Page 1

Instruction Manual
Manual No. 012-07688E
PASPort Dissolved Oxygen
Sensor
Model No.
PS-2108
Page 2

Instruction Manual
Manual No. 012-07688E
PASPort Dissolved Oxygen
Sensor
Model No.
PS-2108
Page 3

®
Model No. PS-2108 Dissolved Oxygen Sensor
Table of Contents
Equipment List .......................................................... 1
Introduction ............................................................. 1
DO Sensor Setup and Calibration...................................... 3
Prepare the Sensor for First Use....................................................................................................3
Set up the Dissolved Oxygen Sensor with DataStudio..................................................................3
Equilibrate the Probe in 100% Humidified Air.............................................................................4
Perform a Single-point Calibration (mg/l Dissolved O2)..............................................................4
Setup to Calibrate in Percent (%) Saturation.................................................................................4
General Sampling Procedure ............................................ 6
Measurements in a Controlled Laboratory Setting........................................................................ 6
Using the Dissolved Oxygen Sensor with other PASCO Sensors:................................................6
Maintenance ............................................................. 7
Changing the Electrolyte solution..................................................................................................7
Replacing the Membrane............................................................ .................................. ...... ...........7
Replacing the O-ring........................................................ ..............................................................8
Storage .................................................................. 9
Short-Term Storage (two weeks or less)........................................................................................9
Long-Term Storage (more than two weeks)..................................................................................9
Troubleshooting ......................................................... 9
Theory of Dissolved Oxygen: ......................................... 10
What is the chemical mechanism by which diatomic oxygen dissolves in water?......................10
Citations:......................................................................................................................................14
Experiment 1:
Introduction to the Operation of the Dissolved O2 Sensor ........ 15
Purpose.........................................................................................................................................15
Materials and Equipment Needed................................................................................................15
Optional Equipment Suggested ...................................................................................................15
Procedure .....................................................................................................................................15
Data Analysis/Questions..............................................................................................................16
Experiment 2:
Photosynthesis and Oxygen Generation with Aquatic Plants ........ 17
Purpose.........................................................................................................................................17
Materials and Equipment Needed................................................................................................17
Procedure .....................................................................................................................................18
During the lab time......................................................................................................................18
Collecting Data ............................................................................................................................19
Sample Data.................................................... ...... .......................................................................19
Analysis .......................................................................................................................................19
i
Page 4

®
Model No. PS-2108 Dissolved Oxygen Sensor
Experiment 3:
Effect of Sodium Sulfite on Dissolved O2 Concentrations ......... 20
Materials and Equipment Needed................................................................................................20
Optional Materials .......................................................................................................................20
Purpose.........................................................................................................................................20
Procedure .....................................................................................................................................20
Questions .....................................................................................................................................21
Experiment 4: Biochemical Oxygen Demand * ....................... 22
Background..................................................................................................................................22
Procedure .....................................................................................................................................22
Experiment 5:
The Effect Of Respiration On Dissolved O2 Concentration ........ 23
Purpose.........................................................................................................................................23
Materials required........................................................................................................................23
Optional materials ........................................................................................................................23
Procedure .....................................................................................................................................23
Questions .....................................................................................................................................24
Appendix A: Tables.................................................... 27
Copyright Notice....................................... ...... ...... .................................. ..... ...... ..........................25
Limited Warranty....................................................... ..................................................................25
Equipment Return........................................................................................................................25
Appendix B: Tables .................................................... 27
Specifications ...............................................................................................................................26
Replacement Parts..................................... ...... .............................................................................26
Technical Support........................................................................................................................26
Appendix C: Tables .................................................... 27
Table 1: Concentration of dissolved oxygen (mg/L) in water at
various temperatures and pressures .............................................................................................27
Table 2: Salinity correction factors for dissolved oxygen in water (based on conductivity)......32
ii
Page 5
Model No. PS-2108 Dissolved Oxygen Sensor
®
PASPort Dissolved Oxygen
Sensor
Model No. PS-2108
Equipment List
Included Equipment Replacement
Model Number*
1. Dissolved Oxygen Probe (1)
2. PASPORT Dissolved Oxygen Sensor box (1)
3. Membrane Replacement Kit (1)
4. Soaker Bottle (1)
5. Electrolyte Solution (1)
6. Syringe for filling cartridge housing (1)
699-06320
CI-6541
R001068
*Use Replacement Model Numbers to expedite replacement orders.
Introduction
The PASCO PS-2108 Dissolved Oxygen Sensor can be used to monitor and explore factors
that affect the concentrations of dissolved oxygen molecules (O2) in aqueous solutions,
