all
clothes from the person’s chest.
If necessary, rip or cut off the
clothing to bare the person’s
chest.
STEP 2: Follow the FRx’s voice instructions
Remove the SMART Pads II case from the carry case. Clean and dry
the patient's skin, and, if necessary, clip or shave excessive chest hair
to ensure good pads contact with the bare skin.
Open the pads case as shown below. Peel off one pad.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
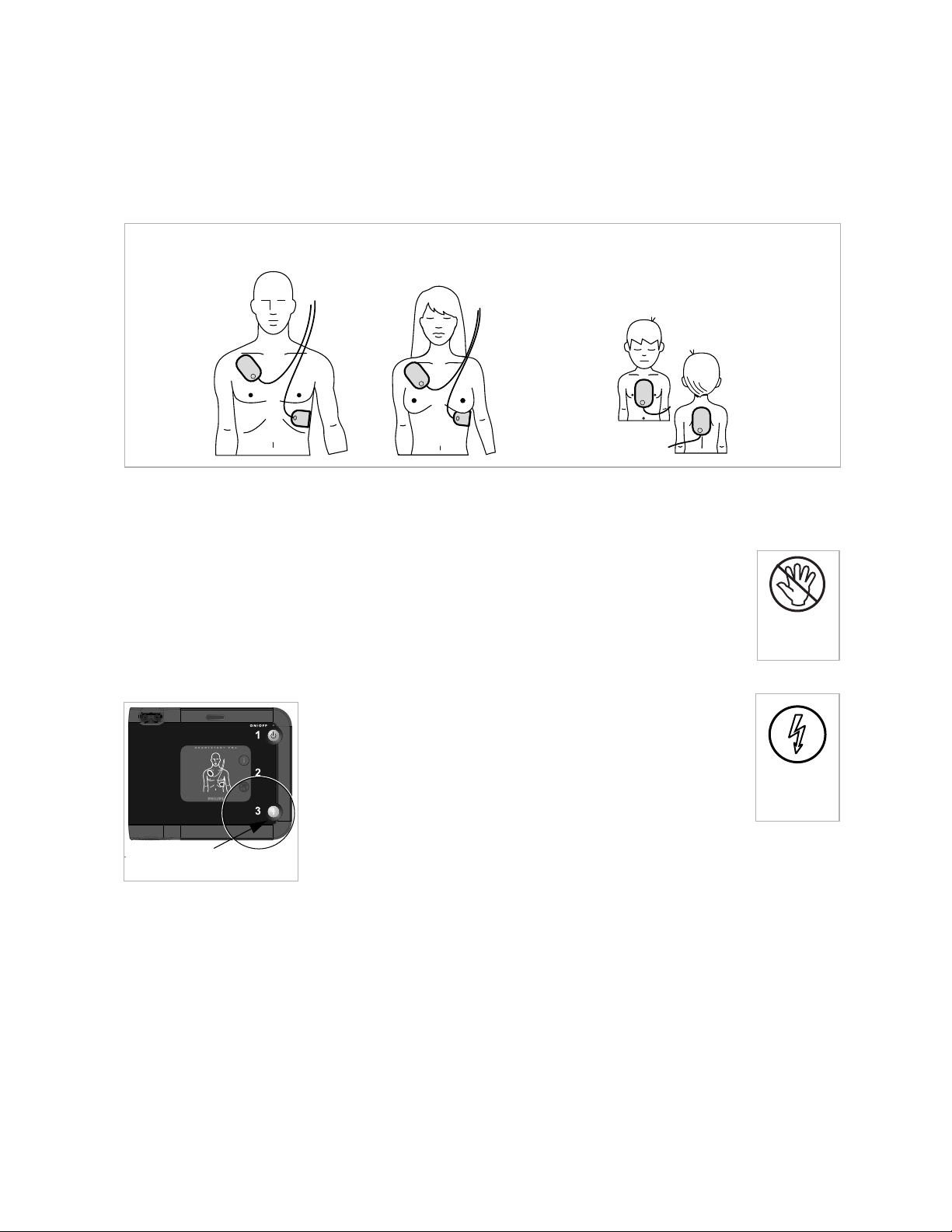
Pads placement is very important. The icons on the pads placement
diagram on the FRx front panel will be flashing, to help guide you.
Place the pad on the patient’s bare skin exactly as shown in the
following drawing. Press the adhesive portion of the pad down firmly.
Then repeat this with the other pad.
Page 17
9
Shock button flashes.
Caution
light
Shock
Button
Where to place pads on adults and children over
55 pounds or 8 years old (anterior-anterior).
Where to place pads on infants or
children under 55 pounds or
8 years old (anterior-posterior).
STEP 3: Press the flashing orange Shock button if
instructed
As soon as the HeartStart FRx detects that the pads are
attached to the patient, the pads icons turn off. The FRx
begins analyzing the patient's heart rhythm. It tells you
that no one should be touching the patient, and the
Philips Medical Systems
Caution light begins flashing as a reminder.
If a shock is needed:
The Caution light stops flashing and stays on, and the
orange Shock button starts flashing. The FRx tells you to
press the flashing orange button. You must press the
Shock button for a shock to be delivered. Before you
press the button, make sure no one is touching the
patient. When you press the Shock button, the FRx tells you that the
shock has been delivered. Then the defibrillator tells you it is safe to
touch the patient, instructs you to begin CPR, and invites you to
press the flashing blue i-button for CPR Coaching if desired.
Page 18

