
OPERATING INSTRUCTIONS
Parr Acid Digestion Bombs
No. 249M
Scope
These instructions are to acquaint the user with the procedures to be followed and the precautions to be taken
when using any ParrAcid Digestion Bomb, except those
designed specifically for microwave heating.(Microwave
bombs are covered by separate instructions, No. 243M.)
Parr PTFE-lined digestion bombs provide a convenient
means for dissolving analytical samples rapidly in strong
acids or alkalis,it is important that the user understand
the capabilities and limitations of the equipment and
will be well aware of the safety precautions to be observed in its operation. Pages 1 through 6 of these instructions apply equally to all Parr Acid Digestion
Bombs with metal bodies and removable PTFE liners.
The user should read these basic instructions carefully
before starting to use any of these bombs. Then turn to
pages 8 through 11 for the special instructions which
apply to each individual model. It must be understood
that the excellent mechanism for sample digestion and
dissolution provided by these bombs can be hazardous if
a bomb is misused. Therefore careful reading and full
compliance with these instructions must be observed in
all applications.
Contents
Scope ......................................................................... 1
Contents.................................................................... 1
Assumption of Risk .................................................. 2
The Nature of PTFE................................................. 2
Potential Hazards .................................................... 2
Sample Selection......................................................3
Loading Limits ......................................................... 3
Acid Selection........................................................... 4
Pressure and Temperature Limits.......................... 4
Heating and Cooling the Bomb ............................... 5
Pre-Treating PTFE Parts ........................................ 5
Liner Maintenance................................................... 5
Liner Lifetimes......................................................... 5
Bomb Maintenance .................................................. 6
References................................................................. 6
Vapor Pressure Tables............................................. 7
Operating Procedure
4745 General Purpose Bomb.................................8
4744 and 4749 General Purpose Bombs...............9
4746 and 4747 High Strength Bombs................. 10
4748 Large Capacity Bomb................................. 11

Assumption of Risk
Potential Hazards
The Parr Instrument Company offers its PTFE-lined
Acid Digestion Bombs to skilled analytical chemists as
an attractive means for digesting and dissolving analytical samples for analysis. Parr designed and manufactures these bombs to be as effective as possible when
used within the limitations prescribed for each individual model. But, since the pressures generated within
these bombs are solely dependent upon the nature of the
materials being treated, the filling level and the amount
of heat applied to promote the reaction, Parr will not be
responsible for personal injuries or damage to the bomb,
to the oven or to other equipment resulting from the use
of these bombs. As with all laboratory operations, the
user must assume responsibility for and institute safety
procedures to protect all personnel from any hazards associated with this equipment. Rigid controls must be established to guarantee that the operator does not
overcharge or overheat the bomb.
The Nature of PTFE
PTFE offers such unique inertness and high temperature usefulness that it is an obvious choice as the material of construction for lining these acid digestion bombs.
PTFE does, however,have two characteristics which
make it somewhat less than perfect for this application,
and the user who understands these deficiencies will be
able to minimize the effect upon his work.
First, PTFE has a tendency to creep or flow under pressure or load. This tendency is present even at room temperature and it is accentuated at higher temperatures.
At operating temperatures below 150ºC the creep effect
will become more pronounced, making it more difficult to
maintain tight seals and resulting in deformation and
shorter life for the PTFE components. The extent of the
creep effect will be roughly proportional to the maximum
operating temperature.
Secondly, PTFE is a porous material. Although the materials and designs used in Parr Acid Digestion Bombs
minimize the effects of this porosity,users of these
bombs can expect to see evidence of vapor migration
across the cover seal and through the wall of the liner itself. Parr is able to minimize these problems by machining these parts from virgin PTFE which has been
molded at an optimum pressure selected to reduce any
porosity to an absolute minimum. The thick walls and
long path seals used in Parr bomb liners also help to
overcome these undesirable properties. Experiments
have shown that the amount of solute lost in this manner during a normal digestion is negligible, but vapor
migration will occur and frequently it will be sufficient
to produce noticeable discoloration on the inner metal
wall of the bomb body and the screw cap.
While many thousands of these bombs have been used
safely and routinely for treating a great variety of samples with different digestion media under a broad range
of operating conditions,it is possible to create conditions
under which these bombs will explode. The bulk of the
reported incidents of this type have been caused by failure of the operator to recognize one or more of the
following potential hazards.
Excessive temperature. When a bomb is overheated,
two factors come into play: (1) the vapor pressure of the
materials in the bomb increases exponentially with temperature and (2) the strength of the materials from
which the bomb is made falls off (again exponentially)
if the bomb is heated above its maximum temperature
limit. Dangerous overheating can be produced by ovens
with defective temperature controls, by water baths boiling dry, or by operator inattention or carelessness.
Excessive pressure. Excessive pressure can be produced not only by overheating, as mentioned above, but
also from uncontrolled gaseous reactions and from high
vapor pressure or explosive materials, or from overloading the bomb as mentioned below.
Excessive loading. When organic materials are treated
in these bombs, they may liberate gases as well as heat.
Since the PTFE liner is an excellent thermal insulator,
this internal heat will be translated into higher internal
temperatures and pressures. The loading limits prescribed for these bombs are purposely conservative to
ensure that the energy released from the sample will not
over-stress the bomb.The user must also remember that
when a water-based solution is heated to 250ºC it expands to fill a space approximately 25 percent larger
than its volume at room temperature. If there is insufficient vapor space in the bomb to accommodate this expansion, the tremendous hydrostatic pressure which
will be generated will destroy the bomb.
Explosive materials. The nitro compounds produced
when nitric acid reacts with certain organic materials
may have explosive properties capable of destroying the
bomb, even when present in quantities well within the
normal recommended charging limits.Consider, for example, what might happen if nitroglycerin were produced by reactions in the bomb. For this reason, fats,
fatty acids, glycerin and similar materials must not be
treated with nitric acid in these bombs, and cellulosic
materials must not be treated with mixed nitric and
sulfuric acids. Similarly, because of its unpredictable
nature, perchloric acid must not be used in these
bombs.
