Page 1
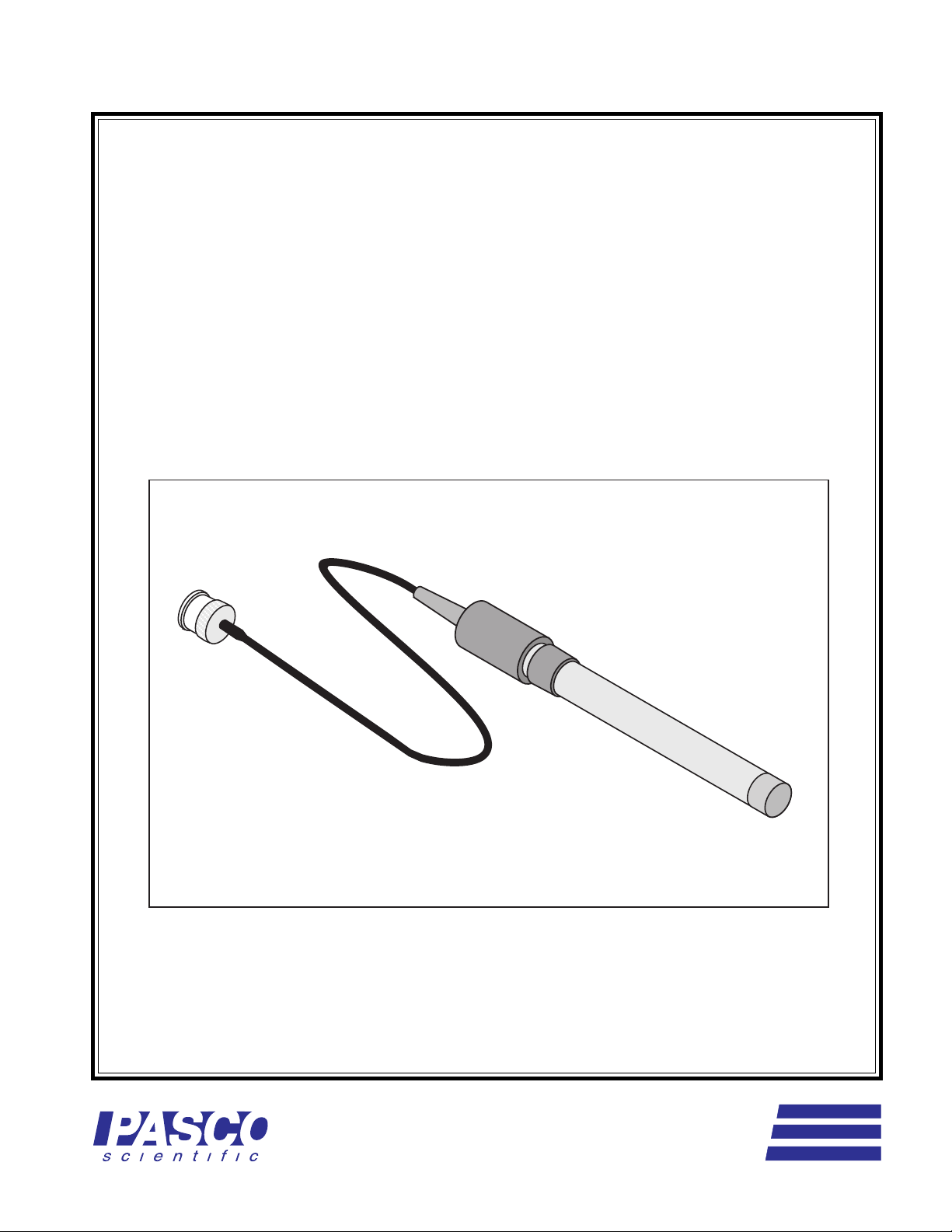
Instruction Manual and
Experiment Guide for the
PASCO scientific
Model CI-6734
SODIUM ION SELECTIVE
ELECTRODE
012-06615A
9/97
© 1997 PASCO scientific $7.50
®
10101 Foothills Blvd. • P.O. Box 619011 • Roseville, CA 95678-9011 USA
Phone (916) 786-3800 • FAX (916) 786-8905 • web: www.pasco.com
better
ways to
teach science
Page 2
Sodium Ion Selective Electrode 012–06615A
Always use eye protection
and gloves when working
with chemicals.
Page 3

