+
User Manual
Senti & Sentiero

Manufacturer
PATH MEDICAL GmbH
Landsberger Straße 65
82110 Germering
Germany
Email info@pathme.de
Telephone +49 89 800 765 02
Fax +49 89 800 765 03
Manual Information
Article number: 100904-EN
Release date: 2019-01
Revision: 0802_MA_Senti&Sentiero_Manual_EN_08-A1
Valid from: Firmware Rev. 2.5, Mira PC Software Rev. 2.0
All mentioned items, products, brands and trademarks are registered or owned by the mentioned
companies.
All information, illustrations, and specifications provided within this manual are based on the latest
product information available at the time of publication. PATH MEDICAL reserves the right to make
changes at any time without notice.
The latest revision of the user manual is available online at www.pathme.de/support.
Errors and omissions excepted.
Copyright Notice
No part of this manual may be reproduced, translated, stored, or transmitted, in any form or by any
means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written
consent of PATH MEDICAL GmbH.
Copyright © 2019 PATH MEDICAL GmbH

Table of Contents
1 Overview .......................................................................................................................................... 5
1.1 Introduction ............................................................................................................................. 5
1.2 Device Versions ....................................................................................................................... 5
1.3 Intended Use ........................................................................................................................... 6
1.4 Performance Characteristics ................................................................................................... 7
2 Explanation of Symbols ................................................................................................................... 9
3 Operational Concept ..................................................................................................................... 11
3.1 Screen Layout ........................................................................................................................ 11
3.2 Online Help ............................................................................................................................ 12
3.3 Test Result Status Icons ......................................................................................................... 12
3.4 Device Hardware ................................................................................................................... 13
3.4.1 On/Off Switch ................................................................................................................ 13
3.4.2 Device Reset .................................................................................................................. 13
3.4.3 Device Sockets ............................................................................................................... 14
3.4.4 Charging the Device ....................................................................................................... 15
3.5 Device Functions ................................................................................................................... 16
3.5.1 User Management ......................................................................................................... 16
3.5.2 Patient Management ..................................................................................................... 16
3.5.3 Device Settings .............................................................................................................. 16
3.5.4 Hardware Tests .............................................................................................................. 17
3.5.5 License Management .................................................................................................... 18
3.5.6 Demo Mode ................................................................................................................... 18
3.5.7 System Information ....................................................................................................... 18
3.5.8 Test Module Information .............................................................................................. 18
3.5.9 Error Handling ............................................................................................................... 19
3.6 Mira PC Software ................................................................................................................... 20
3.7 PATH Service Tool .................................................................................................................. 21
4 Service and Maintenance .............................................................................................................. 23
4.1 General Service Information ................................................................................................. 23
4.2 Routine Maintenance and Calibration .................................................................................. 23
4.3 Repair .................................................................................................................................... 24
5 Cleaning ......................................................................................................................................... 25
6 Accessories .................................................................................................................................... 27

7 Warranty........................................................................................................................................ 29
8 Notes on Safety ............................................................................................................................. 31
8.1 General Usage ....................................................................................................................... 31
8.2 Handling, Transport, and Storage.......................................................................................... 32
8.3 Electrical Safety ..................................................................................................................... 32
8.4 Electromagnetic Compatibility .............................................................................................. 33
8.5 Accessories ............................................................................................................................ 34
8.6 Waste Disposal ...................................................................................................................... 35
9 Technical Specifications ................................................................................................................. 37
9.1 General Device Information .................................................................................................. 37
9.2 Device Characteristics ........................................................................................................... 37
9.3 Power Supply ......................................................................................................................... 38
9.4 Storage, Transport, and Operating Conditions ..................................................................... 38
10 Electromagnetic Compatibility Information .............................................................................. 41

Senti
(Model: SIH100097)
Sentiero
Including:
Sentiero
(Model: SOH100098)
Sentiero Advanced
(Model: SOH100360)
1 Overview
1.1 Introduction
Thank you for purchasing a Senti or Sentiero. This manual is your guide for safely operating and
maintaining your device.
Please read this manual carefully before using Senti or Sentiero the first time. We
recommend taking particular note of the safety (see section 8: Notes on Safety), intended
use (see section 1.3: Intended Use), cleaning (see section 5: Cleaning) and maintenance (see section
4: Service and Maintenance) instructions.
Senti and Sentiero are reliable, easy-to-use, and mobile medical devices. All devices provide easy
navigation via touch-screen and are intended for hearing examinations (see section 1.3: Intended
Use). Some of the mentioned firmware modules in this manual may not be included with your
license. Please contact your distributor if you would like to upgrade your license to include more
modules.
1.2 Device Versions
There are multiple versions available within the Senti and Sentiero device families.
HANDHELD DEVICES:
Senti and Sentiero with PCB revision ≥ 67 differ from Senti and Sentiero with PCB revision <67 in
extended internal memory (e.g. for speech tests). Sentiero Advanced differs from Sentiero in socket
layout and offers the additional ability to conduct acoustically evoked potential (AEP) tests. Sentiero
and Sentiero Advanced with PCB revision ≥ 70 offer the ability to conduct tympanometry and
acoustic reflex measurements when used together with the tympanometry add-on TY-MA (planned
for first quarter 2019).
Page 5 / 44
Senti Desktop
Including:
Senti D. (Model: SID100419)
Senti D. Flex (Model: SID100433)
Sentiero Desktop
(Model: SOD100497)
DESKTOP DEVICES:
Senti Desktop and Senti Desktop Flex differ in sockets. Senti Desktop offers jack plugs and
is calibrated to a specific headphone and/or bone conductor. Senti Desktop Flex offers the
ability to exchange different calibrated transducers. Sentiero Desktop offers the same modules as
Sentiero together with the ability to conduct tympanometry and acoustic reflex measurements.
1.3 Intended Use
Devices of the Senti and Sentiero device families offer different test methods which can be
configured to fit the professional's needs for hearing screening or diagnostics. Devices of the Senti
device family provide multiple psycho-acoustic test procedures including conventional and imagebased pure-tone audiometry (e.g. Audio, MAGIC) and speech tests (e.g. SUN, MATCH). Devices of the
Sentiero device family additionally provide physiological test procedures including transitory evoked
otoacoustic emissions (TEOAE), distortion product otoacoustic emissions (DPOAE), auditory
brainstem responses (ABR; Sentiero Advanced only), auditory steady state responses (ASSR; Sentiero
Advanced only), and auditory impedance and acoustic reflex measurements (Sentiero Desktop,
Sentiero and Sentiero Advanced with tympanometry add-on).
Available psycho-acoustical methods on Senti and Sentiero are especially indicated for use with
cooperative patients starting at the age of two years or adequate development age, which enables
them to do play/interactive audiometry. All other physiological modules are suitable to be used for
all ages elder than infants from 34 weeks (gestational age) that are ready for discharge from the
hospital.
All physiological test methods are especially indicated for use in defining the type and configuration
of hearing loss particularly for individuals whose behavioral audiometric results are deemed
unreliable or to assist in the diagnosis of otologic disorders. Estimation of cochlear hearing thresholds
(DPOAE Threshold) is possible at various frequencies without the need of cooperative interaction
with the patient. Acoustic reflex and tympanometry are featured to evaluate the functional condition
of the middle and outer ear. For each method, several protocols can be configured. The results can
be used to make further recommendations regarding appropriate intervention strategies.
Devices of the Sentiero device family are intended for the following purposes:
Diagnostics, monitoring and follow-up after newborn hearing screening
Pre-school, school, and adult hearing screening
ENT diagnostics based on measurement of
a) Otoacoustic emissions
b) Tympanometry and acoustic reflex (Sentiero Desktop, Sentiero and Sentiero
Advanced with tympanometry add-on)
Page 6 / 44

c) Auditory Brainstem Responses (Sentiero Advanced only)
d) Auditory Steady State Responses (Sentiero Advanced only)
Senti and Sentiero are intended for use by audiologists, ear-nose-throat (ENT) doctors, and
other hearing health care professionals and audiologically trained technicians in a medical
environment. Please consider local regulations regarding the qualification requirements for
performing measurements with a specific test module.
Senti and Sentiero are not intended for operational use by the general public. All test
procedures must be supervised or conducted by qualified personnel. In the United States
of America, Federal law restricts this device to sale by or on the order of a licensed physician.
Senti and Sentiero are intended for indoor-use only and must be operated at defined
environmental conditions. See also operating conditions in section 9: Technical
Specifications and information about environmental conditions regarding electromagnetic
disturbances in section 10: Electromagnetic Compatibility Information. Senti and Sentiero are not
intended for use in oxygen-rich environments.
CONTRAINDICATIONS:
Senti and Sentiero must not be used in cases of external otitis (outer ear canal infection) or
in any case which yields to pain when inserting the ear probe or applying any other
transducer.
SIDE EFFECTS:
There are no known undesirable side effects for devices of the Senti and Sentiero device families.
See also section 8: Notes on Safety.
1.4 Performance Characteristics
All Senti and Sentiero devices are capable of producing acoustic signals which are transmitted to the
patient via an air or bone conduction transducer. All Sentiero devices are capable of recording
acoustic signals from the patient via an ear probe. Sentiero Advanced is capable of recording biopotential signals from the patient via an electrode. Sentiero and Sentiero Advanced with
tympanometry add-on TY-MA and Sentiero Desktop are capable of producing static air pressure. Test
result data is shown on the device display.
Essential performance of Senti/Sentiero devices includes general device operability, correct tone and
pressure presentation, correct signal data recording, and correct result display. Deterioration of
essential performance may result in a device not ready to work properly, in wrong audiological
diagnostics or in acoustic or pressure overexposure.
In order to preserve essential performance, routine maintenance is required (see section 4.2: Routine
Maintenance and Calibration).
Page 7 / 44