2
Sample Selection
Inorganic materials. Most inorganic digestions proceed smoothly without unusual hazards, using not more
than 1.0 gram of sample in a 23 mL bomb, 2.0 grams in a
45 mL bomb and 5.0 grams in the larger,125 mL size. As
in all reactions, the bomb must never be completely filled
as there must always be vapor space above the surface of
the charge.To besure that there is adequate free space,
the total volume of the charge must never exceed twothirds (66%) of the capacity of the cup when working
with inorganic materials. By observing these limits and
taking precautions to prevent overheating, there should
be no unusual hazards in treating inorganic samples
with mineral acids.
Ores, rock samples, glass and other inorganic materials
can be dissolved in Parr acid digestion bombs using
strong mineral acids: HF, HCl, H2SO4, HNO3, Aqua Regia and others.Digestion times for these materials can
vary anywhere from 2 hours to several days. Ordinary
glass materials (SiO2) will mandate the use of HF,sometimes in combination with HCl, or aqua regia. Temperatures in the range of 100 to 150ºC are routinely used.
Alumina is routinely digested using 10% sulfuric acid.
Temperatures used for these samples are typically in excess of 200ºC.It is advantageous, from the standpoint of
minimizing the digestion time, to reduce the sample to
granular or powder form prior to digestion. The increase
in surface area of the sample has a significant impact on
the reaction with the digestion aid.
The following
used for glass and other silicate samples using the Parr
23 mL Acid Digestion Bomb. For the dissolution of glass,
sand and mineral silicate samples, weigh 0.4 g of the
powdered sample into the PTFE liner.Moisten the sample with water and cautiously and 4 mL of 40 to 50% HF
to the liner. Cover the liner and allow it to stand until
the initial reaction has taken place, then seal the bomb.
Place the bomb assembly into a preheated lab oven for 2
hours at 130-150ºC.Remove the bomb from the oven, and
after cooling the room temperature, the bomb may be
opened.
Elements that form insoluble fluorides, such as Al, Ba,
Ca, and Mg can be dealt with effectively by adding 1
gram of boric acid, after cooling, and re-heating again for
1 hour. A blank, used throughout the dissolution and
analysis procedure,should contain the same amount of
HF and boric acid. For example, it is important to have
the boric acid blank subtracted from the sample spectrum in ICPES analysis to account for the boron interferences with other elemental lines.
Organic materials. Many organic materials can be
treated satisfactorily in these digestion bombs but careful attention must be given to the nature of the sample
and to possible explosive reactions with the digestion me-
general digestion procedure can be
dia. In all cases the size of the sample and the amount of
oxidant used must be carefully controlled. For nitric
acid digestions (Carius decompositions) of organic compounds, the dry weight of organic matter must not exceed 0.1 gram in a 23 mL bomb, 0.2 gram in a 45 mL
bomb and 0.5 gram in the 125 mL size. The sample does
not have to be dried before it is placed in the bomb, but
the amount used must not exceed the above limits when
converted to a dry weight basis. The amount of concentrated nitric acid (sp. g 1.42) added to the above charges
must fall within the amounts shown in the table of loading limits. Notice that both minimum and maximum
amounts of acid are specified. If the sample contains less
than the specified maximum amount of dry organic matter, the amount of nitric acid must be reduced proportionately.
As stated above, fats, fatty acids,glycerin and similar materials which form explosive compounds in an intermediate stage must not be treated with nitric acid in these
bombs. Digestions involving other organic materials
must also be handled cautiously since it is impossible to
list all of the potentially dangerous combinations which
might arise. For best protection, the user and his supervisor should study each reaction carefully before proceeding to use the Parr digestion bomb or any other pressure
vessel, asking such questions as: Is the reaction exothermic? What intermediate and final products might be produced and what will be their behavior?
When working with new or unfamiliar materials it is always advisable to run preliminary experiments using
small samples and observing the behavior of the reactants carefully.This initial screening is best conducted in
the heavier 4746 bomb which has a safety rupture disc.
Organic samples are typically treated with concentrated
nitric acid. Nitric - sulfuric mixtures are not recommended for digesting organic samples due to the possibility of forming potentially unstable reaction products.
Digestion with perchloric acid can be dangerous,for the
same reason, and must not be used. Typical heating
Loading Limits
Minimum and
Bomb
No.
4744
4745
4746
4747
4748
4749
Size
mL
45
23
23
23
125
23
Maximum
Inorganic
Sample
2.0 gram
1.0
1.0
1.0
5.0
1.0
Maximum
Organic
Sample
0.2 gram
0.1
0.1
0.1
0.5
0.1
Maximum Nitric
Acid to be used
with an
Organic Sample
5.0-6.0 mL
2.5-3.0
2.5-3.0
12-15
2.5-3.0
3

times range from 1 to 8 hours. Temperatures in the 150
to 200ºC range are generally quite effective.
The following
ganic samples using the Parr 23 mL Acid Digestion
Bomb. Weigh no more than 0.1 g of sample (dry weight
basis) into the PTFE digestion vessel. Add 2.5 mL hot
concentrated nitric acid and let stand open for 15 minutes. Add 0.2 mL of hydrofluoric acid to dissolve siliceous
materials contained in the sample. (In case that the sample does not contain siliceous material, this step can be
omitted.) Close the vessel and place into a preheated
oven (100-150ºC) for 1 to 4 hours,as required until the
solution is clear. If the solution is colloidal, heat until
clear. Remove the vessel from the oven and let cool to
room temperature (2 hours) before opening.Sample
sizes and amount of reagents used are proportional to
the free volume of the PTFE liner.
general digestion procedure for or-
Acid Selection
With the vigorous action that can be achieved in these
bombs at elevated temperatures and pressures, most
organic samples can be decomposed in nitric acid alone
without using mixed nitric and sulfuric acids as in oxidations at atmospheric pressure. Users should always try
nitric acid alone and resist the temptation to add sulfuric acid to the charge. Sulfuric acid may not be needed at
the higher temperature and it may tend to dehydrate the
system and promote the formation of unstable nitro
compounds.
