Page 1

INSTRUCTION MANUAL
Page 2

Inside Front Cover
English version
is blank
Page 3

Table of Contents
Physio-Stim®
Instruction Manual
Prescription Information ...............................................................................1
Orthofix PEMF Stimulation ..........................................................................2
Clinical Success of the Physio-Stim .................................................................2
Device Description .......................................................................................3
Treatment Instructions ..................................................................................4
Device Operation .........................................................................................4
Turning the Device On and Off ................................................................4
Timing of Treatment Sessions ...................................................................5
Charging/Recharging the Battery ..............................................................5
Visual and Audio Indicators ...........................................................................6
Device Application .......................................................................................7
Models 3202 and 3303 ...............................................................................7
Model 3313............................................................................................................8
Models 3314L and 3314R .........................................................................9
Model 3315 ............................................................................................10
Equipment Classification and Device Symbol Descriptions ............................11
Care and Cleaning ......................................................................................12
Travel .......................................................................................................12
Storage .......................................................................................................12
Disposal .....................................................................................................12
Service .......................................................................................................12
Warranty Policy............................................................................................13
Package Contents:
1- Physio-Stim Osteogenesis Stimulator
1- Literature Pack
1- Power Supply
1- Line Cord
THIS DEVICE IS NONSTERILE.
IT DOES NOT REQUIRE STERILIZATION.
U.S. Patent No. 5,743,844
Page 4

1
Prescription Information
Indication
The Physio-Stim® is indicated for the treatment of an established nonunion
acquired secondary to trauma, excluding vertebrae and all flat bones, where
the width of the nonunion defect is less than one-half the width of the bone to
be treated. A nonunion is considered to be established when the fracture site
shows no visibly progressive signs of healing.
Contraindication
Use of this device is contraindicated where the individual has synovial
pseudarthrosis.
Warnings
• The safety and effectiveness of the use of this device on individuals lacking
skeletal maturity has not been established.
• In the presence of a malaligned nonunion, careful consideration of the use
of this device must be undertaken on an individual basis, as treatment with
this device is not intended to alter or affect the degree of malalignment.
• Demand type pacemaker operation may be adversely affected by exposure
to pulsed electromagnetic fields. Physicians should not prescribe a
Physio-Stim for application which may place the treatment transducer in
close proximity to the pacemaker. Further screening by the attending
cardiologist is recommended (such as with an electrocardiogram).
• Animal studies conducted to date do not suggest any long-term adverse
effects from the use of this device. However, long-term effects in humans
are unknown.
• The safety and effectiveness of this device on individuals with a nonunion
secondary to, or in connection with, a pathological condition has not been
established.
Precautions
• Nonunion fractures with gaps in excess of 1 centimeter (cm) have not
been evaluated.
• Although animal reproductive studies performed with this device
demonstrated no adverse findings, the safety of use of this device during
pregnancy and nursing in humans has not been established.
• This device should not be used if there are mental or physical conditions
which preclude patient compliance with physician and device instructions.
Adverse Events
Rare instances of reversible minor discomfort have been reported.
They were: cumbersome or uncomfortable, tingling or pain and
minor skin rash.
Page 5