Page 8 / 44
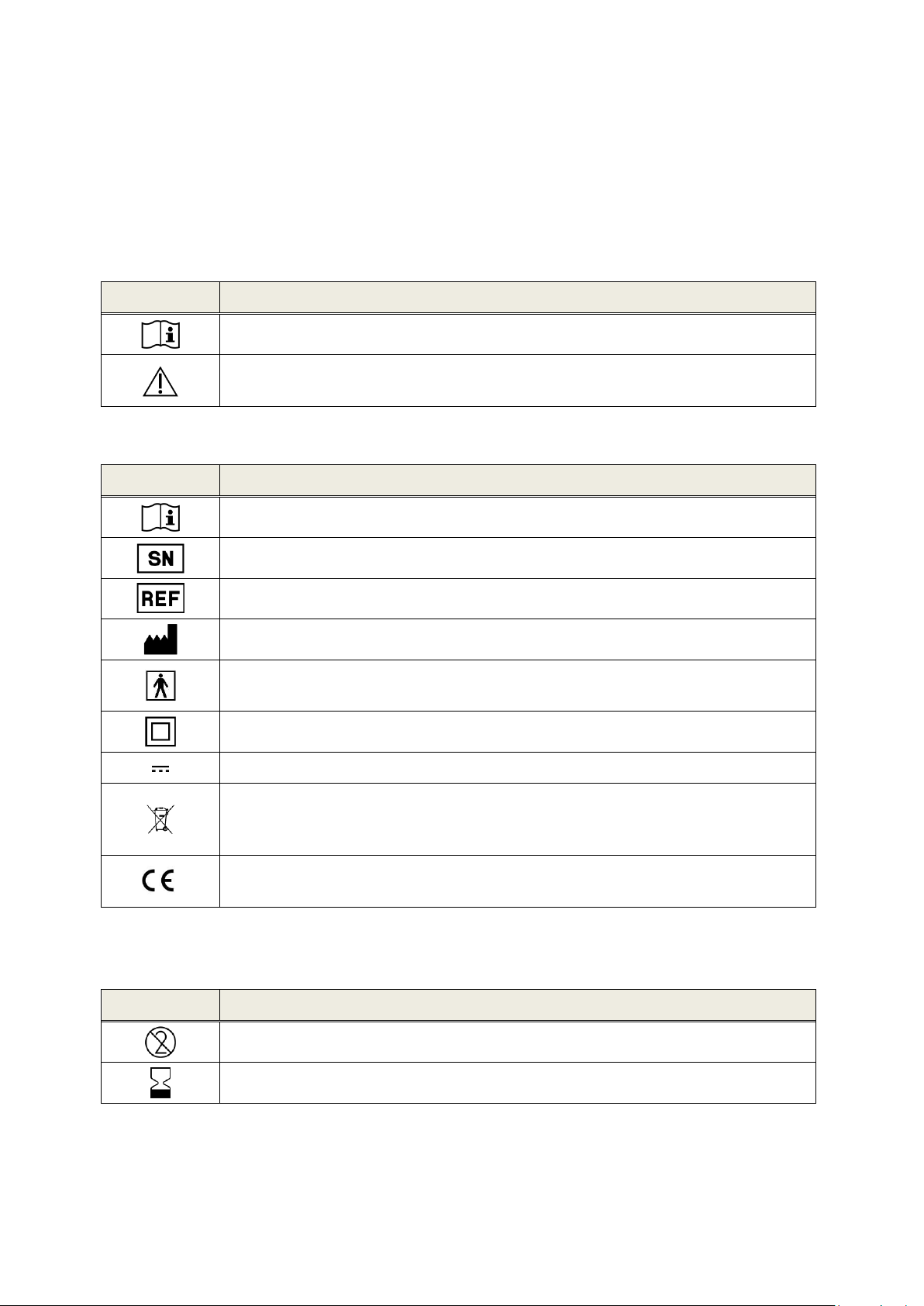
Symbol
Explanation
Important notice: please read for important information.
Warning: please read for safety-relevant information, which may cause risk of
danger to persons and/or device if not followed.
Symbol
Explanation
Consult instruction for use, i.e. this manual.
Serial number
Article number
Manufacturer name and address, production date
Compliance with applied part type BF (body floating) requirements
according to DIN EN 60601-1
Device with safety class II according to DIN EN 60601-1
Direct current input
The device is electronic equipment covered by the directive 2012/19/EC on waste
electrical and electronic equipment (WEEE). When discarded, the item must be
sent to separate collection facilities for recovery and recycling.
CE mark to declare conformity with medical device directive 93/42/EEC. The
number below the CE mark refers to the identifier of the notified body.
Symbol
Explanation
Single use only. Do not reuse the respective item.
Expiration date. Do not use the respective item after the specified date.
2 Explanation of Symbols
This section explains all symbols used within this manual and on the device label.
Symbols within this manual:
Symbols on the device label:
For further symbols, e.g. on accessory labels, please refer to the respective manual or data sheet of
the accessory. Important symbols may include:
Page 9 / 44

Page 10 / 44
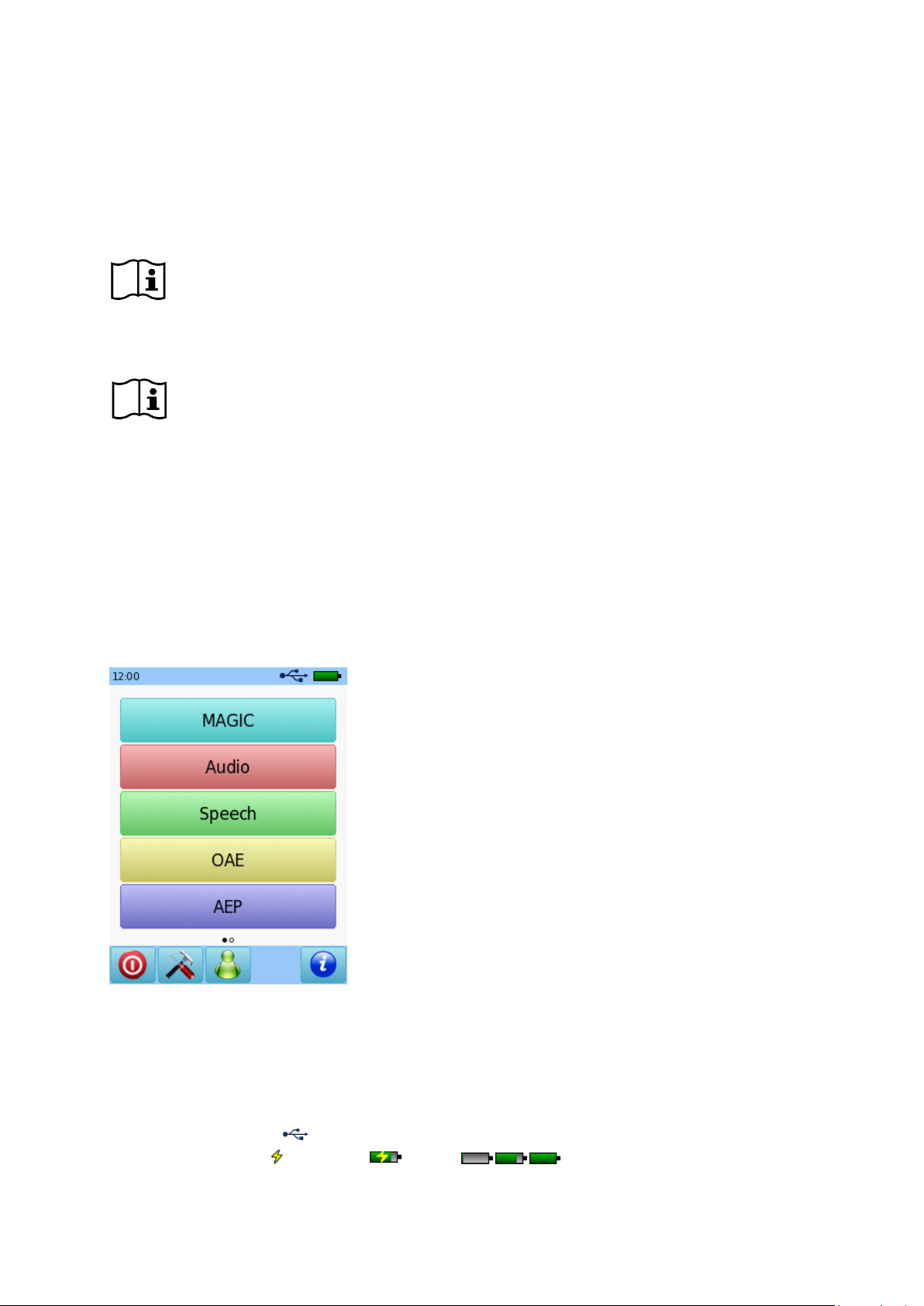
① Header
② Main screen
③ Footer
3 Operational Concept
After switching on the device, the device can be operated via a touch-sensitive display. In the
following the most important device functions and screen elements are explained.
Further information and details about the various test modules, potential clinical
applications and recommendations for combining several test procedures are explained in
the guide for practical application (How-To-Manual). You can download this document from
www.pathme.de/support.
Further technical details as e.g. maximum levels for the various test modules and for all
available transducers and specifications regarding test module parameters are described
in a separate Technical Specification document. You can download this document from
www.pathme.de/support.
Please note that screen shots or references to test modules in this manual may not reflect the actual
test configuration of your device.
3.1 Screen Layout
The device screen is in general split up in three sections (see Figure 1):
Figure 1: Device screen layout
① Header, including the following elements:
- Device time (e.g. 12:00)
- Screen-related information (e.g. selected patient name, selected test module/preset name)
- USB connection ( is shown if USB cable is connected to a PC)
- Battery status ( fully charged charging status indicator from empty to full)
Page 11 / 44