10
I-button
i-button flashes.
If a shock is not needed:
The blue i-button comes on solid, to show that it is safe to touch the
patient. The FRx also tells you to perform CPR if needed. (If CPR is
not needed – for example, if the patient is moving or regaining
consciousness – follow your local protocol until emergency medical
personnel arrive.) Then the FRx invites you to press the flashing blue
i-button for CPR Coaching if desired.
For CPR Coaching:
Press the flashing blue i-button during the first 30 seconds
*
of the patient care pause to activate CPR Coaching.
(If the Infant/Child Key is inserted, the CPR Coaching
provided will be for infant/child CPR.) When the pause is
over, the defibrillator tells you to stop CPR, so it can analyze the
patient’s heart rhythm. The motion caused by CPR can interfere with
analysis, so be sure to stop all motion when instructed.
Philips Medical Systems
Treating infants and children
WARNING: Most cardiac arrests in children are not caused by
heart problems. When responding to cardiac arrest in an infant
or child:
• Provide infant/child CPR while a bystander calls EMS and brings
the FRx.
• If no bystander is available, provide 1-2 minutes of CPR before
calling EMS and retrieving the FRx.
• If you witnessed the child's collapse, call EMS immediately and
then get the FRx.
Alternatively, follow your local protocol.
* The default configuration for the FRx provides CPR Coaching when you press
the i-button in this situation; however, the default setting can be revised by your
Medical Director using Philips software available separately. See Appendix F for
more information.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 19
11
Insert the Infant/Child
Key if available.
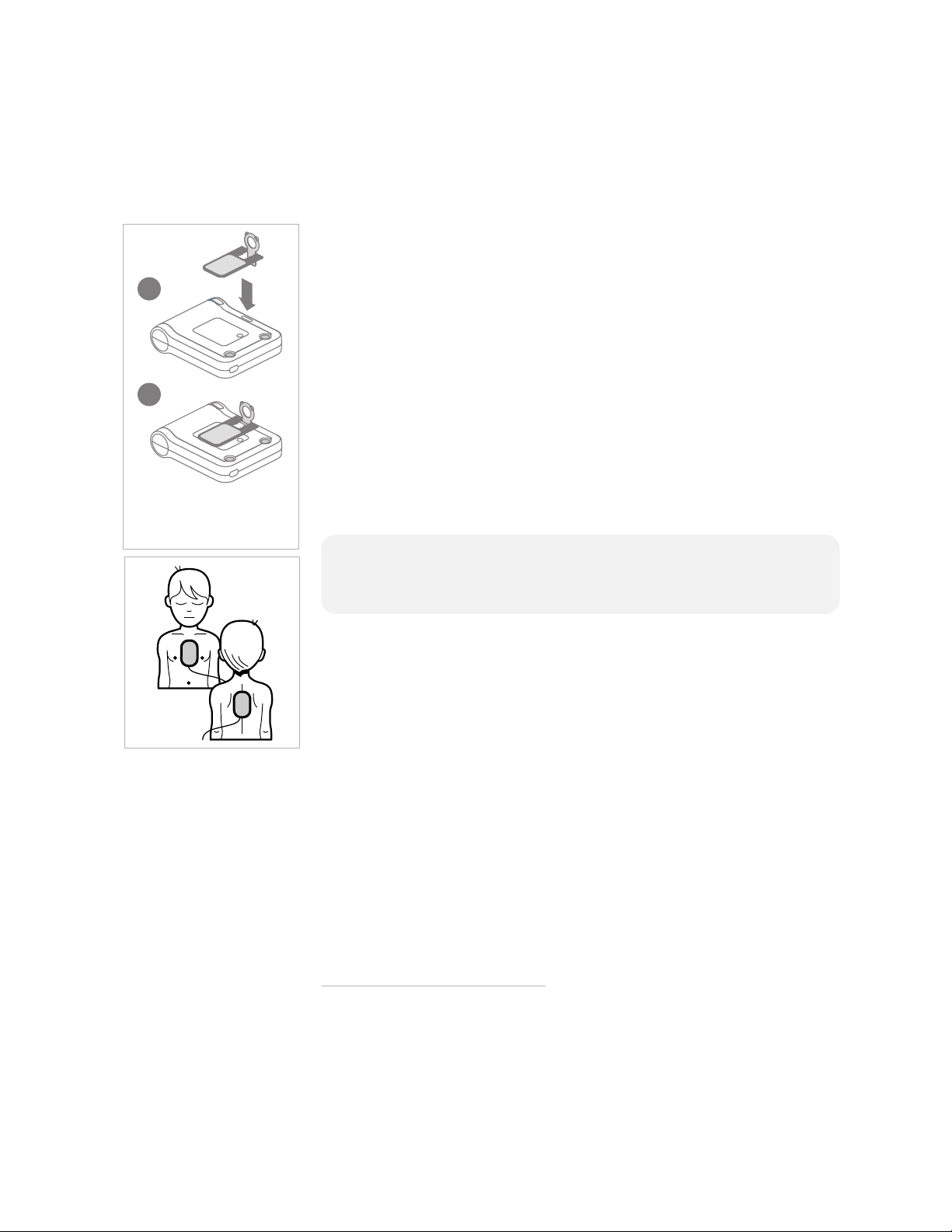
If the victim is under 55 pounds or 8 years old, and you have an
Infant/Child Key:
• Insert the Infant/Child Key into the slot at the top center of the
front panel of the FRx (see illustration at left). The pink portion
of the Key pivots (1) and fits into the slot (2), with the front of
the Key lying flat on the surface of the FRx so the infant/child
pads placement diagram is visible. (The back of the Infant/Child
Key also has a diagram showing how to insert it.)
• Turn on the FRx and follow instructions to remove all clothing
from the torso, to bare both the chest and the back.
• Place the pads on the child’s front and back, as illustrated. It does
not matter which pad is placed on the chest or the back.
NOTE: It does not matter whether you insert the Infant/Child
Key before or immediately after turning on the FRx. However, the
Key should be inserted before placing the pads on the patient.
With the Infant/Child Key inserted, the FRx will announce “Infant/
Philips Medical Systems
Child Mode,” automatically reduce the defibrillation energy from the
*
adult dose of 150 Joules to 50 Joules,
and provide optional infant/
child CPR Coaching.
If the Infant/Child Key is removed during use, the FRx will announce
“Adult Mode.” Any shocks delivered will be at adult energy, and
optional CPR Coaching will be for adult CPR.
* This lower energy level may not be effective for treating an adult.
Page 20
12
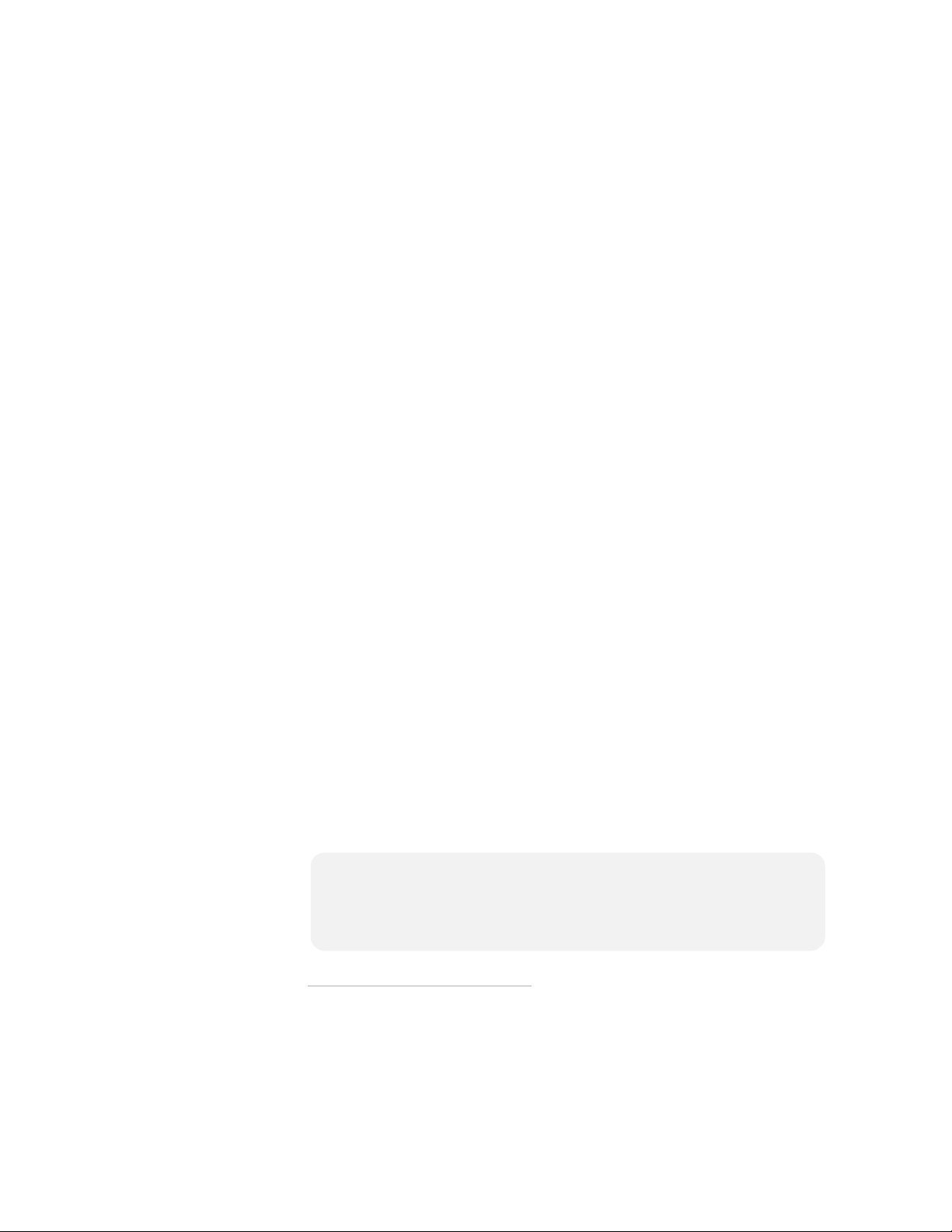
If the victim is under 55 pounds or 8 years old, but you do NOT have an
Infant/Child Key:
• DO NOT DELAY TREATMENT.
• Turn on the FRx and follow instructions to remove all clothing
from the torso, to bare both the chest and the back.
• Place the one pad in the center of the chest between the nipples,
and the other in the center of the back (anterior-posterior).
If the victim is over 55 pounds or 8 years old, or if you are not sure of the
exact weight or age:
• DO NOT DELAY TREATMENT.
• Turn on the FRx without inserting the Key and follow
instructions to remove all clothing from the chest.
• Place the pads as illustrated on each pad (anterior-anterior).
Make sure the pads do not overlap or touch each other.
When emergency medical services arrive
Philips Medical Systems
When Emergency Medical Services (EMS) personnel arrive to care
for the patient, they may decide to apply another defibrillator to
allow monitoring of the patient. Depending on their equipment, the
EMS team may apply different pads. In that case, the SMART Pads II
should be removed. EMS personnel may want a summary of the
last-use data
down the i-button until the FRx beeps.
NOTE: After the EMS team removes the SMART Pads II from the
patient, remove the Infant/Child Key, if used, and install a new
pads set before returning the FRx to service, to be sure it is ready
for use.
* See Chapter 4, “After Using the HeartStart FRx,” for details about data storage.
HEARTSTART FRx 861304 OWNER’S MANUAL
*
stored in the FRx. To hear the summary data, hold
Page 21

13
Reminders
• Remove any medicine patches and residual adhesive from the
patient’s chest before applying the pads.
• Do not place the pads directly over an implanted pacemaker or
defibrillator. A noticeable lump with a surgical scar should
indicate the position of an implanted device.
• Do not allow the pads to contact other electrodes or metal
parts that are in contact with the patient.
• If the pads do not stick well, check that the pads adhesive has not
dried out. Each pad has a layer of adhesive gel. If the gel is not
sticky to the touch, replace the pads with a new set. (For ease of
handling, the pad is designed with a non-gel area around the
connector cable.)
• Keep the patient still and keep any movement around the patient
to a minimum during rhythm analysis. Do not touch the patient
or the pads while the Caution light is on solid or flashing. If the
FRx is unable to analyze due to electrical “noise” (artifact), it will
tell you to stop all movement and remind you not to touch the
patient. If the artifact continues for more than 30 seconds, the
Philips Medical Systems
FRx will pause briefly to allow you to deal with the source of the
noise, then resume analysis.
• The FRx will only deliver a shock if the flashing orange Shock
button is pressed when the instruction is given. If the Shock
button is not pressed within 30 seconds after the instruction, the
FRx will disarm itself, and (for the first CPR interval) give a
reminder to make sure emergency medical services have been
called, then begin a CPR interval. This is designed to minimize
interruption of CPR and help ensure ongoing patient support.
• While waiting for you to press the Shock button, the FRx will
continue to analyze the heart rhythm. If the patient’s rhythm
changes before you press the Shock button, and a shock is no
longer needed, the defibrillator will disarm and tell you a shock is
not advised.
• If for any reason you want to turn off the defibrillator during a
use, you can press the On/Off button – holding it down for at
least one second – to return the device to standby mode.
Page 22