2
Orthofix PEMF Stimulation
Pulsed electromagnetic field (PEMF) osteogenesis stimulation is a safe,
nonsurgical, prescription treatment to heal nonunion fractures and promote
spinal fusion. Electrical currents have been used to heal bones since the
mid-1800s. However, it wasn’t until the 1950s that scientists made an
important discovery. When human bone is bent or broken, it generates an
electrical field. This low-level electrical field activates the body’s own repair
mechanism which, in turn, stimulates bone healing.
Orthofix PEMF osteogenesis stimulators generate a uniform, low-level, pulsed
electromagnetic field similar to the electrical field generated by the body.
The application of PEMF directly to the fracture site helps activate and augment
the body’s natural healing process to enhance bone fusion. Thank you for
including Orthofix in your healing process. To learn more about osteogenesis
stimulation, please visit our website at www.bonestimulation.com.
Clinical Success of the Physio-Stim
The Physio-Stim has been proven safe and effective in clinical studies. In a
prospective, multicenter, controlled clinical study for nonunion fractures,
an overall success rate of 80% was demonstrated among 126 patients
(135 nonunion fractures) who averaged 3 or greater hours of daily treatment.
The average duration of nonunion in these difficult fractures was 2.6 years,
with an average of two prior surgical procedures per fracture. The success rate
of Physio-Stim treatment for nonunion repair demonstrated no statistically
significant change over long-term (four year) follow-up.
Page 6
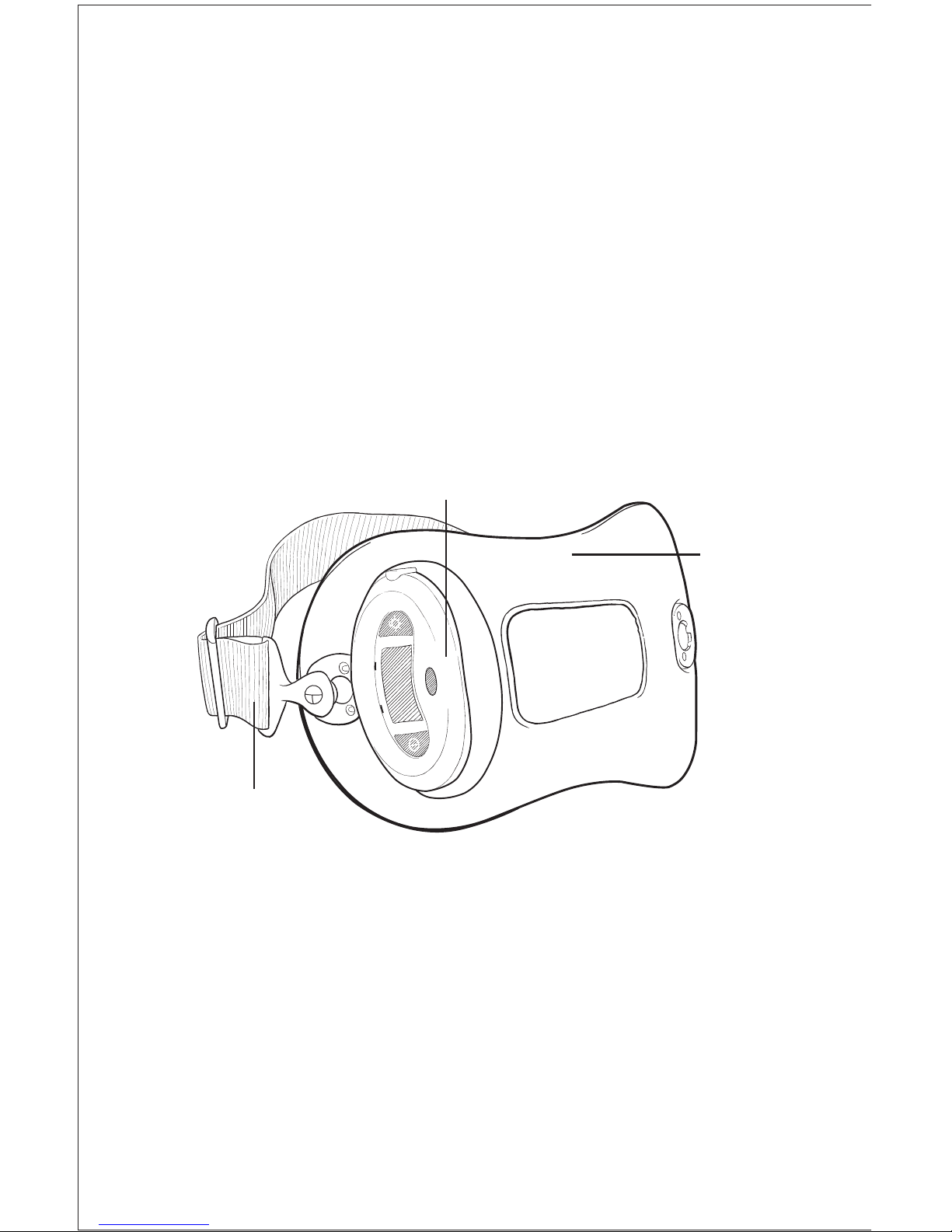
3
Device Description
The Physio-Stim is an external, low-level, PEMF device and has been designed
with patient comfort and convenience in mind. It is a single-piece device that
is lightweight, flexible and portable, allowing freedom of movement during
treatment. A Liquid Crystal Display (LCD) and audible alarm provide
important feedback during treatment such as the operational status, treatment
time remaining, battery capacity, etc. See “Visual and Audio Indicators” for
more information.
The Physio-Stim is comprised of a control unit and treatment transducer.
The control unit contains a micro-processor that generates the Physio-Stim
electrical signal. That signal is converted to a highly uniform, low-energy
magnetic field by the treatment transducer. When the device is centered
over the treatment area, the therapeutic Physio-Stim PEMF signal is delivered
directly to the treatment site.
The Physio-Stim is powered by a rechargeable lithium-ion battery pack.
The LCD and audible alarm will alert the patient when the battery is low and
needs to be recharged. See “Charging/Recharging the Battery” for more
information. To ensure that the device is functioning properly, the Physio-Stim
constantly monitors battery voltage and the electrical signal. If at any time
during treatment the device stops functioning properly, the LCD will display
an appropriate symbol or error code. See “Visual and Audio Indicators” for
more information.
Model: 3313
Control Unit
Treatment
Transducer
Adjustable Strap
Page 7
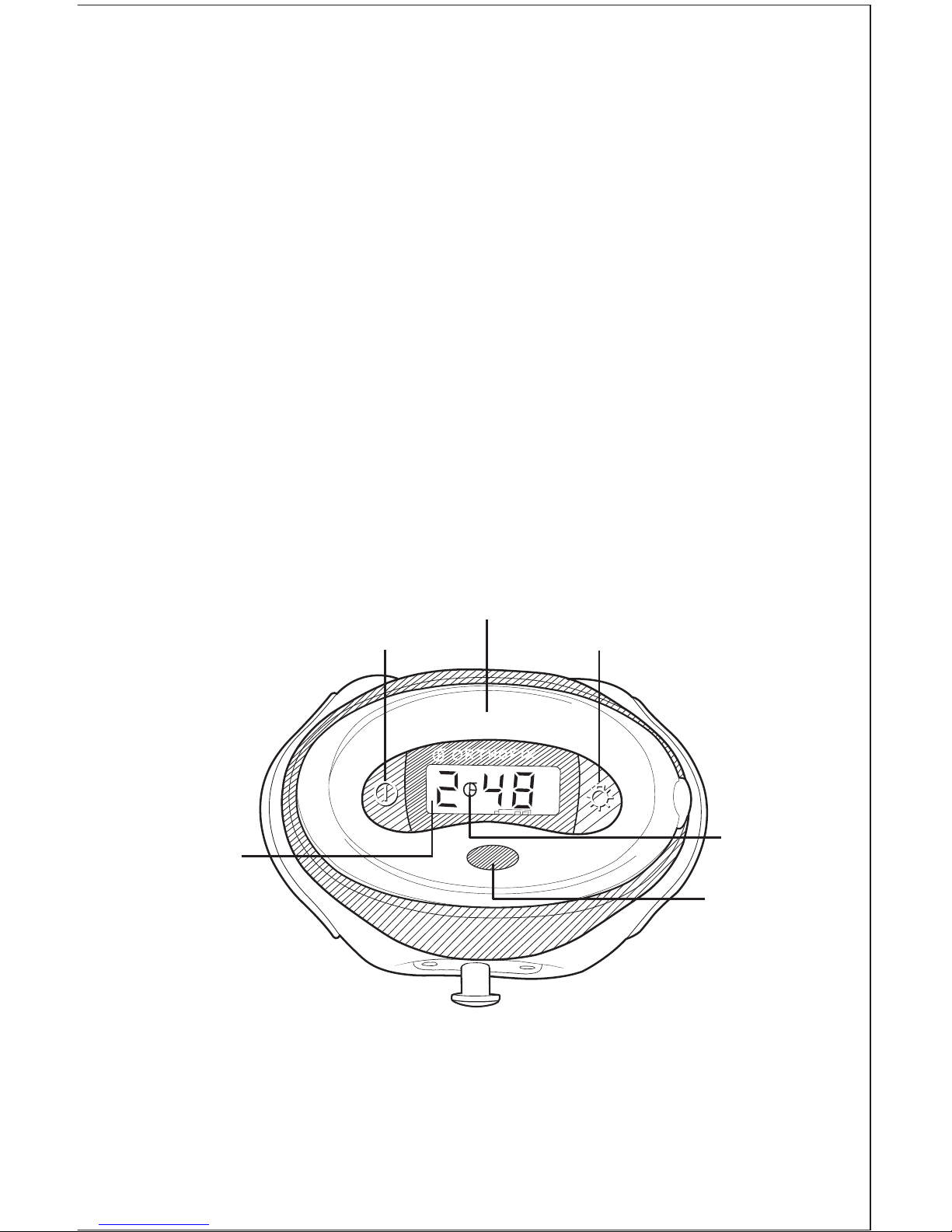
4
Treatment Instructions
The Physio-Stim should be worn for three hours per day. Based upon clinical
data for nonunion, the overall treatment duration ranges between 90 and 180
days based upon specific patient conditions. At the end of the daily treatment
the device will turn itself off. The Physio-Stim may be used at any time of day
that is most convenient and comfortable for the patient. It is lightweight and
adjustable. And because the Physio-Stim is portable, treatment can be
received while the patient is sitting, walking, reclining, sleeping, etc. However,
since each patient is unique, the overall activity level should be based on
physician instructions.
Device Operation
Turning the Device On and Off
The Physio-Stim is turned on and off by pressing the On/Off button on the
control unit of the device. When the device is on, a sequence of status
messages will display momentarily. The LCD should then show the treatment
time remaining and a flashing Orthofix logo. The flashing logo indicates that the
device is on and functioning normally. (If you do not see this on the display,
contact Orthofix Customer Service.) A backlight button is on the control unit.
In low light, press the backlight button for illumination of the LCD.
Control Unit
LCD
Flashing
Orthofix Logo
Infrared Port
(IR Port)
On/Off Button
Backlight Button
Page 8
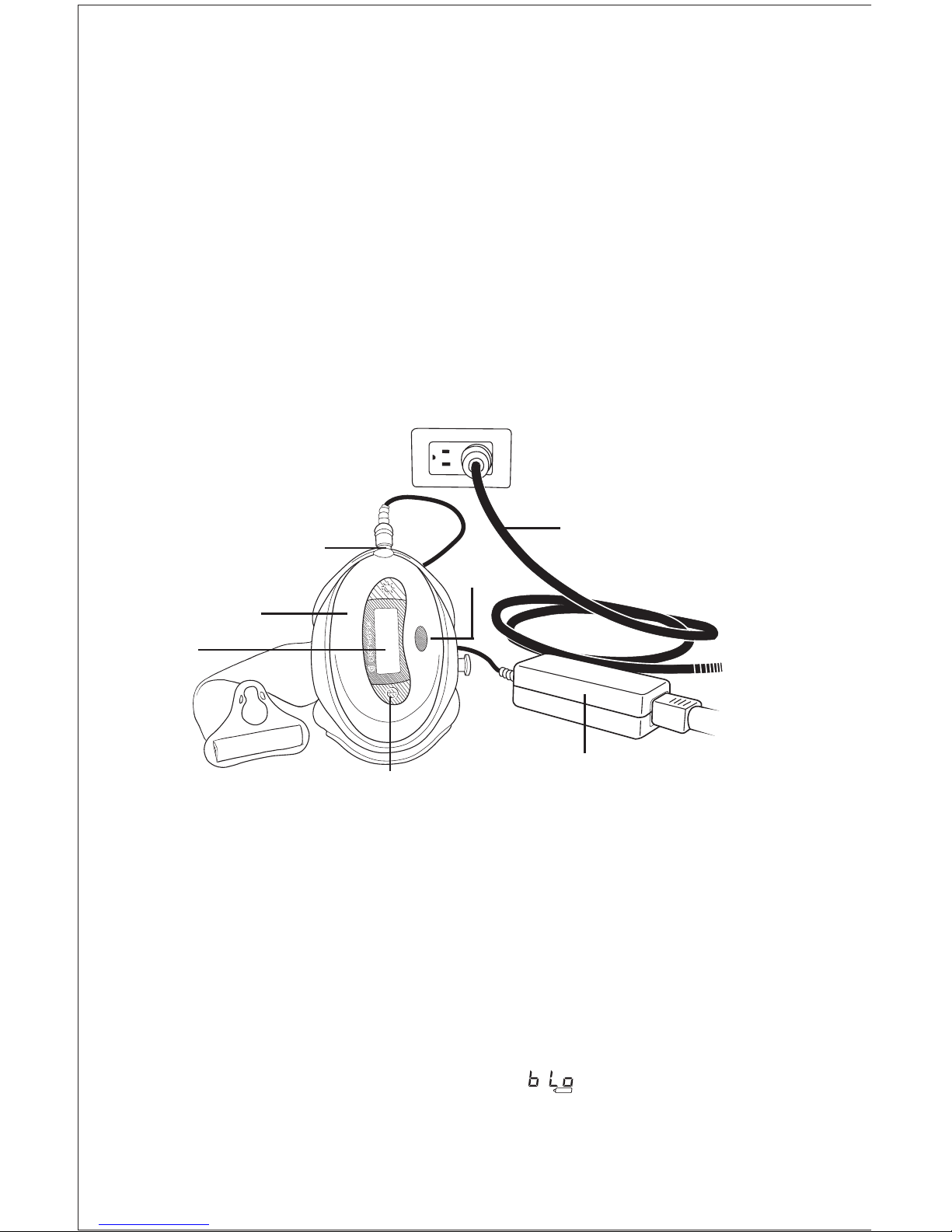
Timing of Treatment Sessions
The Physio-Stim automatically times each treatment session. The timing begins
when the device is turned on. The LCD shows a countdown of the time
remaining in the treatment session. At the end of daily treatment, the device
will turn itself off. To stop treatment prior to the end of a treatment session,
simply press the On/Off button. To resume treatment, press the On/Off
button again. The LCD will display the remaining treatment time.
Note: For the countdown to function correctly, treatment sessions
should be greater than 60 minutes duration.
Charging/Recharging the Battery
The Physio-Stim is powered by a rechargeable lithium-ion battery pack.
A power supply is provided with the device. Use only the Orthofix charging
system to charge the battery. Note: The Physio-Stim battery will require
charging prior to the first use.
5
To charge/recharge the battery, simply plug the barrel connector end of the
power supply into the charger port located on the control unit. Plug the line
cord securely into the power supply. Plug the line cord into any standard AC
wall outlet. A fully discharged battery may require up to 4 hours to charge
completely.
The Physio-Stim battery can be recharged at any time the device is not in use.
It is strongly recommended that the device be recharged after
completing daily treatment.
Note: The Physio-Stim will not deliver treatment while charging.
When the device is on, the Physio-Stim LCD will show a battery capacity
symbol. A flashing battery outline, the symbol and an audible beep
indicate a battery low condition and that the battery needs to be charged.
See “Visual and Audio Indicators” for more information.
LCD
On/Off Button
Control Unit
Power Supply
Line Cord
Charger Port
IR Port
Page 9