Test result OK
Screening test: valid result
Diagnostic test: result within expected range of normal hearing
Test result incomplete, in-between OK and not OK, further test interpretation needed
Test with hearing threshold result (e.g. Audio, MAGIC Audio, and DPOAE Threshold):
result within expected range of mild hearing loss
Test result not OK
Screening test: invalid result
Diagnostic test: result within expected range of moderate to severe hearing loss
② Main screen, including screen-related elements (e.g. test module list, patient list, test data result view)
③ Footer, including control elements (e.g. for browsing to different screens) and online help (see section
3.2: Online Help)
For explanation of symbols please refer to the device online help (see section 3.2: Online Help).
3.2 Online Help
Context-sensitive help screens allow an intuitive handling of the device. Automatically generated
message boxes may additionally present context-sensitive warnings or information.
The context-sensitive help screens are available via the blue information icon, which is
displayed in the footer. The help screens explain the currently available symbols and their
functions.
At some screens, there is an additional information icon, which will provide further
information for the user (e.g. recommendations for measuring DPOAEs in a noisy environment, explanation
of free-field calibration).
3.3 Test Result Status Icons
In the test history list, test results are shown with an overall test result status icon. The icons
correspond to the following definitions:
The test result status icon is meant as a rough hearing status estimator. It is not to be
interpreted as a binding result. A green status icon is not necessarily an indication that the
full auditory system is normal. A full audiologic evaluation should be administered if concerns about
hearing sensitivity persist. A yellow or red status icon should not be assumed to be an indicator of a
lack of auditory function or the presence of pathology. However, it should be followed with full
audiologic diagnostic testing as appropriate. In all cases, the examiner needs to check and interpret
result data within the context of the patient’s case history, considering results from other
measurements and additional influencing factors as appropriate (e.g. environmental conditions
during the test, patient cooperativeness).
Page 12 / 44

3.4 Device Hardware
3.4.1 On/Off Switch
The on/off switch is located at the right side of the device housing for handheld devices and at the
rear panel of the device housing for desktop devices (see Figure 2). The on/off switch can be used to
switch on or off the device. For switching on the device, press the switch briefly. The welcome screen
appears. For switching off the device, press the switch for about 10 seconds.
Alternatively the device can be switched off via the off switch icon in the footer of the device
display.
In addition, the on/off switch can be used in some test modules (e.g. MAGIC, SUN) to show the
footer, which may be hidden in these modules during the test.
Figure 2: On/off switch for handheld (left) and desktop device (right, marked with blue circle)
3.4.2 Device Reset
If the device is stalled (i.e., no reaction when pressing the touch screen), the device can be reset.
After reset, the device can be started again with the on/off switch. The reset button does not reset
any device or test module settings or any other data on the device.
In order to reset the device, for handheld devices, press the reset button on the back side of the
device below the rubber casing (see Figure 3). For desktop devices, press the on/off switch for
several seconds.
Figure 3: Device reset button for handheld device
Page 13 / 44

Socket
Connectable accessory
Blue
Headphones, insert earphones, free-field loudspeaker
Sentiero, Sentiero Advanced: 2nd ear probe, ear coupler cable,
tympanometry add-on (PCB revision ≥ 70 only)
Red
Sentiero, Sentiero Advanced: Ear probe, microphone
Sentiero Advanced: Bone conductor, trigger cable
Grey
Senti, Sentiero: Patient response button, label printer, power supply, bone
conductor
Senti: RS232 cable
White
Sentiero Advanced: Electrode cable, patient response button, label printer,
power supply, modem
USB socket
USB cable with type mini B connector
3.4.3 Device Sockets
Multiple accessories can be connected to the device. This includes e.g. transducers (e.g. headphones,
ear probe), electrode cable, patient response button, label printer, communication cable (RS232,
USB), and power supply unit. For further information see section 6: Accessories.
Desktop devices: When printing via label printer, please make sure that both the device
and the label printer are connected to the power supply unit; otherwise no printout will
be possible.
For handheld devices (see Figure 4 and Figure 5) the sockets can be used as described in Table 1.
Table 1: Device socket overview for handheld devices
Figure 4: Socket panel of handheld devices (from left to right: Senti, Sentiero, Sentiero Advanced)
Figure 5: USB socket of handheld device
Page 14 / 44

Socket
Connectable accessory
Blue
Senti Desktop Flex, Sentiero Desktop: Headphones, insert earphones, free-field
loudspeaker
Sentiero Desktop: 2nd ear probe, trigger cable
Red
Senti Desktop Flex, Sentiero Desktop: Bone conductor
Sentiero Desktop: Ear probe, microphone
①
Senti Desktop: Headphones
Please note that only a headphone specifically calibrated to the device can be used.
②
Senti Desktop: Bone conductor
Please note that only a bone conductor specifically calibrated to the device can be used.
③
Patient response button
④
Label printer, modem
⑤
USB cable with type B connector
⑥
Power supply
⑥ ⑤ ④ ③ ② ①
⑥ ⑤ ④ ③
For desktop devices (see Figure 6) the sockets can be used as described in Table 2.
Table 2: Device socket overview for desktop devices
Figure 6: Socket panel of desktop devices (top: Senti Desktop, bottom: Sentiero Desktop)
Please note that Senti Desktop Flex in comparison to Sentiero Desktop does not contain an additional
pressure outlet socket nearby the blue connector socket in Figure 6 bottom.
3.4.4 Charging the Device
Connect the power supply unit to the device (see section 3.4.3: Device Sockets). For charging the
device, connect the power plug to a power mains socket with appropriate output voltage and
frequency. For more information about power supply units please see section 9: Technical
Specifications and information provided on the power supply unit. The charging process starts
Page 15 / 44

automatically and is finished within about 2 hours (handheld) or 8 hours (desktop), respectively. The
battery status can be derived from the battery status icon symbol: fully charged; charging;
status indicator from empty to full.
3.5 Device Functions
3.5.1 User Management
With the Mira PC software you can activate or de-activate the user management on your device (see
Mira online help for more information). If the user management is activated, after switching on the
device, you will be asked to select a user and to enter the user password. Please follow the
explanations on the device. If you would like to change a user you need to logoff from the device and
restart the device. If the user management is active, you are only enabled to change module
parameters when logged in as administrator.
Please make sure that local data protection requirements are met. When deactivating
users on Senti/Sentiero devices, the device does not provide any inherent access
protection (i.e. no login with password).
3.5.2 Patient Management
After switching on the device (and if applicable after login) a patient can be added, selected from the
list of patients or the test module selection can be called in “Anonymous” mode, i.e. without adding
a patient. It is also possible to delete a single patient or all patients (Device Settings Data Management).
In “Anonymous” mode tests can be conducted and saved to a session. The session can
later be renamed with the appropriate patient data. This may be helpful e.g. for quickly
testing a sleeping child if there is no time to enter the patient data in advance. When
conducting data in “Anonymous” mode, always make sure that you are able to assign the test data
later to the correct patient.
For further information about patient management please see device online help (see section 3.2:
Online Help) on the “Select Patient” screen.
3.5.3 Device Settings
There are multiple options to configure the device to your needs.
The device settings can be reached with the tools button from the main patient selection screen.
The following device settings are available:
- Date and time, date and time format
- Language, sound (key click, result sound), display brightness, energy options, start menu,
keyboard preferences
Page 16 / 44