14
4 After using the HeartStart FRx
After each use
1. Check the outside of the FRx for signs of damage, dirt, or
contamination. If you see signs of damage, contact Philips for
technical support. If the defibrillator is dirty or contaminated,
clean it according to the guidelines in Chapter 5, “Maintaining the
HeartStart FRx.”
2. Plug the cable connector for a new set of SMART Pads II into the
FRx (do not open the pads case). Check supplies and accessories
for damage and expiration dates. Replace any used, damaged or
expired items. For directions on changing the pads and replacing
the battery, please see Chapter 2, “Setting Up the HeartStart
FRx.” The single-use pads must be replaced after being used.
Philips Medical Systems
3. Unless your protocol requires that the battery remain installed,
remove the battery for five seconds, then reinstall it to run the
battery insertion self-test to check the operation of the
defibrillator.
Ready light is blinking.
4. Return the FRx to its storage location so it will be ready for use
when needed.
* If you leave the battery in the FRx after using the defibrillator, then transfer the
last-use data to a computer running HeartStart Event Review software, the
software will calculate the local date and time of the device use. However, if you
remove the battery prior to transferring the data, the software will only show
elapsed time.
HEARTSTART FRx 861304 OWNER’S MANUAL
*
When the test is complete, check that the green
Page 23

15
FRx data storage
The FRx automatically stores data about its last clinical use in its
internal memory. The stored data can be conveniently transferred to
a personal computer or a handheld computer running the
appropriate application in the Philips HeartStart Event Review data
management software suite. Event Review software is for use by
trained personnel only. Information about HeartStart Event Review
is available online www.medical.philips.com/goto/eventreview.
Follow your local protocol with regard to prompt data transfer for
*
medical review after using the FRx.
timing are provided in Event Review documentation.
The information automatically stored by the FRx includes a summary
of last-use data and detailed data about its last clinical use. You can
get a voice summary of information about the last use of the
defibrillator by holding the i-button down until it beeps once. The
Philips Medical Systems
FRx will tell you how many shocks were delivered and how long it
has been since it was turned on. Summary data are available anytime
the defibrillator is ready for use (the battery and pads are installed,
and the defibrillator is not turned on) or while it is actually in use.
Removing the battery erases the summary data for the last use.
Details about data transfer and
Last-use data stored in internal memory include:
• ECG recordings (a maximum of 15 minutes following pads
†
application
* The FRx automatically stores information about its last clinical use in its internal
memory for at least 30 days, so the data can be downloaded to a computer
running appropriate Event Review software. (If the battery is removed during
this period, the defibrillator retains the files. When the battery is reinstalled,
the last-use ECG recording will be kept in defibrillator memory for an additional
30 days.) After this time, the last-use ECG recordings will automatically be
erased to prepare for a future use.
† If ECG recordings from a previous use have not been erased, the maximum time
for new ECG recordings may be less.
)
Page 24

16
• the FRx’s status (entire incident)
• the FRx’s rhythm analysis decisions (entire incident)
• the elapsed time associated with stored events (entire incident)
5 Maintaining the HeartStart FRx
Routine maintenance
The FRx is very simple to maintain. The defibrillator performs a
self-test every day. In addition, a battery insertion self-test is run
whenever a battery is installed in the device. The defibrillator’s
extensive automatic self-test features eliminate the need for any
manual calibration.
WARNING: Electrical shock hazard. Do not open the FRx, remove
its covers, or attempt repair. There are no user-serviceable
components in the FRx. If repair is required, return the FRx to an
authorized service center.
Philips Medical Systems
Reminders:
• Do not leave the defibrillator without a set of pads connected;
the defibrillator will start chirping and the i-button will start
flashing.
• Do not store the FRx with the Infant/Child Key installed.
• The FRx runs daily self-tests. As long as the green Ready light is
blinking, it is NOT necessary to test the defibrillator by initiating
a battery insertion self-test. This uses battery power and risks
draining the battery prematurely.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 25

17
Periodic checks
Other than the checks recommended after each use of the FRx,
maintenance is limited to periodically checking the following:
• Check the green Ready light. If the green Ready light is not
blinking, see Troubleshooting Tips, below.
• Replace any used, damaged or expired supplies and accessories.
• Check the outside of the defibrillator. If you see cracks or other
signs of damage, contact Philips for technical support.
Cleaning the FRx
The outside of the HeartStart FRx can be cleaned with a soft cloth
dampened in soapy water, chlorine bleach (2 tablespoons per quart
or liter of water), ammonia-based cleaners, or 70% isopropyl
(rubbing) alcohol. It is recommended that the carry case be cleaned
with a soft cloth dampened in soapy water.
Philips Medical Systems
Reminders:
• Do not use strong solvents such as acetone or acetone-based
cleaners, abrasive materials, or enzymatic cleaners to clean the
FRx and accessories.
• Do not immerse the FRx in fluids. Do not sterilize the FRx or its
accessories.
Disposing of the FRx
The FRx and its accessories should be disposed of in accordance
with local regulations.
Page 26

18
Troubleshooting tips
The FRx’s green Ready light is your guide to knowing if the
defibrillator is ready for use.
• If the Ready light is blinking: The FRx has passed the battery
insertion self-test and the last periodic self-test and is therefore
ready for use.
• If the Ready light is solid: The FRx is in use or running a self-test.
• If the Ready light is off, the FRx is chirping, and the i-button is
flashing: A self-test error has occurred, there is a problem with
the pads, the Infant/Child Key has been left installed, or the
battery power is low. Press the i-button for instructions.
• If the Ready light is off but the FRx is not chirping and the
i-button is not flashing: there is no battery inserted, the battery is
depleted, or the defibrillator needs repair. Insert/replace battery
and run the self-test. As long as the FRx passes the self-test, you
can be assured it is ready for use.
Philips Medical Systems
More detailed testing and troubleshooting information is available in
Appendix G.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 27

APPENDICES
A Accessories
B Glossary of terms
C Glossary of symbols/controls
D Warnings and precautions
E Technical information
F Configuration
G Testing and troubleshooting
H Additional technical data required for European conformity
19
Philips Medical Systems
Page 28

Notes
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 29

21
A Accessories
Accessories* for the HeartStart FRx Defibrillator 861304 available separately from
your local Philips representative or online at www.medical.philips.com/heartstart
include.
• Batteries (spare is recommended)
• Battery [REF: M5070A]
• Aviation applications battery [REF: 989803139301]
• HeartStart SMART Pads II [REF: 989803139261] (spare is recommended)
• Carry Cases
• FRx carry case [REF: 989803139251]
• Hard-shell carry case [REF: YC]
• Cabinets and Mounts
• Wall mount bracket [REF: M3857A]
• Defibrillator cabinet, semi-recessed [REF: PFE7023D]
• Defibrillator cabinet, wall surface mount [REF: PFE7024D]
• Defibrillator cabinet, basic [REF: 989803136531]
• Infant/Child Key [REF: 989803139311]
Philips Medical Systems
• Fast Response Kit (pouch containing a pocket mask, a disposable razor, 2 pairs
of disposable gloves, a pair of paramedic’s scissors, and an absorbent wipe)
[REF: 68-PCHAT]
• Data Management Software
• HeartStart Configure PDA software [REF: 989803143041]
• HeartStart Case Capture PDA software [REF: 989803143051]
• HeartStart Review Express Connect [REF: 861311 option A01]
• HeartStart Event Review, single-PC license [REF: M3834A]
• HeartStart Event Review, organization-wide license
[REF: 989803141811]
• HeartStart Event Review Pro, single-PC license [REF: 861276 option A01]
• HeartStart Event Review Pro, three-PC license [REF: 861276 option A02]
• HeartStart Event Review Pro, organization-wide license
[REF: 861276 option A03]
APPENDICES
* Certain accessories require a prescription in the Untied States.
Page 30