Do not use perchloric acid. We repeat again the
warning that perchloric acid should not be used in these
bombs because of its unpredictable behavior when
heated in a closed vessel. Also, other reactions which are
highly exothermic or which might be expected to release
large volumes of gas should be avoided.
Pressure and Temperature Limits
Maximum working pressures for each acid digestion
bomb are shown in the individual listings on pages 8
through 11. Extreme care must be exercised to ensure
that pressures do not exceed these prescribed limits.The
user must understand that in acid systems the solubility
of gases such as NO
the temperature rises, having the effect of adding noncondensable gas to the vapor phase in a closed vessel. As
a result, the amount of acid present, the acid concentration and the free head space above the liquid will all
have a bearing on the pressure developed in a closed
bomb. Free head space must therefore be provided in
all procedures,and the volume and concentration of
the acid must be held to a minimum.
Maximum operating temperatures for each acid digestion bomb are shown in the individual listings. But these
limits must be used cautiously because, with certain
acids, pressures higher than the allowable limit will be
generated if the bomb is heated to the listed maximum
temperature. For example,Table I on page 6 shows the
heating only 3 mL of fuming nitric acid to 256ºC in a 23
mL bomb will generate a pressure of 1565 psi which is
well above the 1200 psi limit for the 4745 general purpose bomb. Table II shows that heating 10 mL of concentrated hydrochloric acid to 255ºC in a 23 mL bomb will
generate 2150 psi, which is well above the allowable
limit for the 4744, 4745 and 4749 bombs. But if the acid
is diluted, Table III shows that much lower pressures are
developed at temperatures to 250ºC.Diluting or reducing
the amount of acid in the charge will generally allow the
use of higher temperatures to develop a given pressure.
But in all operations the user must use good judgment in
selecting the operating temperature, and he must control
the heating medium carefully to be sure that both temperature and pressure are held within prescribed limits.
, HCl and SO3will be reduced as
2
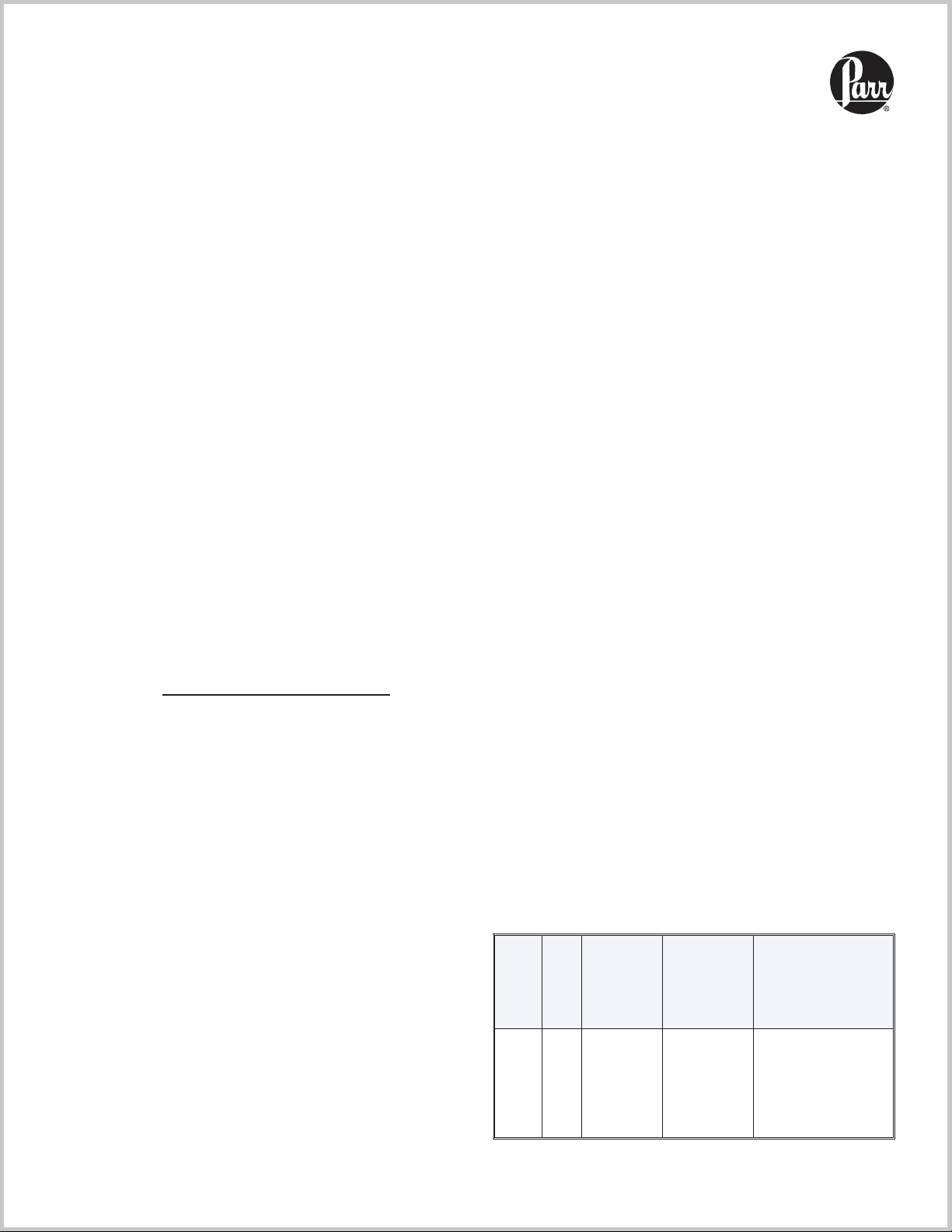
The 4744 and 4749
Bombs can be held
4749 Bomb
with A284AC
Tumbling Ring
4
firmly in an A285AC
Holding Fixture while
tightening the cap with a
264AC2 Hook Spanner
Heating and Cooling the Bomb
Parr metal-jacketed acid digestion bombs can be heated
in a variety of ways, including ovens, water baths, sand
baths, oil baths, mantles or block heaters. In all cases it
is critical to ensure that proper temperature control is
maintained to prevent the build-up of dangerous high
pressures. When working with organic materials the
bomb should be heated in a remote location or behind a
protective barricade to shield all personnel in the laboratory in case the bomb should unexpectedly explode.
