Page 1

INSTRUCTION MANUAL
Model 5212
USA
SPINAL-STIM
MANUAL
SS-1602
DRAFT 5/02/16 - DO NOT DISTRIBUT
FINAL WILL BE PROVIDED ONCE APPROVED
E
Page 2

Assembled in the United States of America
Spinal-Stim Device Patent No.
U.S. 5,743,844
U.S. 6,132,362
U.S. 6,261,221
Page 3

Spinal-Stim® Instruction Manual
Table of Contents
Prescription Information .............................................................................
• Indications ............................................................................................
• Contraindication ...................................................................................
• Warnings ..............................................................................................
• Precautions ...........................................................................................
• Adverse Events .....................................................................................
Device Information ......................................................................................
• Device Description ................................................................................
• Device Life ............................................................................................
Device Operation .........................................................................................
• Turning the Device On and Off.............................................................
• Treatment Instructions .........................................................................
• Timing of Treatment Sessions ...............................................................
• Charging the Battery ............................................................................
• Visual and Audio Indicators ..................................................................
• Wearing the Device ..............................................................................
• Sizing the Device ..................................................................................
Device Accessories .....................................................................................
Device Use and Care .................................................................................
• Care and Cleaning ..............................................................................
• Storage ...............................................................................................
• Travel ..................................................................................................
• Disposal ..............................................................................................
• Service ................................................................................................
Clinical Information ...................................................................................
• Clinical Data Summary .......................................................................
• Adjunct Clinical Trial ...........................................................................
• Failed Fusion Clinical Trial ...................................................................
Equipment Classification ...........................................................................
Compliance Statements ............................................................................
Warranty ...................................................................................................
1
1
1
1
1
1
2
2
2
3
3
4
4
4
6
7
9
11
11
11
11
12
12
12
13
13
13
14
16
17
19
Device Box Components
1 – Spinal-Stim
1 – Power Supply
1 – Literature Pack
Orthofix Patient Services: 800-535-4492 or 214-937-2718
To learn more about Orthofix, please visit our website at www.orthofix.com.
Page 4