22
• Infrared adapter for use with HeartStart Event Review software
[REF: ACT-IR]
• HeartStart FRx Defibrillator Quick Reference Guide [REF: 989803138601]
• Training
• HeartStart Training Pads II (kit containing one set of Training Pads II in
training pads case, adult pads placement guide, Instructions for Use, and
illustrated guide) [REF: 989803139271]
• Replacement Training Pads II (pair of training pads on disposable liner for
use in training pads case provided with HeartStart Training Pads II)
[REF: 989803139291]
• Adult pads placement guide [REF: M5090A]
• Infant/Child pads placement guide [REF: 989803139281]
• HeartStart FRx Defibrillator Instructor's Training Toolkit, NTSC
[REF: 989803139321] or PAL [REF: 989803139331]
• HeartStart FRx Defibrillator Training DVD [REF: 989803139341]
• Internal Manikin Adapter [REF: M5088A]
• External Manikin Adapter, 10 pack [REF: M5089A]
Philips Medical Systems
APPENDICES
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 31

BGlossary of terms
The terms listed in this Glossary are defined in the context of the Philips
HeartStart FRx Defibrillator 861304 and its use.
AED Automated external defibrillator (a semi-automatic defibrillator).
AED mode The standard treatment mode for the HeartStart FRx Defibrillator. It provides
voice instructions guiding the rescuer through applying the adhesive pads, waiting
for rhythm analysis, and delivering a shock if needed.
analysis See “SMART analysis.”
arrhythmia An unhealthy, often irregular, beating of the heart.
artifact Electrical “noise” caused by sources such as muscle movements, CPR, patient
transport, or static electricity that may interfere with rhythm analysis.
23
battery The sealed lithium manganese dioxide battery used to power the HeartStart FRx
Defibrillator. It is provided in a pack that fits into a compartment on the back of
the defibrillator.
Caution light A light on the front of the HeartStart FRx Defibrillator that flashes during rhythm
analysis and is on solid when a shock is advised, as a reminder not to touch the
Philips Medical Systems
configuration The settings for all operating options of the HeartStart FRx Defibrillator, including
CPR Cardiopulmonary resuscitation. A technique for providing artificial respiration and
CPR Coaching Basic verbal instructions for performing cardiopulmonary resuscitation, including
defibrillation Termination of cardiac fibrillation by applying electrical energy.
ECG Electrocardiogram, a record of the electrical rhythm of the heart as detected
fibrillation A disturbance of the normal heart rhythm that results in chaotic, disorganized
patient
treatment protocol. The factory default configuration can be modified by
authorized personnel using HeartStart Event Review software.
heart compressions.
hand placement, rescue breathing, compression depth and timing, provided by the
FRx when the flashing blue i-button is pressed during the first 30 seconds of a
patient care pause.
through defibrillation pads.
activity that cannot effectively pump blood. Ventricular fibrillation (fibrillation in
the lower chambers of the heart) is associated with sudden cardiac arrest.
APPENDICES
Page 32

24
HeartStart Event Review A suite of data management software applications for use by trained personnel to
review and analyze FRx Defibrillator patient use and by authorized personnel to
alter FRx configuration. Information is available from Philips Medical Systems on
the internet at http://www.medical.philips.com/goto/eventreview.
i-button A blue “information” button on the front of the HeartStart FRx Defibrillator. If the
i-button is pressed during the 30 seconds it flashes during a patient care pause, the
FRx provides CPR Coaching;* if the i-button is pressed when it is flashing and the
FRx is chirping, the FRx provides troubleshooting guidance. At other times, if the
i-button is pressed and held until it beeps once, the FRx provides summary
information about its last clinical use and device status. When the i-button is on
solid (not flashing), it indicates the user may safely touch the patient.
Infant/Child Key A “key” recommended for use when defibrillating a potential SCA victim under
55 pounds or 8 years old. When inserted into a dedicated slot on the FRx’s front
panel, the Infant/Child Key illustrates correct pads placement, with lighted icons,
on these young victims. With the Infant/Child Key inserted, the HeartStart FRx
automatically reduces the energy of any shock delivered to 50 J and provides CPR
Coaching, if selected, appropriate for infants and children.
infrared (IR)
communications
A method of sending information using a special part of the light spectrum. It is
used to transmit information between the HeartStart FRx Defibrillator and a
computer running HeartStart Event Review software.
Philips Medical Systems
APPENDICES
shock is not needed, based on analysis of the patient’s heart rhythm.
NSA pause A pause provided by the HeartStart FRx Defibrillator following an No Shock
Advised (NSA) decision. The pause can be configured to a “standard” NSA pause
or a “SMART” NSA pause. During a standard NSA pause the defibrillator
performs no background monitoring of patient rhythm. During a SMART NSA
pause, the defibrillator conducts background monitoring and, if it detects an
artifact-free shockable rhythm, will exit the pause and begin rhythm analysis. If the
HeartStart FRx detects artifact such as that created by CPR, or if the user presses
the i-button for CPR Coaching during a SMART NSA pause, the defibrillator will
not exit the pause for rhythm analysis in order to allow CPR to be completed
uninterrupted.
non-shockable rhythm A heart rhythm that the HeartStart FRx Defibrillator determines is not
appropriate for defibrillation.
* Pressing the i-button for CPR Coaching during a SMART NSA pause turns off
background monitoring.
HEARTSTART FRx 861304 OWNER’S MANUAL
NSA “No Shock Advised,” a decision made by the HeartStart FRx Defibrillator that a
Page 33

On/Off button A green button located on the front of the HeartStart FRx Defibrillator. Pressing
the On/Off button when the defibrillator is in standby mode turns the defibrillator
on; pressing and holding the On/Off button for one second when the defibrillator
is on turns the defibrillator off and disarms the defibrillator. In addition, pressing
the On/Off button stops the battery insertion self-test that automatically runs
when a battery is inserted.
pads See “SMART Pads II.”
patient care pause A defined period to allow CPR. See “NSA pause” and “protocol pause.”
periodic self-tests Daily, weekly, and monthly tests automatically conducted by the HeartStart FRx
Defibrillator when it is in its standby mode. The tests monitor many key functions
and parameters of the defibrillator, including battery capacity, pads readiness, and
the state of its internal circuitry.
protocol A sequence of operations performed by the HeartStart FRx Defibrillator to direct
patient care in the AED mode.
protocol pause A period provided by the HeartStart FRx Defibrillator after a shock series, during
which the responder can administer CPR. The defibrillator does not conduct
background monitoring of the patient’s heart rhythm during this pause.
Quick Shock The ability of the FRx to deliver a defibrillation shock very quickly – typically
within 8 seconds – after the end of a patient care pause.
25
APPENDICES
Philips Medical Systems
A blinking Ready light means the defibrillator is ready for use; a solid Ready light
means the defibrillator is being used.
rhythm analysis See “SMART analysis.”
Shock button An orange button with a lightning bolt symbol on it, located on the front of the
HeartStart FRx Defibrillator. The Shock button flashes when a shock is advised.
You must press the button for the shock to be delivered.
shockable rhythm A heart rhythm that the HeartStart FRx Defibrillator determines is appropriate
for defibrillation, such as ventricular fibrillation and some ventricular tachycardias
associated with sudden cardiac arrest.
SMART analysis The proprietary algorithm used by the HeartStart FRx Defibrillator to analyze the
patient’s heart rhythm and determine whether a shock is advised.
Ready light A green LED showing the readiness for use of the HeartStart FRx Defibrillator.
SMART biphasic
waveform
The patented, low-energy defibrillation shock waveform used by the HeartStart
FRx Defibrillator. It is an impedance-compensated biphasic waveform. It delivers
150 Joules, nominal, into a 50 ohm load; when the Infant/Child Key is inserted, it
delivers 50 Joules, nominal, into a 50 ohm load.
Page 34

26
SMART NSA pause See “NSA pause.”
SMART Pads II The adhesive pads used with the HeartStart FRx Defibrillator to defibrillate
patients of any age or weight. The pads are applied to the patient’s bare skin and
used to detect the patient’s heart rhythm and to transfer the defibrillation shock.
standby mode The operating mode of the HeartStart FRx Defibrillator when a battery has been
installed, and the unit is turned off and ready for use when needed. Shown by
blinking green READY light.
standard NSA pause See “NSA pause.”
APPENDICES
sudden cardiac arrest
(SCA)
waveform See “SMART biphasic waveform.”
The sudden stopping of the heart’s pumping rhythm, accompanied by loss of
consciousness, absence of respiration, and lack of a pulse.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 35

27
C Glossary of symbols/controls
symbol description
On/Off button. Green. Pressing the On/Off button when the defibrillator is in
standby mode turns the defibrillator on; pressing and holding the On/Off button
for one second when the defibrillator is on turns the defibrillator off and disarms
the defibrillator. In addition, pressing the On/Off button stops the battery
insertion self-test that automatically runs when a battery is inserted.
Information button (i-button). Blue. Pressing the i-button while it is flashing
during a patient care pause provides CPR Coaching in default configuration;
pressing it while it is flashing and the defibrillator is chirping provides
troubleshooting guidance. Pressing it until it beeps at other times provides
summary information about the defibrillator’s last clinical use. Pressing it briefly in
standby mode gives device status.
Caution light. Flashes during rhythm analysis, and is on but not flashing when a
shock is advised, as a reminder not to touch the patient.
Shock button. Orange. If a shock is needed, flashes when the defibrillator is
Philips Medical Systems
charged. The defibrillator directs the user to press the Shock button to deliver a
shock to the patient.
APPENDICES
Refer to operating instructions.
Lithium manganese dioxide battery.
TSO C-142 certified battery (989803139301 only)
One battery in package.
Do not crush the battery.
Page 36