At the end of a run the bomb must be cooled to the touch
before attempting to remove the PTFE cup. Cooling must
proceed slowly. Do not submerge the bomb in a sink on
an aluminum plate and run cold water over the plate but
not over the bomb. Cooling can be accelerated by placing
the bomb in the air flow from a small fan. If it is difficult
to remove the PTFE cup after a bomb has been cooled to
the touch, additional cooling in a refrigerator or freezer
may be necessary to shrink the cup sufficiently to remove it from the bomb body.
With use, these liners will absorb nitric and other acids
and become discolored. Generally this will not affect the
usefulness of the liner unless it represents unwanted contamination. Absorbed material can sometimes be driven
out by heating the liner, but care must be taken to prevent deformation as noted above. A 2 or 3 percent solution of the sodium salt of EDTA (ethylenediamine
tetracetic acid) has been found to be effective in removing heavy metal ions from these liners.
If the bomb has been used with nitric acid, the PTFE
liner should be removed, washed thoroughly and stored
outside of the bomb body to prevent possible corrosion of
the metal parts from any residual acid absorbed on the
liner. If there is a long storage period between uses, the
cup may not fit in the cylinder. To bring the cup back to
size, chill both the cup and cover in a refrigerator or
freezer at 0ºC for about an hour.Then slide the cup and
cover into the body and allow the assembly to come to
ambient temperature.
Liner Lifetimes
Pre-Treating PTFE Parts
Before using a new PTFE cup and cover,these parts
should be heated in a bomb with a charge of pure water.
This pre-treatment will help to develop the required
seals and it may prevent annoying leakage in subsequent procedures.The amount of water used in this
pre-treatment should not exceed 40 percent of the capacity of the cup. Thus:
For 23 mL cups...... use 9 mL of pure water
For 45 mL cups...... use 18 mL of pure water
For 125 mL cups ... use 50 mL of pure water
Assemble the bomb as directed in the individual instructions and heat the bomb in an oven at 150ºC for one hour.
Although some digestion procedures may require longer
heating periods,a one hour preliminary treatment
should be sufficient to prepare the PTFE parts for
effective use.
Liner Maintenance
Always handle PTFE liners with care to protect the sealing faces from mechanical damage which would make it
impossible to develop a reliable seal.
Never heat a PTFE liner without slipping it into a bomb
body. If heated outside of the bomb its dimensions will
change and it will no longer fit in the body. If this happens, cooling the liner in a refrigerator or freezer will
usually shrink it back to its original size. Then by alternately heating and cooling the liner in the bomb its dimensions should stabilize. Similarly, if a liner is stuck in
the metal body it usually can be removed by cooling the
bomb.
The lifetime of the PTFE liners used in Parr acid digestion bombs is governed by the unique characteristics of
PTFE as detailed on page 2. Cup lifetimes depend primarily upon the pressures and temperatures to which
they are exposed. Lifetimes as short as 10 to 30 runs, or
as many as 100 runs,have been reported. In general, exposure to high temperatures and/or pressures can be expected to shorten the lifetime of a cup.
A PTFE cup should be considered no longer usable and it
should be replaced if it loses one percent (1%) or more of
its contents when filled half-full with water and heated
for thirty minutes at the intended operating temperature. Continued use of a leaky cup will expose the outer
body to corrosive agents, resulting in loss of strength and
possible bomb failure. When replacing a cup, the cover
must be replaced also. Replacement cups and covers
are readily available.
The A263AC Spanner-Jack holds the 4746 Bomb firmly
during opening and closing operations and provides a
convenient tool for pressing the PTFE cup out of the
body.
5

Bomb Maintenance
References
These bombs have been designed to operate with little or
no maintenance other than careful inspection to ensure
that they have not been deformed by undetected high internal pressures.Individual replacement parts are available. The screw caps are made of either brass or a high
strength bronze to minimize the need for a lubricant on
the screw threads, but a thin film of an antisieze compound will be helpful if a bomb is to be used at temperatures above 200ºC.The several procedures and
precautions listed below should be observed in all
operations.
If the bottom plate is deformed by over pressure, replace
the plate.Then, before proceeding, review the chemistry
of the digestion which caused the over pressure.
If the inside of the metal body becomes discolored it
should be repolished to remove any metallic corrosion
products which might contaminate the liner.
Corrosion discs and rupture discs should be replaced
whenever they show signs of corrosion or wear.
If several acid digestion bombs of the same style are
used in the same laboratory, do not interchange the
cups or other parts of the assembly.
If the bomb or any of its parts should suffer any unusual
damage, contact the Technical Service Department of
Parr Instrument Company to determine the proper
corrective action or repairs which may be required.
1. J. Dolezal, P. Povondra, Z. Sulcek, “Decomposition
Techniques in Inorganic Analysis”, London Iliffe
Books Ltd., New York American Elsevier Publishing
Co., Czech Edition 1966, English Edition 1968, Library of Congress Catalog Card, No. 68-27170.