012–06615A Sodium Ion Selective Electrode
Table of Contents
Introduction......................................................................................................................................... 1
Theory .................................................................................................................................................. 1
Equipment ........................................................................................................................................... 2
Included ........................................................................................................................................ 2
Additional Required...................................................................................................................... 2
Required Solutions.............................................................................................................................. 2
General Preparation ........................................................................................................................... 4
Electrode Preparation.................................................................................................................... 4
Electrode Slope Check Using Science Workshop .......................................................................... 4
Measurement....................................................................................................................................... 5
Measuring Hints............................................................................................................................ 5
Sample Requirements ................................................................................................................... 5
Units of Measurement................................................................................................................... 5
Measurement Procedure .................................................................................................................... 6
Direct Measurement of Sodium ....................................................................................................6
Low Level Sodium Determination ............................................................................................... 7
Electrode Characteristics ................................................................................................................... 8
Reproducibility ............................................................................................................................. 8
Interferences.................................................................................................................................. 8
Temperature Influences................................................................................................................. 9
Electrode Response ..................................................................................................................... 10
Limits of Detection ..................................................................................................................... 10
pH Effects ................................................................................................................................... 10
Electrode Life ............................................................................................................................. 11
Maintenance .......................................................................................................................................11
Electrode Storage ........................................................................................................................ 11
Specifications..................................................................................................................................... 12
Troubleshooting Guide ..................................................................................................................... 12
Glassware/Plastic-ware ............................................................................................................... 12
Electrode ..................................................................................................................................... 12
Standards & Reagents ................................................................................................................. 13
Sample ........................................................................................................................................ 13
Technique .................................................................................................................................... 13
Troubleshooting Hints ...................................................................................................................... 13
Technical Support ............................................................................................................................. 17
i
Page 4
Copyright, Warranty , and Equipment Return
PleaseFeel free to duplicate this manual
subject to the copyright restrictions below.
Copyright Notice
The PASCO scientific 012-06615 A manual is
copyrighted and all rights reserved. However,
permission is granted to non-profit educational
institutions for reproduction of any part of the Sodium
Ion Selective Electrode manual providing the
reproductions are used only for their laboratories and
are not sold for profit. Reproduction under any other
circumstances, without the written consent of PASCO
scientific, is prohibited.
Limited Warranty
PASCO scientific warrants the product to be free from
defects in materials and workmanship for a period of
one year from the date of shipment to the customer.
PASCO will repair or replace at its option any part of
the product which is deemed to be defective in
material or workmanship. The warranty does not
cover damage to the product caused by abuse or
improper use. Determination of whether a product
failure is the result of a manufacturing defect or
improper use by the customer shall be made solely by
PASCO scientific. Responsibility for the return of
equipment for warranty repair belongs to the
customer. Equipment must be properly packed to
prevent damage and shipped postage or freight
prepaid. (Damage caused by improper packing of the
equipment for return shipment will not be covered by
the warranty.) Shipping costs for returning the
equipment after repair will be paid by PASCO
scientific.
Credits
Author: Peter Boyle
Editor: Steve Miller
Equipment Return
Should the product have to be returned to PASCO
scientific for any reason, notify PASCO scientific by
letter, phone, or fax BEFORE returning the product.
Upon notification, the return authorization and
shipping instructions will be promptly issued.
ä
NOTE: NO EQUIPMENT WILL BE
ACCEPTED FOR RETURN WITHOUT AN
AUTHORIZATION FROM PASCO.
When returning equipment for repair, the units must
be packed properly. Carriers will not accept
responsibility for damage caused by improper
packing. To be certain the unit will not be damaged in
shipment, observe the following rules:
➀ The packing carton must be strong enough for the
item shipped.
➁ Make certain there are at least two inches of packing
material between any point on the apparatus and the
inside walls of the carton.
➂ Make certain that the packing material cannot shift in
the box or become compressed, allowing the
instrument come in contact with the packing carton.
Address: PASCO scientific
10101 Foothills Blvd.
P.O. Box 619011
Roseville, CA 95678-9011
Phone: (916) 786-3800
FAX: (916) 786-3292
email: techsupp@pasco.com
web: www.pasco.com
ii
Page 5

