Page 1
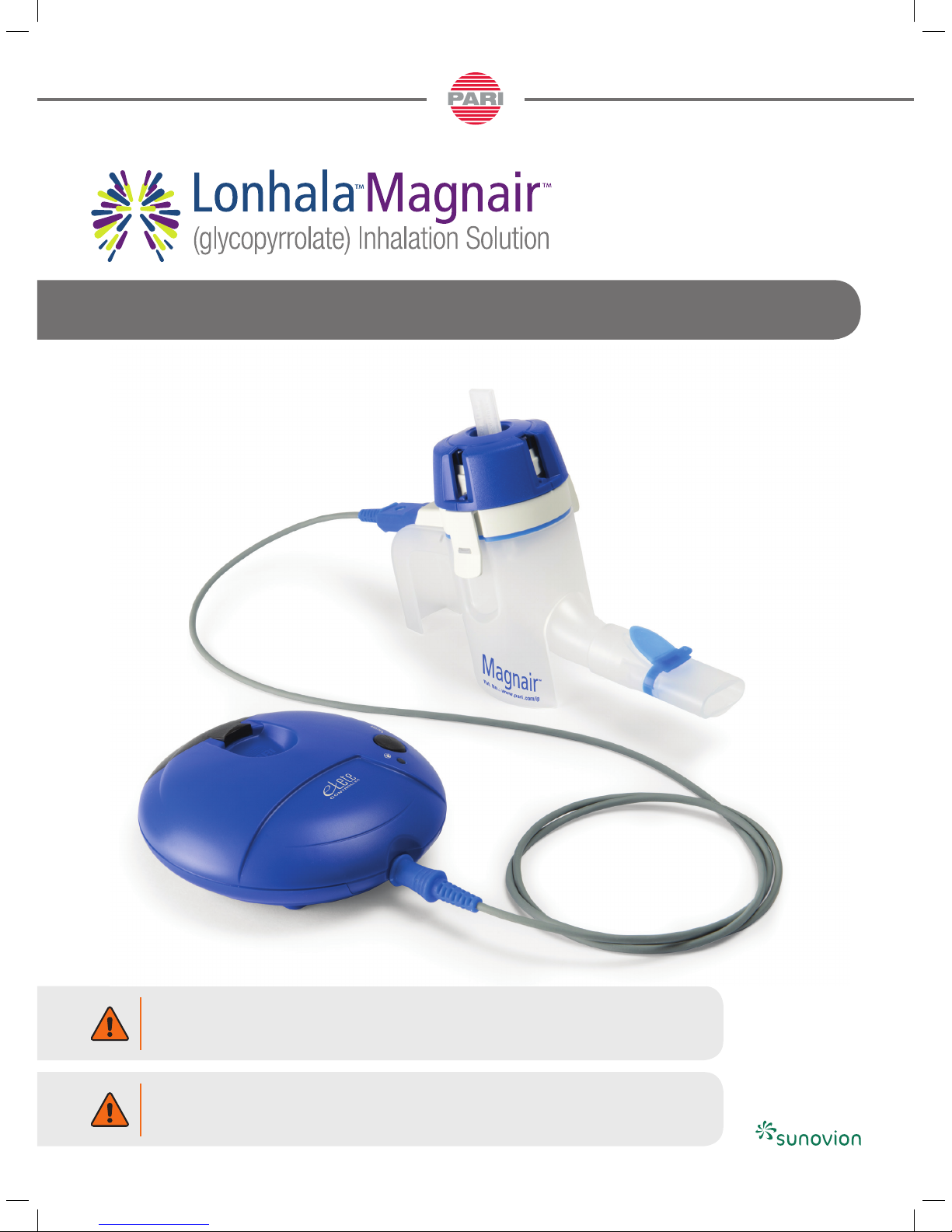
MAGNAIR™ NEBULIZER SYSTEM INSTRUCTIONS FOR USE
For use with LONHALA™ (glycopyrrolate) Inhalation Solution only
Warning
Read and understand the full Patient Information (PI) for information and
warnings related to LONHALA. The PI is contained in the LONHALA box.
Warning
Read and understand these Instructions for Use and all safety precautions
it contains. Improper use can cause serious or fatal injury/illness.
Assembly required. LONHALA vials packaged separately.
Marketed by:
Page 2

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
2
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
TABLE OF CONTENTS
A. Safety Precautions ..................................................................................................................................... 3
B. Getting Started ........................................................................................................................................... 5
C. Steps for Using Batteries or AC Adapter with MAGNAIR ...........................................................................6
D. Assembling Your MAGNAIR ..................................................................................................................... 8
E. Using LONHALA MAGNAIR .................................................................................................................... 11
F. Cleaning the Handset ............................................................................................................................. 15
G. Troubleshooting .......................................................................................................................................20
H.Specications ........................................................................................................................................... 23
Intended Use and Authorized Users
MAGNAIR is intended for single-patient use in the delivery of LONHALA to patients who self-administer
treatments or have treatments administered by a caregiver or health care provider.
MAGNAIR Handset is not sterile.
Page 3