Error message
Recommended actions for troubleshooting
No probe found
Check if the ear probe is properly connected to the device.
If not, connect the ear probe to the device.
Probe failed
1) Check if the ear probe is placed in the correct test cavity.
If not, use the correct test cavity provided with the ear probe.
2) Check if the calibration curves* are within the upper and lower tolerance
limit markers or if both of the calibration curves* are smooth lines.
If not, make sure to use the correct test cavity and check if one or both
channels of the probe tip are clogged. If so, change or clean the probe tip.
* For EP-TE ear probes only one channel is available and hence only one curve is shown
- Test preferences (e.g. BC symbol representation, speech calibration)
For further information about device settings please see device online help (see section 3.2: Online
Help) on the “Settings” screen and its submenus.
3.5.4 Hardware Tests
The main device functions can be tested with the “Functional Checks” option.
The device self-test examines several device properties as internal power supply, codec
function, and memory integrity. If a device property is correctly working, a green
checkmark icon is shown. Otherwise a red “x” icon is shown. If not all device properties are
tested successfully (i.e., not only green checkmarks), please contact your distributor.
The probe test examines ear probe functionality. Please use either the red test cavity (test
cavity for probe tip A) for testing the large probe tip or the blue test cavity (test cavity for probe tip
S) for testing the small probe tip. Do not use other combinations. The probe test either results in a
pass (probe OK) or in one of the error messages shown in Table 3. Please follow the recommended
actions for troubleshooting mentioned in Table 3.
Table 3: Probe test error messages and recommended actions
If the recommended actions in Table 3 or in the online FAQ (www.pathme.de/support/faq) do not
help in solving the problem, please contact your distributor.
The pump unit test examines the functionality of the pneumatic system of Sentiero
Desktop or of the tympanometry add-on connected to Sentiero or Sentiero Advanced. If
the pump unit test fails (red icon), please contact your distributor.
The volume calibration of the device (“tymp calibration”) shall be checked regularly with
the probe fitted to the calibration cavities. The functionality of the device shall also be
checked on an ear known to produce a normal, peaked tympanogram (e.g. to ensure the pump is
operational and its tube is not blocked).
Page 17 / 44

3.5.5 License Management
If you would like to add modules to your device please contact your distributor. You can also use the
demo mode to evaluate the need for additional modules (see section 3.5.6: Demo Mode).
When upgrading your license, you will receive a new license key that needs to be entered on your
device. Before entering a new license key on the device, please make sure that you keep a written
note of the former license key details for potential reinstallation if needed. In order to update your
license key you need to go to the “License Management” screen (Device Settings About Device). The
existing license key and all currently licensed modules are displayed. When correctly entering and
confirming the new license key, the additional modules will be available on the device.
If you order a speech license, you will additionally receive a speech license file, which needs to be
installed on the device via Mira. Please follow the speech file installation instructions that you
receive together with the speech license file.
3.5.6 Demo Mode
You can activate the demo mode a limited number of times. In demo mode, you are able to use all
modules that are available for your device until the end of the day. Please note, that after activating
the demo mode, you cannot change your device date and time until the end of the day. If you are
interested in upgrading your device permanently with a specific module, please contact your
distributor.
3.5.7 System Information
On the system information screen, general information about the device and firmware
version is displayed. Information about connected transducers is also displayed if the
respective transducer has been connected before the system information screen is entered. On the
second page, the next service date of the device and the next calibration dates of the known
transducers are listed. When contacting your distributor regarding any service request (e.g. error
message or module update) this data should be at hand.
3.5.8 Test Module Information
Multiple test modules are available for each device. This includes standard pure-tone audiometry
(Audio), image-based pure-tone audiometry (MAGIC), speech tests (e.g. SUN, MATCH), other
subjective tests (e.g. MAUS, BASD), transient otoacoustic emissions (TEOAE), distortion product
otoacoustic emissions (DPOAE), auditory brainstem responses (ABR), auditory steady-state responses
(ASSR), tympanometry, and acoustic reflex measurements. This list may be subject to change. Please
contact your distributor or check the PATH MEDICAL homepage for an up-to-date list of available
modules and features.
Page 18 / 44

Error
Recommended action for troubleshooting
Black display
The display is automatically deactivated after 2 minutes (time span configurable)
without user activity in order to increase use time without recharging. Touch the
display in order to leave the power saving mode.
No feedback, black
display
After 10 minutes (time span configurable) without user activity the device automatically
powers down completely. Start the device by pressing the on-switch.
No feedback, black
display, device
stalled
If the device does not respond to user action you might need to restart the device by
pressing the reset switch (see section 3.4.2: Device Reset). Charge the battery if
necessary.
When conducting a measurement, please consider the following aspects:
If necessary for appropriate test performance (e.g. Audio, OAE), the device must be used in
a quiet environment (e.g. soundproof cabin, room with low ambient noise). For
measurements with ear probes (e.g. OAE) also a sound insulation headphone can be used. For AEP
measurements (e.g. ABR, ASSR) acoustical noise is less influential on test performance than muscle
artefacts (e.g. patient movement). For AEP measurements also make sure to test in an environment
with low electromagnetic disturbance from electronic devices (e.g. computers, lights, other
electronic medical devices) as electromagnetic radiation may deteriorate AEP test performance. It is
recommended to perform AEP tests in a shielded cabin. Please consider local regulations regarding
requirements for the test environment.
OAEs are most likely not present in ears with sound-conductive hearing loss, since both the
stimulus and the response amplitude are reduced due to the damping of the middle ear.
Please use only the large ear tips together with the large probe tip (PT-A) and the small ear
tips together with the small probe tip (PT-S). A wrong combination of ear tip and probe tip
may deteriorate test performance. See also advice in the accessory box. If in doubt about what
combination is correct, please contact your distributor.
If possible, do not hold the ear probe while it is placed inside the ear during OAE testing.
This may introduce noise into the measurement. Common sources of noise are acoustical
(room noise), biological (patient breathing, moving, talking, chewing, etc.), or physical (ear probe
movement) noise.
For further information and details about the various test modules, potential clinical
applications and recommendations for combining different test procedures please refer to
the How-To-Manual, which can be downloaded from www.pathme.de/support.
3.5.9 Error Handling
If an error occurs with your device please check the below list and proceed as recommended in Table
4. Further information about error handling can be found in section 3.5.4: Hardware Tests or in the
online FAQ (www.pathme.de/support/faq).
Page 19 / 44

Error
Recommended action for troubleshooting
Error message:
“Battery is too low
for testing.”
Connect the device to the power supply unit for charging the battery. It may take a few
minutes until the device is ready for starting a test module again.
Device stops test
and/or shuts down
during test.
Connect the device to the power supply unit for charging the battery. If a test is
stopped due to low battery and the device is shut down, the test data is saved before
shut down.
Error message:
“Remove cable”
Remove the connector cable (e.g. label printer cable, RS-232 cable, modem cable).
Error message:
“Touch screen
error”
The error message appears if there is a permanent pressure on the touch screen during
startup of the device. Check if there is a particle between the display and the
surrounding display frame. Remove the particle with a small and soft tool (e.g. paper
strip).
Error message:
“Calibration/service
interval expired”
The error message appears if the calibration interval of a transducer or the service
interval of the device has expired. Please send the transducer and/or the device to your
service partner.
“Error [Error-ID]”
Device error recognized by device self-test. Contact your service partner for more
information.
Table 4: Errors and recommended actions
If the recommended actions in Table 4 or in the online FAQ do not help in solving the problem,
please contact your distributor.
3.6 Mira PC Software
The latest Mira PC software is available via download from the PATH MEDICAL homepage (see
www.pathme.de/support). Mira includes the latest firmware and speech files for updating the
device. Mira comes with an online help for further information about correct handling.
Mira can be used for administering users, downloading data from the device, uploading and
downloading patient information to and from the device, reviewing and archiving test data, printing
test data to a standard PC printer, and exporting test data in various formats (e.g. GDT, Excel).
Some of the functionality only works with a communication license installed on the device (e.g. data
download from device). You do not need a communication license installed for the following
activities with Mira:
- updating your device to a new firmware
- updating a speech license or speech files
- updating user management on the device
- uploading patients to the device
- pdf export of test data (Direct Print)
Information about Mira error handling can be found at www.pathme.de/support/faq.
Page 20 / 44