012–06615A Sodium Ion Selective Electrode
E=E
S
X
E
X
X
γ
C
1
C
X
Introduction
The PASCO scientific Sodium Ion Selective Electrode is used to quickly, simply, accurately, and economically
measure sodium ions in aqueous solutions.
Theory
The Sodium Ion Selective Electrode is composed of a sodium-selective glass membrane bonded to a glass body.
When the membrane is in contact with a solution containing sodium ions, an electrode potential develops across
the membrane. This electrode potential is measured against a constant reference potential, using an ISE Amplifier
and a ScienceWorkshop computer interface. The level of sodium ions, corresponding to the measured potential, is
described by the Nernst equation.
log
+
0
where:
= measuredelectrodepotential
= referencepotential(a constant)
E
0
mV
S = electrodeslope( ≈ 59
decade
)
= level ofsodiumionsin solution
The activity, X, represents the effective concentration of free sodium ions in the solution. The activity is related to
the free ion concentration, C
, by the activity coefficient, γ by:
ƒ
=
ƒ
Activity coefficients may vary, depending on the total ionic strength, I, determined as:
I =
ΣCXZ
2
2
X
where:
= concentrationofion
X
ZX= charge of ion X
Σ = sum of all of the types of ions in the solution
In the case of high and constant ionic strength relative to the sensed ion concentration, the activity coefficient, γ is
constant and the activity, X, is directly proportional to the concentration.
1
Page 6
Sodium Ion Selective Electrode 012–06615A
To adjust the background ionic strength to a high and constant value, ionic strength adjuster is added to samples
and standards. The recommended ISA for sodium is an ammonium chloride/ammonium hydroxide buffer.
Solutions other than this may be used as ionic strength adjusters as long as ions that they contain do not interfere
with the electrode’s response to sodium ions.
Equipment
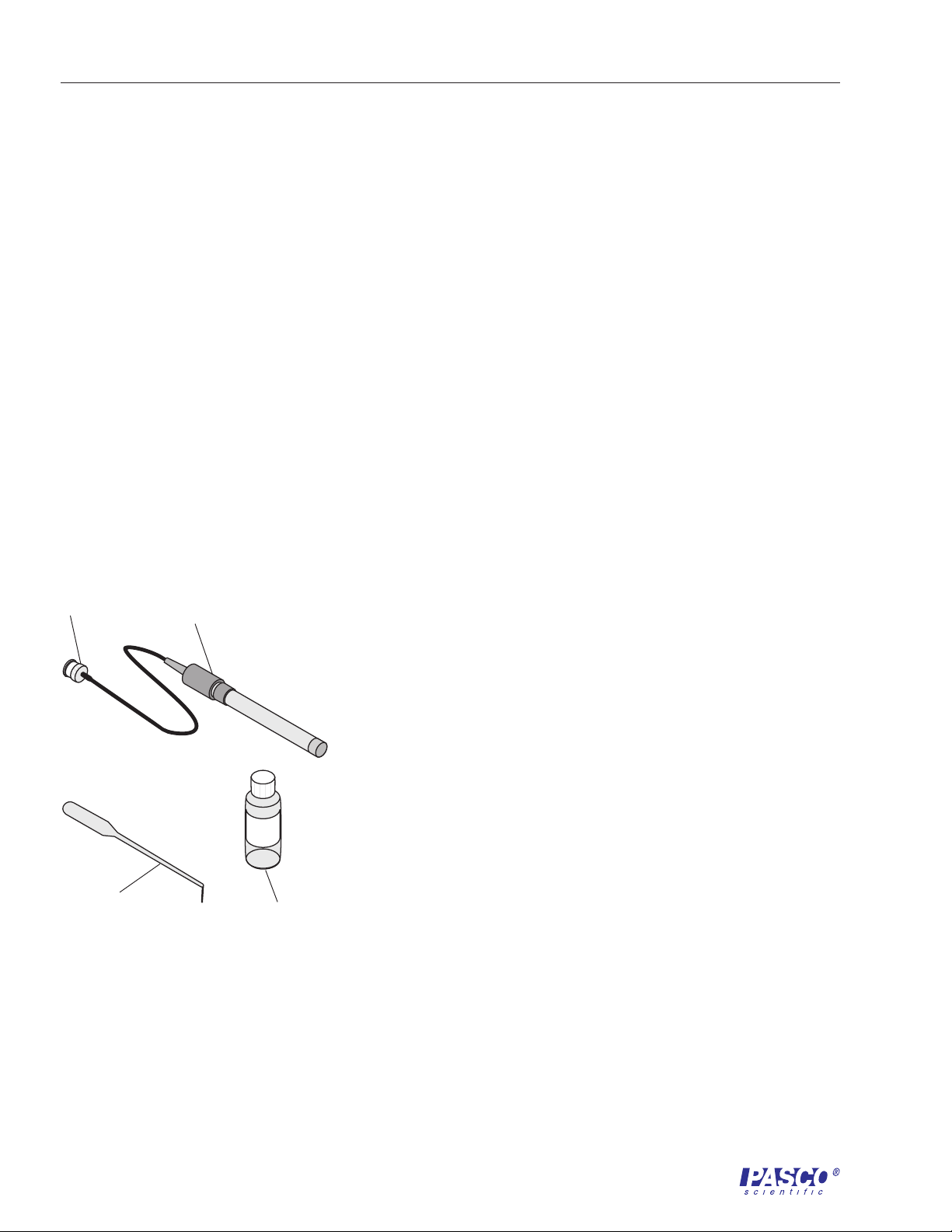
Included:
to ISE
Amplifier
• Sodium Ion Selective
Electrode
• Sodium Ion Selective
Electrode fill solution
• pipette for fill solution
Sodium Ion Selective
Electrode
Additional Required:
Required Equipment
• PASCO CI-6738 ISE (Ion Selective Electrode) Amplifier
• Science Workshop 2.2.5 or higher
• PASCO Science Workshop Computer Interface
• Semilogarithmic 4-cycle graph paper for preparing
calibration curves.
• magnetic stir plate
• Lab-ware made of plastic, not glass, for all low level
measurements.
Required Solutions
The stock solutions described in this section may be created as
described in the text or ordered directly from PASCO. The solutions
available for order, and their respective prices are listed on the ‘ISE
Working Solution Price List’.
• Deionized or distilled water for solution and standard
preparation.
filling pipette
Figure 1.
Included Equipment
2
R
O
R
O
1
e
f
e
r
0
1
3
S
e
n
c
e
Fi
l
l
4
M
K
E
L
P
M
A
n
tio
u
l
o
S
I
C
filling
solution
• Ionic Strength Adjuster (ISA), 4 M NH
Cl/4 M NH4 OH
4
To prepare this solution, half fill a 1000 ml volumetric flask
with distilled water and add 214 grams of reagent-grade
ammonium chloride (NH
concentrated ammonium hydroxide (NH
Cl). Under a hood, add 270 ml of
4
OH), swirl the
4
flask gently to dissolve the solid, and allow to cool. Fill the
flask to the mark with distilled water, cap, and upend
several times to mix the solution.
• Sodium Electrode Storage Solution, 5 M NaCl
To prepare this solution, add 29.2 grams of reagent-grade
sodium chloride (NaCl) to 100 ml of distilled water. To
each 100 ml storage solution, add 2 ml of ISA.
Page 7