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
3
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
These Instructions for Use contain information and safety precautions for MAGNAIRTM (MAGG-nair)
Nebulizer System for use with LONHALATM (lon-HAL-luh) (glycopyrrolate) Inhalation Solution.
• US Federal law restricts this device to sale by or on order of a physician.
Warning
MAGNAIRisdesignedspecicallyforuseonlywithLONHALA. To reduce the risk of severe
or fatal injury/illness, Never use other medications in MAGNAIR. Read and follow all
warnings and instructions in the Instructions for Use prior to using this device.
Read all Dangers and Warnings before using.
Dangers
To reduce the risk of serious or fatal injury from electrocution:
1. Do Not place or store MAGNAIR where it can be in water or fall into water (e.g., near a bathtub or sink).
Do Not place or drop into water or other liquid. Do Not use while bathing.
2. Do Not reach for MAGNAIR if it has fallen into water or other liquid. If using AC adapter, unplug.
Retrieve MAGNAIR only after it has been unplugged.
Warnings
1. The MAGNAIR Handset is intended for Single Patient Use Only. Do not share your MAGNAIR
with anyone else.
2. MAGNAIRisdesignedspecicallyforuseonlywithLONHALA. To reduce the risk of severe or fatal
injury/illness, Never use other medications in MAGNAIR.
3. Read and follow all warnings and instructions in the Instructions for Use prior to using this device.
4. Toreducetheriskofseriousorfatalinjuryfromelectrocution,re,burns,andtoreducetherisk
of damage and malfunction of the unit:
a. Do Not overload wall outlets or use extension cords.
b. Keep all electrical cords away from heated surfaces.
c. Do Not spray liquids onto the housing of the Controller. Liquid may cause damage to the electrical
parts and could lead to a malfunction. In the event that liquids enter the Controller, contact the
Sunovion Customer Service line (1-888-394-7377).
d. Do Not drop or insert any object into any opening on MAGNAIR.
e. Do Not operate where oxygen is being administered in a closed environment, such as an oxygen tent.
Save these Instructions for Use for future reference.
If for any reason you do not understand any part of these Instructions for Use, please contact the
Sunovion Customer Service line 1-888-394-7377 before proceeding with your treatment.
Take special note of all safety precautions marked Danger and Warning.
A. SAFETY PRECAUTIONS
IMPORTANT INFORMATION FOR USE
Page 4
MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
4
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Warnings
5. Always unplug this product from AC power immediately after using and before cleaning.
6. Before use, check your Controller and Handset for proper assembly. All parts must be connected
andrmlyinplace.UseofanimproperlyassembledMAGNAIRcoulddiminishoreliminatethe
effectiveness of the treatment.
7. Use Only adapters and accessories that are authorized for MAGNAIR. Use of unapproved adapters
and accessories can lead to improper treatment, injury, or damage to the Controller.
8. Never operate the Controller if it is improperly or incompletely assembled or damaged.
If you suspect either situation, call the Sunovion Customer Service line (1-888-394-7377).
9. Never operate MAGNAIR if:
a. it has damaged cords or plugs;
b. it is not working properly;
c. the inside of the Controller has been exposed to any liquids.
10. TomaintaintheefciencyofMAGNAIR,removetheAerosolheadfromtheMAGNAIRHandset
body and clean and air-dry all MAGNAIR Handset parts after each treatment. Follow the
instructions in Section F to clean the MAGNAIR Handset properly.
11. Cleaning the MAGNAIR Handset properly will help prevent the Aerosol head from clogging. If the
Aerosol head becomes clogged, the aerosol mist may be reduced, altered, or stopped, which could
increase the nebulization time (up to 15 minutes) and/or diminish the effectiveness of the treatment.
Do Not Stop Treatment until MAGNAIR shuts off.
12. This product contains small parts that may present a choking hazard to small children.