6
Visual and Audio Indicators
The LCD and audible alarms are designed to provide helpful information
to the user. The chart below shows the various displays and alarms and
their meaning.
Physio-Stim LCD Visual and Audio Indicators
Symbol /Alarm Description Meaning
All LCD symbols visible and
continuous audible alarm for
approximately 5 seconds
Countdown timer displays
remaining treatment time
(hours & minutes)
Orthofix logo flashes
Countdown timer
displays three dashes
audible alarm (5 beeps)
Steady symbol
for approximately 5 seconds
Symbols flash / audible alarm
(approximately 1 beep
per second)
Steady symbol indicates
approximate % of charge
Symbol filling repeatedly
indicates charge mode
Continuous audible alarm
Display of any E code
(e.g., E01, E02 . . .)
Power-on self test
Normal treatment
in progress
No treatment time
remaining
Treatment complete
— power off
Battery low —
recharge required
Battery status —
remaining charge
or charging mode
Device locked —
call for service
Error message —
call for service
Page 10
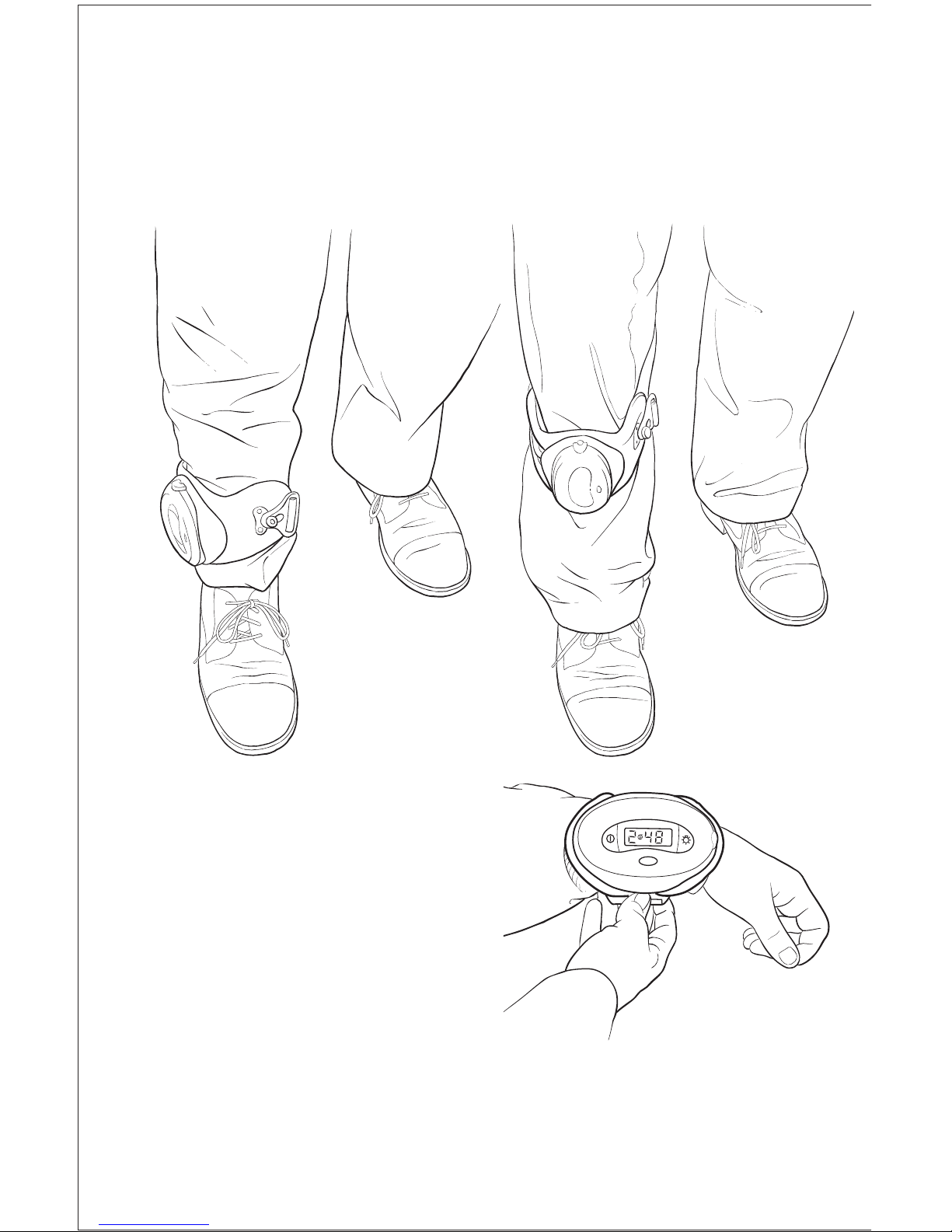
7
Device Application
Models 3202 and 3303
Physio-Stim Models that are “U-shaped” are specifically designed for placement
on a limb (e.g., tibia, femur, radius). These models may be worn over clothing,
or over a cast or external fixation device if present.
To apply
1. Begin with the Physio-Stim unbuckled.
2. Place the Physio-Stim so that
it is centered over the treatment site.
It is suggested that the skin or cast
(if present) be marked to facilitate
placement of the device.
3. Bring the elastic strap around the limb
and fasten the clasp.
4. If strap adjustment is needed, remove
the device and tighten or loosen
the strap until it feels secure and
comfortable.
Page 11