28
symbol description
Do not expose the battery to high heat or open flames. Do not incinerate the
battery.
Do not mutilate the battery or open the battery case.
Do not expose to moisture.
Handle with care.
This side up.
Philips Medical Systems
Defibrillation protection. Defibrillation protected, type BF patient connection.
APPENDICES
Meets IEC 529 class IPx5 for sealing against jetting water and class IP5x for
sealing against solid objects (dust protected).
Certified by the Canadian Standards Association.
Meets the requirements of the European medical device directives 93/42/EEC.
Meets the requirements of the applicable European directive.
Printed on recycled paper.
Storage requirements (refer to associated thermometer symbol).
Transportation requirements (refer to associated thermometer symbol).
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 37

symbol description
&
#
&
#
Environmental (temperature and relative humidity) requirements.
Install the battery in the defibrillator before the date (MM-YYYY) shown on the
associated label.
Reference order number.
Serial number.
Lot number.
Date of manufacture (989803139301 only)
29
APPENDICES
Philips Medical Systems
Class 9 miscellaneous dangerous goods. (Symbol required on outer packaging by
freight carrier regulations to identify shipments containing lithium batteries.)
On HeartStart SMART Pads II (989803139261 only). These pads are disposable
and are for single patient use only.
Contents: one set of two defibrillation pads.
Store the pads at temperatures between 32° and 122° F (0° and 50° C).
This product does not contain natural rubber latex.
This product is not sterile.
Page 38

30
symbol description
Replace pads after 24 hours.
Expiration (refer to associated date code).
Expiration date.
Federal law (USA) restricts this device to sale by or on the order of a physician.
Not for use with Laerdal defibrillator models 911, 1000, 2000, or 3000.
Not for use with HeartStart HS1 defibrillators, including HeartStart Home and
HeartStart OnSite.
Fits Philips HeartStart designated connector ports, including FR2+ and MRx.
Philips Medical Systems
APPENDICES
Pads placement illustration.
For use on infants and children under 55 pounds (25 kg).
Insert Infant/Child Key into slot on FRx.
Dispose of in accordance with your national or local requirements.
Indicates that this device is optimized for Guidelines 2005.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 39

D Warnings and Precautions
It is important to understand how to use your HeartStart FRx Defibrillator safely.
Please read these warnings and precautions carefully.
A warning describes something that could cause serious personal injury or death.
A precaution describes something that could cause minor personal injury,
damage to the FRx, loss of data stored in the FRx, or less chance of successful
defibrillation.
NOTE: The HeartStart FRx Defibrillator is designed to be used only with
Philips-approved accessories. The FRx may perform improperly if nonapproved accessories are used.
Warnings
31
flammable gases If the FRx is used to give a shock in the presence of flammable gases such as in an
oxygen tent, there is a risk of explosion. Move supplemental oxygen and oxygen
delivery devices away from the defibrillation pads. (However, it is safe to use the
FRx on someone wearing an oxygen mask.)
Philips Medical Systems
patient handling Performing CPR or otherwise handling or moving the patient while the FRx is
battery The HeartStart M5070A and 989803139301 batteries are not rechargeable. Do
not try to recharge, open, crush, or burn the battery, or it may explode or catch
fire.
fluids Do not let fluids to get into the FRx. Avoid spilling any fluids on the FRx or its
accessories. Spilling fluids into the FRx may damage it or cause a fire or shock
hazard. Do not sterilize the FRx or its accessories.
accessories Using damaged or expired equipment or accessories may cause the HeartStart
FRx Defibrillator to perform improperly, and/or injure the victim or the user.
analyzing heart rhythm can cause an incorrect or delayed analysis. If the FRx tells
you a shock is advised while you are handling or moving the patient, stop the
vehicle or CPR and keep the patient as still as possible for at least 15 seconds. This
will give the FRx time to reconfirm the analysis before telling you to press the
Shock button.
cell phones The FRx can work correctly when it is fairly close to equipment like emergency
two-way radios and cell phones. Normally, using a cell phone near the patient
should not cause a problem for the FRx. However, it is best to keep such
equipment only as close as necessary to the patient and the FRx.
APPENDICES
Page 40

APPENDICES
32
pads Do not allow the pads to contact other electrodes or metal parts that are in
contact with the patient.
Precautions
device handling The FRx was designed to be sturdy and reliable for many different use conditions.
However, handling the FRx too roughly can damage it or its accessories and will
invalidate the warranty. Check the FRx and accessories regularly for damage,
according to directions.
maintenance Improper maintenance may damage the FRx or cause it to function improperly.
Maintain the FRx according to directions.
skin burns Do not let the pads touch each other or other electrodes, lead wires, dressings,
medicine patches, etc. Such contact can cause electrical arcing and skin burns
during a shock and may also divert the electrical current away from the patient’s
heart. During a shock, air pockets between the skin and pads can cause skin burns.
To help prevent air pockets, make sure pads stick well to the skin. Do not use
dried out pads because they will not provide good contact with the skin.
patient handling Before delivering a shock, it is important to disconnect the patient from other
medical electrical equipment, such as blood-flow meters, that may not incorporate
defibrillation protection. In addition, make sure the pads are not in contact with
metal objects such as a bed frame or stretcher.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 41

E Technical information
HeartStart FRx 861304 Defibrillator specifications
The specifications provided in the following tables are nominal values. Additional
information can be found in the Technical Reference Manual for HeartStart
Defibrillators, located online at www.medical.philips.com.
Physical
category specifications
size 2.4” H x 7.1” D x 8.7” W
(6 cm H x 18 cm D x 22 cm W).
33
weight Approximately 3.5 lbs (1.6 kg) with battery and pads installed.
pads compatibility HeartStart SMART Pads II 989803139261
(In an emergency or during use, HeartStart DP2/DP6 pads may be used.
However, the FRx should not be stored with DP2/DP6 pads installed, as the
daily self-test will not give a “pass” result and the device will chirp.)
Philips Medical Systems
category specifications
temperature and
relative humidity
altitude 0 to 15,000 feet (0 to 4,572 m).
shock/drop abuse
tolerance
vibration Operating: meets MILSTD 810F Fig. 514.5C-17, random.
Environmental
Operating and standby (battery installed, pads connected):
32° to 122° F (0° to 50° C);
10% to 75% RH (non-condensing).
Storage/shipping (with battery and pads case):
-4° to 140° F (-20° to 60° C) for up to 1 week;
0% to 85% RH (non-condensing) for up to 2 days, thereafter 65% RH maximum
Withstands 1 meter drop on any edge, corner, or face of the device onto
masonry surface.
Standby: meets MILSTD 810F Fig. 514.5C-18, swept sine (helicopter).
APPENDICES
Page 42

34
category specifications
sealing Meets IEC 529 class IPx5 for jetting water and class IP5x for solid objects (dust
protected).
APPENDICES
ESD/EMI (radiated and
See Appendix F.
immunity)
aircraft: method Meets RTCA/DO-160D:1997 Section 21 (Category M - Charging).
Controls and indicators
category specifications
controls Green On/Off button
Blue i-button
Orange Shock button
Optional Infant/Child Key accessory
indicators Ready light: green, blinks when the defibrillator is in standby mode (ready for
use); solid when the defibrillator is being used.
i-button: blue, flashes when information is available, on solid during patient care
pause.
Caution light: flashes when the defibrillator is analyzing, comes on solid when
the defibrillator is ready to deliver a shock.
Shock button: orange, flashes when the defibrillator is charged and ready to
deliver a shock.
Pads Placement LEDs: flash when FRx is turned on; off once pads are placed on
patient. Also operates with Infant/Child Key inserted to indicate pads placement
on infants and children under 55 pounds (25 kg) or 8 years old.
Philips Medical Systems
audio speaker Provides voice instructions and warning tones during normal use.
beeper Provides chirps when troubleshooting is needed.
status indicator Status indicator LCD displays device readiness for use.
low battery detection Automatic during daily periodic self-testing.
low battery indicator Alarm chirps and flashing blue in-button.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 43