2. Zdenek Sulcek, Pavel Povondra, “Methods of Composition in Inorganic Analysis”, CRC Press, Boca Raton,
FL 33431, ISBN 0-8493-4963-X, QD75.3.S84 1989
543 88-10572
3. Wesley M. Johnson, John A. Maxwell, “Rock and Mineral Analysis Second Edition, 1981, Vol.27", John
Wiley & Sons, ISSN 0069-2883; v. 27, QE 438.J64
1981 552’.06 81-1659, ISBN 0-471-02743-X AACR2.
4. Jon C. Van Loon, “Selected Methods of Trace Metal
Analysis Biological and Environmental, Samples
Vol. 80", John Wiley & Sons 1981, ISSN 0069-2883;
v. 80, QD139.T7V35 1985 548-85-3279.
6

V aporPressureTab les
Table IV
Table I
Pressure developed at constant volume by 3 mL of fuming (91%) HNO3, Sp.gr. 1.48, in a 23 mL space.
Temperature Pressure
Deg. C psi
133 180
165 380
192 630
219 995
256 1565
285 2245
313 2945
326 3135
Data from: Journal of Research of the National Bureau of
Standards, Vol.30, February 1943, p. 110.
Table II
Pressure developed at constant volume by 10 mL of 36%
HCl in a 23 mL space.
Temperature Pressure
Deg. C psi
178 705
223 1430
255 2150
285 3095
298 3615
Data from: Journal of Research of the National Bureau of
Standards, Vol.33, December 1944, p. 468.
Table III
Pressure developed at constand volume by 10 mL of
aqua regia in a 23 mL space.
Temperature Pressure
Deg. C psi
106 305
155 705
162 765
202 1375
221 1670
236 1960
258 2505
Aqua regia made with 20 volumes, 36% HCl + 1 volume
fuming (91%) HNO3.
Data from: Journal of Research of the National Bureau of
Standards, Vol.33, December 1944, p. 469.
Table V
Saturation pressure of water.
Temperature Pressure
Deg. C psi
125 34
150 69
175 129
200 225
225 370
250 576
275 862
300 1245
325 1747
Data from: Harr, Gallagher and Kell, NBS/NRC Steam
Tables, McGraw-Hill, New York.
Pressure developed at constant volume by 10 mL of
22.9% HCl in a 23 mL space.
Temperature Pressure
Deg. C psi
132 70
159 125
191 265
220 510
250 920
285 1640
315 2250
338 3400
Data from: Journal of Research of the National Bureau of
Standards, Vol.33, December 1944, p. 468.
7
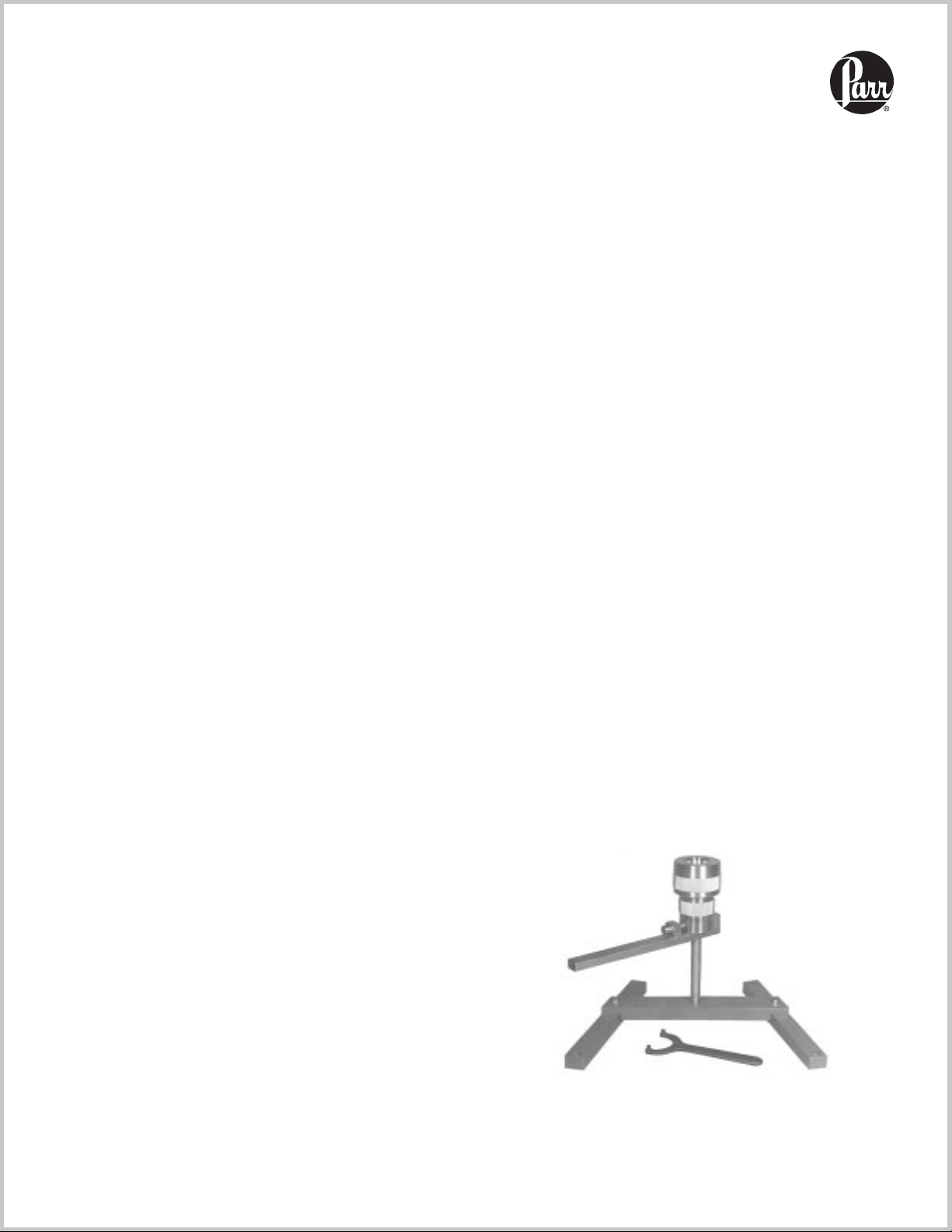
4745
General Purpose
Acid Digestion Bomb
Bomb Number 4745
Size, mL 23
Maximum charge, grams,
Inorganic sample 1.0
Organic sample 0.1
Recommended max. temperature, °C 150
Absolute max. temperature, °C 250
Absolute max. pressure, psig 1200
Operating Procedure
Always keep the bomb upright during assembly and closing operations.Check the 239AC Bottom Disc to be sure
that it is installed with the proper side facing upward to
provide full diameter support for the liner.