8
Model 3313
Physio-Stim Model 3313 may be placed on the collarbone (clavicle) or larger
limb such as the thigh (femur). Model 3313 may be worn over clothing, or
over a cast or external fixation device if present.
To apply on a collarbone
1. Fasten one end of the elastic strap to the end opposite the control unit.
2. Place the Physio-Stim so that it is centered over the treatment site (either
left or right collarbone). The control unit should be in front and visible.
3. Bring the elastic strap around the body under the opposite arm and fasten
to the end adjacent to the control unit.
4. If strap adjustment is needed, remove the device and tighten or loosen the
strap until it feels secure and comfortable.
To apply on a limb
1. Use the short strap provided and attach to the device. Fasten one end
of the elastic strap to either end of the device.
2. Place the Physio-Stim so that it is centered over the treatment site. Gently
bend the device in a U-shape around the limb. It is suggested that the skin
or cast (if present) be marked to facilitate placement of the device.
3. Bring the elastic strap around the limb and fasten the clasp.
4. If strap adjustment is needed, remove the device and tighten or loosen the
strap until it feels secure and comfortable.
Page 12

9
Models 3314L and 3314R
Physio-Stim Models 3314L or 3314R are intended for placement on either the
left or right shoulder (proximal humerus).
To Apply
1. Fasten one end of the elastic strap to the end opposite the control unit.
2. Place the Physio-Stim so that it is centered over the treatment site (left or right
shoulder). The control unit should be in front and visible.
3. Bring the elastic strap around the body under the opposite arm and fasten
on the end of the device next to the control unit.
4. If strap adjustment is needed, loosen or tighten the strap until it feels secure
and comfortable.
Page 13

To Apply
1. Fasten one end of the elastic strap to the device.
2. Place the Physio-Stim so that it is centered over the affected hip (left or right).
3. Bring the elastic strap around the body and fasten on the opposite end
of the device.
4. If strap adjustment is needed, loosen or tighten the strap until it feels secure
and comfortable.
10
Model 3315
Physio-Stim Model 3315 is intended for placement on the hip (proximal femur).
Page 14