The MAGNAIR AC adapter and Connection cord also presents a strangulation hazard.
13. Close supervision is necessary when this product is used near children or the physically or
mentally impaired.
14. Do Not allow pets near the Connection cord or AC adapter as they may chew on and damage them.
15. Do Not use your MAGNAIR while driving or in any situation that takes away your full attention.
16. Do Not disassemble the blue Controller at any time. There are no user serviceable parts inside the
Controller. Contact the Sunovion Customer Service line (1-888-394-7377) for help.
17. Do Not use alcohol for cleaning and disinfection. Some parts will be damaged by alcohol.
18. TheLONHALAvialisforsingleuseonlyandmustnotbereused,relled,orusedinanyotherdevice.
A. SAFETY PRECAUTIONS (cont.)
Page 5
MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
5
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
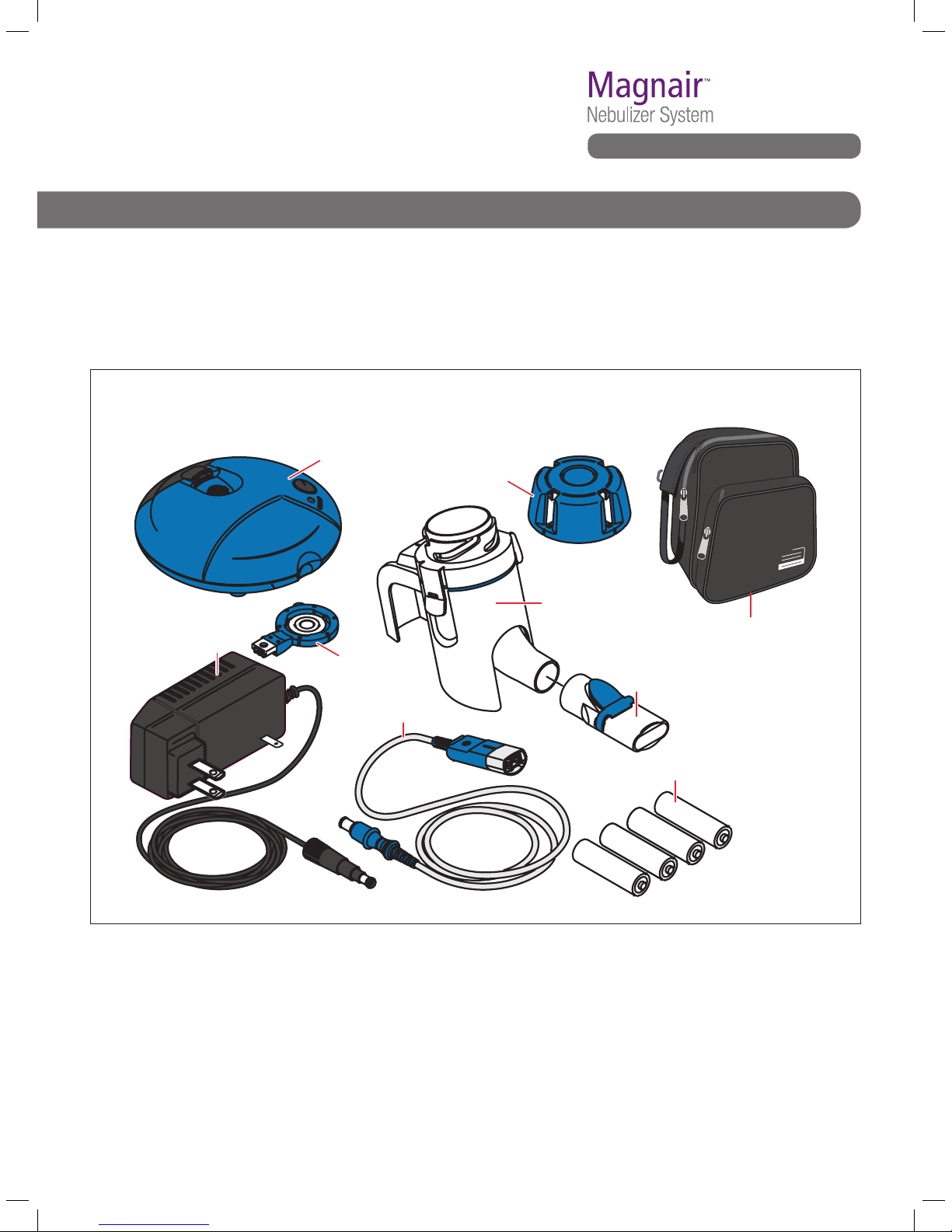
B. GETTING STARTED
Check to make sure you have all of the following
MAGNAIR parts and become familiar with how to
identify each piece. If any parts are missing, call the
Sunovion Customer Service line (1-888-394-7377).
See Fig. B1.
Fig. B1 MAGNAIR Nebulizer System
Controller
Medication cap
Handset body
Mouthpiece
4 AA batteries
AC adapter
Aerosol head
Connection cord
LONHALA vials packaged separately.
Carrying bag
Page 6
MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
6
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
C. STEPS FOR USING BATTERIES OR AC ADAPTER WITH MAGNAIR
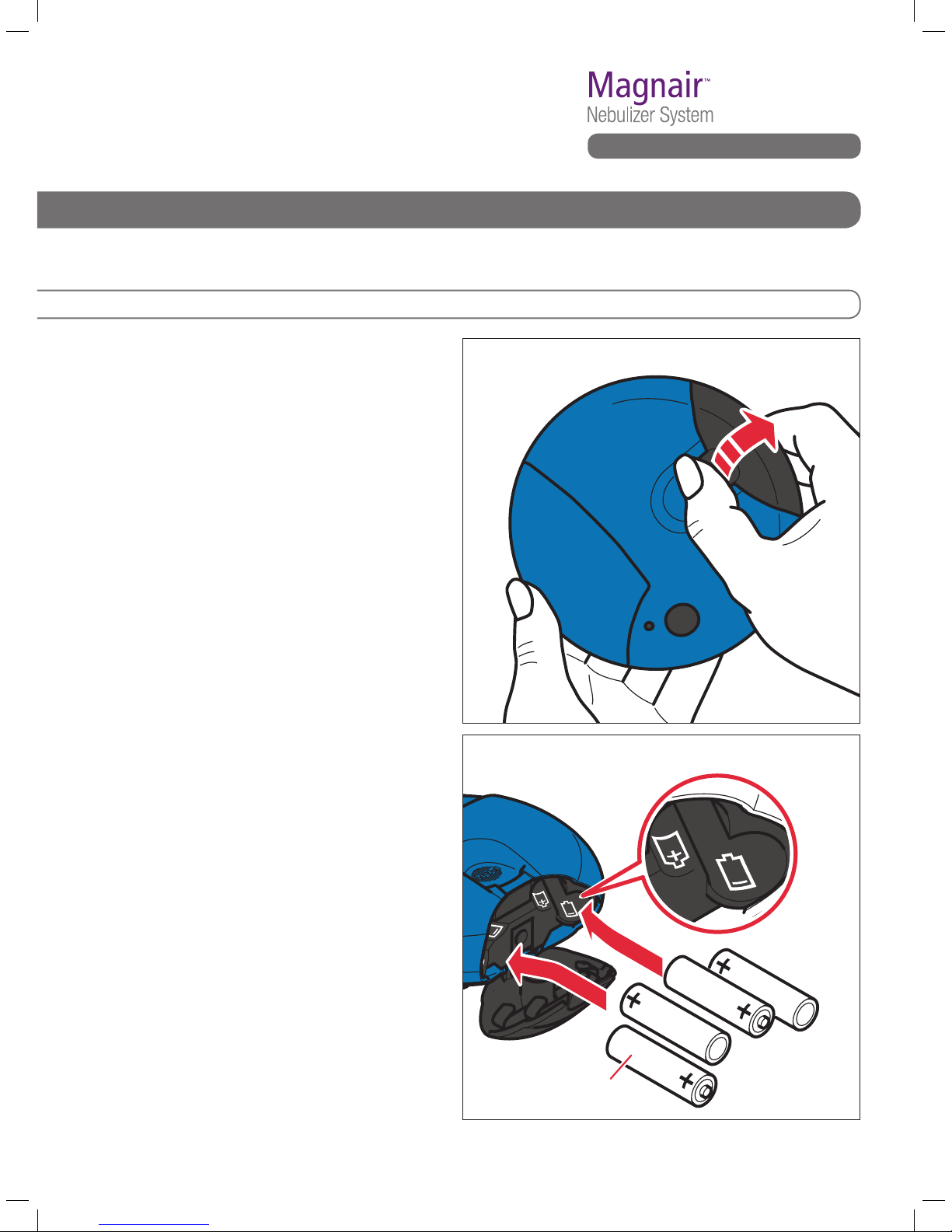
Fig. C1 Open the battery door
of the Controller.
Fig. C2 Load the batteries.
STEPS FOR USING BATTERIES WITH MAGNAIR
MAGNAIR is designed to be used with AA batteries or with the AC adapter.
Four (4) high quality AA batteries will provide
approximately 2 weeks of treatment.
C1. Open the battery door on the Controller
by placing your thumb on the black tab
ofthebatterydoorandrmlypushing
the tab to open the door. Note that the
battery door is designed to have a
tightt.See Fig. C1.
C2. Load the batteries.
Eachbatterychamberhasasmallgure
that shows the proper position of each
battery. Using the battery tips as guides
and starting left-to-right, insert the
batteries: tip out, tip in, tip out, tip in.
See Fig. C2.
4 AA batteries
Page 7
MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
7
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. C4-C5 Plug the AC adapter into the inlet
on the black battery door of
the Controller. Then plug the
AC adapter into the wall outlet.
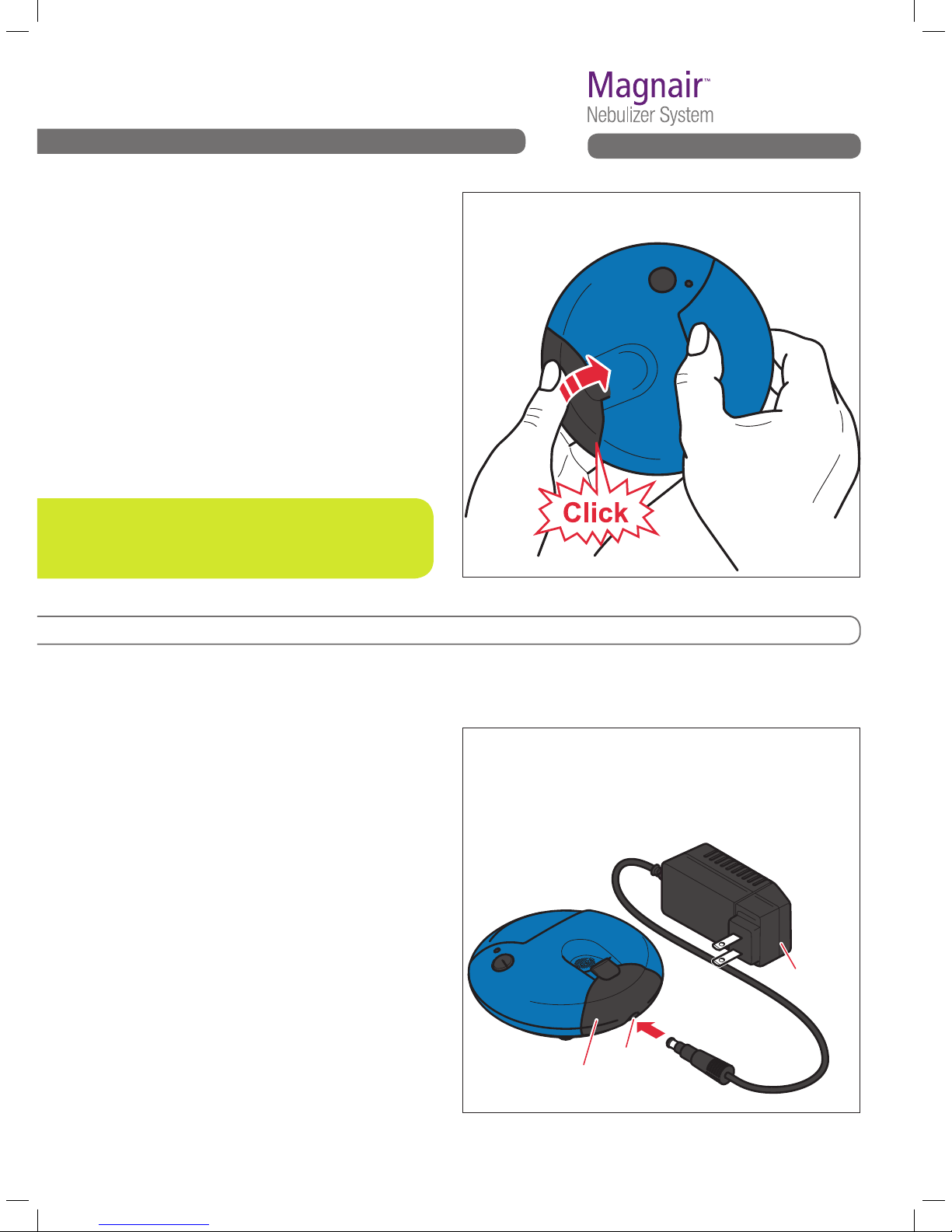
C3. Close the battery door.
To close the battery door, push it closed until
you hear it Click into place. See Fig. C3.
NOTE: Rechargeable and disposable batteries
vary considerably in terms of storage life and
output. If used exclusively, batteries have an
operating life of ~2 weeks, based on high
quality disposable AA batteries that meet the
specicationslistedinSection H and following
the cleaning procedure described in Section F.
To reduce the risk of battery leakage, it is
recommended to remove the batteries if you
plan to store the Controller for more than
30 consecutive days.
C4. Plug the AC adapter into the Controller.
To connect the AC adapter to the Controller,
placetheControlleronaat,stablesurface.
The inlet is located on the underside of the
black battery door. Push the round end of
the AC adapter cord into the inlet. Do Not try
to insert the AC adapter into the blue colored
part of the Controller. See Fig. C4-C5.
If you choose not to use the AC adapter,
it is strongly recommended to have an
extra set of batteries with you at all times.
STEPS FOR USING THE AC ADAPTER WITH MAGNAIR
The AC adapter will automatically adjust to the incoming voltage. The AC adapter will power
MAGNAIR with and without installed batteries.
C5. Plug the AC adapter into the wall outlet.
NOTE: the AC adapter will not charge the
batteries in the Controller. See Fig. C4-C5.
C. STEPS FOR USING BATTERIES OR AC ADAPTER WITH MAGNAIR (cont.)
Black battery door
Inlet
AC adapter
Fig. C3 Close the battery door
of the Controller.
Page 8