Defibrillation waveform
category nominal specifications
35
waveform parameters Biphasic truncated exponential. Waveform parameters are automatically
adjusted as a function of patient defibrillation impedance. In the diagram at left,
D is the duration of phase 1 and E is the duration of phase 2 of the waveform, F
is the interphase delay (500 µs), and Ip is the peak current.
The HeartStart FRx delivers shocks to load impedances from 25 to 180 ohms.
The duration of each phase of the waveform is dynamically adjusted based on
delivered charge, in order to compensate for patient impedance variations, as
shown below:
adult defibrillation
load phase 1 phase 2 peak delivered
resistance (Ω) duration (ms) duration (ms) current (A) energy (J)
25 2.8 2.8 65 128
50 4.5 4.5 40 150
75 6.25 5.0 30 155
100 8.0 5.3 24 157
125 9.65 6.4 21 159
APPENDICES
150 11.5 7.7 18 160
175 12.0 8.0 16 158
pediatric defibrillation
(using Infant/Child Key 989803139311)
Philips Medical Systems
load phase 1 phase 2 peak delivered
resistance (Ω) duration (ms) duration (ms) current (A) energy (J)
25 2.8 2.8 35 43.4
50 4.5 4.5 22 50.2
75 6.3 5.0 16 51.8
100 8.0 5.3 13 52.4
125 9.0 6.0 11 52.3
150 9.0 6.0 10 50.2
175 9.0 6.0 9 48.1
Page 44

36
category nominal specifications
energy Using HeartStart SMART Pads II for adult defibrillation: 150 J nominal (±15%)
into a 50 ohm load.
Using HeartStart SMART Pads II with Infant/Child Key inserted: 50 J nominal
(±15%) into a 50 ohm load. Sample pediatric energy doses:
age energy dose
newborn 14 J/kg
1 year 5 J/kg
2 - 3 years 4 J/kg
4 - 5 years 3 J/kg
6 - 8 years 2 J/kg
Doses indicated are based on CDC growth charts for the 50th percentile
weights for boys.*
* National Center for Health Statistics in collaboration with the National Center for
Chronic Disease Prevention and Health Promotion. CDC growth charts: weight-for-age
percentiles, revised and corrected November 28, 2000. Atlanta, GA: Centers for Disease
Control and Prevention © 2000.
charge control Controlled by Patient Analysis System for automated operation.
shock-to-shock cycle time < 20 seconds typical, including analysis.
Philips Medical Systems
APPENDICES
“charge complete”
indicator
patient care
pause-to-shock time
Shock button flashes, audio tone sounds; device is able to deliver a shock as
soon as a shock is advised.
Quick Shock. 8 seconds, typical, from end of patient care pause to shock
delivery.
disarm (AED mode) Once charged, the HeartStart FRx will disarm if:
• patient’s heart rhythm changes to non-shockable rhythm,
• a shock is not delivered within 30 seconds after the FRx is armed,
• the On/Off button is pressed for one second to turn off the FRx,
• the Infant/Child Key is inserted or removed,
the battery has been removed or is completely depleted, or
• the impedance between pads is out of range.
adult shock delivery vector Via SMART Pads II placed in the anterior-anterior (Lead II) position.
infant/child shock delivery
Via SMART Pads II typically placed in the anterior-posterior position.
vector
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 45

ECG analysis system
category specifications
37
function Evaluates impedance of adhesive pads for proper contact with patient skin, and
evaluates the ECG rhythm and signal quality to determine if a shock is
appropriate.
shockable rhythms Ventricular fibrillation (VF) and some ventricular tachycardias, including
ventricular flutter and polymorphic ventricular tachycardia (VT). The
HeartStart FRx Defibrillator uses multiple parameters to determine if a rhythm
is shockable.
NOTE: Some very low-amplitude or low-frequency rhythms may not be interpreted as
shockable VF rhythms. Also, for safety reasons, some VT rhythms often associated with
circulation may not be interpreted as shockable rhythms.
non-shockable rhythms On detection of any non-shockable rhythm, prompts user to perform CPR if
needed.
pacemaker detection Pacemaker artifact is removed from the signal for rhythm analysis.
artifact detection If electrical “noise” (artifact) is detected that interferes with accurate rhythm
APPENDICES
analysis, analysis will be delayed until the ECG signal is clean.
analysis protocol Depending on results of analysis, either prepares for shock delivery or provides
a pause. For details of protocol, see Appendix F, “Configuration.”
Philips Medical Systems
ECG analysis performance
meets AHA recommendationsb for adult defibrillation
rhythm class
ECG test
samplea size
observed 90% one-sided
performance lower
confidence limit
shockable rhythm —
ventricular fibrillation
shockable rhythm —
ventricular tachycardia
non-shockable rhythm —
normal sinus rhythm
non-shockable rhythm —
asystole
300 sensitivity >90% (87%)
100 sensitivity >75% (67%)
300 specificity >99% (97%)
100 specificity >95% (92%)
Page 46

38
rhythm class
ECG test
samplea size
meets AHA recommendationsb for adult defibrillation
observed 90% one-sided
performance lower
confidence limit
non-shockable rhythm — all
other non-shockable
rhythms
a. From Philips Medical Systems Heartstream ECG rhythm databases.
b. American Heart Association (AHA) AED Task Force, Subcommittee on AED Safety & Efficacy. Automatic External Defibrillators for Public
Access Use: Recommendations for Specifying and Reporting Arrhythmia Analysis Algorithm Performance, Incorporation of New
Waveforms, and Enhancing Safety. Circulation 1997;95:1677-1682.
c. Supraventricular tachycardia (SVT) is specifically included in the non-shockable rhythm class, in accordance with AHA recommendations
and the AAMI standard DF80.
c
450 specificity >95% (88%)
Accessories specifications
HeartStart SMART Pads II 989803139261
category specifications
pads for defibrillation,
pacing, monitoring,
cardioversion
Disposable, adhesive pads with a nominal active surface area of 80 cm
provided in a disposable plastic case, and an integrated 48 inch (121.9 cm),
typical, cable. Pads in case are designed to fit into carry cases.
APPENDICES
SMART Pads II compatibility defibrillator model adult
FRx*
FR2/FR2+
FR/ForeRunner
MRx/XL/XLT/4000
HS1/OnSite/Home
competitive adapters
patient use
yes
yes
yes
yes
no; use M5071A
yes
infant/child patient use
no, use M3870A
manual mode only
no; use M5072A
manual mode only
2
yes
no
each,
b
Philips Medical Systems
* Pre-connectable to FRx defibrillator only.
pads shelf life Pads package is labeled with a use-by date of at least two years from date of
manufacture.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 47

M5070A battery and 989803139301 TSO C-142* battery
category specifications
battery type 9 VDC, 4.2 Ah, lithium manganese dioxide. Disposable, long-life primary cell.
capacity When new, a minimum of 200 shocks or 4 hours of operating time at 25° C
(77° F). (IEC 60601-2-4, 2002)
39
(prior to insertion)
(after insertion)
battery limitations Never charge, short circuit, puncture, deform, incinerate, heat above 60° C, or
maintenance and calibration
requirements for continued
(989803139301 only)
environmental qualification
per RTCA/DO-227, Section
Philips Medical Systems
shelf life
A minimum of 5 years from date of manufacture when stored and maintained
according to directions provided in this document.
standby life
Typically, 4 years when stored and maintained according to directions provided
in this document.
training life Supports 10 hours of use in training mode.
expose contents to water. Remove the battery when discharged.
There are no periodic maintenance or calibration requirements that are
necessary for continued airworthiness of the 989803139301 battery. For
airworthiness
battery maintenance with respect to performance within the AED, please see
Chapter 5. There are no user-serviceable parts in the battery.
Meets following acceptance criteria: No leaking, venting, distortion, fire, or
rupture. Change in open circuit voltage <2%.
2.3
Infant/Child Key 989803139311
category specifications
size 6.3” x 2.4” x 0.2” (16 cm x 6 cm x 0.5 cm).
weight 1.0 oz (29 g).
APPENDICES
material Polycarbonate.
* The conditions and tests required for TSO approval of this article are minimum performance standards. It is the responsibility of those
installing this article either on or within a specific type or class of aircraft to determine that the aircraft installation conditions are within
the TSO standards. TSO articles must have a separate approval for installation in an aircraft. The article may be installed only if performed
under 14 CFR Part 43 or the applicable airworthiness requirements.
Page 48

40
Environmental considerations
By complying with your national or local regulations regarding disposal of electric,
electronic, and battery waste, you can make a positive contribution to our shared
environment.
product information
APPENDICES
defibrillator The defibrillator contains electronic components. Do not dispose of it as
unsorted municipal waste. Collect such electronic waste separately and dispose
of it at an appropriate recycling facility according to your country's or local
regulations.
battery The battery cells contain chemicals. The chemistry used in each battery is
identified by a symbol on the label; symbols are defined in the defibrillator
User's Guide/Instructions for Use/Owner's Manual. Recycle the battery at an
appropriate recycling facility.
pads The used pads may be contaminated with body tissue, fluid, or blood. Dispose
of them as infectious waste. Recycle the case at an appropriate recycling facility.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 49

