Oticon Medical Neuro Instruction Manual
Magnetic Resonance Imaging (MRI) Exam
0459
Instructions for Use
Neuro – The Cochlear Implant System
(2015)
Neurelec S.A.S
2720 Chemin Saint Bernard, 06220 Vallauris France
TEL : +33 (0)4 93 95 18 18, FAX : +33 (0)4 93 95 38 01

Table of content
MRI medical exam ....................................................................................................3
A. MRI exam with implanted magnet in place (at 1,5 Tesla) ........................................... 5
B. MRI exam with magnet removal (3 Tesla) ..................................................................6
C. Dummy removal and magnet replacement ..............................................................11
D. Magnet replacement ...............................................................................................11
2
MRI medical exam
Magnet removal is not required for magnetic field strengths at 1.5 Tesla. For magnetic field
strengths greater than 1.5 Tesla magnet removal is required for MRI compatibility. In order to
insure patient safety during an MRI exam and to
prevent damage to the Neuro Zti, the following instructions must be understood and followed.
Any questions or concerns should be clarified with the manufacturer prior to conducting an MRI
exam with a patient implanted with the Neuro Zti.
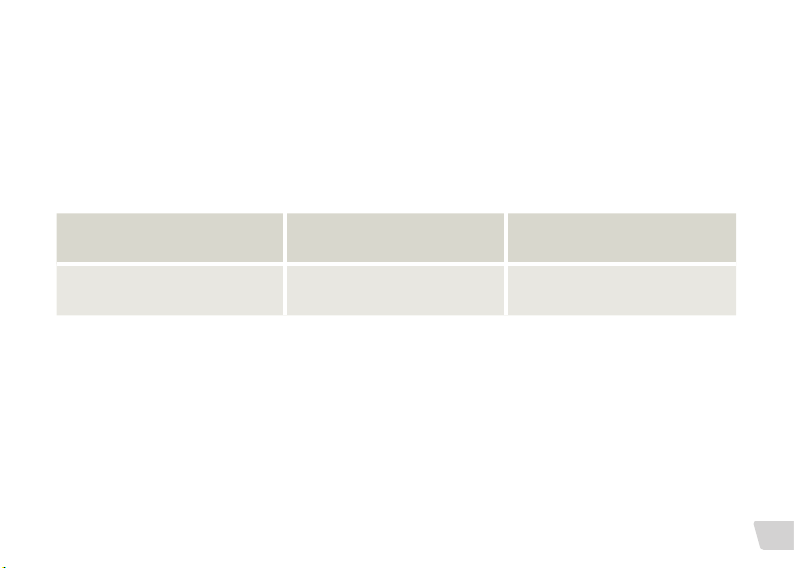
MRI Strength 1.5 Tesla 3 Tesla
Recommended intervention
No surgery required, use standard
head bandage
Remove magnet prior to
MRI exam
Warnings:
1. All external cochlear implant components such as the BTE, antenna, audio streamers, cables
and any other cochlear implant associated accessories are NOT compatible with MRI examination and must be removed prior to entering an MRI examination room.
There is a risk that prolonged exposure to high strength magnetic fields could demagnetize the
implanted magnet held by the Neuro Zti. To reduce the risk of demagnetization, care should be
taken to keep the longitudinal axis of the patient’s head parallel to the scanner’s principal
magnetic field. Loss of magnetization may interfere with antenna retention following an MRI
3

procedure. Should transcutaneous magnetic coupling become compromised, surgical intervention may be required to replace the implanted magnet following the procedures described
below.
2. If the region of medical interest is likely to fall within the projected artifact produced by the
Neuro Zti, the magnet may be removed prior to an MRI exam for MRI strengths at 1.5 Tesla to
minimize artifacts.
3. It is possible that the medical region of interest may be obscured by artifacts produced by the
Neuro Zti even with the implanted magnet removed.
4. It is possible that the patient may experience auditory sensations such as crackling, beeping
and/or a humming sound during an MRI exam. The patient should be advised of this possibility and that it does not indicate device malfunction or damage.
5. If the patient is a bilateral Neuro Zti recipient, the same procedures outlined in this document
must also be followed for the contralateral implant.
6. Head first: Technicians must slide the Neuro Zti recipient head first in the MRI machine
independent of the anatomical region to be scanned. Caution: Entering feet first is contraindicated.
7. Note: To perform an MRI exam, the radiologist should fill out an exam form that can be found
at www.oticonmedical.com. The form must be sent back for approval to the manufacturer that
is mentioned on the cover before performing the MRI exam.
4
 Loading...
Loading...