012–06615A Sodium Ion Selective Electrode
ä
Note: Electrodes must not be stored in distilled water or air. Do not rinse with
distilled water.
• Dilute Electrode Rinse Solution
To prepare this solution from your own laboratory stock, add 20 ml of ISA to a one liter volumetric flask
and fill to the mark with distilled water. Use this solution to rinse the electrode between measurements.
• Sodium Standard, 0.1 M NaCl
To prepare this solution, half fill a one liter volumetric flask with distilled water and add 5.84 grams of
reagent-grade NaCl. Swirl the flask gently to dissolve the solid. Fill the flask to the mark with distilled water,
cap, and upend several times to mix the solution.
• Sodium Standard, 1,000 ppm Na
+
To prepare this solution, half fill a one liter volumetric flask with distilled water and add 2.542 grams of
reagent grade NaCl. Swirl the flask gently to dissolve the solid. Fill the flask to the mark with distilled water,
cap, and upend several times to mix the solution.
• Sodium Standard, 100 ppm Na
+
To prepare this solution, half fill a one liter volumetric flask with distilled water and add 0.254 grams of
reagent grade NaCl. Swirl the flask to dissolve the solid. Fill the flask to the mark with distilled water, cap,
and upend several times to mix the solution.
3
Page 8

Sodium Ion Selective Electrode 012–06615A
General Preparation
fill hole
Electrode Preparation
1. Remove the rubber cap covering the electrode tip and
the rubber insert covering the filling hole of the Sodium
Ion Selective Electrode. Fill the electrode with the
included filling solution to a level just below the fill
hole. No preparation is required with a sealed reference
electrode.
a
rubber
sleeve
rubber cap
2. Connect the Sodium Ion Selective Electrode to the ISE
Amplifier and insert the DIN connector of the ISE
Amplifier into analog channel A or B on a PASCO
Computer Interface (Figures 2b and 2c).
Electrode Slope Check Using ScienceWorkshop
(check electrodes each day)
1. To a 150 ml glass beaker, add 100 ml of distilled water
and 2 ml of ISA. Place the beaker on a magnetic stirrer
and begin stirring at a constant rate. Start the
ScienceWorkshop software, select the Ion Selective
Electrode sensor, open a Digital display, change the
number of digits to the right of the decimal from 1 to 3,
and begin monitoring data. Lower the electrode tip into
the solution.
2. Using a pipette, add 1 ml of 0.1 M or 1,000 ppm sodium
standard to the beaker. When the reading has stabilized,
record the voltage reading indicated in the Digits display.
3. Using a pipette, add 10 ml of the same sodium standard
used above to the beaker. When the reading has stabilized,
record the voltage reading indicated in the Digits display.
4. Determine the difference between the two readings. A
difference of 59 ± 4mV indicates correct electrode
operation assuming the solution temperature is between
20 °C and 25 °C. See the Troubleshooting sections if
the potential change is not within this range.
b
Insert DIN
connector into
analog channel
A or B .
Rotate ring
c
one-quarter
turn to secure
electrode
connector
Figure 2
Equipment Setup. a: filling the electrode
with filling solution; b & c: connecting the
electrode to the ISE Amplifier and to the
computer interface
ISE Amplifier
➤ Note: Slope is defined as the change in potential observed when the concentration
changes by a factor of 10.
4
Page 9