3.7 PATH Service Tool
The PATH Service Tool is only available for authorized distributors and service partners. The latest
PATH Service Tool software is available via download from the PATH MEDICAL homepage via
restricted area login. The PATH Service Tool is needed for servicing devices and for calibrating
transducers. Additional hardware (e.g. CaliPro device, loopback cable) and training from PATH
MEDICAL is required. For further information see separate PATH Service Tool manual or contact
PATH MEDICAL (service@pathme.de).
Page 21 / 44

Page 22 / 44

4 Service and Maintenance
4.1 General Service Information
PATH MEDICAL is committed to customer satisfaction. Please contact your distributor for
ordering supplies, obtaining information on training courses and service contracts, getting
help with device-related problems, suggesting desired features, or finding answers not addressed in
the device online help or associated manuals. General information on your device and on PATH
MEDICAL can be found at www.pathme.de.
Updates to software, firmware and documentation (e.g. user manual) are available on the PATH
MEDICAL homepage. If updates are available, PATH MEDICAL distributors will be informed. It is the
responsibility of the local distributor to inform the end customer. If you are not sure whether your
software, firmware, or documentation is up-to-date please check www.pathme.de/support or
contact your distributor.
Service activities and repairs of the device and its electro-medical accessories must only be
conducted by PATH MEDICAL or its authorized service partners. Authorized service partners are
enabled from PATH MEDICAL with necessary documentation and training in order to conduct
specified service activities and repairs.
PATH MEDICAL reserves the right to decline any responsibility for the safety in operation, reliability,
and capability of the device or accessory if any service activities or repairs were conducted by a nonauthorized service partner (see also section 7: Warranty). If in doubt, please contact PATH MEDICAL
(service@pathme.de) before commissioning a service activity or repair. Please send the device or
accessory in its original packaging to your distributor.
4.2 Routine Maintenance and Calibration
To ensure safe operations and to keep measurements valid, it is stipulated by PATH
MEDICAL to check the device and calibrate its transducers at least once a year or more
frequently if required by local regulations or if there is any doubt about correct system function. A
warning message is shown on the device if the device service date or a transducer calibration date
has expired. Please return the device or accessory immediately to your distributor or service partner.
Free-field loudspeakers need to be calibrated regularly by the user according to device instructions.
Hence, free-field loudspeakers are exempt from the above mentioned annual calibration procedure.
Please note that for all Senti and Sentiero devices (except Senti Desktop), it is easy to
exchange transducers individually and recalibrate them separately. This will help you to
increase uptime and availability of your device.
Page 23 / 44

REGULATORY BACKGROUND:
For the device and all transducers, an annual metrological inspection following §11 Clause 2 of the
medical device operator act (MPBetreibV, Germany) must be conducted by a service partner who is
authorized by PATH MEDICAL. Regarding the Audio module an annual inspection period is stipulated
by DIN EN ISO 8253-1 and by MPBetreibV annex 2. The measurement principle of otoacoustic
emissions (OAE) or acoustically evoked potentials (AEP) is not explicitly described in MPBetreibV.
Therefore, the manufacturer is obliged to define metrological inspection instructions. DIN EN 606456 (OAE) and DIN EN 60645-7 (AEP) both suggest an annual inspection interval.
EXPLANATION:
The device and its accessories contain parts, which are exposed to environmental impacts and
contamination. In order to ensure an accurate measurement function, the fault tolerance provided
by the manufacturer or defined by applicable standards needs to be controlled by specifically
designed instrumentation and defined procedures. Therefore, metrological inspection must be
conducted by authorized service partners instructed and trained by PATH MEDICAL.
For acoustic transducers differences in environmental conditions between the point of
calibration and the point of use may influence the calibration accuracy. For more
information please refer to section 8.2: Handling, Transport, and Storage.
In addition to the annual metrological inspection, a regular visual inspection and a regular
check for correct operation of the device and its accessories is recommended. Guidelines
for routine inspections are provided e.g. in DIN EN ISO 8253-1 for pure-tone audiometry. Before
using the middle ear analyzer module each day, use the calibration volume cavities provided with
your device to check the calibration of the ml/mmho meter. Please follow local regulations or
guidelines.
4.3 Repair
In case a device or accessory is defective or differs in any way from its original setup, PATH MEDICAL
or an authorized service partner will repair, re-calibrate or exchange the device or accessory. All
repairs are subject to parts and material availability. Please contact your distributor to find out about
the lead time of any repair activity.
Prior to sending any equipment for repair, please provide relevant information to your service
partner (e.g. model, serial number, firmware version, contact information, shipping information,
detailed description of experienced issue or defect). This may help in speeding up the repair process
and failure analysis and in excluding issues that can be solved without sending the device. Additional
information may be requested by your service partner.
See also sections 4.1: General Service Information and 7: Warranty.
Page 24 / 44

5 Cleaning
Cleaning the device and its accessories is very important for compliance with hygienic
requirements and to avoid any cross-infection. Please always consider local regulations and
read this section carefully.
Before cleaning the device, the device must be switched off and removed from all connected
components (e.g. power supply unit).
Wipe the surface of the device with a cloth slightly dampened with mild detergent or
normal hospital bactericides or antiseptic solution. The following quantities of chemical
substances are allowed: ethanol: 70-80%, propanol: 70-80%, aldehyde: 2-4%. Do not immerse the
device and make sure that no liquid gets into the device. Dry the device with a lint-free cloth after
cleaning.
Disposable accessories (e.g. ear tips and other accessories marked for single use only on the package
label or data sheet) must be replaced between patients (or ears of the same patient) to avoid crossinfection.
The ear probe test cavity must be used with a disinfected and clean new probe tip. In case of
contamination with pathological material or suspected dirt inside the cavity, please discontinue the
use of the test cavity. For external cleaning, please use a sterile alcohol wipe, typically containing
70% isopropyl alcohol.
It is recommended that parts which are in direct contact with the patient (e.g. headphone cushions)
are subject to standard disinfecting procedures between patients. This includes physical cleaning and
use of recognized disinfectants. The use of hygiene protective covers is recommended for
headphones (if available for the used headphone model).
For further information about cleaning instructions for accessories (e.g. ear probe) please refer to the
respective manual or data sheet of the accessory.
When using a cleaning agent, please refer to the manufacturer's data sheet of the cleaning agent for
the minimum time period in which the wipe has to be in direct contact with the surface of the device
or accessory to ensure effectiveness of cleaning.
The device and its accessories are provided non-sterile and are not intended to be sterilized.
Page 25 / 44

Page 26 / 44

Type
Model examples
Applied part
Max. cable length*
Headphone
HP-[xx]: HDA-280, HDA-300, DD-45, PD-81, ME-70
yes
3.0 m (118’’)
Insert earphone
IP-[xx]: PIEP, IP-30
yes
2.0 m (79’’)
Ear coupler cable
PECC-[xx]
yes
2.0 m (79’’)
Related accessories: ear coupler
Bone conductor
BC-[xx]: B-71, B-81
yes
2.8 m (110’’)
Free-field loudspeaker
JBL Control 2P
no
---
Free-field loudspeaker cable
FFC
no
2.5 m (98’’)
Ear probe
EP-TE, EP-DP, EP-VIP, EP-TY
yes
1.8 m (71’’)
Tympanometry add-on
TY-MA
yes
1,8+0,9 m (71+35’’)
Related accessories:
- probe tips (adult and baby size)
- ear tips (multiple sizes and types)
- test cavity (corresponding to adult and baby size probe tip)
- calibration volume cavity for tympanometer (0.5, 2, 5 ml)
- inspection/cleaning tool
- fixation clip
Microphone (for live speech)
Mic-[xx]
no
0.95 m (37’’)
Electrode cable
Electrode cable
yes
1.8 m (71’’)
Electrode trunk cable
EC-03 (connected to electrode lead cable)
no
1.4 m (55’’)
Electrode lead cable
Multiple configurations (connected to electrode
trunk cable)
yes
0.5 m (20’’)
Related accessories:
- electrode testing device
- electrodes
Label printer
Seiko SLP 650 SE, Able AP1300
no
---
Label printer cable
LP-[xx]
no
1.6 m (63’’)
Related accessories: printout paper rolls
Patient response button
PB-[xx]
yes
1.95 m (77’’)
Sound insulation headphone
Peltor Optime III
no
---
Communication cable
USB
no
2.0 m (79’’)
Communication cable
RS-232
no
1.5 m (59’’)
Related accessories: RS232-to-USB converter
Trigger cable
TIC
no
2.4 m (94’’)
Modem (for pathTrack)
Cinterion EHS6
no
---
Modem cable:
MC-[xx]
no
1.5 m (59’’)
Transportation bag / case
---
no
---
PC software
Mira
no
---
Power supply unit
Sinpro MPU12C-104/MPU12A-104, Sinpro MPU16C104, Friwo FW7662M/12
no
3.2 m (126’’)
6 Accessories
Available accessories for Senti and Sentiero devices include:
* Maximum cable length rounded to next 5 cm step. The actual cable length may vary dependent on the model of the
accessory type. The given cable length is the maximum cable length across all models for the accessory type.
Page 27 / 44