particularly in applications related to ecological studies of water environments. The
Dissolved Oxygen Sensor is specifically designed for use with a PASPort interface or logger,
®
plus DataStudio
of temperature, water movement, inorganic chemicals, organic matter, and living organisms
on levels of dissolved O
ecological surveys of aqueous habitats, including BOD (Biological Oxygen Demand) studies.
The Dissolved Oxygen Sensor is designed for use in aqueous media at temperatures ranging
from 10 °C to 40 °C. For greatest accuracy, the following requirements should be met:
although the unit is temperature compensated, it should be calibrated at approximately the
same temperature as the test solution; the sensor must equilibrate for a short period when the
temperature of the test solution changes-the greater the temperature change, the longer the
period of equilibration required; the test solution should constantly flow past the membrane
of the probe.
data acquisition software. In the laboratory, students can explore the effects
. They can also monitor dissolved O2 levels in the field as a part of
2
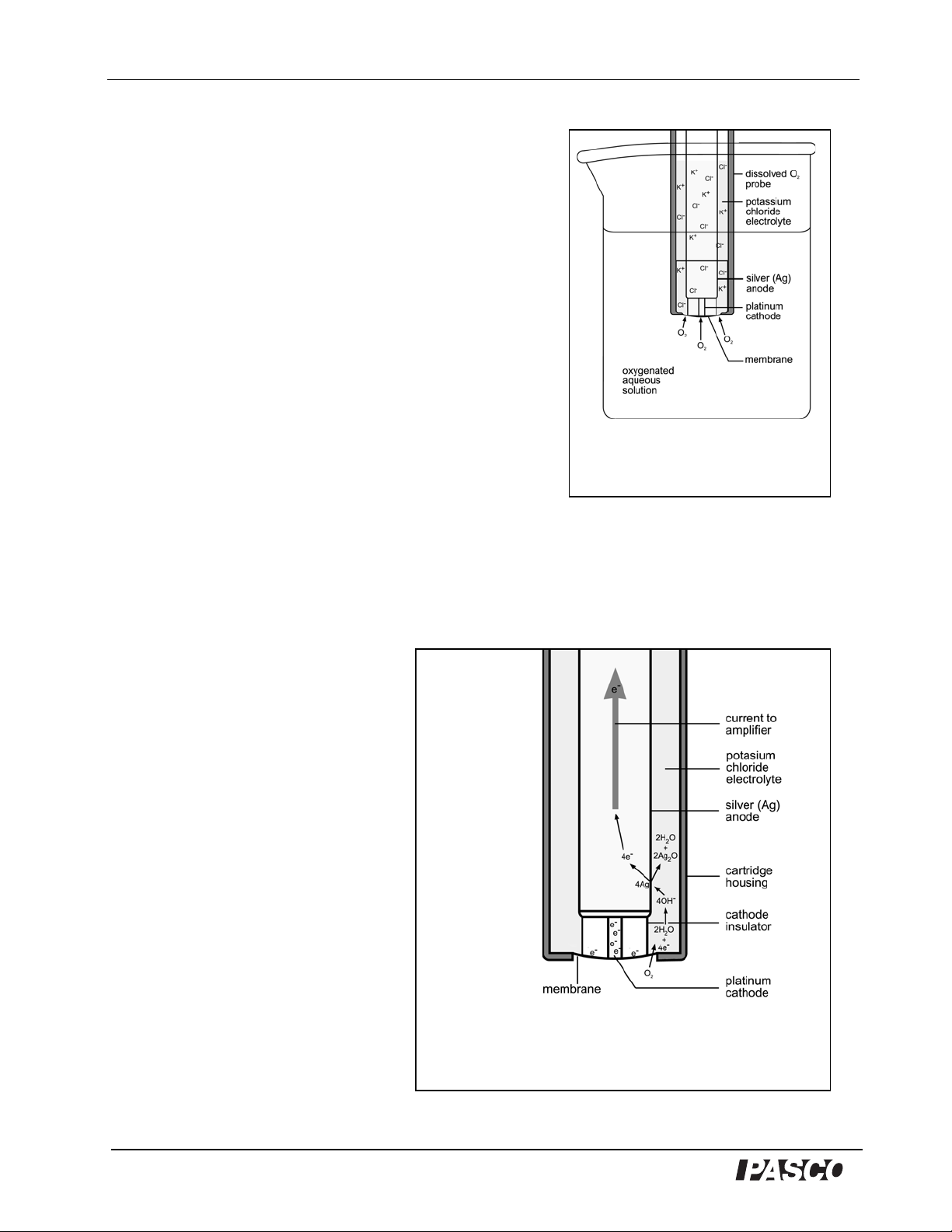
The Dissolved Oxygen Sensor has a polarographic probe composed of a platinum cathode
and a silver (Ag) anode surrounded by a potassium chloride (KCl
*PASPORT sensors require a PASPORT computer interface.
) electrolyte solution.
(aq)
1
Page 6
PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Figure 1
Oxygen molecules pass through the
semipermeable membrane into the
electrolyte surrounding the electrodes
Figure 2
O
2
molecules diffuse across the membrane and react with water
molecules in the presence of electrons from the cathode to form
hydrox ide ions (OH
-
). Hydroxide ions diffuse to the anode and react with
silver (Ag) atoms, forming silver oxide (Ag
2
O), water, and free electrons.
O22H2O4e-4OH
-
Reduction potential = 0.40V
++
The sensor functions by measuring the electric current
produced by a chemical reaction in the probe. The
chemical reaction involves the reduction of oxygen (O2)
molecules and the oxidation of the silver (Ag) atoms of
the anode. A voltage of 0.7 volts is applied across the
electrodes, causing the desired redox reaction (see
below) to be favored. When the dissolved O2 probe is
placed in an aqueous medium, such as deionized water
that contains dissolved O2, the dissolved O2 molecules
diffuse across a thin silicon membrane into the
electrolyte that surrounds the electrodes of the probe
(Figure 1). The membrane is semipermeable, allowing
the dissolved O2 to pass through it, but preventing
passage by most other molecules that might interfere
with the chemical reactions at the elect rodes. The
chemical reactions produce electrons that cause electric
current to flow through the sensor's electric circuit. Since
the rate of diffusion is dependent on the concentration of the dissolved O2, the number of
diffused O2 molecules will vary approximately in direct proportion to the concentration of
dissolved O2 in the test solution. Accordingly, the number of electrons produced by the redox
chemical reactions of the dissolved O2 will be almost directly proportional to the
concentration of dissolved O2 in the test solution.
The following is an overview of the
chemical and electrical processes at
each of the electrodes that are
involved in measuring dissolved O2
with the Dissolved Oxygen Sensor .
As soon as the dissolved O2
molecules pass through the silicon
membrane into the electrolyte
solution, they come into close
proximity to the platinum cathode
(Figure 2). The negative charge
(excess electrons) of the cathode
induces the reduction of the
dissolved O2, forming hydroxide
-
ions (OH
):
1
2
Page 7
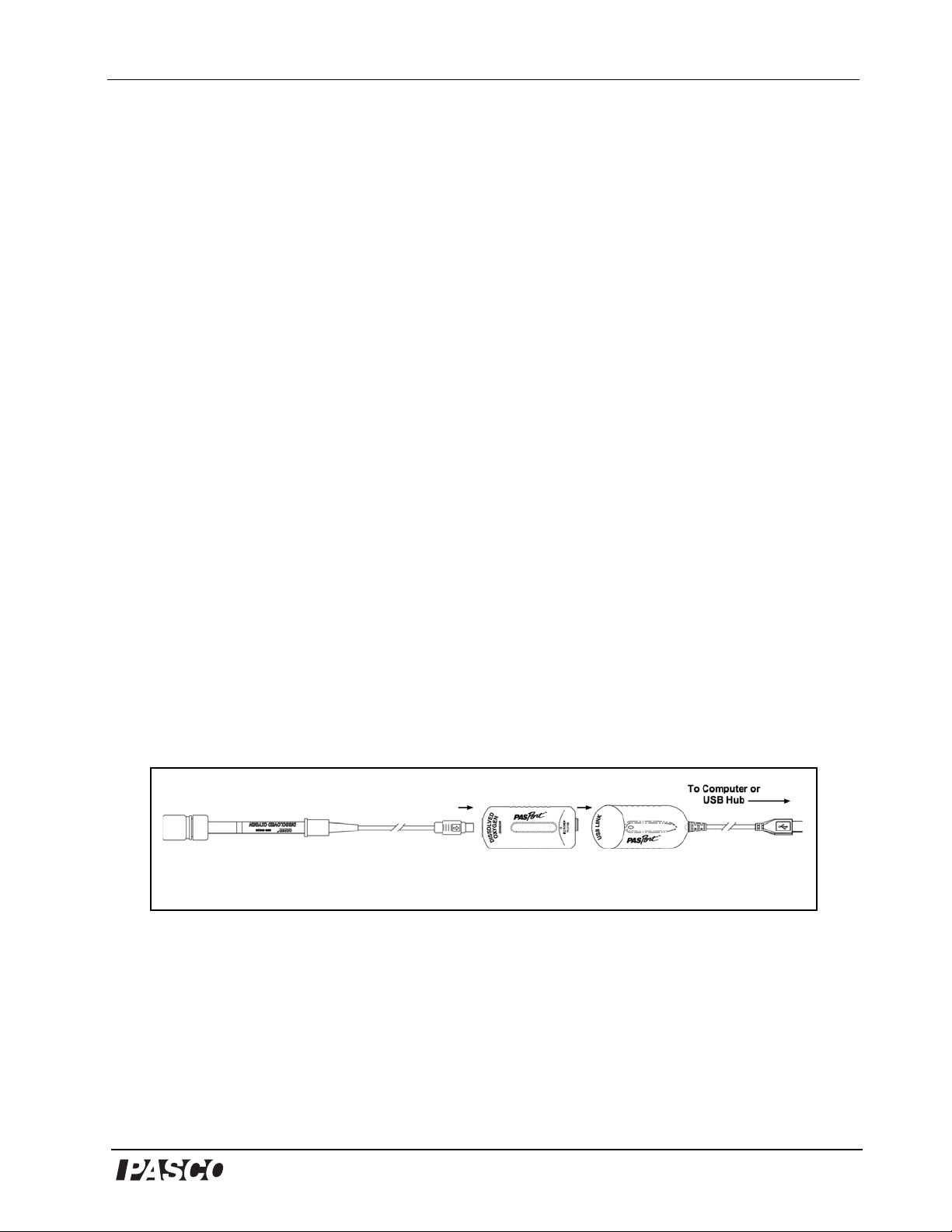
Model No. PS-2108 Dissolved Oxygen Sensor
®
4Ag 4OH
-
2Ag2O2H2O4e
-
Reduction potential = 0.343V
+++
Figure 3
Plug the DO
2
probe into the DO2 sensor box and connect the sensor box to a PASPort USB interface that is
connected to a computer’s USB port or to a USB hub that is connected to the computer.
The negatively charged hydroxide ions diffuse to the silver anode. There they combine with
silver (Ag) atoms from the silver anode, forming silver oxide and releasing electrons that join
the current in the electrode in the following chemical reaction:
The released electrons produce a current that passes from the electrode and is amplified. The
current due to the chemical reactions of the O2 molecules must be corre cted for temperature
variations, since the rate of reaction varies directly with the temperature.
The correction is accomplished through the use of a temperature-sensing thermistor that is
built into the probe. With the temperature sensing thermistor, the temperature of the probe is
monitored, and the gain of the amplifier is automatically adjusted to compensate for the
temperature dependence of the chemical reactions in the probe. A signal representing the
temperature-compensated dissolved O2 concentration of the solution is fed to the computer
interface and displayed by DataStudio in concentration (mg/l) or saturation (%).
DO Sensor Setup and Calibration
Prepare the Sensor for First Use
Prior to the first use of the sensor, you will need to fill the Dissolved Oxygen Sensor electrode
membrane cartridge and cartridge housing with an electrolyte solution. See the maintenance
section for instructions.
Set up the Dissolved Oxygen Sensor with DataStudio.
1. Attach the probe to the sensor box (Figure 3).
2. Plug the sensor box into a PASPort interface or logger connected to a computer.
3. The PASPortal window should open, allowing a choice between DataStudio or EZScreen.
Select DataStudio.
4. The Digits display opens automatically. Open additional or alternate displays as desired.
1
Find a more detailed discussion in Micha el L. Hit ch m an, M e asurement of Dissolved Oxygen, John Wiley and Sons,
New York, 1978, pp. 59-123.
3
Page 8
PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Water level
Shake
vigorously
Soaker
Bottle
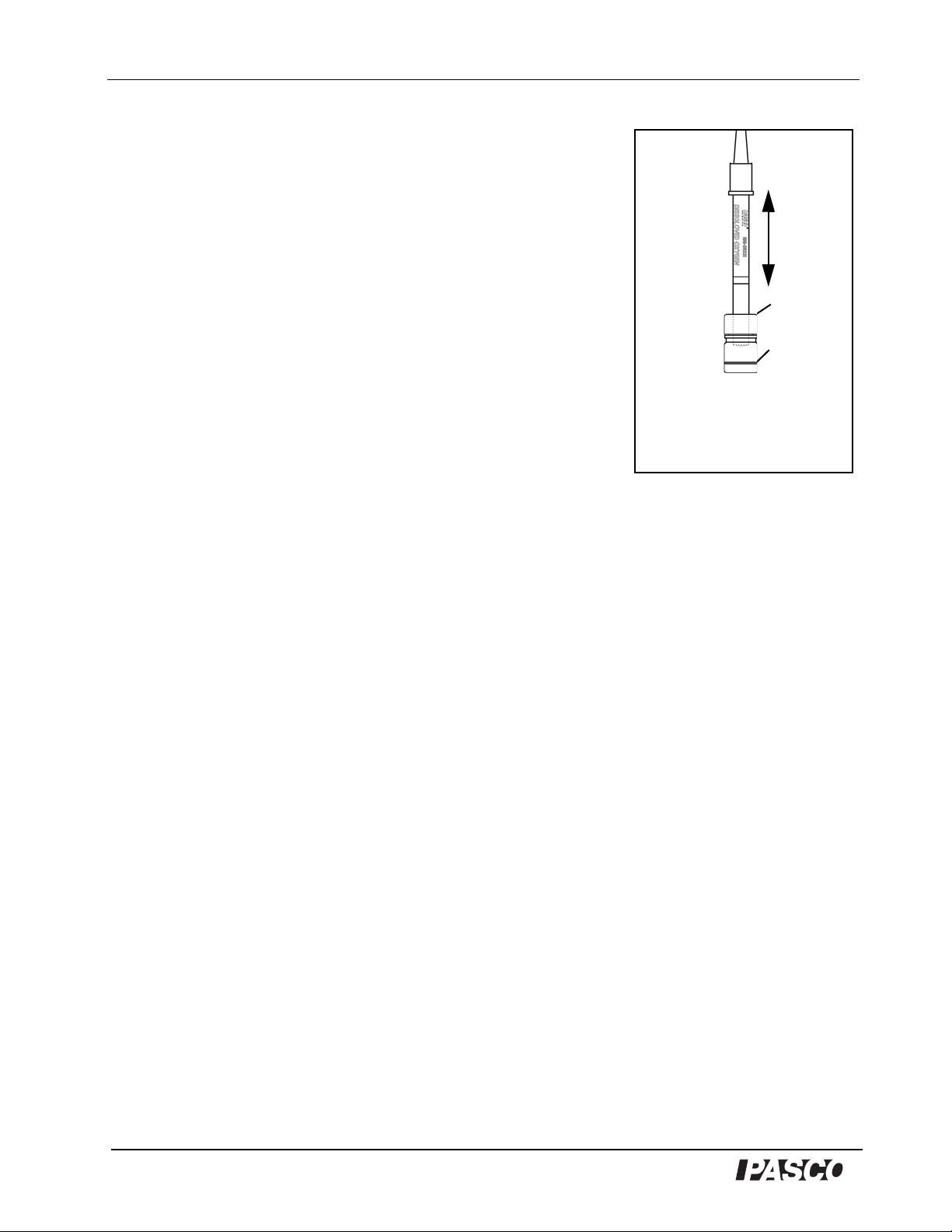
Figure 4
To equilibrate the probe, insert the
end of the probe into a clean soaker
bottle containing ~5 mL of deionized
water. Shake the probe vigorously for
about 10 Sec.
Equilibrate the Probe in 100% Humidified Air
1. Place about 5 mL (to a height of about 1 cm) of deionized
water into a clean soaker bottle. Slip the cap and O-ring of the
soaker bottle over the end of the probe.
2. Insert the probe into the soaker bottle and screw on the lid.
Adjust the height of the end of the probe to about 2 cm above
the top of the water (Figure 4).
3. Shake the soaker bottle vigorously for about 10 seconds.
Shake off any large water drops from the membrane.
Perform a Single-point Calibration (mg/l Dissolved O2)
1. Obtain current barometric pressure and temperature readings
for your location. You can use the PAPSPORT Barometer (PS-
2113) and Temperature Sensor (PS-2131), or the PASPORT Weathe r Sensor (PS-2154) to
take the necessary measurements.
2. Refer to the Solubility table 1 in Appendix C and find the appropriate dissolved oxygen
value for the temperature and barometric pressure at your location.
Example: At a temperature of 25ºC and a barometric pressure of 760 MM. Hg, 8.2 mg of oxygen will
dissolve in one liter of water at 10 0% saturation.
3. In DataStudio, click the Setup button on the toolbar. Make sure mg/L is selected next to
the Calibrate… button in the Dissolved Oxygen Sensor dialog.
4. With the DO2 sensor equilibrated as described above, click the Calibrate… button.
5. Refer to the tables for the value of mg/l dissolved O
in saturated water at the temperature
2
and uncorrected barometric pressure at which you are measuring. (If you do not know the
barometric pressure, assume that it is 760 mm Hg.) Enter the value in the mg/l box. Click
OK.
Setup to Calibrate in Percent (%) Saturation
1. (DataStudio only) Click the Setup button on the DataStudio toolbar. Click the mg/L button
next to the Calibrate… button and select % from the drop-down menu
Note: Always calibrate the probe before measuring absolute (rather than relative) concentratio ns. Calibrate at or
close to the temperature and barometric pressure at which the test solution is to be measured.-
Note: Percent (%) saturation output is valid only at the temperature and barometric pressure at which the probe is
calibrated.
Note: Before taking measurements of dissolved oxygen (DO) concentration (rather than % saturation), change
4
Page 9