Prescription Information
Indications
Spinal-Stim® is a noninvasive electromagnetic bone growth stimulator indicated
as a spinal fusion adjunct to increase the probability of fusion success and as a
nonoperative treatment of salvage of failed spinal fusion, where a minimum of
nine months has elapsed since the last surgery.
Contraindication
Cardiac pacemakers may be adversely affected by exposure to PEMF. Use of
this device is contraindicated where the individual has an implanted cardiac
pacemaker.
Warnings
• Although animal teratological studies performed with the device
demonstrated no adverse findings, the safety of use of this device
during pregnancy and nursing in humans has not been established.
• The safety and effectiveness of the use of this device on individuals
lacking skeletal maturity have not been established.
• Animal studies conducted to date do not suggest any long-term adverse
effects from the use of a similar device. However, long-term effects in
humans are unknown.
Precautions
• This device should not be used if there are mental or physical conditions
which preclude compliance with the physician and device instructions.
• This device has not been evaluated in treating patients with the
following conditions: osseous or ligamentous spinal trauma, spondylitis,
Paget’s disease, moderate to severe osteoporosis, metastatic cancer, renal
disease, and uncontrolled diabetes mellitus.
• The results of premarketing data from the randomized double-masked
cohort indicate that inconsistent users (defined as those patients that
used the device for less than an average of two hours per day) had
success rates similar to those in the placebo group. Therefore, the use of
the device for less than the minimum recommended usage may result in
lower success rates.
Adverse Effects
Rare instances of reversible minor discomfort have been reported. These
were: cumbersome or uncomfortable, minor tingling or pain, minor skin rash,
insomnia, fainting, nausea/diarrhea, and polymenorrhea.
1
Page 5
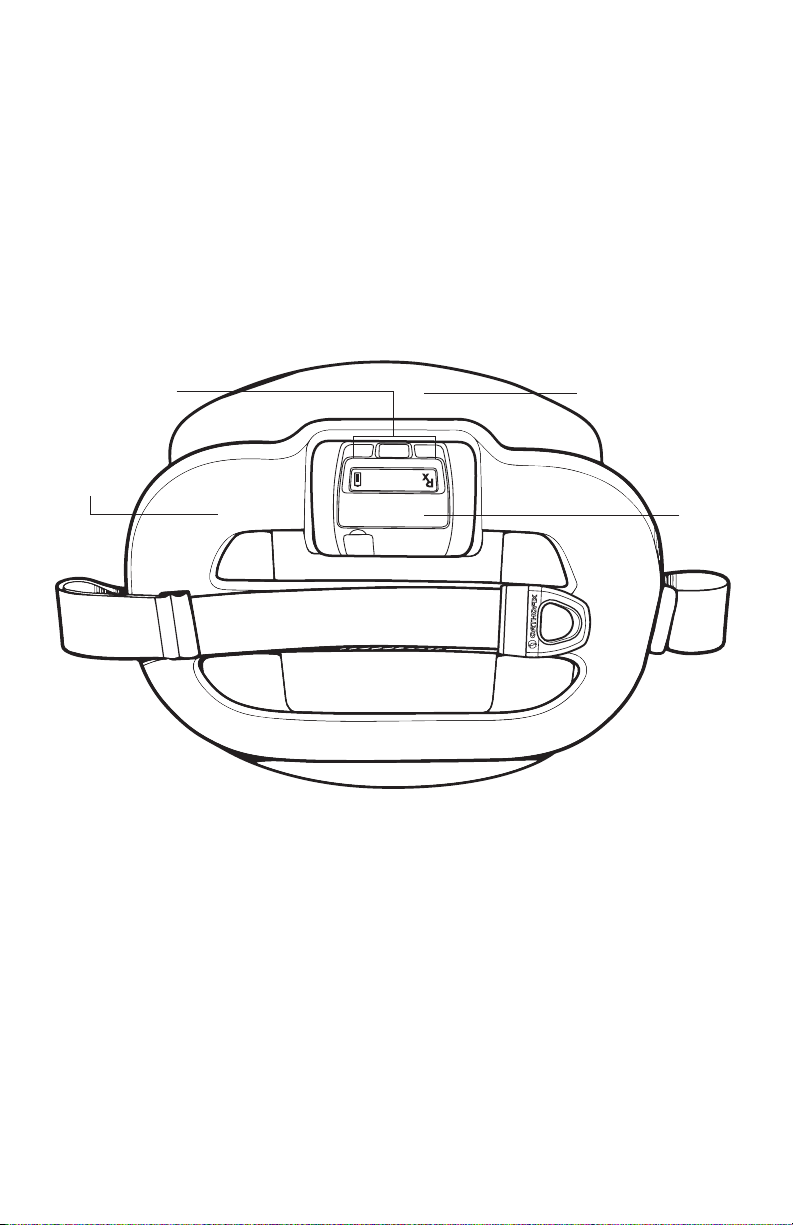
Device Information
Device Description