11
Equipment Classification and Device Symbol Descriptions
Equipment Classifications
• Internally powered equipment
• Type BF applied part
• IEC 529 enclosure rating: IPXO
• Equipment not suitable for use in the presence of a flammable anaesthetic
mixture with air or nitrous oxide.
• Mode of operation: intermittent operation
The use of accessories other than those specified may result in increased
emissions or decreased immunity of the device.
The battery charger is provided with a 3-wire appliance inlet but considered
double insulated with Class II construction throughout.
For safe usage, follow manufacturer instructions when using the product.
Use of the product in any other manner could have harmful effects and/or void
the warranty.
Note: Inspect the device prior to each use for wear or deterioration.
Do not use if the device does not appear to be in suitable condition.
Symbol Meaning
Attention - Refer to Instructions for Use
Type BF Applied Part
On/Off
Backlight Button
Storage Temperature Range
Year of Manufacture for Active Device
Charger Port
Page 15

12
Care and Cleaning
Physio-Stim is a technologically advanced electronic device and should
be handled with appropriate care. Dropping or other mishandling of the
Physio-Stim may cause damage to the device.
DO NOT expose the Physio-Stim to direct sunlight
for long periods of time.
DO NOT expose the Physio-Stim to excessive heat.
Avoid storing the device in areas prone to extreme temperatures
such as an enclosed automobile or trunk.
DO NOT expose the Physio-Stim to excessive moisture.
DO NOT dispose of the Physio-Stim in an incinerator.
DO NOT use solvents to clean the Physio-Stim.
Clean the device by wiping with a soft, damp cloth.
Travel
When traveling by air, it is best to check the Physio-Stim with the luggage. If
the device is taken on board the airplane, it should not be worn when passing
through passenger screening devices. The Physio-Stim could be damaged. The
Physio-Stim user manual should be taken with you to quickly and easily identify
the device for any security personnel.
Storage
Storage temperature range: -10º C to 45º C (14º F to 113º F)
Operating temperature range: +5º C to 40º C (41º F to 104º F)
Relative humidity: Up to 95%, noncondensing
Disposal
The Physio-Stim is for single patient use. Product contains lithium
batteries; do not incinerate. Dispose of device properly to prevent
injury. Please dispose of this product at collection facilities for waste
electrical equipment used in household.
Service
If you have questions concerning the device or require any assistance,
please call 800-535-4492 or 214-937-2000. There are no user serviceable parts.
Notify the manufacturer for any servicing needs.
Page 16

Warranty Policy
Orthofix Inc. warrants the Physio-Stim to be free from defects in materials and
workmanship for one year from the date of first use. Provided that all terms
and conditions of this Limited Warranty are complied with, Orthofix Inc. will
replace defective components.
This Limited Warranty applies to the product only under normal use and does
not cover any damage or defect caused by accident, misuse, abuse, fire, flood,
and acts of God or by any alteration, tampering, repair or attempted repair
by anyone other than Orthofix Inc. This warranty only applies to the patient
for whom the product is prescribed and is not assignable or transferable.
Defective products covered by this Limited Warranty must be returned to
Orthofix Inc. Attention: Orthofix Returns. You must contact your local
distributor to obtain the Return Authorization (RA) number and address prior
to returning the product.
Except as specifically required by applicable law, the foregoing warranty is in
lieu of all other warranties, expressed or implied and Orthofix Inc. specifically
disclaims any and all warranties of merchantability or fitness for a particular
purpose. Under no circumstances shall Orthofix Inc., its authorized representative,
affiliated or subsidiary companies be liable for special, consequential or
incidental damages. The sole remedy with respect to any defective product
shall be limited to replacement.
This Limited Warranty may not be extended or modified except in writing by
Orthofix Inc. No sales person, representative, distributor or physician is
authorized to make or consent to any extension or modification of the terms
of this Limited Warranty.
Manufactured by:
Orthofix Inc.
3451 Plano Parkway
Lewisville, TX 75056-9453
214-937-2000
Patient Services
800-535-4492 toll free
www.orthofix.com
Orthofix Inc.
P/N 20119435 Rev. AA 2014-11-06
PS-1422 PL-US © Orthofix Holdings, Inc.
Page 17

Estimulador del
crecimiento óseo
EDICIÓN PARA LOS EE.UU.
MANUAL DE INSTRUCCIONES
Page 18

Inside Front Cover
Spanish version
Page 19

Índice
Manual de instrucciones de Physio-Stim®
Información de prescripción .............................................................................. 1
Estimulación CEMP de Orthofix ......................................................................... 2
Éxito clínico de Physio-Stim ............................................................................... 2
Descripción del dispositivo ................................................................................ 3
Instrucciones para el tratamiento ....................................................................... 4
Funcionamiento del dispositivo .......................................................................... 4
Cómo encender y apagar el dispositivo ....................................................... 4
Cronometraje de las sesiones de tratamiento ............................................. 5
Cómo cargar/recargar la batería .................................................................. 5
Indicadores visuales y de audio .......................................................................... 6
Aplicación del dispositivo ................................................................................... 7
Modelos 3202 y 3303 ................................................................................... 7
Modelo 3313 ................................................................................................ 8
Modelos 3314L y 3314R ............................................................................... 9
Modelo 3315 .............................................................................................. 10
Clasificación del equipo y descripciones de los símbolos del dispositivo ........ 11
Cuidado y limpieza ........................................................................................... 12
Viajes ................................................................................................................ 12
Almacenamiento ............................................................................................... 12
Eliminación ....................................................................................................... 12
Servicio técnico ................................................................................................. 12
Política de garantía ............................................................................................ 13
Contenido del paquete:
1- Estimulador del crecimiento óseo Physio-Stim
1- Paquete de material impreso
1- Fuente de alimentación
1- Cable de alimentación
ESTE DISPOSITIVO NO ESTÁ ESTERILIZADO.
NO REQUIERE ESTERILIZACIÓN.
Patente de los EE.UU. n.° 5,743,844
Page 20