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
8
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
D. ASSEMBLING YOUR MAGNAIR
Your Handset parts (Medication cap, Aerosol head, Handset body, and Mouthpiece) will be
replaced when you receive your next supply of LONHALA. Discard your old Handset parts and
use the new Handset parts every time you get a LONHALA MAGNAIR Rell Kit.
Warning
Clean the Handset before the rst use
and after every use. (See Section F.)
Inspect all Handset parts to ensure they
are clean and not damaged. Do Not
leave the Aerosol head in the Handset
body after use, and Do Not use dirty
or damaged parts because this can
impair the function of the Handset.
D1. Wash your hands.
Fig. D2 Open the Handset body.
D2. Open the top of the Handset body.
Lift the clasp on top of the Handset
bodyslightlyandipituptoopen.
See Fig. D2.
Fig. D3 Insert the Aerosol head into the
Handset body. Do not touch the
center of the Aerosol head.
D3. Insert the Aerosol head into the Handset
body as shown, taking care Not to touch the
center of the Aerosol head. Hold the Aerosol
head like you would hold a small frying
pan so that the silver text side is facing up
and the brown ring is facing down. Notice
that the Aerosol head has a small tab on
the side. Align the small tab with the
matching notch in the Handset body.
See Fig. D3.
Notch
Text side
Tab Clasp
Clasp
Page 9