Place the sample and the digestion media in the PTFE
cup, attach the cover and push it down firmly with a
twisting motion. Slide the closed liner into the bomb
body and push it down as far as it will go. It may be helpful to push the bottom disc upward to meet the liner and
thereby prevent air binding between the liner and the
disc.
Set the 254AC Pressure Plate on top of the cup cover,
add the 241AC Spring and the 283AC Upper Plate, then
attach the screw cap and turn it down firmly by hand. A
firm twist by hand should be sufficient to develop and
maintain a tight seal. No wrench or spanner is required.
Place the bomb in a temperature controlled oven or
other heating medium and follow the heating and cooling procedures described in the general instructions.
If mixing is required, an optional tumbling ring
(A284AC) can be attached to the bomb body, the
same as offered for the 4744 and 4749 Bombs.
Parts List
Part No. Description
239AC
241AC
243AC
244AC
254AC
A255AC
283AC
Bottom disc
Sprint
Bomb body
Screw cap
Pressure plate, lower
PTFE cup with cover
Pressure plate, upper
8

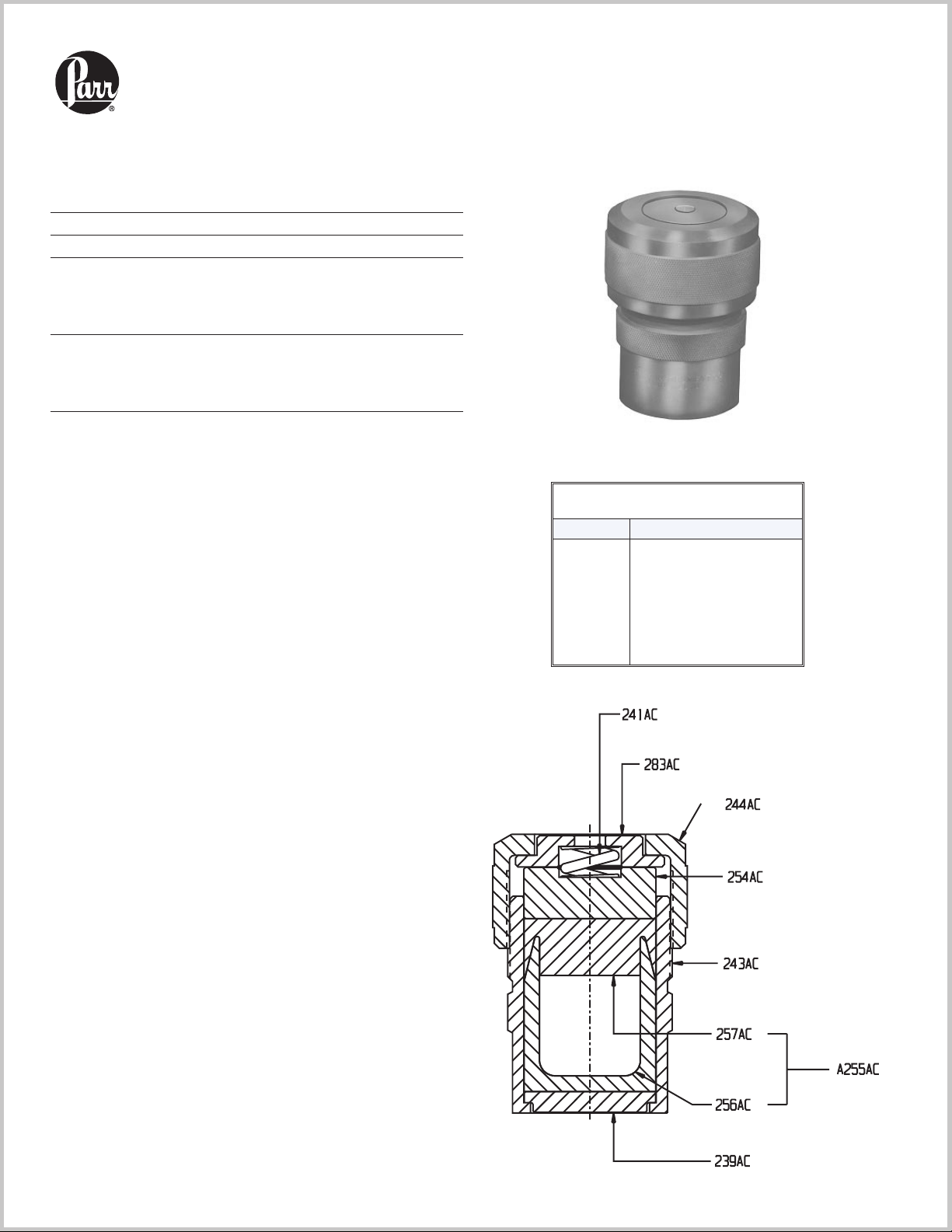
4744 and 4749
45
23
General Purpose
Acid Digestion Bomb
Bomb Number 4744 4749
Size, mL 23 45
Maximum charge, grams,
Inorganic sample 1.0 2.0
Organic sample 0.1 0.2
Recommended maximum
temperature, °C 250 250
Absolute maximum
temperature, °C 250 250
Absolute maximum
pressure, psig 1800 1800
Operating Procedure
point where it is impossible to maintain a tight seal.