FConfiguration
Overview
The Philips HeartStart FRx Defibrillator comes with a factory default configuration
designed to meet the needs of most users. This configuration can only be changed
by using HeartStart Configure version 1.0 or higher, Event Review version 3.2 or
higher, or Event Review Pro 3.1 or higher. This software is for use by trained
personnel. Information about HeartStart data management products is available
online www.medical.philips.com/goto/eventreview. See Appendix A for ordering
information.
Device options
The following table includes the features of FRx operation that are not related to
patient treatment
parameter settings default default description
41
APPENDICES
speaker volume 1, 2, 3, 4,
Philips Medical Systems
auto send periodic
self-test (PST) data
ECG out data On, Off On Enables the ECG data to be broadcast
5, 6, 7, 8
On, Off On Enables the periodic self-test data to be
8 The volume of the FRx’s speaker is set to 8,
highest.
broadcast through the device's infrared
data port.
through the device's infrared data port.
Page 50

42
Patient treatment protocol options
parameter settings default default description
APPENDICES
“call EMS” voice
reminder timing
shock series 1, 2, 3, 4 1 The automatic protocol pause
shock series interval
(minutes)
• At power on (when the
user turns on the FRx)
• At power on and at the
start of the first patient
care pause
• At the start of the first
patient care pause
•No reminder
1.0, 2.0,
∞ (infinity)
At the start of
the first
patient care
pause.
Provides a voice reminder to
make sure emergency medical
services have been called, at
the start of the first patient
care pause.
for CPR is activated each time
a shock is delivered.*
During the protocol pause,
the FRx does not perform
rhythm analysis.
The length of the protocol
pause after a shock series is
completed is determined by
the protocol pause timer
setting.
1.0 A delivered shock must occur
within 1 minute of the
previous shock to be counted
as part of the current shock
series.
NOTE: This parameter is only
applicable when the shock series
is not configured to the default 1
shock.
Philips Medical Systems
* A shock series begins when a shock is delivered after the FRx is turned on. A new shock series begins after a protocol
pause. If shock series is configured for 2 or more, a new shock series also begins if the time since the previous shock
exceeds the shock series interval setting.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 51

parameter settings default default description
43
protocol pause timer
(minutes)
0.5, 1.0, 1.5,
2.0, 2.5, 3.0
2.0 A 2-minute protocol pause for
CPR automatically starts after
voice instruction is given when
a shock series is completed.
After the protocol pause, the
defibrillator returns to rhythm
analysis.
If the user presses the
i-button for optional CPR
coaching, the FRx provides
coaching for 5 cycles of CPR,
starting and ending with
compressions, when the CPR
Coaching parameters are also
set to their default values. The
number of CPR cycles varies
for other protocol pause
timer and CPR Coaching
parameter settings.
NSA pause type • Standard NSA pause: FRx
does not perform rhythm
analysis during the NSA
pause.
Philips Medical Systems
• SMART NSA pause: FRx
conducts background
monitoring during the
SMART NSA pause. If a
potentially shockable
rhythm is detected, FRx
terminates the SMART
NSA pause and resumes
rhythm analysis.
SMART NSA
pause
During a SMART NSA pause,
the defibrillator conducts
background monitoring. If a
potentially shockable rhythm
is detected in a motionless
patient, the defibrillator
terminates the SMART NSA
pause and resumes rhythm
analysis.
NOTE: If the FRx detects CPR in
progress or if the responder has
pressed the i-button for CPR
Coaching, the SMART NSA
APPENDICES
pause will be converted to a
standard NSA pause. During the
standard NSA pause, the
defibrillator does not perform
rhythm analysis.
Page 52

44
parameter settings default default description
APPENDICES
NSA pause timer
(minutes)
0.5, 1.0, 1.5,
2.0, 2.5, 3.0
CPR prompt • CPR1: Instructs the user
to begin CPR.
• CPR2: Instructs the user
that it is safe to touch the
patient and to begin CPR.
• CPR3: Instructs the user
to begin CPR and to press
the i-button for CPR
Coaching.
• CPR4: Instructs the user
that it is safe to touch the
patient, to begin CPR, and
to press the i-button for
CPR Coaching.
2.0 A 2-minute NSA pause for
CPR automatically starts after
voice instruction is given when
no shock is advised (NSA).*
If the user presses the
i-button for optional CPR
coaching, the FRx provides
coaching for 5 cycles of CPR,
starting and ending with
compressions, when the CPR
Coaching parameters are also
set to their default values. The
number of CPR cycles varies
for other NSA pause timer
and CPR Coaching parameter
settings.
CPR4:
Instructs the
user that it is
safe to touch
the patient, to
begin CPR,
and to press
the i-button
for CPR
Coaching.
The CPR reminder voice
instructions provided at the
beginning of a pause interval
assures the user that it is safe
to touch the patient, instructs
the user to begin CPR, and
invites the user to press the
i-button for guidance in the
basic steps of CPR.
NOTE: CPR Coaching is available
only with the CPR3 and CPR4
settings.
Philips Medical Systems
* If the shock series is configured to 2 or more, and a shock has been delivered as part of a series, the length of the first NSA
pause interval within that shock series is determined by the protocol pause timer setting. Otherwise, the length of an NSA
pause is determined by the NSA pause timer setting.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 53

parameter settings default default description
45
CPR Coaching
adult ventilation instruction
yes, no yes Optional CPR Coaching
includes rescue breaths at the
rate determined by the CPR
Coaching compression:
ventilation ratio for adults
when an adult pads set is
installed.
Note: if this parameter is
configured to NO, CPR Coaching
will always be compressions-only
when an adult pads set is
installed.
infant/child ventilation
CPR Coaching
instruction
yes, no yes Optional CPR Coaching
includes rescue breaths at the
rate determined by the CPR
Coaching compression:
ventilation ratio for infants and
children when an infant/child
pads set is installed.
APPENDICES
Note: if this parameter is
configured to NO, CPR Coaching
will always be compressions-only
when an infant/child pads set is
Philips Medical Systems
CPR Coaching
compression:ventilation
ratio
• 30:2 adult and
30:2 infant/child
• 30:2 adult and
30:2 adult and
30:2 infant/
child
15:2 infant/child
• 15:2 adult and
15:2 infant/child
installed.
When the user presses the ibutton for optional CPR
Coaching during a protocol
pause or NSA pause, the FRx
will provide coaching in basic
CPR for cycles of 30
compressions and 2
ventilations for adults,
children, and infants. Pauses
begin and end with
compressions.
Page 54

46
G Testing and troubleshooting
Te s t i n g
The HeartStart FRx Defibrillator automatically tests its battery, connected SMART
Pads II, and internal circuitry every day. It alerts you if it finds a problem. See the
Technical Reference Guide, available online at www.medical.philips.com/heartstart,
for a detailed discussion of the self-tests.
You can also test the defibrillator at any time by removing the battery for five
seconds then reinstalling it. This test takes about one minute. Because the battery
insertion self-test is very detailed and uses battery power, running it more often
than necessary will drain the battery prematurely. It is recommended that you run
the battery insertion self-test only:
• when the defibrillator is first put into service.
• after each time the defibrillator is used to treat a patient.
• when the battery is replaced.
• when the defibrillator may have been damaged.
Philips Medical Systems
APPENDICES
Note: If the FRx turns off when you install the battery instead of running the
battery insertion self-test, check to be sure that the pads case is not open. If
the pads case is open, the FRx assumes that it may be in use and so will not run
the self-test.
If you need to use the defibrillator to treat a victim of SCA while you are running a
battery self-test, press the On/Off button to stop the test and turn on the
HeartStart FRx for use.
Troubleshooting
The FRx’s green Ready light is the signal that tells you if the defibrillator is ready
for use. The defibrillator chirps and the i-button flashes to alert you to a problem.
Recommended action when you need to use the device
If the FRx is chirping and the blue i-button is flashing, it is possible that the
defibrillator still has enough battery power to be used to treat a victim of SCA.
Press the On/Off button.
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 55

If the FRx does not turn on when you press the On/Off button, remove the
battery and replace it with a new battery if available and press the On/Off button
to turn on the defibrillator. If no spare battery is available, remove the installed
battery for five seconds, then reinsert it and run a battery insertion self-test.
If the problem continues, do not use the defibrillator. Attend to the patient,
providing CPR if needed, until emergency medical personnel arrive.
Troubleshooting while the FRx is being used
(green Ready light is solid)
Always follow any instructions the device gives.
defibrillator says: possible cause recommended action
47
... to replace the
battery immediately
The battery is nearly depleted. The
FRx will turn off unless a new battery
Install a new battery immediately.
is installed.
... to plug in pads
connector
... to replace pads
Philips Medical Systems
• The pads connector has been
unplugged.
• The pads have been damaged.
• The pads have been peeled from the
case, but have not been successfully
attached to the patient. There may
• Plug in the pads connector.
• Replace the damaged pads.
• Replace the pads on patient with
new pads to continue with the
rescue.
APPENDICES
be a problem with the pads.
... to press the pads firmly
to the skin
... to make sure the pads
have been removed
from the case
... the pads should not
be touching the
patient’s clothing
... to make sure the pads
connector is fully inserted
• The pads are not properly applied to
the patient.
• The pads are not making good
contact with the patient's bare chest
because of moisture or excessive
hair.
• The pads are touching each other.
• The pads may not have been
removed from the case or may be on
the patient’s clothing.
• Pads connector is not fully inserted.
• Make sure that the pads are sticking
completely to the patient’s skin.
• If the pads are not sticking, dry the
patient's chest and shave or clip any
excessive chest hair.
• Reposition the pads.
• Make sure pads are not in the case
or on patient’s clothing.
• Make sure the pads connector is fully
inserted.
If the voice instruction continues after
you do these things, replace the pads
set.
Page 56