012–06615A Sodium Ion Selective Electrode
Measurement
Measuring Hints
• All samples and standards should be at the same temperature for precise measurement, preferably ambient
temperature. A difference of l °C in temperature will result in about a 2% measurement error. The electrodes
should not be used above 70 °C.
• Constant, but not violent, stirring is necessary for accurate measurement. Magnetic stirrers can generate
sufficient heat to change the solution temperature. To counteract this effect, place a piece of insulating
material, such as a styrofoam sheet, between the stirrer and the beaker.
• Always rinse the electrodes with electrode rinse solution from a wash bottle between measurements. Use
a clean, dry tissue to prevent cross contamination. Never use distilled water.
• Store the electrodes in electrode storage solution between measurements. Do not store in air or distilled
water. Always soak new electrodes overnight in electrode storage solution prior to first use. When making
low level sodium measurements, use a dilute sodium chloride storage solution. Add 1 ml of ISA to 100 ml
of dilute storage solution.
• Plastic lab-ware should be used for low level measurements (< 1 ppm).
• All measurements should be made in basic solution. All samples and standards should be adjusted to a pH
> 9 with ISA.
• For samples with high ionic strength, prepare standards whose composition is similar to the sample.
• Always check to see that the membrane is free from air bubbles after immersion into standard or sample.
Sample Requirements
• The sample measuring range is pH 9–12. Use the ISA recommended to adjust the pH for best accuracy.
Make sure that the samples and standards are at the same temperature. The glass electrode sensing bulb will
not be attacked by most organic solvents.
Units of Measurement
Sodium concentrations are measured in units of parts per million, equivalents per liter, soles per liter, or any other
convenient concentration unit. Table 1 indicates some of the concentration unit conversion factors.
T ABLE 1 Concentration Unit Conversion Factors
ppm Na
229.90 1.0 X 10
+
22.99 1.0 X 10
2.30 1.0 X 10
moles/liter Na
+
-2
-3
-4
5
Page 10

Sodium Ion Selective Electrode 012–06615A
Measurement Procedure
Direct Measurement
Direct measurement is a simple procedure for measuring a large number of samples. A single meter reading is all
that is required for each sample. The ionic strength of samples and standards should be made the same by adjustment
with ISA for all sodium solutions. The temperature of both sample solution and of standard solutions should be the
same.
Direct Measurement of Sodium
ä
Note: A calibration curve is constructed on semilogarithmic paper. The measured
electrode potential (linear axis) is plotted against the standard concentration (log axis). In
the
linear region of the curve, only two standards are necessary to determine a calibration
curve. Calibration solutions close to the anticipated value of the “unknown” should be
chosen. In the
measurement procedures given are for the linear portion of the curve. The nonlinear
portion of the curve requires the use of low level procedures.
nonlinear region, additional points must be measured. The direct
1. By serial dilution of the 0.1 M or 1,000 ppm standards, prepare 100 ml of l0
-2
M, l0
-3
M, and l0-4 M or
100 and 10 ppm sodium standards. Add 2 ml of ISA per 100 ml of standard. Prepare standards with a
composition similar to the samples if the samples have an ionic strength above 0.l M
2. Place the most dilute solution (l0
-4
M or 10 ppm) in a beaker Place the beaker on the magnetic stirrer and
begin stirring at a constant rate. After assuring that ScienceWorkshop is operating, lower the electrode
tip into the solution. When the reading has stabilized, record the voltage reading indicated in the Digits
display.
3. Place the mid-range solution (l0
-3
M or 100 ppm) in a beaker. Place the beaker on the magnetic stirrer
and begin stirring. After rinsing the electrodes with electrode rinse solution, blot dry and immerse the
electrode tip in the solution. When the reading has stabilized, record the voltage reading indicated in the
Digits display.
4. Place the most concentrated solution (l0
-2
M or 1,000 ppm) in a beaker. Place the beaker on the magnetic
stirrer and begin stirring. After rinsing the electrodes with electrode rinse solution, blot dry and immerse
the electrode tip in the solution. When the reading has stabilized, record the voltage reading indicated in
the Digits display.
5. Using the semilogarithmic graph paper, plot the voltage reading (linear axis) against the concentration
(log axis). Extrapolate the curve down to about 5.0Xl0
-5
M. For measurements below this level, follow
the instructions for low-level measurement. A typical calibration curve can be found in Figure 3.
6
Page 11

012–06615A Sodium Ion Selective Electrode
+140
+100
10-fold change
+60
~59 mV
electrode
+20
potential
(mV)
-20
-60
10
0.1 1 10
-6
10
-5
10
(ppm)
- 4
10
100 1,000
-3
10
-2
10
-1
Na+ concentration (M)
Figure 3
Typical sodium electrode calibration curve
6. To a clean, dry 150 ml beaker, add 100 ml of sample and 2 ml of ISA. Place the beaker on the magnetic
stirrer and begin stirring. After rinsing the electrode tip with electrode rinse solution, blot dry and place
the electrode tip in the solution. When the reading has stabilized, record the Voltage reading in the Digits
Display. Using the calibration curve, determine the sample concentration.
7. The calibration should be checked every two hours. Assuming no change in ambient temperature, place
the electrode tip in the mid-range standard. After the reading has stabilized, compare it to the original
reading recorded in step 3 above. A reading difference by more than 0.5 mV or a change in the ambient
temperature will necessitate the repetition of steps 2-5 above. A new calibration curve should be prepared
daily.
Low Level Sodium Determination
This procedure is recommended for solutions with ionic strength less than 1.0x10
less than lxl0
-5
M or 1 ppm. If the solution is high in ionic strength, but low in sodium, use the same procedure, but
prepare a calibration solution with a composition similar to the sample. Use plastic lab-ware for low sodium
measurements.
-2
M and a sodium concentration
1. Using 20 ml of standard ISA, dilute to 100 ml with distilled water.
2. Dilute 20 ml of the outer chamber filling solution to 100 ml with distilled water and fill the reference
electrode if using a double junction reference electrode.
3. Dilute 1 ml of the 0.l M standard to 100 ml to prepare a 1.0Xl0
-3
M standard solution for measurements
in moles per liter. Dilute 10 ml of the 1000 ppm standard solution to 100 ml to prepare a 100 ppm standard
solution for measurements in ppm. Add 1 ml of low level ISA to each 100 ml of standard. Standards should
be prepared fresh daily.
4. Using a 150 ml plastic beaker, add 100 ml of distilled water and 1 ml of low level ISA. Add NH
OH, if
4
necessary, to adjust the pH above 9. Place the beaker on the magnetic stirrer and begin stirring at a constant
rate.
5. Place the electrode tip in the solution. Assure that ScienceWorkshop is operating.
7
Page 12