Model No. PS-2108 Dissolved Oxygen Sensor
®
Cg=k Pg
your software settings back to mg/L.
DataStudio: Click the Setup button on the DataStudio toolbar. Click the % button next to the Calibrate button and
select mg/L from the drop-down menu.
Note: Calibration is not required for measurements of relative DO content, such as before/during/after
experiment relative changes.
2. Click the Calibrate… button to open the Calibrate dialog.
3. Place the probe in 100% humidified air. When the readings stabilize around a value, click
the Set button. Then click OK. It may take a few minutes for the sensor to equilibrate for
calibration. The readings may not completely stabilize at one specific point.
Calibration in 100% humidified air is equivalent to calibrating in 100% air-saturated
water. This is because the oxygen must first pass through the electrolyte solution in the
Dissolved Oxygen Sensor to get to the cathode. The concentration of oxygen in both the
100% air-saturated water and the electrolyte solution will be equivalently proportional to
the partial pressure of oxygen in air, following Henry's law where
Cg = the solubility of the gas (oxygen)
Pg = the pressure of the gas over the solution
This relationship holds true whether the end of the sensor is immersed in the water or
suspended in air.
4. Before taking measurements of dissolved oxygen concentration, change your unit settings
back to mg/L.
5
Page 10

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
General Sampling Procedure
1. Immerse the Dissolved Oxygen probe into the test solution until the silver temperature
compensation band is submerged.
2. Click the Start button on the DataStudio tool bar or select Monitor Data from the
Experiment menu.
3. Gently, swirl the probe for a minute or two until the dissolved oxygen readings stabilize
around a value. As an alternative, use a magnetic stirrer to slowly stir the test solution (do
not stir fast enough to entrain air bubbles or form a large vortex.
Measurements in a Controlled Laboratory Setting
In a laboratory setting, you can make long-term measurements of dissolved oxygen levels by
gently and continuously stirring the solution with a magnetic stir plate and stir bar. Minimize
the surface area of the atmosphere/liquid interface to retard gas exchange with the
atmosphere. A vessel such as an Erlenmeyer flask or a large test tube works well.
Note: DO NOT USE MINERAL OIL, as it is difficult to clean from the membrane.
Using the Dissolved Oxygen Sensor with other PASCO Sensors:
Some PASCO sensors, including the Dissolved Oxygen Sensor, emit electrical signals into
the test solution and may interfere with other sensors taking simultaneous measurements. If
you want to take simultaneous measurements with the Dissolved Oxygen Sensor and another
PASCO sensor (such as the Conductivity Sensor or pH Sensor), conduct controlled
experiments and ensure that no intra-sensor interference occurs under your experimental
conditions.
6
Page 11
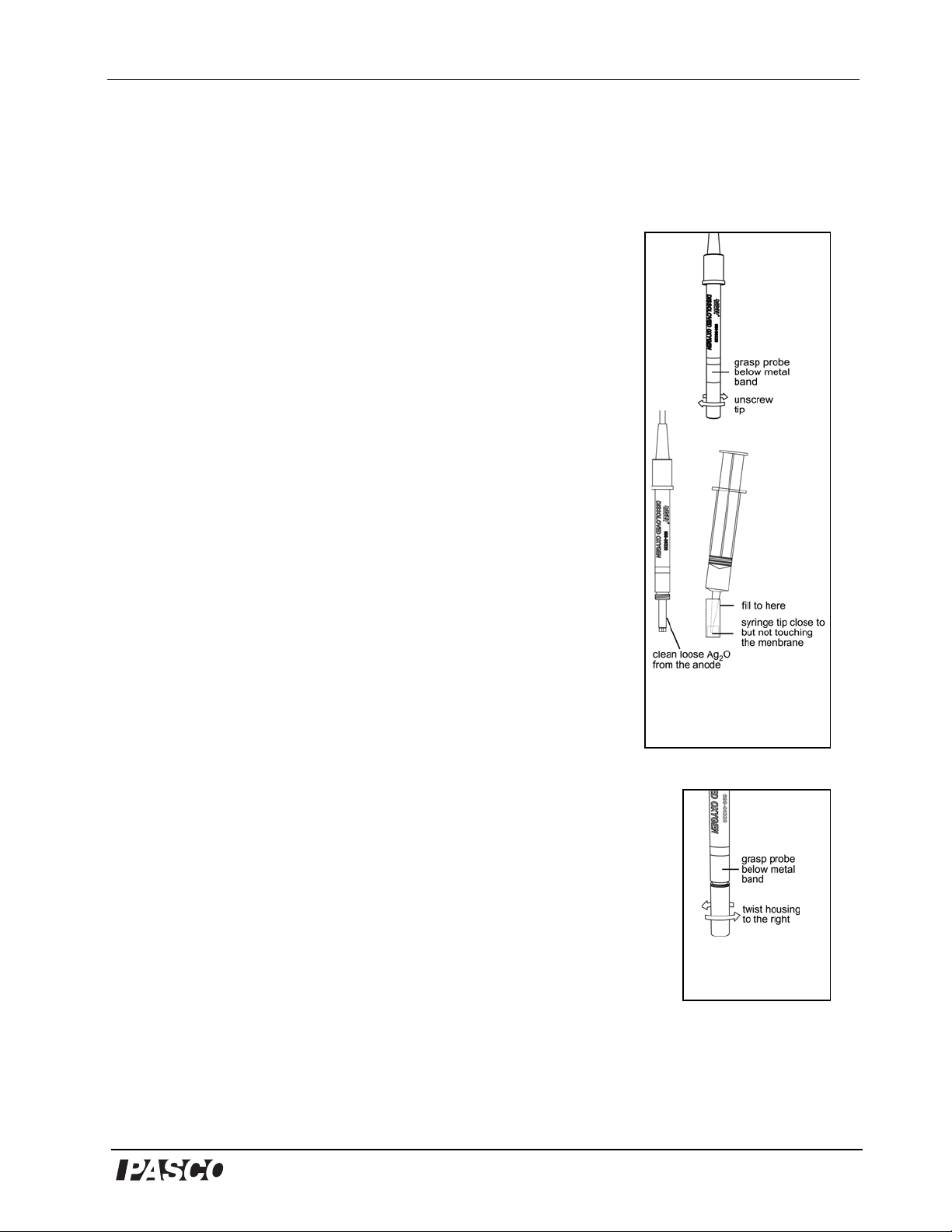
Model No. PS-2108 Dissolved Oxygen Sensor
®
Figure 5
Removing the cartridge
housing and filling with
electrolyte.
Figure 6
Replacing the cartridge
housing.
Maintenance
Changing the Electrolyte solution
The electrolyte solution (probe filling solution) should be
periodically replaced and the silver electrode cleaned to maintain
optimal performance of the probe. If the probe is not performing
optimally, replace the electrolyte solution as follows:
1. Unscrew the end of the probe by turning it to the left, and
remove the cartridge housing (Figure 5).
Always hold the probe below the stainless steel band when
unscrewing the cartridge housing or otherwise applying torque
to the end of the probe.
2. Rinse the electrode with tap water (or deionized water in areas
with hard tap water) and rub it dry with a paper towel, removing
loose silver oxide (Ag2O) from the anode.
3. Rinse and air dry the cartridge housing.
4. Pull about 10 ml of the Polarographic solution into the syringe,
being careful not to introduce air bubbles.
5. Place the tip of the syringe very close to, but not touching the
membrane, and slowly fill the membrane cartridge and cartridge
housing to approximately 5 mm from the top of the housing.
Note: Tap the cartridge housing while filling to avoid introducing air bubbles.
6. Holding the probe in a vertical position, slip the cartridge housing
over the electrode and turn to the right to tighten (Figure 6).
7. Dry with a paper towel or tissue.
Replacing the Membrane
If the silicon membrane becomes torn or otherwise damaged, replace it
as follows:
1. Follow steps 1 through 3 under "Changing the Electrolyte Solution"
above.
7
Page 12
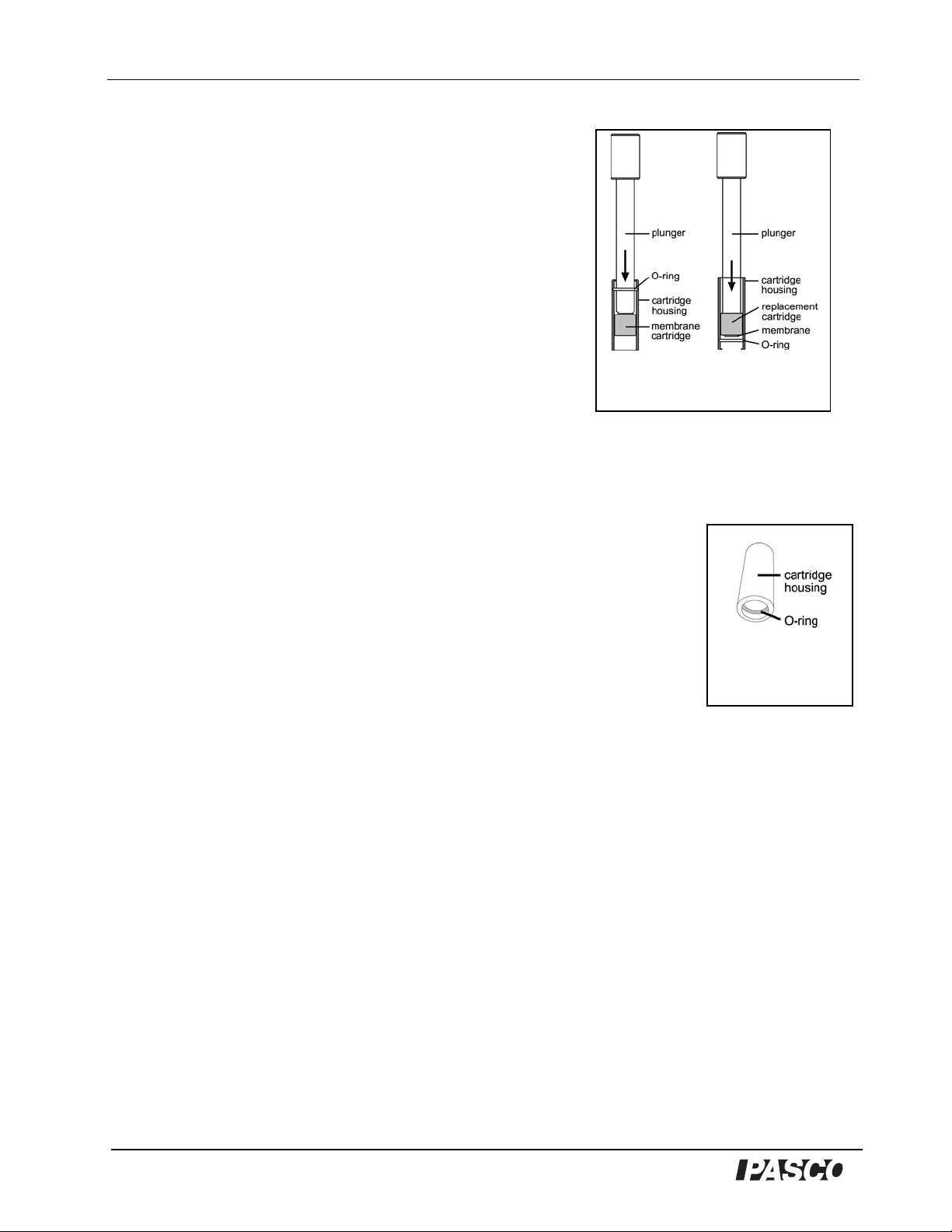
PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Figure 7
Removing and replacing the
membrane cartridge.
A
B
Figure 8
Examine the O-ring for
damage.
2. Use the supplied plunger to push the membrane cartridge
out of the cartridge housing (Figure 7A).
3. Examine the O-ring (Figure 8) and replace it if it is
damaged (See "Replacing the O-ring" below.).
4. Insert a replacement membrane cartridge and use the
plunger to push it down until it is seated at the end of the
housing that has the O-ring (Figure 7B).
5. Fill with ele ctrolyte and reas semble the pr obe as directed
in steps 4 through 7 of "Changing the Electrolyte
Solution."
Replacing the O-ring
The O-ring should rarely if ever require replacing. However, if it develops nicks or splits and
begins to allow leakage of the electrolyte solution from the probe, replace it as follows:
1. Follow steps 1and 2 of the procedure for replacing the membrane
cartridge (above).
2. After removing the membrane cartridge, remove the O-ring with a pair
of fine-tipped tweezers and insert a new O-ring.
3. Insert the membrane cartridge as directed in step 4 of “Replacing the
Membrane”.
4. Fill with electrolyte and reassemble the probe as directed in steps 4
through 7 of "Changing the Electrolyte Solution."
8
Page 13

Model No. PS-2108 Dissolved Oxygen Sensor
®
Storage
Short-Term Storage (two weeks or less)
Store the probe in the plastic storage bag with the tip inserted in the soaker bottle containing
about 10 ml of deionized water.
Long-Term Storage (more than two weeks)
1. Unscrew and remove the cartridge housing by turning it to the left.
Always hold the probe below the stainless steel band when unscrewing the cartridge housing
or otherwise applying torque to the end of the probe.
2. Dispose of the electrolyte and rinse the electrode with tap water (or deionized water in
areas with hard tap water) and dry it with a paper towel, removing loose silver oxide
(Ag2O) from the anode.
3. Rinse the cartridge housing with tap water and allow it to air dry.
4. Rinse the soaker bottle with tap or deionized water and shake dry.
5. Replace the cartridge housing onto the probe and place the dry probe in its storage bag
with the end of the probe in the soaker bottle to protect the membrane from damage.
Troubleshooting
If the Dissolved Oxygen Sensor does not give the expected output, do the following, and
check the function of the sensor after each step until the sensor works properly:
1. Replace the filling solution. See "Changing the Electrolyte Solution."
2. Replace the membrane. See "Replacing the Membrane."
3. Check the O-ring and replace if necessary (See "Replacing the O-ring."
If the Dissolved Oxygen Sensor still does not function properly, contact Technical Support
(See the Technical Support section in the back of this manual.)
9
Page 14

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Induced Dipoles
The electron cloud of an isolated Oxygen
molecule is distributed symmetrically
between the bonded O atoms.
As the negative end of the polar water
molecule approaches…
…the O2 electron cloud is moved away to
reduce repulsion between the O2 cloud and
the negative end of the water molecule.
The O2 molecule itself becomes polar.
A dipole has been induced in the otherwise
nonpolar O2 molecule. The H2O and the O2
are now weakly attracted to one another.
Theory of Dissolved Oxygen:
What is the chemical mechanism by which diatomic oxygen dissolves in water?
This is a particularly eloquent description of the mechanism of oxygen solubility in water:
[from Water on the Web1]
Water, as a polar molecule, induces an accumulation of electron density (dipole moment) at one end of nonpolar
gas molecules such as oxygen (O
approaching a nonpolar O
the bonded O
cloud of the O
positive and negative charges sep arated by a di stance) has been induced in the nonpolar O
and H
2
poles of nearby molecules is termed a dipole- indu ced dipole force. Th e creation of th ese forces then explains the
mechanism by which gases dissolve in water.
atoms. As the negative end of the H2O molecule approaches the oxygen molecule, the electron
2
moves away to reduce the negative-to-negative repulsion. As a result, a dipole (a molecule with
2
O to become weakly attracted to each other . This intermolecular attraction between the oppos itely charged
2
Still images from the Water on the Web animation:
) and carbon dioxide (CO2). In animation, observe a polar water molecule
2
molecule. The electron cloud of O2 is normally distributed symm e trically between
molecule, causing O2
2
10
II. What factors influence the amount of dissolved oxygen in water? There are several
factors that can influence the amount of gaseous diatomic oxygen (as well as other gases) that
will dissolve in water. When speaking of gases dissolving in water, discussion is limited to
gases that do not chemically react with the water.
Page 15

Model No. PS-2108 Dissolved Oxygen Sensor
®
1. Water Temperature effects
• Gases usually dissolve in liquids in an exothermic process. This expression represents that
process:
gas + solvent liquid gas saturated solvent + heat (this will reach an equilibrium)
• Le Chatelier's Principle predicts that an added stress to one side of this equilibrium will
cause a shift in equilibrium in order to accommodate that stress, thereby driving the
equilibrium point to the left in the expression below:
GAS + solvent liquid gas saturated solvent +heat
• Experimental results support this theory:
This graph was constructed using DataStudio, drawing on data taken from Table HY-DO-1 in
the GLOBE protocol for Dissolved Oxygen.
2
11
Page 16

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
P=kC
C1
P1
------ -
C2
P2
------ -=
9mg/L
1012 milibars
--------------------------------------- -
790 milibars 7 mg/L
2. Overlying Gas Pressure Effects
• Henry's Law states that the solubility of a gas in a liquid is affected by the pressure of the
gas above the liquid-gas interface.
P=gas pressure (or partial pressure)
C=concentration of dissolved gas
k = Henry’s Law Constant (differs with temperature, gas, and solvent)
• Changing pressures, while maintaining the same temperature, gas, and solvent allows one
to draw a relationship that excludes Henry's Law Constant:
• If a gas-liquid system starts at equilibrium and the partial pressure of a gas is increased
(with other factors such as temperature remaining unchanged) the amount of gas that is
dissolved in the liquid at the new equilibrium will increase.
For example:
P1 = 1012 millibars (approximately sea level)
C1 = 9 mg / L
P2 = 790 millibars (approximately 6700 ft or 2050 m above sea level)
• The most significant way in which this effect manifests itself is the reduced maximum
possible dissolved oxygen at higher altitudes. This is the reason why barometric pressure
at the sample site is important. Higher altitudes lead to a decrease in atmospheric pressure
(all atmospheric gases), which leads to a decrease in the partial pressure of oxygen (as well
as all of the other atmospheric gases). Please note the weather services often report
barometric pressure for a location, which is normalized for the same local conditions as if
reported at sea level, that is, the values are corrected for altitude.
• The decrease in atmosphere pressure with increase in altitude is not linear. Please see the
altitude correction table in order to compensate for altitude differences.
3. Hydrostatic Pressure
• Water under significant pressure may hold a higher amount of dissolved gas, compared to
water under less pressure. Plumbing systems are commonly under significant pressure.
The gases dissolved in water that is in a plumbing system may be at equilibrium at that
pressure, but when the pressure is released or decreased by an act such as drawing the
water from a tap into a container at atmospheric pressure, the solution may be
supersaturated. This phenomenon is similar to opening a can of carbonated beverage or
soda pop. However, the dissolved gases may take several seconds to several hours to reach
12
Page 17