Spinal-Stim stimulator is an external device that generates a Pulsed
Electromagnetic Field (PEMF) as a nonsurgical, prescription treatment
to increase the chances of a successful fusion. The device is lightweight,
adjustable, and portable, including a rechargeable battery that allows
freedom of movement during treatment. A Liquid Crystal Display (LCD) and
audible indicators provide important feedback during treatment. See “Device
Operation” for more information.
Front
Treatment
Coil
LCD
2:00
Back Treatment Coil
Control
Unit
Model 5212
Spinal-Stim contains a Control Unit and Treatment Coils in one integrated
device. A micro-processor generates Spinal-Stim’s electrical signal, which is a
highly uniform, low-energy magnetic field sent from the treatment coils. When
the coils are centered over the treatment area, the therapeutic Spinal-Stim PEMF
signal is delivered through clothing and skin directly to the fusion site.
To learn more about bone growth stimulation, please visit our patient website
at www.bonestimulation.com.
Device Life
Spinal-Stim provides daily treatments for up to 365 days. The physician
determines the overall length of treatment (months/weeks) on an individual
basis according to fusion healing progress.
2
Page 6
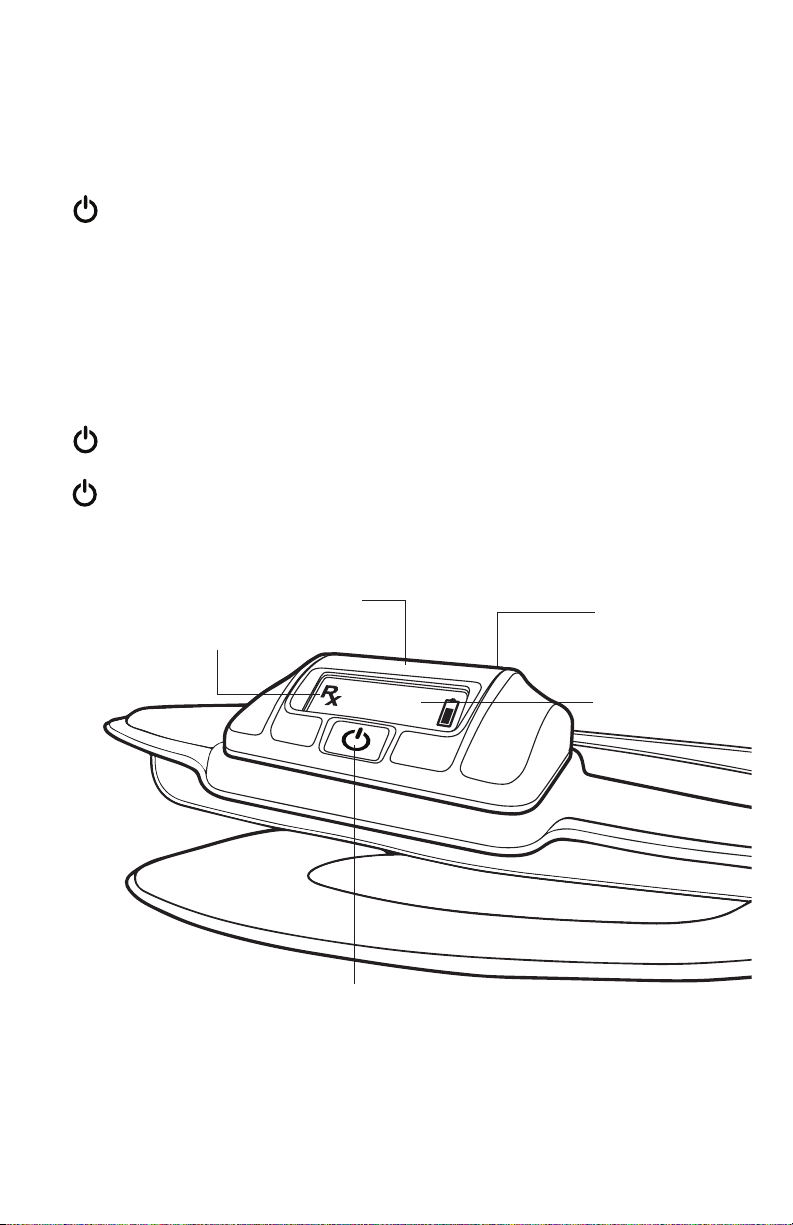
Device Operation
2:00
Turning the Device On and Off
Spinal-Stim can be turned on by pressing and holding the On/Off
Button on the Control Unit of the device until it beeps.
When the device is turned on, a status screen will display the number
of days since the first use, the treatment status, and the
compliance percentage.
The LCD will show the prescribed treatment time remaining and the
battery status.
The flashing semicolon on the LCD screen and On/Off button indicate that
the device is on and delivering treatment.
Spinal-Stim can be turned off by pressing and holding the On/Off Button
on the Control Unit of the device until it beeps.
The On/Off Button on the Control Unit doubles as a Backlight to light up
the LCD. In low light, press the On/Off Button to light up the LCD.
Treatment Indicator
Control Unit
On/Off Button
Charging
Port
LCD
3
Page 7