When this happens, the cup and cover must be replaced.
Any attempt to force a seal by over tightening the screw
cap might crack the cap.
4749
Always keep the bomb upright during assembly and closing operations.Check the 277AC Bottom Disc to be sure
that it is installed with the proper side facing upward to
provide full diameter support for the liner.Place sample
and digestion media in the PTFE cup, add the cover and
slide the liner into the bomb body. Push it down as far as
it will go. It may be helpful to push the bottom disc upward to meet the liner and thereby prevent air binding
between the liner and disc.
Place the 286AC Corrosion Disc and the 287AC Rupture
Disc on top of the liner.Notice that two discs are required, with the thinner (corrosion) disc next to the
PTFE cover and the thicker (rupture) disc on the outside
of the sandwich next to the blowout opening. Add the
241AC Spring with upper and lower pressure plates,
then attach the screw cap and turn it down firmly by
hand. Additional closing force applied with a hook spanner will be required to seal these bombs, but avoid over-
tightening. Set the bomb in the A285AC Holding
Fixture and tighten the cap not more than one-eight turn
with the 264AC2 Hook Spanner.Any further tightening
in excess of one-eighth turn will destroy the seal on the
liner and it may put an excessive load on the screw cap,
causing it to deform and possibly crack and fail.
Place the bomb in a temperature controlled oven or other
heating medium and follow the heating and cooling procedure described in the general instructions. If mixing is
required an optional tumbling ring (A284AC) can be attached to the bomb body.With this ring in place,the
bomb will roll smoothly when placed in a powered roller,
providing good agitation during long digestion procedures.
After extended use, the tapered rim on the PTFE cup
will become thin and the cover may be deformed to a
Parts List
Part No. Description
241AC
264AC2
276AC
276AC2
277AC
278AC
A280AC
A280AC2
282AC
283AC
A284AC
A285AC
286AC
287AC
Spring
Hook spanner
Bomb body (23 mL)
Bomb body (45 mL)
Bottom disc
Screw cap
PTFE cup with cover (23 mL)
PTFE cup with cover (45 mL)
Pressure plate, lower
Pressure plate, upper
Tumbling ring
Holding fixture
Corrosion disc, .002"
Rupture disc, .003"
4744
9
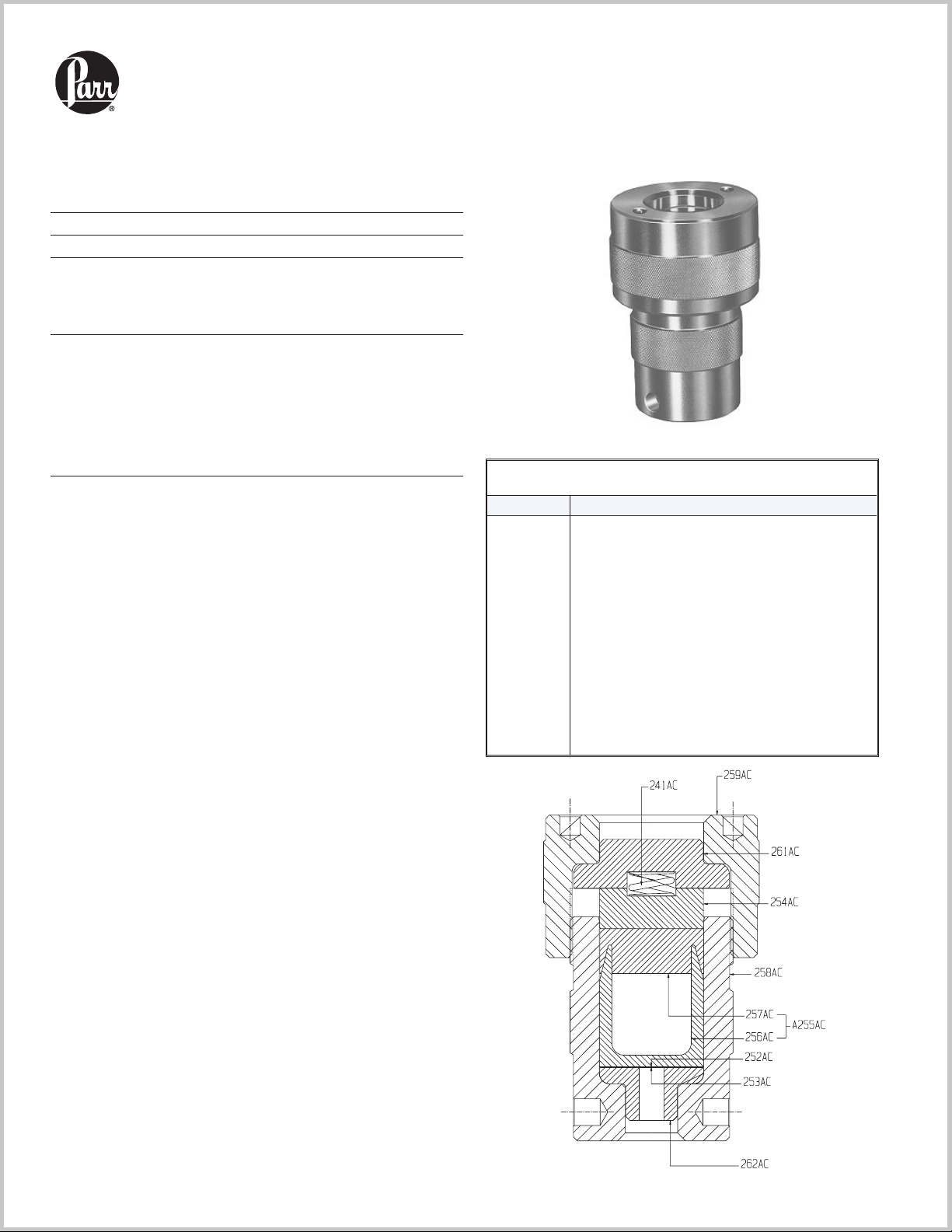
4746 and 4747
High Strength
Acid Digestion Bomb
Bomb Number 4746 4747
Size, mL 23 23
Maximum charge, grams,
Inorganic sample 1.0 1.0
Organic sample 0.1 0.1
Recommended maximum
temperature, °C 275 275
Absolute maximum
temperature, °C 275 275
Absolute maximum
pressure, psig 5000 3300
Operating Procedure
Always keep the bomb upright during assembly and closing operations.Check the 262AC Bottom Orifice in the
4746 Bomb, or the 262AC2 Solid Plug in the bottom of
the 4747 Nickel Bomb, to be sure that it is in place. Then
drop the 253AC Rupture Disc and the 252AC Corrosion
Disc into the body of the 4746 Bomb. When in place, the
thinner (corrosion) disc should be on top of the thicker
(rupture) disc in the bottom sandwich next to the blowout orifice. (No discs are used in the 4747 Nickel Bomb.)