Sodium Ion Selective Electrode 012–06615A
6. Add increments of the l.0xl0
-3
M or 100 ppm standard as given in Table 2 below.
7. After the reading has stabilized, record the Voltage reading in the Digits display after each addition.
T ABLE 2: Step-wise Calibration for Low Level Sodium Measurements
Added
Concentration
Step Pipette Volume (ml) ppm M
1 A 0.1 0.10 l.0 X l0
2 A 0.1 0.20 2.0 X l0
3 A 0.2 0.40 4.0 X l0
4 A 0.2 0.60 6.0 X l0
5 A 0.4 0.99 9.9 X 10
6 B 2.0 2.91 2.9 X l0
7 B 2.0 4.76 4.8 X l0
-6
-6
-6
-6
-6
-5
-5
Pipette A = 1 ml graduated pipette
Pipette B = 2 ml pipette
Solutions: additions of 100 ppm or 1.0 X l0
-3
M standard to 100 ml of solution prepared in step 3 above
8. On semilogarithmic graph paper, plot the millivolt reading (linear axis) against the concentration (log
axis) as in Figure 3.
9. Rinse the electrodes with electrode rinse solution and blot dry.
10. To a 150 ml plastic beaker add 100 ml of sample and 1 ml of low level ISA. Place the beaker on the
magnetic stirrer and begin stirring. Adjust the pH, if necessary, to above 9. Lower the electrode tip into
the solution. After the reading has stabilized, record the Voltage reading in the Digits Display and
determine the concentration from the low level calibration curve.
11. Prepare a new low level calibration curve daily. Check the calibration curve every two hours by repeating
steps 3-8.
Electrode Characteristics
Reproducibility
Electrode measurements reproducible to +2% can be obtained if the electrode is calibrated every hour. Factors such
as temperature fluctuations, drift, and noise limit reproducibility. Reproducibility is independent of concentration
within the electrode’s operating range.
Interferences
Table 3 lists some common cations that, if present in high enough levels, will cause electrode interferences and
measurement errors or electrode drift when using the sodium ion electrodes.
Most samples do not contain or contain very little of the cations shown in Table 3. The ammonium ion (NH
4
in the recommended ISA, will not result in an error if all samples and standards have the same level of ISA present.
8
+
), found
Page 13

012–06615A Sodium Ion Selective Electrode
Electrode drift and slow response could indicate the presence of high interference from the ions listed. Soak the
electrodes in electrode storage solution when this happens to restore proper response. See Measuring Hints
section.
TABLE 3: Levels of Interfering Ions Resulting in a 10% Error at Specified Levels of Sodium
Interference 1.0X10
Li
K
Rb
NH
Ag
Tl
+1
+1
+1
+1
4
+1
+1
5X10
1X10
3X10
3X10
3X10
5X10
-4
M 1.0X10
-4
M 5X10
-2
M 1X10
-1
M3 M
-1
M3 M
-9
M 3X10
-2
M 5X10
-3
M 1.0X10
-3
M 5X10
-1
M1 M
-8
M 3X10
-1
M
Interference 1 ppm 10 ppm 100 ppm
+1
Li
K
Rb
NH
Ag
Tl
+1
+1
+1
4
+1
+1
1.5 ppm 15 ppm 150 ppm
17 ppm 170 ppm 1,700 ppm
1.1X104 ppm 1.1X105 ppm
1.8X103 ppm 1.8X104 ppm
0.0001 ppm 0.001 ppm 0.01 ppm
4.5X103 ppm 4.5X104 ppm
Temperature Influences
-2
M
-2
M
-7
M
Samples and standards should be at the same temperature, since electrode potentials are influenced by changes in
temperature. A l °C difference in temperature results in a 2% error at the l0
-3
M level. Because of solubility equilibria
on which the electrode depends, the absolute potential of the reference electrode changes slowly with temperature.
The slope of the electrode, as indicated by the factor “S” in the Nernst equation, also varies with temperature. Table
4 indicates the variation of theoretical slope with temperature.
Provided that temperature equilibria has occurred, the sodium ion electrodes can be used at temperatures from -5 °
to 70 °C. Room temperature measurements are recommended, since measurements at temperatures markedly
different from room temperature may require equilibrium times up to one hour. The electrode should not be used
at temperatures above 70 °C, since damage to the membrane may result.
T ABLE 4: T emperature vs. V alues for the Electrode Slope
Temperature ( ºC) “S”
0 54.20
10 56.18
20 58.16
25 59.16
30 60.15
40 62.13
50 64.11
9
Page 14