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
9
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. D4 Close the Handset body.
D4. Close the Handset body, making sure
the Aerosol head is properly inserted
and the tab is aligned with the notch
and level with the Handset body. You
may hear a Click. If you do not
close your Handset body completely
or align the Aerosol head correctly,
your medication may leak and you
will not get your full treatment.
See Fig. D4.
Do not force the top of the Handset
body closed. If the top of the Handset
body cannot close (no Click is heard),
make sure that the Aerosol head is
seated correctly and level.
D. ASSEMBLING YOUR MAGNAIR (cont.)
Fig. D5 Attach the Mouthpiece.
D5. Attach the Mouthpiece to the
Handset body. Make sure the Blue
valve is pressed down into the slot on
the Mouthpiece and is positioned on the
top of the Mouthpiece before attaching
the Mouthpiece onto the Handset body.
See Fig. D5.
Blue valve
Page 10

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
10
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. D6a Connect the Connection
cord to the Controller.
D6a. Connect the Controller to the Handset
body using the Connection cord.
• One end of the Connection cord has
a blue round connector. Insert the
Connection cord into the inlet on the
blue side of the Controller. Push the
Connection cord in as far as it will go.
You may hear a slight Click if inserted
correctly. See Fig. D6a.
Fig. D6b Connect the other end of the
Connection cord to the Handset body.
D6b. Connect the Controller to the
Handset body using the
Connection cord as shown.
• The other end of the Connection cord
is blue and gray. Insert the Connector
(with gray mark facing up) into
the Handset body as far as it will
go, ensuring the gray mark on the
Connection cord lines up with the
blue mark on the Handset body.
• Check that the ends of the Connection
cord are fully inserted into the
Controller and the Handset body.
If they are not, the Handset may not
work properly. See Fig. D6b.
Gray mark
D. ASSEMBLING YOUR MAGNAIR (cont.)
Blue inlet
Connection
cord
Blue mark
Connection cord
Page 11

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
11
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
E. USING LONHALA MAGNAIR
Fig. E1 Remove the LONHALA vials
and separate them.
Fig. E2 Insert the LONHALA vial into
the bottom of the Medication
cap until it Clicks.
E1. With clean hands, open the foil
pouch enough to remove the
2 LONHALA vials.
Manually separate by twisting apart the
2 LONHALA vials and return 1 vial back
to the opened foil pouch and store in
the Carrying bag to be used at the next
treatment. Discard the vial if not used
within 7 days. See Fig. E1.
E2. Insert 1 LONHALA vial into the
Medication cap.
The Medication cap has a top and a bottom.
InsertaLONHALAvialwiththeattabrst
through the bottom of the Medication cap
and press it in as far as it will go. You
should hear a Click if the LONHALA
vial was inserted correctly. See Fig. E2.
Warning
MAGNAIR will only operate with
vials containing LONHALA. Do
not try to use any other type of
medication with your MAGNAIR
or use the vials in any other
type of device.
You should be able to hear a Click when
the LONHALA vial is inserted correctly.
Medication cap: top
Medication cap: bottom
LONHALA
vial
Page 12

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
12
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. E3a Do not touch the part
that pierces the vial.
Fig. E3b Place the Medication cap
with LONHALA vial
on top of the
Handset body.
E3. Prepare to attach the Medication cap.
Do Not touch the part of the Handset body
that pierces the LONHALA vial.
See Fig E3a.
Make sure the Aerosol head is installed
before attaching the Medication cap
because your medicine could leak and
you will not get your full treatment.
Place the Medication cap with the
LONHALA vial on top of the Handset body.
See Fig. E3b.
Turn the Medication cap in a clockwise
direction. As you turn the Medication cap,
the LONHALA vial will open and you should
hear a Click. The notch (at the base of the
opening) in the Medication cap should line
up with the blue line on the Handset body.
See Fig. E3c.
E. USING LONHALA MAGNAIR (cont.)
Fig. E3c Turn the Medication cap onto the
Handset body until you hear a Click.
Warning
Do Not Loosen or remove the Medication cap or unclasp the Handset until treatment is
completed because your medicine could leak and you will not get your full treatment.
Line up notch in cap with
blue line on Handset body.
Page 13