Treatment Instructions
• Spinal-Stim should be worn each day for the number of hours
prescribed by a physician (a minimum of 2 hours/day).
• Spinal-Stim may be used at any time of day that is most convenient for
the patient.
• The device is programmed to reset the treatment clock daily at midnight
Central Standard Time, unless adjusted by a physician or Orthofix
representative for a different time zone.
• Hours worn prior to the reset time will be logged and stored in the device
for monitoring daily use compliance.
• The overall treatment duration (number of months/weeks) will vary based
on specific patient conditions as determined by a physician.
• Because Spinal-Stim is lightweight and portable, treatment can be
received while sitting, walking, reclining, sleeping, etc. However, since
each patient is unique, the overall activity level should be based on
physician instructions.
Timing of Treatment Sessions
• Spinal-Stim tracks the treatment time; this tracking (or timing)
begins when the device is turned on and at least one minute of
treatment is complete.
• The LCD shows a countdown of the daily treatment time remaining.
•
To stop treatment at any point, simply press and hold the On/Off Button
until you hear a beep.
• To resume treatment, press the On/Off button again.
• The countdown will resume at the remaining treatment time.
• When daily treatment is completed, the device will automatically
turn off.
Charging the Battery
Spinal-Stim is powered by a rechargeable lithium-ion battery pack. A power
supply to charge the battery is provided with the device. Use only the Orthofix
power supply to charge the battery (Part no. Orthofix 20110412).
To ensure that the device is functioning properly, Spinal-Stim constantly
monitors battery voltage and the electrical signal. The LCD will display a
battery capacity symbol and the device will beep to alert the patient when
the battery is low and will soon need to be recharged.
Spinal-Stim should be charged before the first use and every day after
completing treatment. The device will not deliver treatment while charging.
4
Page 8
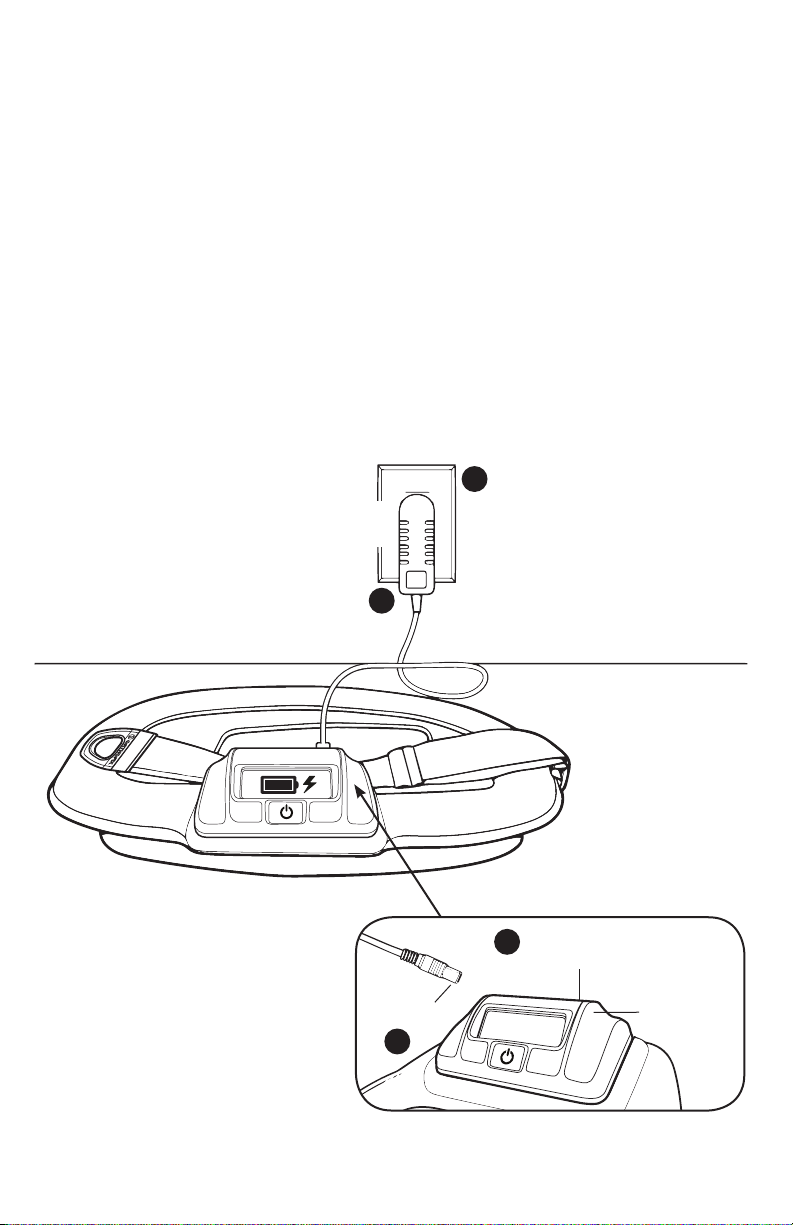
Follow these steps to recharge the battery:
1. Open the Charging Port Cover.
2. Plug the Charging Connector into the Charging Port located on the
Control Unit.
3. Plug the power supply into any standard AC Wall Outlet.
4. The LED on the power supply will light up green as an indicator that the
AC Wall Outlet is delivering power.
5. The Control Unit LCD will display a battery symbol filling to verify that
the device is charging. When the battery reaches a complete charge,
a check mark symbol will be displayed next to the battery symbol. In
addition, the device will beep once to alert the patient.
6. If the battery is fully depleted, it may require up to 4 hours to charge
completely.
7. After charging is complete, remove the Charging Connector and replace
the Charging Port Cover.
3
Power
Supply
AC Wall Outlet
4
Charging
Connector
2
5
Charging Port
1
Cover
Control Unit
Page 9