Model No. PS-2108 Dissolved Oxygen Sensor
®
equilibrium.
A famous, naturally occurring case of supersaturation and subsequent gas release (in this
case it was dissolved carbon dioxide gas) was the Lake Nyos tragedy, which took place in
Cameroon (1986).
4. Salinity.
• Salt and other solids dissolved in water will affect the total amount of gas that a liquid
solvent can dissolve. Given otherwise identical conditions, fresh water can hold more
dissolved oxygen than salt water. When measuring dissolved oxygen in salt water (in
excess of 1000 mg/L dissolved salts), calibrate the probe in a sample of the salt water in
which the DO measurement is desired. For situations in which the salt content is not
constant (such as estuarine environments) one must calibrate the probe using fresh water.
For each DO reading, one must also make an accurate determination of dissolved salt
content in order to account for the change in oxygen solubility in waters of varying salinity.
• To obtain theoretical maximum DO in saline waters, consult the following table for the
theoretical maximum dissolved oxygen at saturation-the low calibration value will remain
at 0 mg/L DO (table from Ambient W ater Quality Criteria, 19973). For values not found in
the table, use the nomogram found on page 28 in Hitchman, 1978.
Solubility of oxygen in water (fresh and sa line) exposed to water-saturated air at sea level
760 mm Hg (101.3 kPa)
Chlorinity (freshwater)
Temp .
(°C)
0.0 14.621 13.728 12.888 12.097 11.355 10.657
1.0 14.216 13.356 12.545 11.783 11.066 10.392
2.0 13.829 13.000 12.218 11.483 10.790 10.139
3.0 13.460 12.660 11.906 11.195 10.526 9.897
4.0 13.107 12.335 11.607 10.920 10.273 9.664
5.0 12.770 12.024 11.320 10.656 10.031 9.441
6.0 12.447 11.727 11.046 10.404 9.799 9.228
7.0 12.139 11.442 11.783 10.162 9.576 9.023
8.0 11.843 11.169 10.531 9.930 9.362 8.826
9.0 11.559 10.907 10.290 9.707 9.156 8.636
10.0 11.288 10.656 10.058 9.493 8.959 8.454
11.0 11.027 10.415 9.835 9.287 8.769 8.279
12.0 10.777 10.183 9.621 9.089 8.586 8.111
13.0 10.537 9.961 9.416 8.899 8.411 7.949
14.0 10.306 9.747 9.218 8.716 8.242 7.792
0 g/L Cl- 5.0 g/L Cl- 10.0 g/L Cl- 15.0 g/L Cl- 20.0 g/L Cl- 25.0 g/L Cl-
4
13
Page 18

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Chlorinity (freshwater)
Tem p.
(°C)
15.0 10.084 9.541 9.027 8.540 8.079 7.642
16.0 9.870 9.344 8.844 8.370 7.922 7.496
17.0 9.665 9.153 8.667 8.207 7.770 7.356
0 g/L Cl- 5.0 g/L Cl- 10.0 g/L Cl- 15.0 g/L Cl- 20.0 g/L Cl- 25.0 g/L Cl-
5. Surface Area and Mixing/Turbulence
• The surface area of the water/gas interface may affect the rate at which gas dissolves into
the water, but ultimately, it will not affect the total amount of gas dissolved at static (nonturbulent) equilibrium. Situat ions in whic h t urbulence is constant (such as the outfall from
a dam spillway) can host a dynamic equilibrium in which water remains supersaturated at a
given point in the stream.
• Stirring a water sample in a laboratory setting has a drastic effect on the behavior of the
DO probe. The nature of a Clark-type probe is that it consumes oxygen in its immediate
environment (leading to an apparent localized drop in DO level). Stirring the sample,
while taking data, is highly recommended in order to obtain faithful DO readings. It is
crucial to maintain a sufficient flow rate across the membrane of the sensor . Flow of about
1 cm per second is recommended. Do not stir to the point of causing excessive turbulence
in the water.
• Allow the probe to reach equilibrium in experiments that are not conducive to stirring.
There will be an initial drop (in most cases up to 60 seconds) on apparent readings until a
local equilibrium is established.
Citations:
1. WOW. 2003. Water on the Web - Monitoring Minnesota Lakes on the Internet and Training
Water Science Technicians for the Future - A National On-line Curriculum using Advanced
Technologies and Real-Time Data. (http://wow.nrri.umn.edu). University of MinnesotaDuluth, Duluth, MN 55812
2. Table HY-DO-1 from Globe Dissolved Oxygen Protocol (http://www.globe.gov/tctg/
hydro_prot_do.pdf?sectionId=151)
3. Ambient Water Quality Criteria for Dissolved Oxygen, February, 1997. Water
Management Branch Environment and Lands Headquarters Division, Ministry Of
Environment, Lands and Parks, Canada
4. Hitchman, Michael L.1978. Measurement of Dissolved Oxygen. John Wiley and Sons,
New York. 255 p.
14
Page 19

Model No. PS-2108 Dissolved Oxygen Sensor
®
Figure 1
Gently stir the probe in the
test solution
Experiment 1: Introduction to the Operation of the
Dissolved O
Sensor
2
Purpose
The purpose of the experiment is to explore the basic operation of the Dissolved Oxygen
Sensor: how to set it up, how to calibrate it, and how to obtain the most accurate
measurements with it.
Materials and Equipment Needed
• Dissolved Oxygen Sensor (PS-2108)
• 600 ml beaker
• PASCO ScienceWorkshop
• 400 ml deionized water saturated with air
• PASPort Interface and DataStudio software
• Reference tables for dissolved oxygen
• aquarium pump (optional)
Optional Equipment Suggested
T o s aturate deionized water with air, fill a clean container one-third full wit h deionized water,
seal it, and shake vigorously for 10 seconds. Alternatively, bubble air through the deionized
water for 15 minutes using an aquarium pump.
Procedure
1. Set up and calibrate the Dissolved Oxygen Sensor in DataStudio
according to the calibration procedure detailed in this manual.
2. Place the probe in the test solution and stir it gently for thirty
seconds.
3. Click t he Start button on the DataStudio toolbar to begin recording
data (Data Run #1).
4. Being careful to keep the stainless steel band submerged to at least
1 cm under the surface of the water, gently stir the probe in the test
solution until the dissolved O
readings stabilize.
2
5. Click the Stop button on the DataStudio toolbar to end data
recording.
15
Page 20

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Figure 2
Allow the probe to rest
undisturbed in the test solution
6. Click the Start button on the DataStudio toolbar to begin recording data (Data Run #2).
7. Allow the probe to rest undisturbed in the test solution and
observe the sensor reading.
8. After the sensor reading stabilizes at the new level, gently move
the probe back and forth through the test solution and observe
the sensor reading.
9. After the sensor reading stabilizes at the new level, repeat steps
6 and 7 several times.
10. . Click the Stop button to end data recording. Save the recorded
data.
Note: The concentration of dissolved O2 should be about the level of the high
value that you entered dur ing cali brat ion. If not , check to be sure that ther e are no lar ge air bubbles on the b ottom
of the probe. If the value is still significantly (mo re than 10 %) different than the high value, re-calibrate the
sensor.
Data Analysis/Questions
1. Why does the measured dissolved O2 concentration decline when you leave the probe
undisturbed?
2. Why does measured dissolved O2 concentration stop declining while the probe is
undisturbed?
3. Why does the measured dissolved O2 concentration rise when you move the probe
through the test solution?
4. Why does the measured dissolved O
concentration stop rising while the probe is moving
2
through the test solution?
5. Which measurement is the most accurate representation of the dissolved O2 concentration
of the test solution?
6. Explain why it is important to move a steady flow of the test solution by the membrane of
the Dissolved Oxygen Sensor while taking dissolved O2 concentration measurements.
16
Page 21

Model No. PS-2108 Dissolved Oxygen Sensor
®
Experiment 2: Photosynthesis and Oxygen Generation
with Aquatic Plants
Purpose
Explore the change in level of dissolved oxygen in water associated with photosynthesis of
aquatic plants.
Materials and Equipment Needed
• 2-PASPort DO sensors PS-2108
• 2-PASPort USB Links PS-2100
• USB equipped computer running DataStudio software
• 100 watt incandescent lamp (or equivalent)
• Lab stand(s)
• Masking Tape
• Paper
• 2-Test Tubes (25mm x 150mm)
• 1-1000 mL beaker
• 1 healthy sprig of Elodea
• Deionized or clean fresh water
1
Background
Plants carry out the process of photosynthesis when conditions are appropriate. Plants take in
reactants including carbon dioxide, water, and energy in the form of light. A waste product
from the photosynthetic process is gaseous oxygen, which is released to the environment.
For aquatic plants, this gas is released into the water, increasing the amount of dissolved
oxygen present in the water.
The dissolved oxygen sensor is well-suited to monitor changing levels of molecular oxygen
in the aqueous environment of the Elodea plant.
Tips for success:
• Set up and run the experiment far ahead of time, so that the procedure is familiar.
• Use fresh Elodea (the growing tip will give the best results).
• Allow water to reach equilibrium, in terms of the dissolved gases (this may take up to 24
hours)
• Submerge the DO sensor so that the metallic band is below the surface of the water.
• Use a full beaker of water for the water bath to control thermal fluctuations.
• Set up: (Complete steps 1-4 in advance)
1. or other PASPort interface
17
Page 22

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Figure 1
Equipment setup.
Procedure
1. Perform a test run on the DO sensor(s) to ensure that the
membrane is intact.
2. Arrange equipment so that the two large test tubes are
nearly full of water.
3. Clamp test tubes in place vertically, with the beaker as a
water bath (to minimize temperature fluctuations).
4. With no plants in place, allow the entire system to reach
equilibrium for about three to four hours (it is not harmful
to exceed this time).
During the lab time
1. Insert each DO sensor into its respective test tube. Perform a virtual calibration in
DataStudio so that the DO mg/L value is set at the same level for each sensor (7 mg/L is a
good starting point). Absolute calibration is less important in this particular situation, since
the goal is to measure relative change, instead of absolute dissolved oxygen.
2. Conduct a test run for approximately 30 seconds to ensure that the DO probes are reading
values that are reasonably close to each other.
3. Remove the DO sensors. Do not allow the probe tip to contact other surfaces (it helps to
clamp the probes to a rack, and then lif t the entir e rac k until the probes ar e clear of the test
tubes).
4. Gently and carefully insert a sprig (about 2-3 cm long) of the healthy green tip of the
Elodea plant in one test tube (experiment). Insert a control, such as a plastic Elodea plant,
in the other test tube (optional). Ta ke care to avoid stirring or agitating the water in the
test tubes more than necessary.
5. Reinsert the DO sensors until the tip of the sensor is close to, but not touching the plant.
6. Use tape and a folded piece of paper to form a light mask around the upper part of the
beaker, such that it's lower edge is even with the bottom of the DO sensor probe tip.
7. Position the light about one meter away from the beaker, so that it can illuminate the
plants through the side of the beaker (horizontally). Turn the light on for a momentary
check of positioning then turn it off.
18
Page 23

Model No. PS-2108 Dissolved Oxygen Sensor
®
Collecting Data
8. Begin collecting data. Allow the system to take in ambient light for 60 seconds, and then
cover the entire set up with an opaque cloth, such as a rubberized lab apron. After fifteen
minutes, remove the cover and turn on the light. Continue to collect data for another
fifteen minutes. Stop data collection.
9. If desired, conduct additional data runs in a similar manner.
Sample Data
Analysis
1. Discuss the reasons for use of a control in this experiment.
2. Why was it not necessary to calibrate the sensors to an absolute standard?
19
Page 24

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Figure 3
Stir test solution with probe
while adding sodium sulfite.
Experiment 3: Effect of Sodium Sulfite on Dissolved
O
Concentrations
2
Materials and Equipment Needed
• Dissolved Oxygen Sensor
• PASPort interface or logger
• 2 M sodium sulfite (25.2 g Na2SO3/100 ml)
• aquarium pump or large bottle
• PASPort USB Interface and DataStudio software
• 600 ml beaker
• 400 ml deionized water
• pipet
Optional Materials
• hot plate/stir plate and magnetic stir bar
CAUTION: Sodium sulfite is a potential skin irritant. Use safety glasses and avoid s kin contact. Skin that
contacts the solution should be rinsed liberally with water.
Purpose
The purpose of the experiment is to explore the effect of the inorganic chemical, sodium
sulfite on dissolved O2 concentrations. Sodium sulfite (Na2SO3) is a commonly used
chemical in photographic developers, paper making, dyeing, bleaching, and engraving.
Procedure
1. Set up and calibrate the Dissolved Oxygen Sensor.
2. Saturate the deionized water with air by vigorously shaking the
water in a bottle or by bubbling with the aquarium pump.
3. Monitor the dissolved O
concentrations of the deionized water
2
while stirring gently with the dissolved O2 probe.
4. When the meter reading stabilizes, record for 30 seconds.
5. After 30 seconds, add 1 ml of the 2 M Na
solution into the
2SO3
water.
6. Continue stirring the solution and recording until the reaction stops.
7. Stop recording and save your data.
20
Page 25

Model No. PS-2108 Dissolved Oxygen Sensor
®
Questions
1. Describe the effect of sodium sulfite on dissolved O2 concentrations.
2. Discuss some potential environmental effects of untreated effluent from industries or
businesses such as paper mills and photo labs that may use sodium sulfite.
21
Page 26

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Experiment 4: Biochemical Oxygen Demand
1
Background
Biochemical oxygen demand (BOD) is an important measure of water quality. BOD is a
measurement of the quantity of oxygen used by microorganisms in the aerobic oxidation of
organic matter. In rivers and streams with high BOD levels, much of the available dissolved
oxygen is consumed by aerobic bacteria, robbing other aquatic organisms of the oxygen they
need to live. This is a condition that may be exacerbated by excessive nitrogenous wastes in
runoff water.
Procedure
1. Measure the dissolved O2 concentration and water temperature in a waterway, preferably on
site using the PASPort logger of choice, with the dissolved oxygen sensor.
2. Colle ct a sample of the water in an airtight, black bottle, and incubate the water sam p le at
room temperature for 5 days.
3. Bring the water sample to approximately the same temperature as the first sample and
measure the dissolved O2 concentration.
4. Calculate the BOD level: mg/l dissolved O2 (original sample) - mg/l dissolved O2 (5-day
old sample).
5. Score the BOD as follows:
4 (excellent): less than 2 mg/l
3 (good): 2- 4mg/l
2 (fair): 4.1-10 mg/l
1 (poor): greater than 10 mg/l
1.Adapted from a 1996 publication by Stevens Institute of Technology
22
Page 27

Model No. PS-2108 Dissolved Oxygen Sensor
®
Figure 1
Transfer activated yeast solution to the
test beaker.
Experiment 5: The Effect Of Respiration On Dissolved
O
Concentration
2
Purpose
The purpose of the experiment is to explore the effect of respiration on dissolved O2
concentrations.
Materials required
• Dissolved Oxygen Sensor (PS-2108)
• 400 ml deionized water
• PASPort interface ha rdware and DataStudio software
• aquarium pump or large bottle
• 1 package rapid active yeast
• 600 ml beaker (2)
• table sugar
Optional materials
• hot plate/stir plate and magnetic stir bar
Procedure
1. Follow the dir ections on the yeast package to activate the
yeast in a beaker using tap water and sugar.
2. Set up and calibrate the Dissolved Oxygen Sensor.
3. Stir 1 teaspoon sugar into the deionized water and
saturate it with air.
4. Monitor the dissolved O
concentrations of the
2
deionized sugar water while stirring gently with the
dissolved O
probe.
2
5. When the reading stabilizes, click the Start button in
DataStudio and record data for 30 seconds.
6. After 30 seconds, transfer about 1 teaspoon of the activated yeast solution into the water.
7. Continue stirring with the probe and recording data for about 10 minutes or until the
oxygen level stabilizes.
23
Page 28