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
13
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. E4 Insert the Mouthpiece into your
mouth. Do not tilt the Handset.
E4. Insert the Mouthpiece into your mouth.
• Sit in an upright position and relax.
This makes inhaling easier.
• Hold the Handset body with your hand
then place the Mouthpiece into your
mouth and seal your lips around it.
• Do Not tilt the Handset. Make sure
the Handset is level.
• Do Not cover the blue valve with
your lips.
• Do Not Loosen or remove the Medication
cap until your treatment is complete
because your medication could leak and
you will not get your full treatment.
See Fig. E4.
E5. Turn on the Controller.
• Press the On/Off button to start
your treatment.
• A green LED light beside the On/Off
button will light up and a single Beep
will be heard to indicate proper
functioning. See Fig. E5.
E. USING LONHALA MAGNAIR (cont.)
Fig. E5 Turn on the Controller.
On/Off button
Page 14

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
14
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. E6 Inhale and exhale normally.
Fig. E7 Your treatment should
take about 2 to 3 minutes.
E6. Breathe in (inhale) and breathe out
(exhale) normally through the Mouthpiece.
The Mouthpiece should remain in your
mouth throughout the treatment period.
Do Not breathe through your nose.
While you are exhaling, you may see the
blueapliftandsomemistescape.
See Fig. E6.
• Continue inhaling and exhaling through
the Mouthpiece until the Controller
Beeps and shuts off.
• To pause your treatment, press the
On/Off button. To continue your
treatment, press the On/Off button
again. See Fig. E5.
E7. At the end of treatment, the Controller
will automatically shut off.
When all of the medication has been
delivered, you will hear 2 Beeps,
the green LED light will turn off, and the
Controller will automatically shut off.
Your treatment should take 2 to 3 minutes.
See Fig. E7.
Warning
If you do not clean the Handset parts after every use, your treatment time might take more than
3 minutes. If the treatment is taking longer than 3 minutes, continue with your treatment until the
Controller shuts off to make sure that you get your full treatment.
E. USING LONHALA MAGNAIR (cont.)
2-3
minutes
Page 15

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
15
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
F. CLEANING THE HANDSET
Warning
To ensure proper operation and to reduce the risk of severe or fatal injury/illness.
Important: Rinse and clean the MAGNAIR Handset parts after every use. Use clear liquid
dishwashing soap. Do Not use white dishwashing soap (e.g., Ivory or Dove) or antibacterial
liquid dish soaps, as these may contain additives harmful to the Aerosol head. Do Not leave the
Aerosol head in your Handset. Do Not wash the Controller, Connection cord, or AC adapter.
Caution
• Do Not put MAGNAIR parts
into a microwave oven.
• Do Not clean the MAGNAIR
parts in a dishwasher.
• Do Not clean the Aerosol head
and Handset parts with brushes
or abrasives.
CLEANING THE HANDSET
Fig. F1 Disconnect the Handset.
F1. Disconnect the Handset from the
Connection cord. See Fig. F1.
Fig. F2 Remove the Medication cap.
F2. Turn the Medication cap in a
counterclockwise direction as
shown, to remove from the
Handset body. See Fig. F2.
Page 16

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
16
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. F3 Remove the LONHALA vial.
F3. Remove the LONHALA vial.
Place the top of the Medication cap into
the palm of your hand and push up as
shown to remove the LONHALA vial.
Throw away the LONHALA vial into
wastebasket. See Fig. F3.
F. CLEANING THE HANDSET (cont.)
Fig. F4 Remove the Mouthpiece.
F4. Remove the Mouthpiece from the
Handset body by giving it a gentle twist
and pull to separate from the Handset
body. See Fig. F4.
Fig. F5 Loosen the blue valve.
F5. Loosen the blue valve. Carefully
loosen the blue valve from the slot in the
Mouthpiece. Make sure the valve is still
attached on one side to the Mouthpiece.
See Fig. F5.
Page 17

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
17
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fig. F6 Remove the Aerosol head from
the Handset body. Do not touch
the center of the Aerosol head.
F6. Remove the Aerosol head from the
Handset body by lifting the clasp on the side
of the Handset. Then remove the Aerosol
head by lifting the handle.
Do Not touch the center of the Aerosol head.
See Fig. F6.
F. CLEANING THE HANDSET (cont.)
Fig. F7 Rinse all Handset parts
with warm running water for
about 10 seconds.
F7. Rinse each of the disassembled Handset parts
well under warm (above 105°F) running tap
water. (approx. 10 seconds). See Fig. F7.
F8. Wash all Handset parts in warm
(above 105°F), soapy water made by
adding a few drops (~¼ teaspoon) of clear
liquid dishwashing soap into a bowl (~1 quart)
of clean warm tap water. Swish the Handset
parts around in the soapy water to clean.
See Fig. F8.
• Do Not wash the Connection cord, Controller,
and AC adapter.
• Do Not use white dish soap or antibacterial
hand soaps because they may clog the
Aerosol head.
• Do Not use a brush or abrasive to clean any
of the Handset parts because it may
damage them.
Fig. F8 Wash all Handset parts
in warm soapy water for
about 10 seconds.
Set aside the Aerosol head to be
cleaned separately in Step F10.
Clasp
Page 18

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
18
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
F9. Rinse the Handset parts well under
running warm tap water (approximately
10 seconds). See Fig. F9.
F. CLEANING THE HANDSET (cont.)
Fig. F9 Rinse Handset parts well with warm
running water for about 10 seconds.
F10. Clean the Aerosol head following the
instructions in Steps 7 through 10.
10A. Rinse both sides of the Aerosol head
well with warm running water for about
10 seconds on each side.
10B. Wash the Aerosol head by holding
the handle and swishing it back and forth
in the warm soapy water for about
10 seconds.
10C. Rinse both sides of the Aerosol head
well with warm running water for about
10 seconds on each side.
Rinsing the Aerosol head well
helps prevent clogging and ensures
proper operation.
• Do Not add dishwashing liquid directly onto the Aerosol head, add to water only.
• Do Not use a brush or abrasive to clean the Aerosol head because it may damage it.
Fig. 10A Rinse Aerosol head with warm running water for about 10 seconds.
Fig. 10B Wash Aerosol head in warm soapy water for about 10 seconds.
Fig. 10C Rinse Aerosol head with warm running water for about 10 seconds.
10A 10B 10C
Page 19