The above list of accessories may be subject to change. Accessories may be available only upon
request, may be replaced by comparable equipment, or may be discontinued without prior notice.
Please contact your distributor for an up-to-date list of available accessories.
Please note that the same accessory may be available with different connectors and therefore
different article numbers for different devices (see section 3.4.3: Device Sockets). When asking your
distributor about accessories please always refer to your device (Senti, Sentiero, Sentiero Advanced,
Senti Desktop, Senti Desktop Flex, and Sentiero Desktop).
Page 28 / 44

7 Warranty
PATH MEDICAL warrants that the supplied device and its accessories are free from defects in material
and workmanship and, when properly used, will perform in accordance with applicable specifications
during the defined warranty period.
Please note that the warranty between the end user and the distributor cannot be managed by PATH
MEDICAL as it is not under PATH MEDICAL's responsibility. Nevertheless, PATH MEDICAL encourages
all regional distributors to provide at least the warranty stated by law or stated by the following
rules.
For the device a one year warranty period is provided. For the rechargeable battery pack, the touch
screen and wearing parts (e.g. ear probe) a six months warranty period is provided. The warranty
period starts at the date of shipment. In case longer warranty periods are defined by law, these
warranty periods take precedence.
This warranty is only valid for devices and accessories purchased from an authorized distributor. This
warranty is not valid in cases of breakage, malfunction due to manipulation or unintended usage,
negligence, non-observance of manufacturer’s instructions including cleaning instructions, crashes or
accidents, damages by external causes (e.g. flood, fire) or damages due to shipment (see also
disclaimer of warranty). This warranty is not valid for normal deterioration of wearing parts and
cosmetic damages (e.g. scratches). Opening the device case or any accessory housing voids this
warranty as well as modifications or changes in the device or accessory not approved in writing by
PATH MEDICAL.
This warranty includes material and labor costs and has to be in accordance with the manufacturer
specifications. PATH MEDICAL reserves the right to credit, repair or replace (with a new or
refurbished product) an “in-warranty” device or accessory at its sole option.
When suspecting a warranty case, please inform your distributor about the defect. Send the device
or accessory together with an error description to your distributor. Mailing expenses are not
refundable and are to be paid by the customer. Please send the device or accessory in its original
packaging to your distributor.
See also section 4.1: General Service Information.
Page 29 / 44

DISCLAIMER OF WARRANTY:
The warranty contained herein is exclusive. PATH MEDICAL disclaims all other warranties
expressed or implied, including, but not limited to, any implied warranty of
merchantability or fitness for a particular purpose or application. PATH MEDICAL shall not be liable
for any incidental, indirect, special or consequential damages whether resulting from the purchase,
use, misuse or inability to use of the device or accessory or relating in any way to the defect in or
failure of the device or accessory, including, but not limited to, claims based upon loss of use, lost
profits or revenue, environmental damage, increased expenses of operation, cost of replacement
goods. PATH MEDICAL's warranty and liability is directed to the distributor and limited to the
regulations in the respective distribution contract and German law. The end user shall address
warranty claims only to the authorized distributor from whom the device was purchased. PATH
MEDICAL reserves the right to refuse warranty claims against products or services that are obtained
and/or used in contravention of the laws of any country.
Page 30 / 44

Follow relevant regulations in your facility regarding maintenance and calibration of
audiometric equipment. This includes regular servicing of the device and calibration of
transducers. See section 4: Service and Maintenance.
Do not try to open or service the device and its components yourself. Return the device
to the authorized service partner for all service.
Do not operate the device if its power supply is connected to the device and shows a
damaged cord or plug. Likewise, this is true for any accessory with a separate power
supply (e.g. label printer).
The device is capable of producing high stimulus levels for diagnostic purposes. Always
make sure to use only stimulus levels, which will be acceptable for the patient. Do not
present high stimulus levels to a patient if it could cause a hearing damage.
Do not change a transducer during a test. This may result in wrong stimulus output and
potential wrong test results.
The patient is allowed to operate the device during self-controlled tests (e.g. MAGIC)
according to instructions from qualified personnel. Do not allow children, handicapped
persons (e.g. mentally handicapped subjects) or other persons who may need assistance
to operate the device without adequate supervision. Supervision by qualified personnel is
recommended for all subjects at all times.
Senti Desktop: The transducers supplied with the device are calibrated to a specific
device. In order to ensure proper stimulus calibration and output, always check that the
connected transducer matches the transducer specified in the system information screen
on the device. Failure to do so may result in a mismatch of the stimulus level displayed on
the device compared to the actual stimulus level delivered to the patient. This may result
in over or under-estimation of hearing. It can also result in higher than expected stimulus
levels being delivered to the patient which may damage hearing. This does not apply to
the flexibly exchangeable transducers for all other Senti and Sentiero devices.
The device needs to be operated in a quiet environment, so that measurements are not
influenced by ambient noises. This may be determined by an appropriately skilled person
trained in acoustics. DIN EN ISO 8253-1 section 11 defines maximum ambient noise levels
for audiometric hearing testing. If not followed, measurement data may not reliably
represent the actual hearing status. See also section 3.5.8: Test Module Information.
For AEP measurements the device needs to be operated in an environment with low
electromagnetic disturbance. It is recommended to perform AEP tests in a shielded cabin.
If not followed, measurement data may be deteriorated by electrical noise.
8 Notes on Safety
In order to allow safe performance of Senti and Sentiero (handheld and desktop) please
read the following notes on safety carefully and follow the provided instructions. If not
followed, risks of danger to persons and/or the device may be the consequence. Retain this manual
for later use and make sure to hand over this manual to any person who uses this device. Applicable
local government rules and regulations must be followed at all times.
8.1 General Usage
Page 31 / 44

For calibrated transducers differences in environmental conditions between the point of
calibration and the point of use may influence the calibration accuracy. For more
information please refer to section 8.2: Handling, Transport, and Storage.
There are no device parts, which can be serviced during use with a patient. There are no
device parts, which can be serviced by the patient when the patient is an intended
operator (e.g. MAGIC). See also section 4: Service and Maintenance.
Do not drop or otherwise cause undue impact to the device or any accessory. If any
damage is suspected (e.g. loose parts inside device), do not use the device or accessory
anymore and return it to your local service partner for repair and/or calibration.
Do not modify the device and its components in any way without written consent of the
manufacturer. Failure to do so may result in a reduced level of safety of the system
and/or degradation of functionality.
Do not transport, store or operate the device at environmental conditions exceeding
those stated in section 9: Technical Specifications. If the device is moved from a cold
location to a warmer one, there will be a risk of condensation. If condensation occurs, the
device must be allowed to achieve normal temperature before it is switched on.
Make sure that any platform, table, cart, or other surface used during the operation,
transport, or temporary or permanent storage of the device and its components is
adequate, sturdy, and safe. PATH MEDICAL is not responsible for any injury or damage
that may result from inadequate, poorly constructed, or unapproved transports, carts, or
operating surfaces.
Do not allow any fluid to infiltrate the device. Do not immerse the device in fluids as e.g.
cleaning agents.
Dust particles may corrupt the touch pad. Please make sure to keep the touch pad clear
of dust particles.
Do not put excessive pressure on the device display or allow any item to puncture the
device display.
Do not place the device next to a radiator or any other heat source.
The power supply is specified as a part of the device. Do not use any power supply other
than the ones defined in section 9: Technical Specifications. Other power supplies made
for other electronic devices such as notebook computers or printers may cause damage
to the device. Likewise, using the Senti/Sentiero power supply on other types of devices
may cause damage to those devices.
Avoid accidental contact between connected but unused applied parts and other
conductive parts including those connected to protective earth. Conductive parts of
electrodes and their connectors including the neutral electrode are not allowed to
contact other conductive parts and earth.
8.2 Handling, Transport, and Storage
8.3 Electrical Safety
Page 32 / 44