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
8. Stop recording and save the data.
Questions
1. What is the evidence that the yeast cells are alive and respiring during the experiment?
2. What happens to the yeast cells when the dissolved O2 concentration reaches zero? (Hint:
Look carefully at the beaker that contains the concentrated yeast solution and think about
how yeast is used in industry.)
24
Page 29

Model No. PS-2108 Dissolved Oxygen Sensor
®
Appendix A: Copyright and Warranty Information
Copyright Notice
The PASCO scientific 012-07688D manual is copyrighted and all rights are reserved.
However, permission is granted to non-profit educational institutions for reproduction of any
part of the Dissolved Oxygen Sensor manual providing the reproductions are used only for
their laboratories and are not sold for profit. Reproduction under any other circumstances,
without the written consent of PASCO scientific, is prohibited.
Limited Warranty
PASCO scientific warrants the product to be free from defects in materials and workmanship
for a period of one year from the date of shipment to the customer. PASCO will repair or
replace at its option any part of the product which is deemed to be defective in material or
workmanship. The warranty does not cover damage to the product caused by abuse or
improper use. Determination of whether a product failure is the result of a manufacturing
defect or improper use by the customer shall be made solely by PASCO scientific.
Responsibility for the return of equipment for warranty repair belongs to the customer.
Equipment must be properly packed to prevent damage and shipped postage or freight
prepaid. (Damage caused by improper packing of the equipment for return shipment will not
be covered by the warranty.) Shipping costs for returning the equipment after repair will be
paid by PASCO scientific.
Equipment Return
Should the product have to be returned to PASCO scientific for any reason, notify PASCO
scientific by email, letter, phone, or fax BEFORE returning the product. Upon notification,
the return authorization and shipping instructions will be promptly issued.
NOTE: NO EQUIPMENT WILL BE ACCEP TE D FOR RETURN WITHOUT AN AUTHORIZATION FROM
PASCO.
When returning equipment for repair, the units must be packed properly. Carriers will not
accept responsibility for damage caused by improper packing. To be certain the unit will not
be damaged in shipment, observe the following rules:
1. The packing carton must be strong enough for the item shipped.
2. Make certain there are at least two inches of packing material between any point on the
apparatus and the inside walls of the carton.
3. Make certain that the packing material cannot shift in the box or become compressed,
allowing the instrument come in contact with the packing carton.
25
Page 30

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Appendix B: Specifications, Replacement Parts,
Technical Support
Specifications
• Cathode: platinum
• Anode: silver/silver chloride
• Response: 98% in 60 seconds
• Stability: Better than 2%
• Fully temperature compensated: 10 °C - 40 °C
• Temperature range: 0 °C - 60 °C
• Output (medium saturated with air): 240 -320 nA
• Membrane: 1 ml silicon
• Probe body: PVC
Replacement Parts
Order from PASCO (1-800-772-8700)
Replacement kit (part number CI-6541) includes:
• Three dissolved O2 probe membrane cartridge
• three O-rings
• bottle of dissolved O2 probe filling solution (contains enough solution to refill the probe
about ten times)
• 10 ml syringe
• Plunger tool
Technical Support
For assistance with the PS-2108 Dissolved Oxygen Sensor or any other PASCO products,
contact PASCO as follows:
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Phone: (800) 772-8700 (toll-free within U.S.)
Phone: (916) 786-3800
FAX: (916) 786-3292
Web: www.pasco.com
Email: support@pasco.com
26
Page 31

Model No. PS-2108 Dissolved Oxygen Sensor
®
Appendix C: Tables
Table 1: Concentration of dissolved oxygen (mg/L) in water at various temperatures
and pressures
From R. F. Weiss (1970). Temp ° C, temperature in degrees Celsius;
atmospheric pressures from 695-600 millimeters mercury (27.36-23.62 “Hg) begin after 40° C
Conversion Factors: 1.0 “Hg = 25.4 mmHg = 33.86 mb or hPa
Temp. Atmospheric pressure, in millimeters of mercury and (in ch e s of mercury)
795
790
785
780
775
770
765
760
755
750
745
740
735
730
725
720
715
710
°C
(31.30
(31.10
(30.91
(30.71
(30.51
(30.31
(30.12
(29.92
(29.72
(29.53
(29.33
(29.13
(28.94
(28.74
(28.54
(28.35
)
)
)
)
)
)
)
)
)
)
)
)
)
)
)
0.0 15.3 15.2 15.1 15.0 14.9 14.8 14.7 14.6 14.5 14.4 14.3 14.2 14.1 14.0 13.9 13.8 13.7 13.6 13.5 13.4
0.5 15.1 15.0 14.9 14.8 14.7 14.6 14.5 14.4 14.3 14.2 14.1 14.0 13.9 13.8 13.7 13.6 13.5 13.4 13.3 13.2
1.0 14.8 14.7 14.7 14.6 14.5 14.4 14.3 14.2 14.1 14.0 13.9 13.8 13.7 13.6 13.5 13.4 13.3 13.2 13.2 13.1
1.5 14.6 14.5 14.5 14.4 14.3 14.2 14.1 14.0 13.9 13.8 13.7 13.6 13.5 13.4 13.3 13.2 13.2 13.1 13.0 12.9
2.0 14.4 14.3 14.3 14.2 14.1 14.0 13.9 13.8 13.7 13.6 13.5 13.4 13.3 13.3 13.2 13.1 13.0 12.9 12.8 12.7
(28.15
)
)
(27.95
)
705
(27.76
)
(27.56
700
)
2.5 14.2 14.2 14.1 14.0 13.9 13.8 13.7 13.6 13.5 13.4 13.3 13.3 13.2 13.1 13.0 12.9 12.8 12.7 12.6 12.5
3.0 14.1 14.0 13.9 13.8 13.7 13.6 13.5 13.4 13.3 13.3 13.2 13.1 13.0 12.9 12.8 12.7 12.6 12.5 12.5 12.4
3.5 13.9 13.8 13.7 13.6 13.5 13.4 13.3 13.3 13.2 13.1 13.0 12.9 12.8 12.7 12.6 12.6 12.5 12.4 12.3 12.2
4.0 13.7 13.6 13.5 13.4 13.3 13.3 13.2 13.1 13.0 12.9 12.8 12.7 12.6 12.6 12.5 12.4 12.3 12.2 12.1 12.0
4.5 13.5 13.4 13.3 13.3 13.2 13.1 13.0 12.9 12.8 12.7 12.7 12.6 12.5 12.4 12.3 12.2 12.1 12.1 12.0 11.9
5.0 13.3 13.3 13.2 13.1 13.0 12.9 12.8 12.7 12.7 12.6 12.5 12.4 12.3 12.2 12.2 12.1 12.0 11.9 11.8 11.7
5.5 13.2 13.1 13.0 12.9 12.8 12.7 12.7 12.6 12.5 12.4 12.3 12.2 12.2 12.1 12.0 11.9 11.8 11.7 11.7 11.6
6.0 13.0 12.9 12.8 12.8 12.7 12.6 12.5 12.4 12.3 12.3 12.2 12.1 12.0 11.9 11.8 11.8 11.7 11.6 11.5 11.4
6.5 12.8 12.8 12.7 12.6 12.5 12.4 12.3 12.3 12.2 12.1 12.0 11.9 11.9 11.8 11.7 11.6 11.5 11.5 11.4 11.3
7.0 12.7 12.6 12.5 12.4 12.4 12.3 12.2 12.1 12.0 12.0 11.9 11.8 11.7 11.6 11.6 11.5 11.4 11.3 11.2 11.1
7.5 12.5 12.4 12.4 12.3 12.2 12.1 12.0 12.0 11.9 11.8 11.7 11.6 11.6 11.5 11.4 11.3 11.3 11.2 11.1 11.0
8.0 12.4 12.3 12.2 12.1 12.1 12.0 11.9 11.8 11.7 11.7 11.6 11.5 11.4 11.3 11.3 11.2 11.1 11.0 11.0 10.9
8.5 12.2 12.1 12.1 12.0 11.9 11.8 11.8 11.7 11.6 11.5 11.4 11.4 11.3 11.2 11.1 11.1 11.0 10.9 10.8 10.7
9.0 12.1 12.0 11.9 11.8 11.8 11.7 11.6 11.5 11.5 11.4 11.3 11.2 11.2 11.1 11.0 10.9 10.8 10.8 10.7 10.6
9.5 11.9 11.9 11.8 11.7 11.6 11.6 11.5 11.4 11.3 11.2 11.2 11.1 11.0 10.9 10.9 10.8 10.7 10.6 10.6 10.5
10.0 11.8 11.7 11.6 11.6 11.5 11.4 11.3 11.3 11.2 11.1 11.0 11.0 10.9 10.8 10.7 10.7 10.6 10.5 10.4 10.4
10.5 11.7 11.6 11.5 11.4 11.4 11.3 11.2 11.1 11.1 11.0 10.9 10.8 10.8 10.7 10.6 10.5 10.5 10.4 10.3 10.2
11.0 11.5 11.4 11.4 11.3 11.2 11.2 11.1 11.0 10.9 10.9 10.8 10.7 10.6 10.6 10.5 10.4 10.3 10.3 10.2 10.1
11.5 11.4 11.3 11.2 11.2 11.1 11.0 11.0 10.9 10.8 10.7 10.7 10.6 10.5 10.4 10.4 10.3 10.2 10.2 10.1 10.0
12.0 11.3 11.2 11.1 11.0 11.0 10.9 10.8 10.8 10.7 10.6 10.5 10.5 10.4 10.3 10.3 10.2 10.1 10.0 10.0 9.9
12.5 11.1 11.1 11.0 10.9 10.8 10.8 10.7 10.6 10.6 10.5 10.4 10.4 10.3 10.2 10.1 10.1 10.0 9.9 9.9 9.8
13.0 11.0 10.9 10.9 10.8 10.7 10.7 10.6 10.5 10.4 10.4 10.3 10.2 10.2 10.1 10.0 10.0 9.9 9.8 9.7 9.7
13.5 10.9 10.8 10.7 10.7 10.6 10.5 10.5 10.4 10.3 10.3 10.2 10.1 10.1 10.0 9.9 9.8 9.8 9.7 9.6 9.6
14.0 10.8 10.7 10.6 10.6 10.5 10.4 10.4 10.3 10.2 10.1 10.1 10.0 9.9 9.9 9.8 9.7 9.7 9.6 9.5 9.5
27
Page 32

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Temp. Atmospheric pressure, in millim e ter s of m ercury and (inc hes of mercury)
795
790
785
780
775
770
765
760
755
750
745
740
735
730
725
720
715
710
705
°C
(31.30
(31.10
(30.91
(30.71
(30.51
(30.31
(30.12
(29.92
(29.72
(29.53
(29.33
(29.13
(28.94
(28.74
(28.54
(28.35
(28.15
)
)
)
)
)
)
)
)
)
)
)
)
)
)
)
)
14.5 10.6 10.6 10.5 10.4 10.4 10.3 10.2 10.2 10.1 10.0 10.0 9.9 9.8 9.8 9.7 9.6 9.6 9.5 9.4 9.4
15.0 10.5 10.5 10.4 10.3 10.3 10.2 10.1 10.1 10.0 9.9 9.9 9.8 9.7 9.7 9.6 9.5 9.5 9.4 9.3 9.3
15.5 10.4 10.4 10.3 10.2 10.2 10.1 10.0 10.0 9.9 9.8 9.8 9.7 9.6 9.6 9.5 9.4 9.4 9.3 9.2 9.2
16.0 10.3 10.2 10.2 10.1 10.0 10.0 9.9 9.8 9.8 9.7 9.7 9.6 9.5 9.5 9 .4 9.3 9.3 9.2 9.1 9.1
16.5 10.2 10.1 10.1 10.0 9.9 9.9 9.8 9.7 9.7 9.6 9.5 9.5 9.4 9.4 9.3 9.2 9.2 9.1 9.0 9.0
17.0 10.1 10.0 10.0 9.9 9.8 9.8 9.7 9.6 9.6 9.5 9.4 9.4 9.3 9.3 9.2 9.1 9.1 9.0 8.9 8.9
17.5 10.0 9.9 9.9 9.8 9.7 9.7 9.6 9.5 9.5 9.4 9.3 9.3 9.2 9.2 9.1 9.0 9.0 8.9 8.8 8.8
18.0 9.9 9.8 9.8 9.7 9.6 9.6 9.5 9.4 9.4 9.3 9.3 9.2 9.1 9.1 9.0 8.9 8.9 8.8 8.7 8.7
18.5 9.8 9.7 9.7 9.6 9.5 9.5 9.4 9.3 9.3 9.2 9.2 9.1 9.0 9.0 8.9 8.8 8.8 8.7 8.7 8.6
19.0 9.7 9.6 9.6 9.5 9.4 9.4 9.3 9.3 9.2 9.1 9.1 9.0 8.9 8.9 8.8 8.8 8.7 8.6 8.6 8.5
19.5 9.6 9.5 9.5 9.4 9.3 9.3 9.2 9.2 9.1 9.0 9.0 8.9 8.9 8.8 8.7 8.7 8.6 8.5 8.5 8.4
20.0 9.5 9.4 9.4 9.3 9.3 9.2 9.1 9.1 9.0 8.9 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.5 8.4 8.3
20.5 9.4 9.3 9.3 9.2 9.2 9.1 9.0 9.0 8.9 8.9 8.8 8.7 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.3
21.0 9.3 9.2 9.2 9.1 9.1 9.0 8.9 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.5 8.4 8.4 8.3 8.2 8.2
(27.95
)
)
(27.76
)
700
(27.56
)
21.5 9.2 9.2 9.1 9.0 9.0 8.9 8.9 8.8 8.7 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.3 8.2 8.1 8.1
22.0 9.1 9.1 9.0 9.0 8.9 8.8 8.8 8.7 8.7 8.6 8.5 8.5 8.4 8.4 8.3 8.2 8.2 8.1 8.1 8.0
22.5 9.0 9.0 8.9 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.5 8.4 8.3 8.3 8.2 8.2 8.1 8.0 8.0 7.9
23.0 9.0 8.9 8.8 8.8 8.7 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.3 8.2 8.1 8.1 8.0 8.0 7.9 7.9
23.5 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.5 8.4 8.4 8.3 8.2 8.2 8.1 8.1 8.0 8.0 7.9 7.8 7.8
24.0 8.8 8.7 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.9 7.8 7.8 7.7
24.5 8.7 8.7 8.6 8.5 8.5 8.4 8.4 8.3 8.3 8.2 8.1 8.1 8.0 8.0 7.9 7.9 7.8 7.7 7.7 7.6
25.0 8.6 8.6 8.5 8.5 8.4 8.3 8.3 8.2 8.2 8.1 8.1 8.0 8.0 7.9 7.8 7.8 7.7 7.7 7.6 7.6
25.5 8.5 8.5 8.4 8.4 8.3 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.9 7.8 7.8 7.7 7.7 7.6 7.6 7.5
26.0 8.5 8.4 8.4 8.3 8.3 8.2 8.1 8.1 8.0 8.0 7.9 7.9 7.8 7.8 7.7 7.6 7.6 7.5 7.5 7.4
26.5 8.4 8.3 8.3 8.2 8.2 8.1 8.1 8.0 8.0 7.9 7.8 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.4
27.0 8.3 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.9 7.8 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.3 7.3
27.5 8.2 8.2 8.1 8.1 8.0 8.0 7.9 7.9 7.8 7.8 7.7 7.7 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2
28.0 8.2 8.1 8.1 8.0 8.0 7.9 7.9 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2
28.5 8.1 8.0 8.0 7.9 7.9 7.8 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.1 7.1
29.0 8.0 8.0 7.9 7.9 7.8 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0
29.5 8.0 7.9 7.9 7.8 7.8 7.7 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0
30.0 7.9 7.8 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9
30.5 7.8 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9
31.0 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8
31.5 7.7 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7
32.0 7.6 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7
32.5 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6
33.0 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6
28
Page 33