1
Información de prescripción
Indicación
Physio-Stim® es un dispositivo indicado para el tratamiento de un defecto de
consolidación causado por algún traumatismo, excluidas las vértebras y todo
hueso plano, donde el ancho del defecto de consolidación es menor que la
mitad del ancho del hueso a tratar. Un defecto de consolidación se considera
establecido cuando el lugar de la fractura no muestra señales de progreso
visible de curación.
Contraindicación
El uso de este dispositivo está contraindicado en individuos con pseudoartrosis
sinovial.
Advertencias
• No se ha establecido la seguridad y efectividad del uso de este dispositivo en
personas que no son esqueléticamente maduras.
• En presencia de un defecto de consolidación por mala alineación, el uso de
este dispositivo se debe someter a una consideración cuidadosa e individual,
ya que el tratamiento con este dispositivo no pretende alterar o afectar el
grado de desalineación.
• La operación de implantación de un tipo de marcapasos a demanda puede
verse afectada por la exposición a campos electromagnéticos pulsados
(CEMP). Los médicos no deberán prescribir la aplicación de un Physio-Stim
que requiera situar el transductor del tratamiento cerca del marcapasos. Se
recomienda que el cardiólogo que atiende al paciente le haga exámenes más
detallados (como un electrocardiograma).
• Los estudios en animales realizados hasta la fecha no sugieren ningún efecto
adverso a largo plazo proveniente del uso de este dispositivo. No obstante,
se desconocen los efectos a largo plazo en humanos.
• No se ha establecido la seguridad y efectividad de este dispositivo en
personas con un defecto de consolidación causado por, o relacionado con,
una afección patológica.
Precauciones
• No se han evaluado las fracturas con defecto de consolidación que tienen
espacios mayores de 1 centímetro (cm).
• Si bien los estudios de reproducción en animales realizados con el dispositivo
no demostraron hallazgos adversos, no se ha establecido la seguridad del uso
de este dispositivo durante el embarazo y la lactancia en humanos.
• Este dispositivo no debe usarse si existen afecciones mentales o físicas que
impidan el cumplimiento de las instrucciones del médico y del dispositivo.
Eventos adversos
En raras ocasiones, se han informado molestias menores reversibles. Estas han
sido: incomodidad o molestias, hormigueo o dolor y leve erupción cutánea.
Page 21

2
Estimulación CEMP de Orthofix
La estimulación del crecimiento óseo mediante campos electromagnéticos
pulsados (CEMP) es un tratamiento de prescripción seguro y no quirúrgico
para sanar las fracturas que no consolidan y favorecer la fusión espinal. Las
corrientes eléctricas se han usado para la consolidación ósea desde mediados
del siglo XIX. No obstante, no fue sino hasta en la década de 1950 que los
científicos hicieron un importante descubrimiento. Cuando un hueso humano
se tuerce o fractura, genera un campo eléctrico. Este campo eléctrico de bajo
nivel activa el mecanismo de reparación propio del cuerpo, que a su vez
estimula la consolidación ósea.
Los estimuladores del crecimiento óseo mediante CEMP de Orthofix generan
un campo electromagnético pulsado de bajo nivel y uniforme similar al campo
eléctrico generado por el cuerpo. La aplicación del CEMP directamente en
el lugar de la fractura ayuda a activar y reforzar el proceso de consolidación
natural del cuerpo, a fin de mejorar la fusión ósea. Gracias por incluir a
Orthofix en su proceso de consolidación. Si desea conocer más acerca de
la estimulación del crecimiento óseo, visite nuestro sitio web en
www.bonestimulation.com.
Éxito clínico de Physio-Stim
En estudios clínicos, se ha comprobado que Physio-Stim es seguro y efectivo.
En un estudio clínico prospectivo, multicéntrico y controlado para fracturas
con defecto de consolidación, se demostró una tasa de éxito general del
80% en 126 pacientes (135 fracturas con defecto de consolidación) que se
sometieron al tratamiento durante 3 horas o más diarias en promedio. La
duración promedio de no consolidación en estas difíciles fracturas era de 2.6
años, con un promedio de dos años antes de los procedimientos quirúrgicos
por fractura. La tasa de éxito del tratamiento con Physio-Stim para la reparación de un defecto de consolidación demostró que no hay un cambio significativo desde el punto de vista estadístico a través del seguimiento a largo plazo
(cuatro años).
Page 22

3
Descripción del dispositivo
El estimulador del crecimiento óseo Physio-Stim es un dispositivo CEMP de
bajo nivel y externo que ha sido diseñado teniendo en cuenta la comodidad y
conveniencia del paciente. Es un dispositivo de una sola pieza ligero, flexible
y portátil, lo que proporciona libertad de movimiento durante el tratamiento.
Una pantalla de cristal líquido (LCD) y una alarma audible proporcionan
importante información al paciente durante el tratamiento, como el estado
operativo, el tiempo de tratamiento restante, la capacidad de la batería, etc.
Para obtener más información, consulte “Indicadores visuales y de audio”.
Physio-Stim incluye una unidad de control y un transductor de tratamiento. La
unidad de control contiene un microprocesador que genera la señal eléctrica
de Physio-Stim. El transductor de tratamiento convierte dicha señal en un
campo magnético de baja energía altamente uniforme. Cuando el dispositivo
está centrado sobre el área de tratamiento, la señal CEMP terapéutica de
Physio-Stim es aplicada directamente en el lugar de tratamiento.
Physio-Stim funciona con un paquete de baterías de ion-litio recargables.
La LCD y la alarma audible advertirán al paciente cuando la batería esté baja
y deba ser recargada. Para obtener más información, consulte “Cómo cargar/
recargar la batería”. A fin de asegurar que el dispositivo esté funcionando
correctamente, Physio-Stim monitorea constantemente la tensión de la batería
y la señal eléctrica. Si en algún momento durante el tratamiento el dispositivo
deja de funcionar correctamente, la LCD mostrará el símbolo o código de
error correspondiente. Para obtener más información, consulte “Indicadores
visuales y de audio”.
Modelo: 3313
Unidad de control
Transductor de
tratamiento
Correa ajustable
Page 23

4
Instrucciones para el tratamiento
Physio-Stim debe usarse durante tres horas al día. Sobre la base de los datos clínicos
de defectos de consolidación, la duración general del tratamiento oscila entre 90 y 180
días, según las condiciones específicas del paciente. Al final del tratamiento diario, el
dispositivo se apagará solo. Physio-Stim puede usarse en el momento del día que sea más
conveniente y cómodo para el paciente. Es ligero y ajustable. Dado que Physio-Stim es
portátil, el tratamiento puede recibirse mientras está sentado, caminando, reclinado,
durmiendo, etc. No obstante, dado que cada paciente tiene características únicas, el nivel
de actividad general debe estar basado en las instrucciones del médico.
Funcionamiento del dispositivo
Cómo encender y apagar el dispositivo
Physio-Stim se enciende y se apaga presionando el botón On/Off (Encendido/Apagado)
que se encuentra en la unidad de control del dispositivo. Cuando el dispositivo está
encendido, aparecerá momentáneamente una secuencia de mensajes de estado. Luego,
la LCD mostrará el tiempo de tratamiento restante y un logotipo intermitente de
Orthofix. El logotipo intermitente indica que el dispositivo está encendido y funciona normalmente. (Si no ve este logotipo intermitente en la pantalla, comuníquese con el departamento de Servicio al cliente de Orthofix). La unidad de control tiene un botón
de luz de fondo. Cuando haya poca luz, presione el botón de luz de fondo para que la
LCD se ilumine.
Unidad de
control
LCD
Logotipo intermitente de Orthofix
Puerto para luz
infrarroja
(Puerto IR)
Botón On/Off
Botón de luz de
fondo
Page 24