Do not use the device during the application of high-frequency surgical devices, cardiac
pacemakers, defibrillators or other electrical stimulators. This may result in burns at the
site of electrodes and possible damage to the applied parts.
Do not use the device in close proximity to shortwave or microwave therapy equipment
as it may produce instability in the applied parts.
If the device is used during surgery, the connectors must not touch conductive items
including ground.
When using the power supply unit Sinpro MPU16C-104 (protection class I), in order to
avoid risk of electrical shock, the power supply unit must only be connected to a supply
mains with protective earth.
Do not connect the label printer, RS232, or modem cable to the device during testing.
If a connection is established from the device to a standard PC which is powered through
the mains network, special precautions must be taken in order to maintain medical
safety. A standard USB cable can only be used if the connected PC is outside the patient’s
close range or if the PC is running on battery, is medically approved, or is powered via a
medically approved safety transformer. In all other cases, a galvanic separator must be
inserted in the USB connection.
The use of Senti/Sentiero devices next to other electronic equipment or with other
electronic equipment in a stacked form should be avoided, as this could result in
improper operation (Senti/Sentiero: e.g. occurrence of unwanted noise). Electronic
equipment may include e.g. mobile phones, pagers, walkie-talkies, or RFID systems. If
such an application cannot be avoided, Senti/Sentiero and the other electronic devices
should be observed to make sure they are working properly. It may be necessary to
implement suitable corrective measures (e.g. new orientation or positioning of
Senti/Sentiero or shielding). Please also refer to section 10: Electromagnetic Compatibility
Information.
Portable radio frequency communications equipment (radio equipment) including their
accessories such as antenna cables and external antennas should not be used closer than
30 cm (12’’) to Senti/Sentiero and its accessories.
During testing it is recommended to keep low-power radio equipment (≤ 2 W) at a
distance of at least 3 m (118’’) from Senti/Sentiero and its accessories.
It is recommended to keep very strong sources of radio frequency emissions (e.g. highpower transmitting antennas from radio or TV stations) at a distance of at least 2 km
(6560 ft.) from Senti/Sentiero (minimum required distance depends on signal power and
directional characteristics of the sender).
Failure to do so may result in a reduction of device performance.
Use of other accessories than the ones specified or provided by PATH MEDICAL may
result in higher electromagnetic emission or reduced immunity to interference of the
device and may result in improper device operation.
8.4 Electromagnetic Compatibility
Page 33 / 44

The probe tip of the ear probe must not be inserted into an ear without a disposable ear
tip properly affixed to the probe tip. Make sure that the ear tip size corresponds to the
patient’s ear canal size.
Ear probes or insert earphones must not be used in cases of external otitis (outer ear
canal infection) or in any case which yields to pain for the patient when inserting the ear
probe or insert earphone.
Disposable accessories (e.g. ear tips and other accessories marked for single use only on
the package label or data sheet) must be replaced between patients (or ears of the same
patient) to avoid cross-infection. Do not clean or reuse these items.
Do not connect any accessories other than those provided by PATH MEDICAL. Other
accessories are not compatible with the device and may result in device damage or
improper functionality of the device. If connecting accessories which do not comply with
the same safety requirements as this product, this may lead to a reduction in the overall
system safety level.
Cleaning the device and its accessories is very important for compliance with hygienic
requirements and to avoid any cross-infection. For further information please refer to
section 5: Cleaning.
Always handle cables and transducers with care. Do not excessively bend or twist any
cable. The cable may break and hence deteriorate overall device functionality or reduce
the overall system safety level. Do not drop, throw or hit any transducer on a hard object.
Sensitive parts (e.g. ear probe microphone and loudspeakers) may get damaged and
deteriorate measurement performance. Do not use a cable or transducer if any damage is
suspected.
Keep small parts (e.g. ear tips) out of patient’s range (especially children) in order to
prevent accidental swallowing.
No parts may be eaten, burnt, or in any other way used for purposes other than
audiometry.
Inspect the transducer channels of the insert earphone and/or ear probe (including probe
tip and ear tip) before use. A blocked loudspeaker channel may yield lower stimulus
levels or prevent successful calibration. A blocked microphone channel may yield lower
response levels or prevent successful calibration. If in doubt conduct a probe test (see
section 3.5.4: Hardware Tests).
The sockets are intended to connect to the respective accessories (e.g. transducer,
electrode cable, power supply unit, label printer). Do not connect any other item to these
sockets. For correct connections see section 3.4.3: Device Sockets.
Do not try to insert any plug into a device socket with excessive force. A plug fits only into
a device socket if the mechanical coding of the plug is corresponding to the device socket.
Color-codes help finding the correct device socket. For desktop devices, please also check
the icons on the back panel of the device for correct insertion. See section 3.4.3: Device
Sockets.
When pulling a plug out of a socket always pull at the plug and not at the cable to avoid
cable break.
Do not expose the label printout to sunlight or heat. Printing on thermal paper fades with
exposure to light or heat.
8.5 Accessories
Page 34 / 44

The device includes a NiMH (handheld) or Li-Ion (desktop) rechargeable battery pack. In
case the battery pack cannot be charged anymore or in case of any other suspected
defect of the battery pack, the battery pack must be replaced by an authorized service
partner. The service partner is responsible for the correct disposal and storage of the
battery pack. Do not dispose of the batteries in your normal household waste bin. Please
follow your local regulations for proper disposal.
Within the European Union, the device must not be disposed of in your normal
household waste bin since electronic waste may contain hazardous substances. The
device is electronic equipment covered by the Directive 2012/19/EC on waste electrical
and electronic equipment (WEEE). Please follow your local regulations for proper disposal
of the device and its accessories.
8.6 Waste Disposal
Page 35 / 44

Page 36 / 44

Device classification (93/42/EEC)
Device classification (MDR Canada)
Class II a
Class II
Application part classification
Application parts
Type BF (body floating)
Headphones, insert earphones, ear probe, tympanometry add-on, ear
coupler cable, bone conductor, electrode cable, patient response button
Ingress protection rating (IP code)
Handheld: IP30
Desktop: IP40
Applied standards
DIN EN ISO 389-1, DIN EN ISO 389-2, DIN EN ISO 389-3, DIN
EN ISO 389-4, DIN EN ISO 389-5, DIN EN ISO 389-8
(transducer calibration), DIN EN ISO 10993-1 (biocompatibility),
DIN EN ISO 15223-1 (manual), DIN EN 60601-1 (electrical
safety), DIN EN 60601-1-2 (EMC), DIN EN 60601-1-4 (PEMS),
DIN EN 60601-1-6 (usability), DIN EN 60601-2-40 (AEP
equipment), DIN EN 60645-1 (pure-tone audiometry), DIN EN
60645-5 (tympanometry), DIN EN 60645-6 (OAE), DIN EN
60645-7 (ABR), DIN EN 62304 (software lifecycle)
Device dimension
Handheld: ca. 209 x 98 x 52 mm (8.22 x 3.86 x 2.05’’)
Desktop: ca. 150 x 210 x 45 mm (5.91 x 8.27 x 1.77’’)
Device weight (including battery pack)
Handheld: ca. 500 g
Desktop: ca. 475 g
Display properties
240 x 320 pixel, graphic LCD
Handheld: 3.5‘‘, Desktop: 5.0‘‘
Maximum power consumption from
battery
Handheld: ca. 5 V, 0.4 A = 2 W
Desktop: ca. 4 V, 0.5 A = 2 W
Typical power consumption from
power supply unit during charging
Handheld: ca. 9 V, 1.0 A = 9 W
Desktop: ca. 12 V, 0.17 A = 2 W
9 Technical Specifications
This section provides a summary of the most important technical specifications. Further
technical details are described in a separate Technical Specification document, which can
be downloaded from www.pathme.de/support/.
9.1 General Device Information
9.2 Device Characteristics
Page 37 / 44