Model No. PS-2108 Dissolved Oxygen Sensor
®
Temp. Atmospheric pressure, in millimeters of mercury and (in ch e s of mercury)
770
765
760
755
750
745
740
735
730
725
720
715
710
705
795
790
785
780
775
(30.51
(30.31
(30.12
(29.92
(29.72
(29.53
(29.33
(29.13
(28.94
(28.74
(28.54
(28.35
(28.15
°C
(31.30
(31.10
(30.91
)
)
33.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5
34.0 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.7 6.6 6.6 6.5 6.5
34.5 7.3 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.5 6.4
35.0 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3
35.5 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3
36.0 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2
36.5 7.1 7.0 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2
37.0 7.0 7.0 6.9 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1
37.5 7.0 6.9 6.9 6.8 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1
38.0 6.9 6.9 6.8 6.8 6.7 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0
38.5 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0
39.0 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 6.0
39.5 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 6.0 5.9
40.0 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.9
(30.71
)
)
)
)
)
)
)
)
)
)
)
)
)
)
(27.95
)
)
(27.76
)
700
(27.56
)
Temp.
695
690
685
°C
(27.36
(27.17
)
)
0.0 13.3 13.2 13.1 13.0 12.9 12.8 12.8 12.7 12.6 12.5 12.4 12.3 12.2 12.1 12.0 11.9 11.8 11.7 11.6 11.5
0.5 13.1 13.1 13.0 12.9 12.8 12.7 12.6 12.5 12.4 12.3 12.2 12.1 12.0 11.9 11.8 11.7 11.6 11.5 11.4 11.3
1.0 13.0 12.9 12.8 12.7 12.6 12.5 12.4 12.3 12.2 12.1 12.0 11.9 11.8 11.7 11.6 11.6 11.5 11.4 11.3 11.2
1.5 12.8 12.7 12.6 12.5 12.4 12.3 12.2 12.1 12.0 12.0 11.9 11.8 11.7 11.6 11.5 11.4 11.3 11.2 11.1 11.0
2.0 12.6 12.5 12.4 12.3 12.2 12.2 12.1 12.0 11.9 11.8 11.7 11.6 11.5 11.4 11.3 11.2 11.1 11.1 11.0 10.9
2.5 12.4 12.4 12.3 12.2 12.1 12.0 11.9 11.8 11.7 11.6 11.5 11.4 11.4 11.3 11.2 11.1 11.0 10.9 10.8 10.7
3.0 12.3 12.2 12.1 12.0 11.9 11.8 11.7 11.7 11.6 11.5 11.4 11.3 11.2 11.1 11.0 10.9 10.9 10.8 10.7 10.6
3.5 12.1 12.0 11.9 11.8 11.8 11.7 11.6 11.5 11.4 11.3 11.2 11.1 11.1 11.0 10.9 10.8 10.7 10.6 10.5 10.4
4.0 12.0 11.9 11.8 11.7 11.6 11.5 11.4 11.3 11.3 11.2 11.1 11.0 10.9 10.8 10.7 10.7 10.6 10.5 10.4 10.3
4.5 11.8 11.7 11.6 11.5 11.5 11.4 11.3 11.2 11.1 11.0 10.9 10.9 10.8 10.7 10.6 10.5 10.4 10.3 10.3 10.2
5.0 11.6 11.6 11.5 11.4 11.3 11.2 11.1 11.1 11.0 10.9 10.8 10.7 10.6 10.5 10.5 10.4 10.3 10.2 10.1 10.0
5.5 11.5 11.4 11.3 11.2 11.2 11.1 11.0 10.9 10.8 10.7 10.7 10.6 10.5 10.4 10.3 10.2 10.2 10.1 10.0 9.9
6.0 11.4 11.3 11.2 11.1 11.0 10.9 10.9 10.8 10.7 10.6 10.5 10.4 10.4 10.3 10.2 10.1 10.0 9.9 9.9 9.8
6.5 11.2 11.1 11.0 11.0 10.9 10.8 10.7 10.6 10.6 10.5 10.4 10.3 10.2 10.1 10.1 10.0 9.9 9.8 9.7 9.7
7.0 11.1 11.0 10.9 10.8 10.7 10.7 10.6 10.5 10.4 10.3 10.3 10.2 10.1 10.0 9.9 9.9 9.8 9.7 9.6 9.5
(26.97
)
680
(26.77
)
Atmospheric pressure, in millim e ter s of m ercury and (inc hes of mercury)
675
670
665
660
655
650
645
(26.57
)
(26.38
)
(26.18
)
(25.98
)
(25.79
)
(25.59
)
(25.39
)
640
(25.20
635
(25.00
630
(24.80
)
625
(24.61
)
620
(24.41
)
615
(24.21
)
610
(24.02
)
605
(23.82
)
600
(23.62
)
7.5 10.9 10.9 10.8 10.7 10.6 10.5 10.5 10.4 10.3 10.2 10.1 10.1 10.0 9.9 9.8 9.7 9.7 9.6 9.5 9.4
8.0 10.8 10.7 10.6 10.6 10.5 10.4 10.3 10.2 10.2 10.1 10.0 9.9 9.9 9.8 9.7 9.6 9.5 9.5 9.4 9.3
29
Page 34

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Temp.
695
690
685
°C
(27.36
(27.17
)
)
8.5 10.7 10.6 10.5 10.4 10.4 10.3 10.2 10.1 10.0 10.0 9.9 9.8 9.7 9.7 9.6 9.5 9.4 9.3 9.3 9.2
9.0 10.5 10.5 10.4 10.3 10.2 10.2 10.1 10.0 9.9 9.8 9.8 9.7 9.6 9.5 9.5 9.4 9.3 9.2 9.2 9.1
9.5 10.4 10.3 10.3 10.2 10.1 10.0 10.0 9.9 9.8 9.7 9.7 9.6 9.5 9.4 9.4 9. 3 9.2 9.1 9.0 9.0
10.0 10.3 10.2 10.1 10.1 10.0 9.9 9.8 9.8 9.7 9.6 9.5 9.5 9. 4 9.3 9.2 9.2 9.1 9.0 8.9 8.9
10.5 10.2 10.1 10.0 9.9 9.9 9.8 9.7 9.7 9.6 9.5 9.4 9.4 9.3 9.2 9.1 9.1 9.0 8.9 8.8 8.8
11.0 10.1 10.0 9.9 9.8 9.8 9.7 9.6 9.5 9.5 9.4 9.3 9.2 9.2 9.1 9.0 9.0 8.9 8.8 8.7 8.7
11.5 9.9 9.9 9.8 9.7 9.6 9.6 9.5 9.4 9.4 9.3 9.2 9.1 9.1 9.0 8.9 8.8 8.8 8.7 8.6 8.6
12.0 9.8 9.8 9.7 9.6 9.5 9.5 9.4 9.3 9.2 9.2 9.1 9.0 9.0 8.9 8.8 8.7 8.7 8.6 8.5 8.5
12.5 9.7 9.6 9.6 9.5 9.4 9.4 9.3 9.2 9.1 9.1 9.0 8.9 8.9 8.8 8.7 8.6 8.6 8.5 8.4 8.4
13.0 9.6 9.5 9.5 9.4 9.3 9.3 9.2 9.1 9.0 9.0 8.9 8.8 8.8 8.7 8.6 8.5 8.5 8.4 8.3 8.3
13.5 9.5 9.4 9.4 9.3 9.2 9.1 9.1 9.0 8.9 8.9 8.8 8.7 8.7 8.6 8.5 8.5 8.4 8.3 8.2 8.2
14.0 9.4 9.3 9.3 9.2 9.1 9.0 9.0 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.2 8.2 8.1
14.5 9.3 9.2 9.2 9.1 9.0 8.9 8.9 8.8 8.7 8.7 8.6 8.5 8.5 8.4 8.3 8.3 8.2 8.1 8.1 8.0
15.0 9.2 9.1 9.1 9.0 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.2 8.2 8.1 8.0 8.0 7.9
15.5 9.1 9.0 9.0 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.8
(26.97
)
680
(26.77
)
Atmospheric pressure, in millimeters of mercury and (inches of mercury)
675
670
665
660
655
650
645
(26.57
)
(26.38
)
(26.18
)
(25.98
)
(25.79
)
(25.59
)
(25.39
)
640
(25.20
635
(25.00
630
(24.80
)
625
(24.61
)
620
(24.41
)
615
(24.21
)
610
(24.02
)
605
(23.82
)
600
(23.62
)
16.0 9.0 8.9 8.9 8.8 8.7 8.7 8.6 8.5 8.5 8.4 8.3 8.3 8.2 8.1 8.1 8.0 7.9 7.9 7.8 7.7
16.5 8.9 8.8 8.8 8.7 8.6 8.6 8.5 8.4 8.4 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.8 7.8 7.7 7.7
17.0 8.8 8.7 8.7 8.6 8.5 8.5 8.4 8.3 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.8 7.8 7.7 7.6 7.6
17.5 8.7 8.6 8.6 8.5 8.5 8.4 8.3 8.3 8.2 8.1 8.1 8.0 7.9 7.9 7.8 7.7 7.7 7.6 7.6 7.5
18.0 8.6 8.6 8.5 8.4 8.4 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.9 7.8 7.7 7.7 7.6 7.5 7.5 7.4
18.5 8.5 8.5 8.4 8.3 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.8 7.8 7.7 7.7 7.6 7.5 7.5 7.4 7.3
19.0 8.4 8.4 8.3 8.3 8.2 8.1 8.1 8.0 7.9 7.9 7.8 7.8 7.7 7.6 7.6 7.5 7.4 7.4 7.3 7.3
19.5 8.4 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.9 7.8 7.7 7.7 7.6 7.6 7.5 7.4 7.4 7.3 7.2 7.2
20.0 8.3 8.2 8.2 8.1 8.0 8.0 7.9 7.8 7.8 7.7 7.7 7.6 7.5 7.5 7.4 7.4 7.3 7.2 7.2 7.1
20.5 8.2 8.1 8.1 8.0 7.9 7.9 7.8 7.8 7.7 7.6 7.6 7.5 7.5 7.4 7.3 7.3 7.2 7.2 7.1 7.0
21.0 8.1 8.1 8.0 7.9 7.9 7.8 7.8 7.7 7.6 7.6 7.5 7.5 7.4 7.3 7.3 7.2 7.2 7.1 7.0 7.0
21.5 8.0 8.0 7.9 7.9 7.8 7.7 7.7 7.6 7.6 7.5 7.4 7.4 7.3 7.3 7.2 7.1 7.1 7.0 7.0 6.9
22.0 8.0 7.9 7.8 7.8 7.7 7.7 7.6 7.5 7.5 7.4 7.4 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.8
22.5 7.9 7.8 7.8 7.7 7.6 7.6 7.5 7.5 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 6.9 6.9 6.8 6.8
23.0 7.8 7.7 7.7 7.6 7.6 7.5 7.5 7.4 7.3 7.3 7.2 7.2 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7
23.5 7.7 7.7 7.6 7.6 7.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.0 7.0 6.9 6.9 6.8 6.7 6.7 6.6
24.0 7.7 7.6 7.5 7.5 7.4 7.4 7.3 7.3 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.7 6.7 6.6 6.6
24.5 7.6 7.5 7.5 7.4 7.4 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5
25.0 7.5 7.5 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.4
25.5 7.4 7.4 7.3 7.3 7.2 7.2 7.1 7.1 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.4 6.4
26.0 7.4 7.3 7.3 7.2 7.2 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.5 6.5 6.4 6.4 6.3
26.5 7.3 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3
30
Page 35

