Page 1
Total Hardness ezSample™ Field Titrator (EZ-2338)
20–200 ppm (mg/L) CaCO3
Safety Information
Read the MSDS before performing this test procedure. Wear safety glasses and disposable gloves.
Sample Pretreatment
If the sample is turbid, it must be filtered prior to performing this test procedure.
Test Procedure
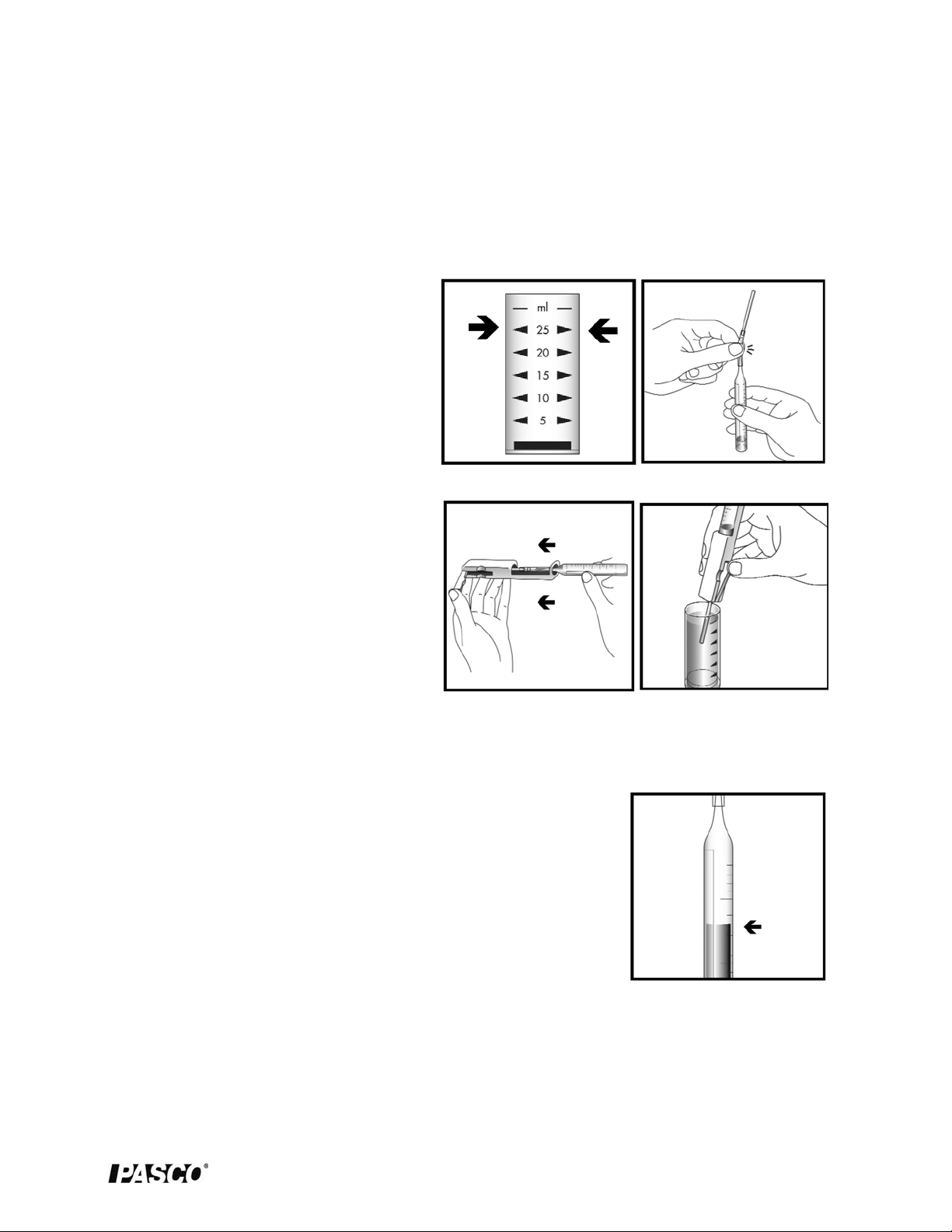
1. Fill the sample cup to the 25 mL mark
with the sample (Figure 1).
2. Gently snap the tip of the ezSample
Snap Vial (ampoule) at the black snap
ring (Figure 2).
NOTE: When the tip is snapped, the flexible
tubing will remain in place on the tapered
neck of the ampoule.
3. Lift the control bar and insert the
ampoule assembly into the titrator
(Figure 3).
NOTE: The rigid sample pipe will extend
approximately 1.5 inches beyond the body of
the titrator.
4. Hold the titrator with the sample pipe
in the sample and press the control bar
firmly, but briefly, to pull in a small
amount of sample (Figure 4). The
contents will turn a BLUE color.
Figure 1
Figure 3
Figure 2
Figure 4
NOTE: NEVER press the control bar unless the sample pipe is immersed in the
sample.
5. With the sample pipe in the sample, press the control bar again
briefly to allow another small amount of sample to be drawn into the
ampoule.
6. After each addition, rock the entire assembly to mix the contents of
the ampoule. Watch for a color change from BLUE to
7. Repeat steps 6 and 7 until a permanent color change occurs.
8. When the color of the liquid in the ampoule changes to PINK,
PINK.
remove the ampoule from the Titrator. Hold the ampoule in a vertical
position and read the scale opposite the liquid level (Figure 5).
Figure 5
NOTE: Divide ppm calcium carbonate by 17.16 to convert results to grains per gallon.
1
012-10114
Page 2

Test Method Description
The total hardness ezSample test method employs ethylenediaminetetraacetic acid (EDTA) titrimetric
chemistry.
magnesium ions. Calmagite is used as the endpoint indicator. Results are expressed as calcium
carbonate (CaCO
NOTE: Because the ampoules have nonlinear scales, the accuracy of the ezSample field titrator kit varies with the
analyte concentration. At the low end of the test range, the accuracy is ± 5%. At the high end of the range, the
accuracy falls to ± 20%.
1,2
In an alkaline solution, EDTA forms a chelated soluble complex with calcium and
1,2,3
3).
References
1. Method 2340C. APHA Standard Methods, 20th ed., p. 2-37, (1998).
2. Method 130.2. EPA Methods for Chemical Analysis of Water &Wastes. (1983).
2
012-10114
 Loading...
Loading...