Visual and Audio Indicators
The LCD and audible beeps are designed to provide helpful information
to the user. The screens, symbols, and beeps are explained below.
Compliance Screen
170/185 = 91.9%
Treatment Screen
1:59
Treatment Complete
Charging Screen
Charging Complete
Low Battery Warning Screen
1:59
Battery must be charged
to turn on
Compliance Screen – Displays a compliance percentage
which is calculated by the number of full treatments days
completed over the number of available treatment days.
u
The treatments days available begin once the device has
been delivered to the patient and a minute of treatment
time has been established.
Treatment Screen – displays the treatment time remaining
in hours and minutes. The timer counts down to zero until
u
daily treatment is complete.
Daily Prescribed Treatment complete
u
Battery Charging – Battery symbol filling repeatedly
u
verifies that the device is charging.
Charging Complete – Indicates when the battery is
u
fully charged.
Low Battery – Displays along with three fast beeps
u
when recharging is recommended.
E12345678
E12345678
Device Expired
Exception Screen
E123
800-535-4492
Battery Empty – Indicates that the battery must be
u
charged before treatment may continue.
Device Expired – Display of a closed lock indicates the
u
device has been available for treatment for 365 days
and will no longer provide treatment.
Exception Codes – Display of ERROR, any E codes
(e.g., E01, E02), along with three slow beeps. Contact
u
Patient Services at 800-535-4492 or 214-937-2718.
6
Page 10