Model No. PS-2108 Dissolved Oxygen Sensor
®
Temp.
695
690
685
°C
(27.36
(27.17
)
)
27.0 7.2 7.2 7.1 7.1 7.0 7.0 6.9 6.9 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2
27.5 7.2 7.1 7.1 7.0 7.0 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2
28.0 7.1 7.1 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.1 6.1
28.5 7.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.2 6.2 6.1 6.1 6.0
29.0 7.0 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.2 6.2 6.1 6.1 6.0 6.0
29.5 6.9 6.9 6.8 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9
30.0 6.9 6.8 6.8 6.7 6.7 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9
30.5 6.8 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8
31.0 6.7 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8
31.5 6.7 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7
32.0 6.6 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7
32.5 6.6 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6
33.0 6.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6
33.5 6.5 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5
34.0 6.4 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5
34.5 6.4 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4
35.0 6.3 6.3 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4
(26.97
)
680
(26.77
)
Atmospheric pressure, in millim e ter s of m ercury and (inc hes of mercury)
675
670
665
660
655
650
645
(26.57
)
(26.38
)
(26.18
)
(25.98
)
(25.79
)
(25.59
)
(25.39
)
640
(25.20
635
(25.00
630
(24.80
)
625
(24.61
)
620
(24.41
)
615
(24.21
)
610
(24.02
)
605
(23.82
)
600
(23.62
)
35.5 6.2 6.2 6.2 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.3
36.0 6.2 6.1 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.3 5.3
36.5 6.1 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.3 5.3 5.2
37.0 6.1 6.1 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.3 5.3 5.3 5.2
37.5 6.0 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.3 5.3 5.3 5.2 5.2
38.0 6.0 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.3 5.3 5.3 5.2 5.2 5.1
38.5 6.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.4 5.3 5.3 5.2 5.2 5.1 5.1
39.0 5.9 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.4 5.3 5.3 5.2 5.2 5.1 5.1 5.0
39.5 5.9 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.4 5.3 5.3 5.2 5.2 5.1 5.1 5.0 5.0
40.0 5.8 5.8 5.7 5.7 5.6 5.6 5.5 5.5 5.4 5.4 5.4 5.3 5.3 5.2 5.2 5.1 5.1 5.0 5.0 5.0
31
Page 36

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Table 2: Salinity correction factors for dissolved oxygen in water (based on
conductivity)
From R. F. Weiss (1970). Temp ° C, temperature in degrees Celsius;
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 0 1000 2000 3000 4000 5000 6000 7000 8000 9000 10000 11000 12000 13000 14000 15000 16000
0.0 1.000 0.996 0.992 0.989 0.985 0.981 0.9 77 0.973 0.969 0.965 0.961 0.957 0.953 0.950 0.946 0.942 0.938
1.0 1.000 0.996 0.992 0.989 0.985 0.981 0.9 77 0.973 0.969 0.965 0.962 0.958 0.954 0.950 0.946 0.942 0.938
2.0 1.000 0.996 0.992 0.989 0.985 0.981 0.9 77 0.973 0.970 0.966 0.962 0.958 0.954 0.950 0.946 0.942 0.938
3.0 1.000 0.996 0.993 0.989 0.985 0.981 0.9 77 0.974 0.970 0.966 0.962 0.958 0.954 0.951 0.947 0.943 0.939
4.0 1.000 0.996 0.993 0.989 0.985 0.981 0.9 78 0.974 0.970 0.966 0.962 0.959 0.955 0.951 0.947 0.943 0.939
5.0 1.000 0.996 0.993 0.989 0.985 0.981 0.9 78 0.974 0.970 0.966 0.963 0.959 0.955 0.951 0.947 0.944 0.940
6.0 1.000 0.996 0.993 0.989 0.985 0.982 0.9 78 0.974 0.970 0.967 0.963 0.959 0.955 0.952 0.948 0.944 0.940
7.0 1.000 0.996 0.993 0.989 0.985 0.982 0.9 78 0.974 0.971 0.967 0.963 0.959 0.956 0.952 0.948 0.944 0.941
8.0 1.000 0.996 0.993 0.989 0.986 0.982 0.9 78 0.975 0.971 0.967 0.963 0.960 0.956 0.952 0.949 0.945 0.941
9.0 1.000 0.996 0.993 0.989 0.986 0.982 0.9 78 0.975 0.971 0.967 0.964 0.960 0.956 0.953 0.949 0.945 0.941
10.0 1.000 0.996 0.993 0.989 0.986 0.982 0.979 0.975 0.971 0.968 0.964 0.960 0.957 0.953 0.949 0.946 0.942
11.0 1.000 0.996 0.993 0.989 0.986 0.982 0.979 0.975 0.971 0.968 0.964 0.961 0.957 0.953 0.950 0.946 0.942
12.0 1.000 0.997 0.993 0.989 0.986 0.982 0.979 0.975 0.972 0.968 0.965 0.961 0.957 0.954 0.950 0.946 0.943
13.0 1.000 0.997 0.993 0.990 0.986 0.983 0.979 0.975 0.972 0.968 0.965 0.961 0.958 0.954 0.950 0.947 0.943
14.0 1.000 0.997 0.993 0.990 0.986 0.983 0.979 0.976 0.972 0.969 0.965 0.961 0.958 0.954 0.951 0.947 0.943
15.0 1.000 0.997 0.993 0.990 0.986 0.983 0.979 0.976 0.972 0.969 0.965 0.962 0.958 0.955 0.951 0.947 0.944
16.0 1.000 0.997 0.993 0.990 0.986 0.983 0.979 0.976 0.972 0.969 0.966 0.962 0.958 0.955 0.951 0.948 0.944
17.0 1.000 0.997 0.993 0.990 0.986 0.983 0.980 0.976 0.973 0.969 0.966 0.962 0.959 0.955 0.952 0.948 0.945
18.0 1.000 0.997 0.993 0.990 0.987 0.983 0.980 0.976 0.973 0.969 0.966 0.963 0.959 0.956 0.952 0.949 0.945
19.0 1.000 0.997 0.993 0.990 0.987 0.983 0.980 0.976 0.973 0.970 0.966 0.963 0.959 0.956 0.952 0.949 0.945
20.0 1.000 0.997 0.993 0.990 0.987 0.983 0.980 0.977 0.973 0.970 0.966 0.963 0.960 0.956 0.953 0.949 0.946
21.0 1.000 0.997 0.993 0.990 0.987 0.984 0.980 0.977 0.973 0.970 0.967 0.963 0.960 0.957 0.953 0.950 0.946
22.0 1.000 0.997 0.993 0.990 0.987 0.984 0.980 0.977 0.974 0.970 0.967 0.964 0.960 0.957 0.953 0.950 0.947
23.0 1.000 0.997 0.994 0.990 0.987 0.984 0.980 0.977 0.974 0.971 0.967 0.964 0.960 0.957 0.954 0.950 0.947
24.0 1.000 0.997 0.994 0.990 0.987 0.984 0.981 0.977 0.974 0.971 0.967 0.964 0.961 0.957 0.954 0.951 0.947
25.0 1.000 0.997 0.994 0.990 0.987 0.984 0.981 0.977 0.974 0.971 0.968 0.964 0.961 0.958 0.954 0.951 0.948
26.0 1.000 0.997 0.994 0.990 0.987 0.984 0.981 0.978 0.974 0.971 0.968 0.965 0.961 0.958 0.955 0.951 0.948
27.0 1.000 0.997 0.994 0.991 0.987 0.984 0.981 0.978 0.975 0.971 0.968 0.965 0.962 0.958 0.955 0.952 0.948
28.0 1.000 0.997 0.994 0.991 0.987 0.984 0.981 0.978 0.975 0.972 0.968 0.965 0.962 0.959 0.955 0.952 0.949
29.0 1.000 0.997 0.994 0.991 0.988 0.984 0.981 0.978 0.975 0.972 0.969 0.965 0.962 0.959 0.956 0.952 0.949
32
Page 37

Model No. PS-2108 Dissolved Oxygen Sensor
®
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 0 1000 2000 3000 4000 5000 6000 7000 8000 9000 10000 11000 12000 13000 14000 15000 16000
30.0 1.000 0.997 0.994 0.991 0.988 0.985 0.981 0.978 0.975 0.972 0.969 0.966 0.962 0.959 0.956 0.953 0.950
31.0 1.000 0.997 0.994 0.991 0.988 0.985 0.982 0.978 0.975 0.972 0.969 0.966 0.963 0.959 0.956 0.953 0.950
32.0 1.000 0.997 0.994 0.991 0.988 0.985 0.982 0.979 0.975 0.972 0.969 0.966 0.963 0.960 0.957 0.953 0.950
33.0 1.000 0.997 0.994 0.991 0.988 0.985 0.982 0.979 0.976 0.973 0.969 0.966 0.963 0.960 0.957 0.954 0.951
34.0 1.000 0.997 0.994 0.991 0.988 0.985 0.982 0.979 0.976 0.973 0.970 0.967 0.963 0.960 0.957 0.954 0.951
35.0 1.000 0.997 0.994 0.991 0.988 0.985 0.982 0.979 0.976 0.973 0.970 0.967 0.964 0.961 0.957 0.954 0.951
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 17000 18000 19000 20000 21000 22000 23000 24000 25000 26000 27000 28000 29000 30000 31000 32000 33000
0.0 0.934 0.930 0.926 0.922 0.918 0.914 0. 910 0.905 0.901 0.897 0.893 0.889 0.885 0.881 0.877 0.873 0.869
1.0 0.934 0.930 0.926 0.922 0.918 0.914 0. 910 0.906 0.902 0.898 0.894 0.890 0.886 0.882 0.878 0.874 0.870
2.0 0.935 0.931 0.927 0.923 0.919 0.915 0.911 0.907 0.903 0.899 0.895 0.891 0.887 0.883 0.879 0.875 0.871
3.0 0.935 0.931 0.927 0.923 0.919 0.915 0.911 0.907 0.903 0.899 0.895 0.891 0.887 0.883 0.879 0.875 0.871
4.0 0.935 0.932 0.928 0.924 0.920 0.916 0. 912 0.908 0.904 0.900 0.896 0.892 0.888 0.884 0.880 0.876 0.872
5.0 0.936 0.932 0.928 0.924 0.920 0.917 0. 913 0.909 0.905 0.901 0.897 0.893 0.889 0.885 0.881 0.877 0.873
6.0 0.936 0.933 0.929 0.925 0.921 0.917 0. 913 0.909 0.905 0.902 0.898 0.894 0.890 0.886 0.882 0.878 0.874
7.0 0.937 0.933 0.929 0.925 0.922 0.918 0. 914 0.910 0.906 0.902 0.898 0.894 0.891 0.887 0.883 0.879 0.875
8.0 0.937 0.933 0.930 0.926 0.922 0.918 0. 914 0.911 0.907 0.903 0.899 0.895 0.891 0.887 0.884 0.880 0.876
9.0 0.938 0.934 0.930 0.926 0.923 0.919 0. 915 0.911 0.907 0.904 0.900 0.896 0.892 0.888 0.884 0.880 0.877
10.0 0.938 0.934 0.931 0.927 0.923 0.919 0.916 0.912 0.908 0.904 0.900 0.897 0.893 0.889 0.885 0.881 0.877
11.0 0.939 0.935 0.931 0.927 0.924 0.920 0.916 0.912 0.909 0.905 0.901 0.897 0.894 0.890 0.886 0.882 0.878
12.0 0.939 0.935 0.932 0.928 0.924 0.920 0.917 0.913 0.909 0.906 0.902 0.898 0.894 0.890 0.887 0.883 0.879
13.0 0.939 0.936 0.932 0.928 0.925 0.921 0.917 0.914 0.910 0.906 0.902 0.899 0.895 0.891 0.887 0.884 0.880
14.0 0.940 0.936 0.933 0.929 0.925 0.922 0.918 0.914 0.911 0.907 0.903 0.899 0.896 0.892 0.888 0.884 0.881
15.0 0.940 0.937 0.933 0.929 0.926 0.922 0.918 0.915 0.911 0.907 0.904 0.900 0.896 0.893 0.889 0.885 0.882
16.0 0.941 0.937 0.934 0.930 0.926 0.923 0.919 0.915 0.912 0.908 0.904 0.901 0.897 0.893 0.890 0.886 0.882
17.0 0.941 0.938 0.934 0.930 0.927 0.923 0.920 0.916 0.912 0.909 0.905 0.901 0.898 0.894 0.891 0.887 0.883
18.0 0.942 0.938 0.934 0.931 0.927 0.924 0.920 0.917 0.913 0.909 0.906 0.902 0.899 0.895 0.891 0.888 0.884
19.0 0.942 0.938 0.935 0.931 0.928 0.924 0.921 0.917 0.914 0.910 0.906 0.903 0.899 0.896 0.892 0.888 0.885
20.0 0.942 0.939 0.935 0.932 0.928 0.925 0.921 0.918 0.914 0.911 0.907 0.903 0.900 0.896 0.893 0.889 0.886
21.0 0.943 0.939 0.936 0.932 0.929 0.925 0.922 0.918 0.915 0.911 0.908 0.904 0.901 0.897 0.893 0.890 0.886
22.0 0.943 0.940 0.936 0.933 0.929 0.926 0.922 0.919 0.915 0.912 0.908 0.905 0.901 0.898 0.894 0.891 0.887
23.0 0.944 0.940 0.937 0.933 0.930 0.926 0.923 0.919 0.916 0.912 0.909 0.905 0.902 0.898 0.895 0.891 0.888
33
Page 38