Sodium Ion Selective Electrode 012–06615A
Electrode Response
Plotting the electrode potential against the sodium concentration on semilogarithmic paper results in a straight line
with a slope of about 59 mV per decade. (Refer to Figure 1.)
The time needed to reach 99% of the stable electrode potential reading, the electrode response time, varies from
one minute or less for sodium concentrations above 1.0X10
to Figure 4.)
+125
+100
+75
+50
-5
M to several minutes near the detection limit. (Refer
10
-3
M to 10
-2
M NaCl
electrode
potential
(mV)
+25
-25
-50
-75
-3
10
0
-3
10
-3
10
1234
M to 10
-6
M NaCl
M to 10
M to 10
-4
M NaCl
-5
M NaCl
time (minutes)
Figure 4
Typical sodium electrode time response to step changes in NaCl
Limits of Detection
The upper limit of detection in pure sodium solutions is 1 M. In the presence of other ions, the upper limit of detection
is above l0
developing at the reference electrode and the salt extraction effect influence this upper limit. Some salts may extract
into the electrode membrane at high salt concentrations, causing deviation from the theoretical response. Either
dilute samples between l M and l0
Free sodium ion concentration down to l.0Xl0
below l0
occur from glassware due to removal from container walls.
-1
M sodium, but two factors influence this upper limit. Both the possibility of a liquid junction potential
-1
M or calibrate the electrode at 4 or 5 intermediate points.
-6
M or 0.1 ppm can be measured in basic solutions. For measurements
-5
M or 1 ppm, use plastic lab-ware (and low level procedures) since a significant pickup of sodium may
pH Effects
The electrode response to sodium ions is greatly influenced by the pH of the solution. Hydrogen ion interferes with
measurements of low level sodium ion measurements, although the electrode can be used over a wide pH range.
(See Figure 5.)
10
Page 15

012–06615A Sodium Ion Selective Electrode
-1
M Na
+
+180
10
+160
electrode
potential
(mV)
+140
+120
+100
+80
+60
+40
+20
H+ Ion
Interference
0
468 12
10
-2
M Na
+
-3
-4
M Na
M Na
102
+
+
14
10
10
pH
Figure 5
Electrode potential behavior vs. solution pH in pure NaCl solution at 25 °
The edge of the shaded area (the straight line) shown in Figure 5 indicates a minimum pH at which dilute sodium
measurements can be made with less than 10% hydrogen ion interference.
The pH should be adjusted to a pH greater than 9 by the addition of ISA to all standards and samples for optimal
results over the entire concentration range of sodium. Additional ammonium hydroxide may be necessary to adjust
the pH to the desired level in some cases.
Electrode Life
The sodium electrode will last six months in normal laboratory use. On-line measurements might shorten
operational lifetime to several months. In time, the response time will increase end the calibration slope will decrease
to the point calibration is difficult and electrode replacement is required.
Maintenance
Electrode Storage
The Sodium Ion Selective Electrode should be stored in the sodium electrode storage solution, never in air or in
distilled water. A more dilute sodium chloride solution (with pH adjusted through the use of ISA) may be used for
storage before low level measurements. For longer storage (longer than two weeks), rinse and dry the sensing glass
and cover the glass tip with any protective cap shipped with the electrode. The reference portion of the combination
electrode (or the outer chamber of the reference electrode) should be drained of filling solution, if refillable, and
the rubber insert placed over the filling hole.
11
Page 16