Wearing the Device
Spinal-Stim can be worn over bracing and clothing. Proper treatment does
not require direct contact with the body. However, the coils must be centered
around the fusion site to be effective. Users can gently bend and shape the
treatment coils to fit more comfortably around the body.
2:00
7
Page 11

The following is the suggested method for
wearing Spinal-Stim:
1. Rest the Back Coil of the device
against the back of a chair and
the Front Coil against the left arm
of the chair. Let the Velcro® Strap
hang over the right arm of the chair.
2:00
3. Pull the Front Coil toward you and
let it rest on top of your legs.
2. Sit in the chair.
2:00
4. Locate the Velcro Strap and pull
it snugly across your body and
attach it to the Velcro Panel
on the Front Coil.
8
Page 12

Sizing the Device
For minor size adjustments, adjust the placement of the
front Velcro Strap. For further adjustments, follow the
steps below.
1. Place Spinal-Stim around the body to determine how
much adjustment is needed.
• Note: when properly adjusted, the coils should be
centered on the body. The Control Unit should be
in front, LCD facing up.
2. If a significant size adjustment is needed, lay the unit flat with
the outside of the device Velcro Panel facing up.
Control Unit
2:00
Velcro Panel
Strap Lock
2:00
Velcro Strap
Side Clip
Back Clip
Front Coil
3. To adjust the Back Coil, open the Strap Lock.
9
Back Coil
Strap Lock
Back Coil
Page 13

4. If more strap length is needed to make the device bigger, slide the Back Clip
toward the Strap Lock. Pull the excess strap through the Strap Lock.
5. If less strap length is needed to
make the device smaller, push the
desired amount of strap through
the Strap Lock. Slide the Back Clip
7. On the back coil, adjust the
Velcro Strap by pulling more or
less elastic strap through the
Side Clip.
away from the Strap Lock to
tighten the excess strap.
6. Close the Strap Lock.
8. When properly adjusted, the Spinal-Stim
straps will be approximately the same
length on each side.
10
Page 14

Device Accessories
Certain body types may benefit from the use of suspenders with Spinal-Stim.
Please contact Patient Services at 800-535-4492 or 214-937-2718 to
order suspenders.
An accessory available to the patient is a user friendly mobile application which
allows the patient to easily monitor their device use. This may be downloaded to
the patient’s smartphone. Reference the Patient Guide to the Orthofix Stim App.
Device Use and Care
• Spinal-Stim is a technologically advanced electronic device and should be
handled with care. Dropping or other mishandling of Spinal-Stim may
damage the device and it may stop working.
• For safe usage, follow manufacturer instructions when using Spinal-Stim.
• Use of the device in any other manner could have harmful effects and/or
void the warranty.
• The use of accessories other than those specified may result in increased
emissions or decreased immunity of the device.
• Inspect the device prior to each use for wear or deterioration.
• Do not use the device if it does not appear to be in suitable condition.
• Do not attempt to open or disassemble Spinal-Stim as there are no user
serviceable parts inside.
• CAUTION: STRANGULATION HAZARD – Keep the Power Supply cord out of
the reachof children.
Care and Cleaning
When cleaning the Spinal-Stim device, follow these instructions:
• Clean the device by wiping surfaces with a damp, soft cloth
(wet with water only). Do not sterilize Spinal-Stim.
• DO NOT expose Spinal-Stim to excessive moisture.
• DO NOT use solvents or alcohol-based liquids (anti-bacterial
cleaners, hand sanitizers, perfume, etc.) to clean Spinal-Stim.
Storage
Unpacked Storage:
Temperature range: within -25°C to 60°C, in up to 93% relative humidity
non-condensing.
Packed Storage, Shipping, and Transport:
Temperature range: -40°C and 60°C, between 10% and 100% relative humidity
including condensation at pressures between 500hPA and 1060hPA in a
safe manor.
Operating Environment:
Temperature range: within +5° C to +40°C,
15-93% relative humidity non-condensing, and 700-1060 hPA.
11
Page 15

Spinal-Stim is designed for a storage life of twelve months plus one year
of usage.
• DO NOT expose Spinal-Stim to direct sunlight for long periods of time.
• DO NOT expose Spinal-Stim to excessive heat or cold.
• Avoid storing the device in areas prone to extreme temperatures, such as
an enclosed automobile or trunk.
Travel
When traveling by air, it is recommended to pack Spinal-Stim with checked
luggage. If taken onboard the airplane, it should be turned off when passing
through security screening equipment, as the device could be damaged. The
Spinal-Stim instruction manual should be taken with you to quickly and easily
identify the device for security personnel. Do not wear or operate Spinal-Stim
while onboard the airplane.
Disposal
After treatment is complete and a physician advises you to discontinue use,
you may dispose of the device according to your local governing ordinances
or recycling plans. You may also contact Orthofix Patient Services regarding
recycling.
Spinal-Stim is a Class III medical device (prescription only) that cannot be
sanitized or used by another person.
Dispose of the device properly to prevent injury.
DO NOT dispose of Spinal-Stim in an incinerator. This device
contains lithium batteries.
Service
If you have questions concerning the device or require any assistance, please
call 800-535-4492 (U.S. only) or 214-937-2718. There are no user serviceable
parts. Notify Orthofix for any servicing needs.
Spinal-Stim has not been evaluated with regard to use with specific
implantable electronic medical devices. Please consult your physician prior
to use of the Spinal-Stim with implantable electronic medical devices.
12
Page 16