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
19
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Warning
Contamination and moisture may affect the Aerosol head and encourage the growth of bacteria.
Therefore, it is important to remove the Aerosol head from the Handset body and clean the
Handset parts after every use. If Handset parts are still dirty after cleaning, then soak and rinse
as described in step F11.
F11. Inspect all Handset parts to make sure
they are completely clean. If any Handset
parts are still dirty, soak the parts in warm
soapy water for 5 more minutes. Rinse
well with warm running water until clean.
Fig. F12 Air-dry all Handset parts.
F12. Air-dry all Handset parts.
Remove excess water by shaking all
parts. Place all Handset parts on a dry,
clean, lint-free towel and allow them to
air-dry. Do Not dry using a paper towel.
Do Not touch the center of the
Aerosol head. See Fig. F12.
F13. Store disassembled Handset parts.
After the Handset parts are completely
dry, place them in the provided
Carrying bag or a dry, dust-free
environment for storage.
Do Not put the Handset parts back
together until ready to use again for
your next treatment of LONHALA.
Warning
A damp environment encourages the growth of bacteria. Make sure all Handset parts are
dried properly.
CARING FOR THE CONTROLLER, CONNECTION CORD AND AC ADAPTER
• Make sure the Controller is off. Remove the Connection cord and AC adapter cord from the Controller.
• Remove the AC adapter from the wall socket.
• Clean the Controller housing, the Connection cord, and AC adapter with a damp cloth.
Warning
• Never let the Controller come in contact with water and never use cleaning agents.
• If liquid does get into the Controller, contact the Sunovion Customer Service line (1-888-394-7377).
F. CLEANING THE HANDSET (cont.)
Page 20

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
20
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
G. TROUBLESHOOTING
CONTROLLER FEEDBACK INDICATORS
Controller
AUDIBLE SIGNAL
Controller
VISUAL SIGNAL
Conditions Action Required
1
1 Brief Beep at start
of dose.
Solid green LED for
duration of dose.
Normal: generating
aerosol mist, no errors
detected.
No action required.
MAGNAIR is on and
working properly.
2
1 Brief Beep at start
of dose.
Flashing orangegreen LED.
Low battery power. Replace batteries or
use AC adapter.
3
1 Brief Beep at start
of dose followed by a
2-tone Beep.
Green LED followed
byashingorange-
green and then
shuts off.
No connection
detected.
Two Beeps occurred
immediately after
starting.
No drug detected.
Two-tone Beep
occurred
10-30 seconds
after starting.
Check connection
between the Controller
and the Handset.
Checktoconrma
LONHALA vial has
been inserted and the
Handset clasp is
closed properly.
Tilt the Handset from
side to side then tap
the Handset to dislodge
LONHALA and restart
the Controller.
4
1 Brief Beep. LED starts green,
then turns orange
and shuts off.
Treatment interrupted.
The Controller On/Off
button has been
intentionally or
unintentionally pressed.
Press the Controller
On/Off button to
resume treatment.
5
2 Brief Beeps. LEDashesgreen
for 2 seconds, and
then shuts off.
Normal: end of dose. No action required.
LONHALA vial contents
are empty.
6
2-tone Beep. LEDashes
orange-green.
Maximum nebulization
time reached
(15 minutes).
Aerosol head clogged.
Press On/Off to
resume treatment
if medication is still
present. Clean the
Aerosol head.
Page 21

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
21
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
POTENTIAL FAULTS AND POSSIBLE CAUSES / RESOLUTIONS
Fault Possible Causes / Resolutions
1
MAGNAIR cannot be turned on –
no green LED and no Beep.
No power
The batteries are discharged. Replace them or use the
AC adapter.
The batteries are not inserted correctly. Remove batteries
and reinsert following diagram on the battery compartment.
The AC adapter is not connected correctly. Check the
wall connection and the Controller connection.
2
TheLEDashesorange-green,
there is a 2-tone Beep and the
Controller turns off.
No connection between Handset and Controller
Check the connection between the Controller and
the Handset.
3
No aerosol mist appears when
MAGNAIRisrstturnedon,or
MAGNAIR turns off after a
few seconds.
Low power
Check for bad batteries.
Missing or improper insertion/assembly of LONHALA vial
ConrmthatanewLONHALAvialhasbeeninserted.
Tap MAGNAIR slightly to move the liquid to the bottom of
the LONHALA vial. If aerosol mist is still not generated after
MAGNAIR has been restarted, the LONHALA vial may not
have been pierced or is empty. Replace the vial if empty.
If partial dose has been received, contact your health care
provider for instructions.
4
Theindicatorlight(LED)ashes
orange-green during operation.
Low battery power
Replace the batteries or use the AC power.
5
Mist appears but the Controller
turns off prematurely.
Low battery power
Replace the batteries or use the AC power.
The Handset was not being held upright
Hold the Handset upright and press the On/Off button.
Insufcient medication
If partial dose has been received and/or medication spills,
call your health care provider.
If Handset clasp is broken and/or does not close properly,
call Sunovion Customer Service (1-888-394-7377).
G. TROUBLESHOOTING (cont.)
Page 22