Sodium Ion Selective Electrode 012–06615A
Specifications
Concentration Range: Saturated to 1.0Xl0
pH Range: 5 to 12 (depending on pH level)
-6
M (0.02 ppm)
Temperature Range: -5
Resistance: < 200 mohm
Reproducibility: ±2%
Storage: store in 5 M NaCl with added ISA
Size: length = 110 mm
°C
– 70 °C
diameter = 12 mm
cable length = 1 m
Troubleshooting Guide
The goal of troubleshooting is the isolation of a problem through checking each of the system components in turn:
the meter, the glassware, the electrodes, the standards and reagents, the sample, and the technique.
Glassware/Plastic-ware
Clean glassware is essential for good measurement. Be sure to wash the glassware/plastic-ware well with a mild
detergent and rinse very well with distilled or deionized water. Clean glassware will drain without leaving water
droplets behind.
Electrode
The electrodes may be checked by using the procedure found in the sections entitled Electrode Slope Check.
1. Be sure to use distilled or deionized water when following the procedures given in Electrode Slope
Check.
2. If the electrode fails to respond as expected, see the sections Measuring Hints and Electrode Response.
Repeat the slope check.
3. If the electrode still fails to respond as expected, substitute another Sodium Ion Selective Electrode (if
available) that is known to be in good working order for the questionable electrode.
4. If the problem persists, the reagent may be of poor quality, interferences in the sample may be present,
or the technique may be faulty. (See Standards & Reagents, Sample, and Technique sections below.)
5. If another electrode is not available for test purposes, or if the electrode in use is suspect, review the
instruction manual and be sure to:
- Clean and rinse the electrode thoroughly.
- Prepare the electrode(s) properly.
- Use the proper filling solution.
- Adjust the pH and the ionic strength of the solution by the use of the proper ISA.
- Measure correctly and accurately.
12
- Review Troubleshooting Hints.
Page 17

012–06615A Sodium Ion Selective Electrode
Standards & Reagents
Whenever problems arise with the measuring procedure that has been used successfully in the past, be Sure to check
the standard and reagent solutions. If in doubt about the credibility of any of the solutions, prepare them again. Errors
may result from contamination of the ISA, incorrect dilution of standards, poor quality distilled/deionized water,
or a simple mathematical miscalculation.
Sample
Look for possible interferences, complexing agents, or substances which could affect the response or physically
damage the sensing electrode (or the reference electrode) if the electrodes work perfectly in the standard, but not
in the sample.
Try to determine the composition of the samples prior to testing to eliminate a problem before it starts. (See
Measuring Hints, Sample Requirements, and Interferences.)
Technique
Be sure that the electrode’s limit of detection has not been exceeded. Be sure that the analysis method is clearly
understood and is compatible with the sample.
Refer to the instruction manual again. Reread General Preparation and Electrode Characteristics.
If trouble still persists, call PASCO Technical Support.
Troubleshooting Hints
Symptom Possible Causes Next Step
Out of Range Reading defective electrode check electrode operation
electrodes not plugged in unplug electrodes and reseat electrodes
properly
reference electrode not filled replenish reference filling solution
electrodes not in solution put electrodes in solution
air bubble on membrane remove bubble by re-dipping electrode
13
Page 18

Sodium Ion Selective Electrode 012–06615A
Symptom Possible Causes Next Step
Noisy or Unstable electrode exposed to interferences soak overnight in electrode storage solution
Readings (readings
continuously or rapidly defective electrode replace electrode
changing)
ISA not used use recommended ISA
stirrer not grounded ground stirrer
Drift (reading slowly samples and standards at different allow solutions to come to room temperature
changing in one direction) temperatures before measurement
electrode exposed to interferences soak overnight in electrode storage solution
incorrect reference filling solution use recommended filling solution
pH too acidic adjust pH with ISA
ISA not used use recommended ISA
Low Slope or No Slope standards contaminated or prepare fresh standards
incorrectly made
standard used as ISA use ISA
electrode exposed to interferences soak overnight in electrode storage solution
pH too acidic adjust pH with ISA
air bubble on membrane remove bubble by re-dipping probe
14
Page 19

012–06615A Sodium Ion Selective Electrode
Symptom Possible Causes Next Step
“Incorrect Answer” incorrect scaling of semilog paper plot voltage potential on the linear axis.
(but calibration curve On the log axis, be sure concentration
is good) numbers within each decade are increasing
with increasing concentration.
incorrect sign be sure to note sign of millivolt reading
correctly
incorrect standards prepare fresh standards
wrong units used apply correct conversion factor:
1.0X10
-3
M = 23.0 ppm as Na
+1
sample pH too acidic adjust pH with ISA
15
Page 20

Sodium Ion Selective Electrode 012–06615A
16
Page 21

T echnical Support
Feedback
If you have any comments about the product or
manual, please let us know. If you have any
suggestions on alternate experiments or find a problem
in the manual, please tell us. PASCO appreciates any
customer feedback. Your input helps us evaluate and
improve our product.
To Reach PASCO
For technical support, call us at 1-800-772-8700
(toll-free within the U.S.) or (916) 786-3800.
fax: (916) 786-3292
e-mail: techsupp@pasco.com
web: www.pasco.com
Contacting Technical Support
Before you call the PASCO Technical Support staff,
it would be helpful to prepare the following
information:
➤ If your problem is with the PASCO apparatus,
note:
- Title and model number (usually listed on the
label);
- Approximate age of apparatus;
- A detailed description of the problem/
sequence of events (in case you can’t call
PASCO right away, you won’t lose valuable
data);
- If possible, have the apparatus within reach
when calling to facilitate description of
individual parts.
➤ If your problem relates to the instruction manual,
note:
- Part number and revision (listed by month and
year on the front cover);
- Have the manual at hand to discuss your
questions.
Page 22

 Loading...
Loading...