Cronometraje de las sesiones de tratamiento
Physio-Stim cronometra automáticamente cada sesión de tratamiento.
El cronometraje comienza al encender el dispositivo. La LCD exhibe una cuenta
regresiva del tiempo restante de la sesión de tratamiento. Al final del tratamiento
diario, el dispositivo se apagará solo. Para interrumpir el tratamiento antes de que
finalice una sesión de tratamiento, simplemente presione el botón On/Off. Para
reanudar el tratamiento, presione el botón On/Off nuevamente. La LCD mostrará
el tiempo de tratamiento restante. Nota: para que la cuenta regresiva funcione
correctamente, las sesiones de tratamiento deben durar más de 60 minutos.
Cómo cargar/recargar la batería
Physio-Stim funciona con un paquete de baterías de ion-litio recargables.
El dispositivo se suministra con una fuente de alimentación. Use únicamente el
cargador Orthofix para cargar la batería. Nota: La batería de Physio-Stim debe
recargarse antes de usarla por primera vez.
5
Para cargar/recargar la batería, simplemente enchufe el conector cilíndrico de la
fuente de alimentación en el puerto para el cargador que se encuentra en la unidad
de control. Enchufe el cable de alimentación en la fuente de alimentación de modo
que quede bien fijado. Enchufe el cable de alimentación en cualquier tomacorriente
de CA común de pared. Una batería totalmente descargada puede tardar hasta
4 horas en cargarse por completo.
La batería de Physio-Stim se puede recargar en cualquier momento en que no
se esté usando el dispositivo. Se recomienda categóricamente recargar el
dispositivo después de completar el tratamiento diario. Nota: Physio-Stim
no aplicará el tratamiento mientras se esté cargando la batería.
Cuando el dispositivo esté encendido, la LCD de Physio-Stim mostrará un símbolo
de la capacidad de la batería. La figura de una batería intermitente, el símbolo
y un tono audible indican que la batería está baja y que debe recargarla. Para obtener
más información, consulte “Indicadores visuales y de audio”.
LCD
Botón On/Off
Unidad de control
Fuente de alimentación
Puerto para
cargador
Puerto
para luz
infrarroja
Cable de alimentación
Page 25

6
Indicadores visuales y de audio
La LCD y las alarmas audibles están diseñadas para proporcionar información
útil al usuario. El cuadro que figura a continuación muestra los diversos
símbolos y las alarmas, y su significado.
Indicadores visuales de la LCD e indicadores de audio de Physio-Stim
Símbolo/Alarma Descripción Significado
Todos los símbolos de la LCD
visibles y alarma audible continua
durante aproximadamente
5 segundos
El cronómetro de cuenta regresiva
muestra el tiempo de tratamiento
restante (horas y minutos)
El logotipo de Orthofix parpadea
El cronómetro de cuenta regresiva
muestra tres rayas
Alarma audible (5 tonos)
Símbolo fijo durante
aproximadamente 5 segundos
Los símbolos parpadean/alarma
audible (aproximadamente 1 tono
por segundo)
El símbolo fijo indica el % de
carga aproximado
Si el símbolo se llena una y otra
vez, indica modo de carga
Alarma audible continua
Aparece cualquier código E
(p. ej., E01, E02. . .)
Autoprueba de
encendido
Tratamiento normal en
proceso
No hay tiempo de tratamiento restante
Tratamiento finalizado/
apague
Batería
baja: recargue
Estado de la batería —
carga restante o modo
de carga
Dispositivo bloqueado —
llame al servicio técnico
Mensaje de error —
llame al servicio técnico
Page 26

7
Aplicación del dispositivo
Modelos 3202 y 3303
Los modelos de Physio-Stim que tienen forma de “U” están diseñados
específicamente para colocarse en una extremidad (por ejemplo, la tibia, el
fémur, el radio). Estos modelos se pueden usar sobre la ropa, o sobre un yeso
o dispositivo de fijación externo, si lo hay.
Aplicación
1. Comience con el Physio-Stim
desabrochado.
2. Coloque el Physio-Stim de manera
tal que quede centrado sobre el lugar
del tratamiento. Se sugiere marcar la
piel o el yeso (si lo hay) para facilitar la
colocación del dispositivo.
3. Ponga la correa elástica alrededor
de la extremidad y abróchela con la
hebilla.
4. Si se necesita ajustar la correa, retire
el dispositivo y apriete o afloje la correa hasta que esté segura y resulte
cómoda.
Page 27

8
Modelo 3313
El modelo 3313 de Physio-Stim se puede colocar en la clavícula o en un hueso largo
de una extremidad tal como el muslo (fémur). El modelo 3313 se puede usar sobre
la ropa, o sobre un yeso o un dispositivo de fijación externa, si lo hay.
Para aplicación en la clavícula
1. Abroche un extremo de la correa elástica al extremo opuesto de la unidad
de control.
2. Coloque el Physio-Stim centrado sobre el lugar del tratamiento (en la
clavícula izquierda o en la derecha). La unidad de control deberá estar hacia
delante y visible.
3. Coloque la correa elástica alrededor del cuerpo y por debajo del otro brazo
y abróchela al extremo que se encuentra adyacente a la unidad de control.
4. Si es necesario ajustar la correa, retire el dispositivo y apriete o afloje la
correa hasta que quede segura y resulte cómoda.
Para aplicación en una extremidad
1. Utilice la correa corta que viene incluida y ajústela al dispositivo. Abroche un
extremo de la correa elástica a cualquier extremo del dispositivo.
2. Coloque el Physio-Stim de tal manera que quede centrado sobre el lugar
del tratamiento. Doble con cuidado el dispositivo hasta ponerlo en forma de
U alrededor de la extremidad. Se sugiere marcar la piel o el yeso (si lo hay)
para facilitar la colocación del dispositivo.
3. Coloque la correa elástica alrededor de la extremidad y abróchela con la
hebilla.
4. Si es necesario ajustar la correa, retire el dispositivo y apriete o afloje la
correa hasta que quede segura y resulte cómoda.
Page 28

9
Modelos 3314L y 3314R
Los modelos 3314L o 3314R de Physio-Stim están diseñados para ponérselos
en el hombro izquierdo o el derecho (húmero proximal).
Aplicación
1. Abroche un extremo de la correa elástica al extremo opuesto de la unidad de
control.
2.
Coloque el Physio-Stim centrado sobre el lugar del tratamiento (en el hombro
izquierdo o en el derecho). La unidad de control deberá estar hacia delante y visible.
3. Coloque la correa elástica alrededor del cuerpo y por debajo del otro brazo y
abróchela en el extremo que se encuentra adyacente a la unidad de control.
4. Si es necesario ajustar la correa, afloje o apriete la correa hasta que quede segura
y cómoda.
Page 29