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
22
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
Fault Possible Causes / Resolutions
6
MAGNAIR will not switch off
automatically.
Turn MAGNAIR off by pressing the On/Off button.
7
Aerosol mist continuously
escapes in large volumes
from the opening slots in the
Medication cap.
Improper Handset assembly. Check that the Handset
hasbeenassembledcorrectlyandtheBluevalveap
is pushed down.
8
The Medication cap will not
close.
LONHALA vial is not seated
properly. Press the LONHALA
vial base toward the cap
until you hear it snap
into place.
9
The LONHALA vial has been
inserted incorrectly.
If the LONHALA vial has been inserted incorrectly, the
following steps must be carried out:
1. Open the Medication cap.
2. If the LONHALA vial has been opened, throw away the
LONHALA vial, rinse and dry the Handset parts, then
insert a new vial.
3. If the LONHALA vial has not been opened, place the
Medication cap back on the Handset and turn the
Medication cap containing your LONHALA vial in a
clockwise direction until it clicks, then restart your
treatment by pressing the On/Off button.
10
Increasing or long inhalation
time.1 Treatment time may vary
up to 15 minutes if the Aerosol
head has become clogged.
The Aerosol head may be clogged. Clean the Aerosol
head by soaking it in soapy water for 5 minutes and rinse
both sides well (Section F ).
11
Top of Handset body has
become detached from bottom
of Handset body.
Line up the hinge part on the
top of the Handset body with
the hinge portion of the bottom
oftheHandsetbody.Pressrmly
until the parts snap together. You
should hear a Click.
Click
1
If the fault cannot be eliminated after following these steps, call the Sunovion Customer Service line
(1-888-394-7377) immediately.
G. TROUBLESHOOTING (cont.)
POTENTIAL FAULTS AND POSSIBLE CAUSES / RESOLUTIONS
Page 23

MAGNAIR questions? Contact the Sunovion Customer Service line (1-888-394-7377).
23
Instructions for Use
For use with LONHALATM
(glycopyrrolate)
Inhalation Solution
H. SPECIFICATIONS
ELECTRICAL
AC adapter
Input . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100 V-240 V, 50 Hz/60 Hz
Output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.5 V
Batteries
Disposable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 x 1.5 V (high quality alkaline or photography-grade)
Rechargeable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 x 1.2 V (Ni-Cd)
OPERATIONAL
Temperature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41° to 104°F (5° to 40°C)
Relative humidity (non-condensing) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15% to 93%
Air pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 to 15 PSI (700 to 1060 hPa)
Aerosol output . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.85-1.15 mL
Aerosol output rate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.4 mL/min
MECHANICAL
MAGNAIR Handset weight, without medication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .approx. 2.6 oz (73 g)
MAGNAIR Controller weight (with batteries) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . approx. 7.6 oz (220 g)
Nebulizer dimensions (W x H x D) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.4 in. x 4.7 in. x 7.19 in. (6 x 12 x 18 cm)
Controller dimensions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H 1.6 in. (4 cm), Ø 4.6 in. (11.6 cm)
TRANSPORT / STORAGE
Temperature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . -13° to 158° F (-25 to +70°C)
Relative humidity (non-condensing) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0 to 93%
Air pressure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 to 15 PSI (500 to 1060 hPa)
HANDSET MATERIALS
Polypropylene, polyoxymethylene, polyamide, silicone, stainless steel, thermoplastic elastomers.
Does not contain any natural rubber (latex).
DISPOSAL
The MAGNAIR parts and batteries must be thrown away in accordance with local (state, county, or municipal) regulations.
PERFORMANCE CHARACTERISTICS
LONHALA (glycopyrrolate) Inhalation Solution, 25 mcg/mL Mean
1
95% Condence Range
2
Delivered dose by breath simulation (mcg) 14.20 11.11–17.29
Delivered dose by breath simulation (% Label Claim) 56.80 44.45–69.16
MMAD3(μm)byNGI
4
3.71 2.92–4.49
CoarseParticles(Dia.>5μm)byNGI(mcg)
CoarseParticles(Dia.>5μm)byNGIin%ofDeliveredDose
5.83
27.72
2.32–9.33
11.20–44.24
FineParticles(Dia.≤5μm)byNGI(mcg)
FineParticles(Dia.≤5μm)byNGIin%ofDeliveredDose
15.20
72.28
11.46–18.93
55.77–88.79
Extra-FineParticles(Dia.<1μm)byNGI(mcg)
Extra-FineParticles(Dia.<1μm)byNGIin%ofDeliveredDose
0.11
0.55
0.03–0.20
0.15–0.94
GSD5 by NGI 1.66 1.49–1.83
1
n=15 devices from 3 device lots; 5 devices tested per drug product batch x 3 batches drug product
2
95%CondenceRange:Two-sidedtoleranceinterval,Proportionoftotalpopulation=0.95,
Condence(1-Alpha)=0.95
3
MMAD:MassMedianAerodynamicDiameter
4
NGI:NextGenerationImpactor
5
GSD:GeometricStandardDeviation
Page 24

Patented. See www.pari.com/ip.
Manufactured by:
PARI Respiratory Equipment, Inc., 2412 PARI Way, Midlothian, VA 23112
©2017 Sunovion Pharmaceuticals Inc. All rights reserved.
For Sunovion customer service, call 1-888-394-7377.
578D2000 Rev B 12/2017
902011R01
LONHALA and are trademarks of Sunovion Pharmaceuticals Inc. MAGNAIR is a trademark of PARI Pharma GmbH,
used under license.
SUNOVION and are registered trademarks of Sumitomo Dainippon Pharma Co., Ltd.
Sunovion Pharmaceuticals Inc. is a U.S. subsidiary of Sumitomo Dainippon Pharma Co., Ltd.
PARI and are registered trademarks of PARI GmbH.
eFlow is a registered trademark of PARI Pharma GmbH.
Made under license from The Technology Partnership plc.
Page 25

Colors:
Magenta
Yellow
Black
Cyan
PMS 2603
This is a computer generated laser proof. It is not to be used as a comparison of final printed color.
It is the ultimate responsibility of the Supplier/Contractor to provide Sunovion printed packaging
components with barcodes that can be read by the end user. It is the Supplier’s option to use the
codes as supplied or to replace with a new code that is modified to better meet press requirements.
Document Information:
File name: 902011R01_05Dec17_r67.indd
MID: 902011R01
Software: InDesign CC2015
Flat Size: 16.5 in x 11 in
Folded Size: 8.25 in x 11 in
PARI A/N: 578D2000-B
Design Firm
Job: 19702
Op: dl/rs/tk/dl/rs/dl
dl/sb/rs/eb/rs/dl
Barcode Information:
N/A
LONHALA MAGNAIR IFU- 8.25 x 11
designteam@perigord-as.com
 Loading...
Loading...