Place the sample and the digestion media in the PTFE
cup, attach the cover and push it down firmly with a
twisting motion. Slide the closed liner into the bomb
body and push it down as far as it will go. It may be helpful to push the bottom plug upward to meet the liner and
thereby prevent air binding between the liner and disc.
Set the 254AC Pressure Plate on top of the PTFE cover,
add the 241AC Spring and the 261AC Top Cap, then attach the screw cap and turn it down firmly by hand. A
firm twist by hand should be sufficient to develop a tight
seal, but a slight additional force applied with a 264AC
Face Spanner will be helpful. However, do not tighten
the cap more than one-eight turn with the spanner.
Place the bomb in a temperature controlled oven or
other heating medium and follow the heating and cooling procedures described in the general instructions.After use at the maximum operating temperature the
PTFE liner may be deformed sufficiently to make it difficult to remove the liner from the bomb after cooling.To
overcome this problem an A263AC Spanner Jack Assembly can be used to push the liner out of the bomb with a
smooth, uniform pressure without damaging the cup.
This spanner jack will also be helpful for holding the
bomb when removing the screw cap with a 264AC Face
Spanner.
Parts List
Part No. Description
241AC
252AC
253AC
254AC
254ACCN
A255AC
258ACAB
258ACCN
259ACBC
261ACAB
262AC
262AC2
A263AC
264AC
Spring
Corrosion disc, .002"
Rupture disc, .010"
Pressure plate, stainless
Pressure plate, nickel
PTFE cup with cover
Bomb body, stainless
Bomb body, nickel
Screw cap, bronze, plated
Top cap, stainless
Bottom orifice, stainless
Bottom plug, nickel
Spanner jack assembly with 265AC spanner
Face spanner only
10

4748
Large Capacity
Acid Digestion Bomb
Bomb Number 4748
Size, mL 125
Maximum charge, grams,
Inorganic sample 5.0
Organic sample 0.5
Recommended max. temperature, °C 250
Absolute max. temperature, °C 250
Absolute max. pressure, psig 1900
Operating Procedure
There must always be adequate free space above the
charge in the 4748 Bomb. For inorganicsamples which
do not generate gases, leave at least 33% of the capacity
of the cup as free space, and increase the space to 50% or
more if the charge tends to liberate gases. For organic
samples, leave at lease 50% free space for non-oxidizing
reactions and 66% to 75% if an oxidizing medium is used.
For Carius and similar decompositions use not more
than 15 mL or less than 12 mL of 70% nitric acid with a
0.5 gram sample. For smaller samples, use 2.5 to 3.0 mL
of nitric acid for each 0.1 gram of dry organic matter.
Slide the filled cup into the bomb and raise the bottom
plate slightly to release any trapped air.Place the 310AC
(thinner) corrosion disc on top of the PTFE cover and the
311AC (thicker) rupture disc on top of the corrosion disc.
NOTE: the PTFE cover will rupture and digestion media
will be sprayed from the opening in the top plate if the
corrosion and rupture discs are not included in the assembly. Add the 306AC pressure plate and two 309AC
Bellville spring washers, topped by the 307AC compression ring.
Back all of the compression screws out of the screw cap
until they are slightly recessed, then advance one screw
until one full thread is observable on the underside. Attach the screw cap to the cylinder and turn it down by
hand as far as it will go.Then, using the TX31SK Allen
wrench furnished with the bomb, tighten the socket head
screws firmly in a criss-cross pattern, moving in sequence around the circle.Repeat the tightening sequence
four times for a final torque of approximately 5 ft-lbs on
each screw.
Place the bomb in a temperature controlled oven and follow the heating and cooling procedure described in the
general instructions.After digestion, allow the bomb to
cool to ambient temperature on an aluminum plate or a
metal table top. It is not good practice to cool the bomb in
cold water or in a freezer. The internal forces will some-
times distort the liner, making it difficult to remove the
liner from the bomb body. Usually the liner can be dislodged by pressing the uncovered bomb against a brass
or plastic projection about 3 cm in diameter and 5 cm
high. A firm tap against the projection may be required.
In extreme cases it may be necessary to cool the bomb in
a refrigerator or freezer to shrink the liner.
After extended use the tapered rim on the PTFE cup will
become thin and the cover may be deformed to a point
Parts List
Part No. Description
301AC
302AC
A305AC
306AC
307AC
A308AC
308ACF
309AC
310AC
311AC
Bottom plate
Bomb body
PTFE cup with cover
Pressure plate
Compression ring
Screw cap with cap screws
Cap screw, 3/8-24 x 3/4, soc. hd.
Bellville spring washer
Corrosion disc, .002", stainless
Rupture disc, .003", Inconel
11

Printed in USA
11/97
PARR INSTRUMENT COMP ANY
211 Fifty-Third Street
Moline, Illinois 61265 USA
309/762-7716 800/872-7720
Fax 309/762-9453
http://www.parrinst.com parr@parrinst.com
 Loading...
Loading...