Aplicación
1. Abroche un extremo de la correa elástica al dispositivo.
2. Coloque el Physio-Stim centrado sobre la cadera afectada (izquierda o
derecha).
3. Coloque la correa elástica alrededor del cuerpo y abróchesela en el
extremo opuesto del dispositivo.
4. Si es necesario ajustar la correa, afloje o apriete la correa hasta que quede
segura y cómoda.
10
Modelo 3315
El modelo 3315 de Physio-Stim está diseñado para colocárselo en la cadera (fémur
proximal).
Page 30

11
Clasificación del equipo y descripciones de los símbolos del dispositivo
Clasificaciones del equipo
• Equipo con alimentación interna
• Pieza aplicada tipo BF
• Clasificación de la caja según la IEC 529: IPXO
• Equipo no apto para usar en presencia de una mezcla anestésica inflamable
con aire u óxido nitroso.
• Modo de funcionamiento: funcionamiento intermitente
El uso de accesorios que no sean los especificados puede provocar un aumento
de las emisiones o una disminución de la inmunidad del dispositivo.
El cargador de la batería incluye una entrada de 3 hilos, pero se considera que
tiene doble aislamiento con construcción Clase II en todo el cargador.
Para un uso seguro, siga las instrucciones del fabricante al usar el producto.
Usar el producto de alguna otra forma puede generar efectos perjudiciales
o anular la garantía.
Nota: antes de cada uso, inspeccione que el dispositivo no haya sufrido
desgaste ni deterioro. No use el dispositivo si no parece estar en buenas
condiciones.
Símbolo Significado
Atención – Consulte las instrucciones de uso
Pieza aplicada tipo BF
On/Off
Botón de luz de fondo
Rango de temperaturas de almacenamiento
Año de fabricación del dispositivo activo
Puerto para cargador
Page 31

12
Cuidado y limpieza
Physio-Stim es un dispositivo electrónico tecnológicamente avanzado y se debe
manipular con el debido cuidado. Dejar caer el Physio-Stim o manipularlo en
forma indebida puede dañar el dispositivo.
NO exponga el Physio-Stim a la luz solar directa durante períodos
prolongados.
NO exponga el Physio-Stim a calor excesivo. Evite almacenar el
dispositivo en áreas expuestas a temperaturas extremas, como un
automóvil o maletero cerrado.
NO exponga el Physio-Stim a humedad excesiva.
NO deseche el Physio-Stim en un incinerador.
NO use disolventes para limpiar el Physio-Stim. Limpie el dispositivo
con un paño suave y húmedo.
Viajes
Cuando se viaje en avión, lo mejor es registrar el Physio-Stim con el equipaje. Si
el dispositivo se lleva a bordo del avión, no debe llevarse puesto cuando se pase
por los controles de inspección de seguridad. El Physio-Stim se podría dañar. Se
debe llevar también el manual de instrucciones del Physio-Stim a fin de identificar
el dispositivo con rapidez y facilidad ante cualquier personal de seguridad.
Almacenamiento
Rango de temperaturas de almacenamiento: -10º C a 45º C (14º F a 113º F)
Rango de temperaturas de funcionamiento: +5º C a 40º C (41º F a 104º F)
Humedad relativa: hasta 95%, sin condensación
Eliminación
El Physio-Stim es para uso de un solo paciente. El producto contiene
baterías de litio, no incinerar. Disponga del dispositivo correctamente para
prevenir lesiones. Por favor de dar este producto a los depósitos de equipo
electrónico obsoletos de uso en casa.
Servicio técnico
Si tiene alguna pregunta en relación con el dispositivo o necesita cualquier tipo
de asistencia, sírvase llamar al 800-535-4492 o al 214-937-2000. El dispositivo no
tiene piezas que puedan ser reparadas por el usuario. Notifique al
fabricante si necesita servicio técnico.
Page 32

Política de garantía
Orthofix Inc. garantiza que Physio-Stim no tendrá defectos en los materiales ni en
la mano de obra durante un año a partir de la fecha en que se use por primera vez.
Siempre y cuando se cumplan todos los términos y condiciones de esta garantía limitada,
Orthofix Inc. reemplazará los componentes defectuosos.
Esta Garantía limitada se aplica al producto únicamente en condiciones de uso normal, y
no cubre ningún daño ni defecto provocado por un accidente, uso indebido, maltrato,
incendio, inundación ni casos fortuitos, ni por ninguna alteración, manipulación,
reparación o intento de reparación por parte de algún tercero que no pertenezca
a Orthofix Inc. Esta garantía se aplica únicamente al paciente al que se le recete el
producto, y no se puede ceder ni transferir. Los productos defectuosos cubiertos por
esta Garantía limitada deben ser devueltos a Orthofix Inc. Atención: Orthofix Returns.
Antes de devolver el producto, debe comunicarse con su distribuidor local para obtener
el número y dirección de Autorización de devolución (RA).
Excepto que la ley aplicable especifique lo contrario, la garantía precedente
reemplaza todas las demás garantías, expresas o implícitas, y Orthofix Inc. se exime
específicamente de toda responsabilidad por cualesquiera y todas las garantías de
comerciabilidad o aptitud para un fin en particular. Orthofix Inc., sus representantes
autorizados, afiliadas o compañías subsidiarias no serán responsables, bajo ninguna
circunstancia, por daños especiales, consecuentes ni incidentales. El único recurso para
subsanar un producto defectuoso será el reemplazo.
Esta Garantía limitada no podrá ser extendida ni modificada, excepto que Orthofix
Inc. lo haga por escrito. Ningún vendedor, representante, distribuidor ni médico está
autorizado a realizar ni permitir ninguna extensión ni modificación de los términos de
esta Garantía limitada.
13
Page 33

BLANK
Page 34

Orthofix Inc.
Edición para los EE.UU.
N.º de Ref. 20119435 Rev. AA 2014-11-06
Impreso en los EE.UU.
PS-1422 PL-US © Orthofix Holdings, Inc.
Fabricado por:
Orthofix Inc.
3451 Plano Parkway
Lewisville, TX 75056-9453
214-937-2000
Servicio al cliente
800-535-4492 (línea gratuita)
www.orthofix.com
Page 35

Inside Back Cover
is blank
Page 36

Orthofix Patient Services
800-535-4492
www.orthofix.com
www.bonestimulation.com
Manufactured by:
Orthofix Inc.
3451 Plano Parkway
Lewisville, TX 75056 USA
Tel (214) 937-2000
Orthofix Inc.
P/N 20119435 Rev. AA 2014-11-06
PS-1422 PL-US © Orthofix Holdings, Inc.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
 Loading...
Loading...