48
defibrillator says: possible cause recommended action
APPENDICES
... to stop all motion • The patient is being moved or
jostled.
• The environment is dry and
movement around the patient is
causing static electricity to interfere
with ECG analysis.
• Radio or electrical sources are
interfering with ECG analysis.
... the shock was not
delivered
• The pads may not be making good
contact with the patient’s skin.
• The pads may be touching each
other.
• The pads may be damaged.
... the shock button was
not pressed
Shock has been advised but the shock
button has not been pressed within 30
seconds.
• Stop CPR; do not touch the patient.
Minimize patient motion. If the
patient is being transported, stop the
vehicle.
• Responders and bystanders should
minimize motion, particularly in dry
environments that can generate
static electricity.
• Check for possible causes of radio
and electrical interference and turn
them off or remove them from the
area.
• Press the pads firmly to the patient's
chest.
• Make sure the adhesive pads are
correctly positioned on the patient.
• Replace the pads if necessary.
When next prompted, press the Shock
button to deliver shock.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 57

Troubleshooting while the FRx is not being used
(green Ready light is not on)
Press the blue i-button to check defibrillator status, and follow any instructions the
device gives.
behavior possible cause recommended action
49
chirps or
i-button flashes
• The battery power is low or the pads
need to be replaced.
• The pads may be damaged or the
adhesive dried out.
• Press the blue i-button. Replace the
battery or pads if instructed.
• Replace the pads with a new set
and do not open the case until pads
are needed in an emergency.
• The pads case may be open.
• The defibrillator may have been turned
off without a pads set installed.
• Make sure the pads case is closed.
• Make sure the pads are properly
installed. (See Chapter 2 for
directions.)
• The Training Pads II set has been left in
the defibrillator.
• The Infant/Child Key may have been
• Remove the Training Pads II set
and replace it with a set of SMART
Pads II.
• Remove the Infant/Child Key.
APPENDICES
left installed.
• The defibrillator has been stored
outside the recommended
Philips Medical Systems
temperature range.
• Remove the battery for five
seconds then reinstall it to start
the battery insertion self-test. If it
fails, insert a new battery to repeat
the test. If it fails again, do not use
the defibrillator. If it passes, store
the defibrillator within the
recommended temperature range.
• The defibrillator has detected an error
during a self-test or cannot perform a
• Contact Philips for service if
needed.
self-test, or the Shock button is
damaged.
no chirping
and/or
i-button does not flash,
or
no response to
pressing i-button
• The battery is missing or completely
depleted.
• The defibrillator may have been
physically damaged.
• Remove the battery for five
seconds then reinstall it to start
the battery insertion self-test. If it
fails, insert a new battery and
repeat the test. If it fails again, do
not use the defibrillator.
• Contact Philips for service.
Page 58

50
H Additional technical data required for European conformity
Electromagnetic conformity
Guidance and manufacturer’s declaration: The HeartStart FRx is intended for use
in the electromagnetic environment specified in the tables below. The customer or
user of the HeartStart FRx should assure that it is used in such an environment.
Electromagnetic emissions
emissions test compliance electromagnetic environment – guidance
APPENDICES
RF
CISPR 11
Group 1
Class B
The FRx uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely
to cause any interference in nearby electronic equipment.
The FRx is suitable for use in all establishments, including
domestic establishments and those directly connected to
the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 59

Electromagnetic immunity
51
immunity test IEC 60601
electrostatic discharge
(ESD)
IEC 61000-4-2
power frequency
(50/60 Hz) magnetic field
IEC 61000-4-8
test level
± 6 kV contact
± 8 kV air
3 A/m 3 A/m Power frequency magnetic fields should
compliance
level
± 6 kV contact
± 8 kV air
electromagnetic environment - guidance
There are no special requirements with
respect to electrostatic discharge.
be at levels characteristic of a typical
location in a typical commercial/hospital
environment.
There are no special requirements for
non-commercial/non-hospital
environments.
radiated RF
IEC 61000-4-3
10 V/m
80 MHz to 2.5
GHz
[E1] V/m Portable and mobile RF communications
equipment should be used no closer to
any part of the HeartStart FRx, including
cables, than is absolutely necessary.
The recommended separation distances
for various transmitters and the AED
are shown in the following table.
Interference may occur in the
vicinity of equipment marked
Philips Medical Systems
NOTE 1.At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2.These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
with the following symbol:
a
b,c
APPENDICES
a. Generally, AEDs are sometimes susceptible to interference generated by patient and/or responder motion in environments in which a high
static electric field is present (e.g., low humidity, synthetic carpets, etc.). As a safety measure, Philips AEDs incorporate a patented method
to sense possible corruption of the ECG signal by such interference and to respond by directing the user to stop all motion. In these cases,
it is important to minimize movement in the vicinity of the patient during rhythm analysis in order to ensure that the signal being analyzed
accurately reflects the patient’s underlying heart rhythm.
b. The ISM (industrial, scientific and medical) bands between 150 kHz and 80 MHz are 6,765 MHz to 6,795 MHz; 13,553 MHz to 13, 567 MHz;
26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz.
c. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio,
AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment
due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which
the HeartStart FRx is used exceeds the applicable RF compliance level above, the HeartStart FRx should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the HeartStart.
Page 60

52
Recommended separation distances between portable and mobile RF
communications equipment and the HeartStart FRx Defibrillator
The HeartStart FRx Defibrillator is intended for use in an electromagnetic
environment in which radiated RF disturbances are controlled. The customer or
the user of the FRx can help prevent electromagnetic interference by maintaining a
minimum distance between portable and mobile RF communications equipment
(transmitters) and the FRx as recommended below, according to the maximum
output power of the communications equipment.
separation distance according to frequency of transmitter (m)
rated maximum output
power of transmitter (W)
80 MHz to 800 MHz
d = 0.6√ P
800 MHz to2.5 GHz
d = 1.15√ P
0.01 0.06 0.115
0.1 0.19 0.36
1 0.6 1.15
10 1.9 3.64
100 6.0 11.5
APPENDICES
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m)
can be determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power
rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1. At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2. The ISM (industrial, scientific and medial) bands between 150 kHz and 80 MHz are 6,765 MHz to 6,795 MHz;
13,553 MHz to 13, 567 MHz; 26,957 MHz to 27,283 MHz; and 40,66 MHz to 40,70 MHz.
NOTE 3. An additional factor of 10/3 is used in calculating the recommended separation distance for transmitters in the
ISM frequency bands between 150 kHz and 80 MHz and in the frequency range 80 MHz to 2.5 GHz to decrease the
likelihood that mobile/portable communications equipment could cause interference if it is inadvertently brought into
patient areas.
NOTE 4. These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
NOTE 5. Transmitters/antenna of this power-level are most likely mounted on an emergency vehicle chassis. The distances
cited here are for open field. For an external antenna, the separation distance is most likely shorter.
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 61

Shock cycle timing
The FRx’s Quick Shock feature allows it to deliver a shock within 8 seconds,
typical, following a CPR pause. From shock to shock, the FRx takes <20 seconds,
typical, including analysis. After 15 shocks, the FRx takes <30 seconds from
analyzing to ready-to-shock. After 200 shocks, the FRx takes <40 seconds from
initial power-on to ready-to-shock.
53
APPENDICES
Philips Medical Systems
Page 62

Notes
Philips Medical Systems
HEARTSTART FRx 861304 OWNER’S MANUAL
Page 63

Intentionally blank.
Philips Medical Systems
Page 64

POWER TO SAVE A LIFE
Philips Medical Systems is part of
Royal Philips Electronics
Philips Medical Systems
United States
Philips Medical Systems
2301 Fifth Avenue, Suite 200
Seattle, WA, USA 98121
(800) 263-3342
Canada
Philips Medical Systems
281 Hillmount Road
Markham, Ontario
L6C 2S3
(800) 291-6743
Europe, Middle East, and Africa
Philips Medizin Systeme Boeblingen GmbH
Cardiac and Monitoring Systems
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
+49 7031 463 2254
Latin America
Philips Medical Systems
1550 Sawgrass Corporate Parkway, Suite 300
Sunrise, FL 33323, USA
(954) 835-2660
REF: 989803138731
Asia Pacific
Philips Electronics Hong Kong Ltd.
30th Floor, Hopewell Centre,
17, Kennedy Road, Wanchai,
Hong Kong
(852) 2821 5888
 Loading...
Loading...