Input rating of power supply units
Sinpro MPU12C-104: 100-240 V, AC, 47-63 Hz, 0.16-0.29 A
Sinpro MPU12A-104: 100-240 V, AC, 47-63 Hz, 0.16-0.29 A
Sinpro MPU16C-104: 100-240 V, AC, 47-63 Hz, 0.18-0.33 A
Friwo FW7662M/12: 100-240 V, AC, 50-60 Hz, 0.15 A
Output rating of power supply units
Handheld: 9V, ≥1.2 A
Desktop: 9-12 V, ≥0.4 A
Rechargeable battery pack
Handheld: 4.8 V (NiMH)
Desktop: 3.7 V (Li-Ion)
Maximum operating time with fully
charged batteries
ca. 6-8 hours (dependent on usage)
Maximum charging cycles
500-1000 (life time > 2 years for normal usage)
Maximum charging time:
Handheld: ca. 2 hours
Desktop: ca. 8 hours
9.3 Power Supply
For medical applications the following power supply units are exclusively allowed when used with
Senti and Sentiero devices:
- Sinpro MPU12C-104, MPU12A-104
- Sinpro MPU16C-104
- Friwo FW7662M/12 (GPP6) – for desktop devices only
For Senti and Sentiero do not use any power supply unit other than the ones mentioned
above. Failure to do so may reduce electrical safety and may damage the device.
When using the power supply unit Sinpro MPU16C-104 (protection class I), in order to avoid
risk of electrical shock, the power supply unit must only be connected to a supply mains
with protective earth.
9.4 Storage, Transport, and Operating Conditions
For storage and transport, please keep the device and its accessories in the provided
carrying case or a similar closable container in order to protect all components against
external forces and environmental impacts as e.g. mechanical stress (scratches), dust or moisture.
Extreme storage and operating conditions may result e.g. in breakage of the touch screen display (at
extremely low temperatures) or in impairment of the device and/or transducer calibration.
If the device is moved from a cold location to a warmer one, there will be a risk of
condensation. In this case, the device must be allowed to achieve normal room
temperature before it is switched on. Also make sure that the below operating conditions are
fulfilled.
Page 38 / 44

Transport temperature
-20 to 60 °C (-4 to 140 °F)
Storage temperature
0 to 40 °C (32 to 104 °F)
Relative air humidity
10 to 90 % non-condensing
Barometric pressure
50 to 106 kPa
Temperature
10 to 40 °C (50 to 104 °F)
Relative air humidity
20 to 90 % non-condensing
Barometric pressure
70* to 106 kPa
Air pressure at point of calibration pc
Air pressure at point of use pu
98 to 104 kPa
< 92 kPa
92 to 98 kPa
< pc – 6 kPa
<92 kPa
< pc – 6 kPa or > pc + 6 kPa
TRANSPORT AND STORAGE CONDITIONS:
OPERATING CONDITIONS:
* In the following cases a transducer recalibration at the point of use is recommended:
See also DIN EN 60645-1 5.3 and Soares et al.: “Audiometer: Correction factor for atmospheric pressure”, InterNoise 2016.
Page 39 / 44

Page 40 / 44

Emitted interference
measurement
Compliance
Electromagnetic environment
High-frequency emission according
to CISPR11
Group 1
The medical electric device uses high-frequency (HF)
energy only for internal operation. Hence, its HF
emissions are very low and it is unlikely that adjacent
electronic devices are disturbed.
Class B
The medical electric device may be used in all
establishments, including those in residential
environments and those that are directly connected to
a public power network that also supplies buildings
used for residential purposes.
Emission of harmonic components
according to IEC 61000-3-2
Class A
---
Emission of voltage fluctuation /
flicker according to IEC 61000-3-3
Compliant
---
Tests for immunity
to interference
IEC 60601 test level
Concurrent level
Electromagnetic environment
Electrostatic discharge
(ESD) according to IEC
61000-4-2
± 8 kV contact
discharge
± 15 kV air discharge
± 8 kV contact
discharge
± 15 kV air discharge
To reduce ESD effects, the ground
floor shall consist of wood,
concrete or ceramic tiles.
Fast transient electric
disturbance; bursts
according to IEC
61000-4-4
± 2 kV for power lines
± 1 kV for input and
output lines
± 2 kV for power lines
± 1 kV for input and
output lines
The quality of supply voltage shall
correspond to typical hospital or
commercial environment.
Impulse voltage,
surges according to
IEC 61000-4-5
± 1 kV voltage
outer conductor –
outer conductor
± 1 kV voltage
outer conductor –
outer conductor
The quality of supply voltage shall
correspond to typical hospital or
commercial environment.
10 Electromagnetic Compatibility Information
Electromagnetic compatibility (EMC) as stated by standard DIN EN 60601-1-2 (Medical electrical
equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard:
Electromagnetic compatibility - Requirements and tests) and 60601-2-40 (Medical electrical equipment - Part 2-40:
Particular requirements for the safety of electromyographs and evoked response equipment) was certified by an
accredited laboratory. Requirements from DIN EN 60601-1-2:2016-05 (see tables below, see also
section 8.4: Electromagnetic Compatibility) are applicable to all devices delivered from 01/2019
(Handheld: PCB Rev. ≥ 70 with connector board, Desktop: PCB Rev. ≥ 333; for previous PCB versions
please refer to the previous manual version or contact PATH MEDICAL). Information on the full
report is available from PATH MEDICAL upon request.
The user must take care that the device is used in an environment with electromagnetic
radiation as specified in Table 5 and in Table 6.
Table 5: Compliance with electromagnetic emission guidelines and resulting requirements for
electromagnetic environment
Page 41 / 44

Tests for immunity
to interference
IEC 60601 test level
Concurrent level
Electromagnetic environment
±2 kV voltage outer
conductor – earth
±2 kV voltage outer
conductor – earth (for
Sinpro MPU16C)
Voltage drop, short
interruption and
fluctuation of supply
voltage according to
IEC 61000-4-11
0 % UT (>95 % UT drop)
for ½ and 1 period
0 % UT for 300 periods
70 % UT (30 % UT drop)
for 30 periods
0 % UT (>95 % UT drop)
for ½ and 1 period
0 % UT for 300 periods
70 % UT (30 % UT drop)
for 30 periods
The quality of supply voltage shall
correspond to typical hospital or
commercial environment.
If the user of the medical electric
device also demands continued
proper functioning of the device
during an interruption of energy
supply, the connection of the
device to an uninterrupted power
supply (UPS) or battery is
recommended.
Magnetic field at
mains frequency
(50/60 Hz) according
to IEC 6000-4-8
30 A/m
30 A/m
Magnetic fields at the mains
frequency shall correspond to
typical hospital or commercial
environment.
Note: UT is the mains AC voltage before applying the test level.
Tests for immunity
to interference
IEC 60601 test level
Concurrent level
Electromagnetic environment
Conducted highfrequency disturbance
according to IEC
61000-4-6
3 V
(150 kHz – 80 MHz)
6 V
(ISM frequencies)
3 V
6 V
Portable and mobile radio units
shall not be used closer than 30
cm (12‘’) to the device and its
components (i.e. connected
cables).
Radiated highfrequency disturbance
according to IEC
61000-4-3
3 V/m
(80 MHz – 2.7 GHz)
9-28 V/m*
(wireless RF
communication)
3 V/m
9-28 V/m*
Portable and mobile radio units
shall not be used closer than 30
cm (12‘’) to the device and its
components (i.e. connected
cables).
* Wireless RF communication frequencies and levels:
28 V/m: 450 MHz, ±5 kHz FM, 1 kHz sine; 810 MHz, 50% PM at 18 Hz; 870 MHz, 50% PM at 18 Hz; 930 MHz,
50% PM at 18 Hz; 1720 MHz, 50% PM at 217 Hz; 1845 MHz, 50% PM at 217 Hz; 1970 MHz, 50% PM at 217 Hz;
2450 MHz, 50% PM at 217 Hz;
27 V/m: 385 MHz, 50% PM at 18 Hz;
9 V/m: 710 MHz, 50% PM at 217 Hz; 745 MHz, 50% PM at 217 Hz; 780 MHz, 50% PM at 217 Hz; 5240 MHz,
50% PM at 217 Hz; 5500 MHz, 50% PM at 217 Hz; 5785 MHz, 50% PM at 217 Hz;
Table 6: Compliance with immunity to interference tests and resulting requirements for
electromagnetic environment
The user must take care, that the device is used in an environment with minimum distances
to potential radiators as described in Table 7.
Table 7: Minimum distance to potential radiators
Page 42 / 44

The device is intended for use in an environment in which high-frequency disturbances are
controlled.
Page 43 / 44

Contact information from distributor/service partner:
PATH MEDICAL GmbH
Landsberger Straße 65
82110 Germering
Germany
Tel.: +49 89 800 765 02 Fax: +49 89 800 765 03 Internet: www.pathme.de
 Loading...
Loading...