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 17000 18000 19000 20000 21000 22000 23000 24000 25000 26000 27000 28000 29000 30000 31000 32000 33000
24.0 0.944 0.941 0.937 0.934 0.930 0.927 0.923 0.920 0.917 0.913 0.910 0.906 0.903 0.899 0.896 0.892 0.889
25.0 0.944 0.941 0.938 0.934 0.931 0.927 0.924 0.921 0.917 0.914 0.910 0.907 0.903 0.900 0.896 0.893 0.889
26.0 0.945 0.941 0.938 0.935 0.931 0.928 0.925 0.921 0.918 0.914 0.911 0.907 0.904 0.901 0.897 0.894 0.890
27.0 0.945 0.942 0.938 0.935 0.932 0.928 0.925 0.922 0.918 0.915 0.911 0.908 0.905 0.901 0.898 0.894 0.891
28.0 0.946 0.942 0.939 0.936 0.932 0.929 0.926 0.922 0.919 0.915 0.912 0.909 0.905 0.902 0.898 0.895 0.892
29.0 0.946 0.943 0.939 0.936 0.933 0.929 0.926 0.923 0.919 0.916 0.913 0.909 0.906 0.903 0.899 0.896 0.892
30.0 0.946 0.943 0.940 0.936 0.933 0.930 0.927 0.923 0.920 0.917 0.913 0.910 0.907 0.903 0.900 0.896 0.893
31.0 0.947 0.943 0.940 0.937 0.934 0.930 0.927 0.924 0.920 0.917 0.914 0.911 0.907 0.904 0.901 0.897 0.894
32.0 0.947 0.944 0.941 0.937 0.934 0.931 0.928 0.924 0.921 0.918 0.914 0.911 0.908 0.905 0.901 0.898 0.895
33.0 0.947 0.944 0.941 0.938 0.935 0.931 0.928 0.925 0.922 0.918 0.915 0.912 0.908 0.905 0.902 0.899 0.895
34.0 0.948 0.945 0.941 0.938 0.935 0.932 0.929 0.925 0.922 0.919 0.916 0.912 0.909 0.906 0.903 0.899 0.896
35.0 0.948 0.945 0.942 0.939 0.935 0.932 0.929 0.926 0.923 0.919 0.916 0.913 0.910 0.906 0.903 0.900 0.897
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 34000 35000 36000 37000 38000 39000 40000 41000 42000 43000 44000 45000 46000 47000 48000 49000 50000
0.0 0.865 0.861 0.856 0.852 0.848 0.844 0.8 40 0.836 0.832 0.828 0.823 0.819 0.815 0.811 0.807 0.803 0.799
1.0 0.866 0.862 0.857 0.853 0.849 0.845 0.8 41 0.837 0.833 0.829 0.825 0.821 0.816 0.812 0.808 0.804 0.800
2.0 0.867 0.862 0.858 0.854 0.850 0.846 0.8 42 0.838 0.834 0.830 0.826 0.822 0.818 0.814 0.809 0.805 0.801
3.0 0.867 0.863 0.859 0.855 0.851 0.847 0.8 43 0.839 0.835 0.831 0.827 0.823 0.819 0.815 0.811 0.807 0.803
4.0 0.868 0.864 0.860 0.856 0.852 0.848 0.8 44 0.840 0.836 0.832 0.828 0.824 0.820 0.816 0.812 0.808 0.804
5.0 0.869 0.865 0.861 0.857 0.853 0.849 0.8 45 0.841 0.837 0.833 0.829 0.825 0.821 0.817 0.813 0.809 0.805
6.0 0.870 0.866 0.862 0.858 0.854 0.850 0.8 46 0.842 0.838 0.834 0.830 0.826 0.822 0.818 0.814 0.810 0.806
7.0 0.871 0.867 0.863 0.859 0.855 0.851 0.8 47 0.843 0.839 0.835 0.831 0.828 0.824 0.820 0.816 0.812 0.808
8.0 0.872 0.868 0.864 0.860 0.856 0.852 0.8 48 0.844 0.840 0.837 0.833 0.829 0.825 0.821 0.817 0.813 0.809
9.0 0.873 0.869 0.865 0.861 0.857 0.853 0.8 49 0.845 0.842 0.838 0.834 0.830 0.826 0.822 0.818 0.814 0.810
10.0 0.874 0.870 0.866 0.862 0.858 0.854 0.850 0.846 0.843 0.839 0.835 0.831 0.827 0.823 0.819 0.815 0.811
11.0 0.874 0.871 0.867 0.863 0.859 0.855 0.851 0.848 0.844 0.840 0.836 0.832 0.828 0.824 0.820 0.817 0.813
12.0 0.875 0.871 0.868 0.864 0.860 0.856 0.852 0.849 0.845 0.841 0.837 0.833 0.829 0.825 0.822 0.818 0.814
13.0 0.876 0.872 0.869 0.865 0.861 0.857 0.853 0.850 0.846 0.842 0.838 0.834 0.830 0.827 0.823 0.819 0.815
14.0 0.877 0.873 0.869 0.866 0.862 0.858 0.854 0.851 0.847 0.843 0.839 0.835 0.832 0.828 0.824 0.820 0.816
15.0 0.878 0.874 0.870 0.867 0.863 0.859 0.855 0.852 0.848 0.844 0.840 0.836 0.833 0.829 0.825 0.821 0.817
16.0 0.879 0.875 0.871 0.867 0.864 0.860 0.856 0.853 0.849 0.845 0.841 0.838 0.834 0.830 0.826 0.822 0.819
17.0 0.879 0.876 0.872 0.868 0.865 0.861 0.857 0.854 0.850 0.846 0.842 0.839 0.835 0.831 0.827 0.824 0.820
34
Page 39

Model No. PS-2108 Dissolved Oxygen Sensor
®
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 34000 35000 36000 37000 38000 39000 40000 41000 42000 43000 44000 45000 46000 47000 48000 49000 50000
18.0 0.880 0.877 0.873 0.869 0.866 0.862 0.858 0.855 0.851 0.847 0.843 0.840 0.836 0.832 0.829 0.825 0.821
19.0 0.881 0.877 0.874 0.870 0.867 0.863 0.859 0.855 0.852 0.848 0.844 0.841 0.837 0.833 0.830 0.826 0.822
20.0 0.882 0.878 0.875 0.871 0.867 0.864 0.860 0.856 0.853 0.849 0.845 0.842 0.838 0.834 0.831 0.827 0.823
21.0 0.883 0.879 0.876 0.872 0.868 0.865 0.861 0.857 0.854 0.850 0.846 0.843 0.839 0.836 0.832 0.828 0.825
22.0 0.884 0.880 0.876 0.873 0.869 0.866 0.862 0.858 0.855 0.851 0.848 0.844 0.840 0.837 0.833 0.829 0.826
23.0 0.884 0.881 0.877 0.874 0.870 0.866 0.863 0.859 0.856 0.852 0.849 0.845 0.841 0.838 0.834 0.830 0.827
24.0 0.885 0.882 0.878 0.874 0.871 0.867 0.864 0.860 0.857 0.853 0.850 0.846 0.842 0.839 0.835 0.832 0.828
25.0 0.886 0.882 0.879 0.875 0.872 0.868 0.865 0.861 0.858 0.854 0.851 0.847 0.843 0.840 0.836 0.833 0.829
26.0 0.887 0.883 0.880 0.876 0.873 0.869 0.866 0.862 0.859 0.855 0.852 0.848 0.844 0.841 0.837 0.834 0.830
27.0 0.887 0.884 0.880 0.877 0.874 0.870 0.867 0.863 0.860 0.856 0.853 0.849 0.845 0.842 0.838 0.835 0.831
28.0 0.888 0.885 0.881 0.878 0.874 0.871 0.867 0.864 0.860 0.857 0.853 0.850 0.846 0.843 0.839 0.836 0.832
29.0 0.889 0.886 0.882 0.879 0.875 0.872 0.868 0.865 0.861 0.858 0.854 0.851 0.848 0.844 0.841 0.837 0.834
30.0 0.890 0.886 0.883 0.879 0.876 0.873 0.869 0.866 0.862 0.859 0.855 0.852 0.849 0.845 0.842 0.838 0.835
31.0 0.890 0.887 0.884 0.880 0.877 0.873 0.870 0.867 0.863 0.860 0.856 0.853 0.850 0.846 0.843 0.839 0.836
32.0 0.891 0.888 0.884 0.881 0.878 0.874 0.871 0.868 0.864 0.861 0.857 0.854 0.851 0.847 0.844 0.840 0.837
33.0 0.892 0.889 0.885 0.882 0.879 0.875 0.872 0.868 0.865 0.862 0.858 0.855 0.851 0.848 0.845 0.841 0.838
34.0 0.893 0.889 0.886 0.883 0.879 0.876 0.873 0.869 0.866 0.863 0.859 0.856 0.852 0.849 0.846 0.842 0.839
35.0 0.893 0.890 0.887 0.883 0.880 0.877 0.874 0.870 0.867 0.863 0.860 0.857 0.853 0.850 0.847 0.843 0.840
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 51000 52000 53000 54000 55000 56000 57000 58000 59000 60000 61000 62000 63000 64000 65000 66000 67000
0.0 0.795 0.790 0.786 0.782 0.778 0.774 0. 770 0.766 0.761 0.757 0.753 0.749 0.745 0.741 0.737 0.732 0.728
1.0 0.796 0.792 0.788 0.783 0.779 0.775 0. 771 0.767 0.763 0.759 0.755 0.751 0.746 0.742 0.738 0.734 0.730
2.0 0.797 0.793 0.789 0.785 0.781 0.777 0. 773 0.768 0.764 0.760 0.756 0.752 0.748 0.744 0.740 0.736 0.732
3.0 0.798 0.794 0.790 0.786 0.782 0.778 0. 774 0.770 0.766 0.762 0.758 0.754 0.750 0.746 0.741 0.737 0.733
4.0 0.800 0.796 0.792 0.788 0.784 0.780 0. 775 0.771 0.767 0.763 0.759 0.755 0.751 0.747 0.743 0.739 0.735
5.0 0.801 0.797 0.793 0.789 0.785 0.781 0. 777 0.773 0.769 0.765 0.761 0.757 0.753 0.749 0.745 0.741 0.737
6.0 0.802 0.798 0.794 0.790 0.786 0.782 0. 778 0.774 0.770 0.766 0.762 0.758 0.754 0.750 0.746 0.742 0.738
7.0 0.804 0.800 0.796 0.792 0.788 0.784 0. 780 0.776 0.772 0.768 0.764 0.760 0.756 0.752 0.748 0.744 0.740
8.0 0.805 0.801 0.797 0.793 0.789 0.785 0. 781 0.777 0.773 0.769 0.765 0.761 0.757 0.753 0.749 0.745 0.742
9.0 0.806 0.802 0.798 0.794 0.790 0.787 0. 783 0.779 0.775 0.771 0.767 0.763 0.759 0.755 0.751 0.747 0.743
10.0 0.807 0.804 0.800 0.796 0.792 0.788 0.784 0.780 0.776 0.772 0.768 0.764 0.760 0.757 0.753 0.749 0.745
11.0 0.809 0.805 0.801 0.797 0.793 0.789 0.785 0.781 0.778 0.774 0.770 0.766 0.762 0.758 0.754 0.750 0.746
35
Page 40

PASPort Dissolved Oxygen Sensor Model No. PS-2108
®
Temp. Conductivity, in microsiemens per centimeter at 25 degrees Celsius
°C 51000 52000 53000 54000 55000 56000 57000 58000 59000 60000 61000 62000 63000 64000 65000 66000 67000
12.0 0.810 0.806 0.802 0.798 0.794 0.791 0.787 0.783 0.779 0.775 0.771 0.767 0.763 0.760 0.756 0.752 0.748
13.0 0.811 0.807 0.804 0.800 0.796 0.792 0.788 0.784 0.780 0.777 0.773 0.769 0.765 0.761 0.757 0.753 0.750
14.0 0.812 0.809 0.805 0.801 0.797 0.793 0.789 0.786 0.782 0.778 0.774 0.770 0.766 0.763 0.759 0.755 0.751
15.0 0.814 0.810 0.806 0.802 0.798 0.795 0.791 0.787 0.783 0.779 0.776 0.772 0.768 0.764 0.760 0.756 0.753
16.0 0.815 0.811 0.807 0.804 0.800 0.796 0.792 0.788 0.785 0.781 0.777 0.773 0.769 0.766 0.762 0.758 0.754
17.0 0.816 0.812 0.809 0.805 0.801 0.797 0.794 0.790 0.786 0.782 0.778 0.775 0.771 0.767 0.763 0.760 0.756
18.0 0.817 0.814 0.810 0.806 0.802 0.799 0.795 0.791 0.787 0.784 0.780 0.776 0.772 0.769 0.765 0.761 0.757
19.0 0.819 0.815 0.811 0.807 0.804 0.800 0.796 0.792 0.789 0.785 0.781 0.777 0.774 0.770 0.766 0.763 0.759
20.0 0.820 0.816 0.812 0.809 0.805 0.801 0.797 0.794 0.790 0.786 0.783 0.779 0.775 0.771 0.768 0.764 0.760
21.0 0.821 0.817 0.814 0.810 0.806 0.802 0.799 0.795 0.791 0.788 0.784 0.780 0.777 0.773 0.769 0.766 0.762
22.0 0.822 0.818 0.815 0.811 0.807 0.804 0.800 0.796 0.793 0.789 0.785 0.782 0.778 0.774 0.771 0.767 0.763
23.0 0.823 0.820 0.816 0.812 0.809 0.805 0.801 0.798 0.794 0.790 0.787 0.783 0.779 0.776 0.772 0.768 0.765
24.0 0.824 0.821 0.817 0.814 0.810 0.806 0.803 0.799 0.795 0.792 0.788 0.785 0.781 0.777 0.774 0.770 0.766
25.0 0.826 0.822 0.818 0.815 0.811 0.808 0.804 0.800 0.797 0.793 0.789 0.786 0.782 0.779 0.775 0.771 0.768
26.0 0.827 0.823 0.820 0.816 0.812 0.809 0.805 0.802 0.798 0.794 0.791 0.787 0.784 0.780 0.776 0.773 0.769
27.0 0.828 0.824 0.821 0.817 0.814 0.810 0.806 0.803 0.799 0.796 0.792 0.789 0.785 0.781 0.778 0.774 0.771
28.0 0.829 0.825 0.822 0.818 0.815 0.811 0.808 0.804 0.801 0.797 0.794 0.790 0.786 0.783 0.779 0.776 0.772
29.0 0.830 0.827 0.823 0.820 0.816 0.812 0.809 0.805 0.802 0.798 0.795 0.791 0.788 0.784 0.781 0.777 0.774
30.0 0.831 0.828 0.824 0.821 0.817 0.814 0.810 0.807 0.803 0.800 0.796 0.793 0.789 0.786 0.782 0.779 0.775
31.0 0.832 0.829 0.825 0.822 0.818 0.815 0.811 0.808 0.804 0.801 0.797 0.794 0.790 0.787 0.783 0.780 0.776
32.0 0.833 0.830 0.826 0.823 0.820 0.816 0.813 0.809 0.806 0.802 0.799 0.795 0.792 0.788 0.785 0.781 0.778
33.0 0.834 0.831 0.828 0.824 0.821 0.817 0.814 0.810 0.807 0.803 0.800 0.797 0.793 0.790 0.786 0.783 0.779
34.0 0.836 0.832 0.829 0.825 0.822 0.818 0.815 0.812 0.808 0.805 0.801 0.798 0.794 0.791 0.788 0.784 0.781
35.0 0.837 0.833 0.830 0.826 0.823 0.820 0.816 0.813 0.809 0.806 0.803 0.799 0.796 0.792 0.789 0.785 0.782
36
 Loading...
Loading...