Clinical Information
Clinical Data Summary
Spinal-Stim was studied in human clinical trials to evaluate its safety and
effectiveness as a therapy added to standard post-surgical care (referred to as
the “adjunct clinical trial”). A separate phase of the clinical trial (referred to as
the “failed fusion clinical trial”) examined patients with fusions that had not
healed (pseudarthrosis) after a lumbar fusion surgery. The patients in both
clinical studies had risk factors.
Adjunct Clinical Trial
Spinal-Stim has been tested in a clinical study involving 54 surgeons at
31 centers. This clinical investigation contained a prospective randomized
double-masked trial of PEMF efficacy. Spinal-Stim was tested as a surgical
adjunct in patients undergoing a first attempt at lumbar fusion. At one year
postoperative, patients using active devices on a consistent daily regimen (an
average of at least two hours per day) developed solid fusion in 92.2% of
the cases.1 Patients consistently using placebo (inactive) devices developed
solid fusion in 67.9% of the cases. This 35% increase in treatment effect is
statistically significant, and is realized regardless of:
At one year after the fusion surgery, patients using active devices on a
consistent daily regimen (an average of at least two hours per day) developed
solid fusion in 92% of the cases. Patients consistently using placebo devices
developed solid fusion in 68% of the cases.
• Number of levels • Vertebral level
• Graft type • Smoking
• Internal fixation • Age
• Gender
The success rate for patients in the randomized double-masked phase for
whom success or failure status is known at four years after treatment with the
Spinal-Stim for all subjects (consistent and inconsistent users combined) was
63% (n=88) as compared with 83% in this phase of the clinical trial (i.e., one
year postoperative).
13
Page 17

Adjunct Clinical Trial: Overall Success Rate
100
80
60
40
% Patients Fused
20
0
92%
68%
Active Placebo
Consistent users (n=64) of the device in this phase had an initial success
rate of 92.2% with a success rate of 70% after four years (a 24% reduction).
Inconsistent users (n=34) and the entire placebo group (n=97) had an
initial success rate of 65% with a success rate of 50% after four years (again,
a 24% reduction). Long-term follow-up data indicates the success rate
differentials between active and placebo units are maintained over time.
Long-term, consistent Spinal-Stim users benefit with a 40% increase in fusion
success, when compared to inconsistent and placebo device users. Based
on this analysis, the reduction in long-term success rates appears unrelated
to treatment with the Spinal-Stim. During this four year period, 10% of the
original patients in the randomized double-masked phase were lost to
follow-up and are not reflected in these success rates.
Failed Fusion Clinical Trial
Spinal-Stim was also tested for nonoperative salvage in patients presenting
with established pseudarthrosis of lumbar fusion in an open trial. Without
additional regrafting of fusion surgery, 67% of these cases reached a
successful fusion with consistent (an average of at least 2 hours per day)
PEMF treatment.2
Spinal-Stim reduced smoking and multi-level fusion as risk factors in failed
fusion patients. Consistent users showed a 67.2% success rate in non-smokers
and a slightly lower 66.7% success rate in smokers. Users with failed single
level fusions showed a 68% success rate and a slightly lower 66% success
rate for failed multi-level fusions.
14
Page 18

Failed Fusion Clinical Trial: Overall Success Rate
100
80
67%
60
40
% Patients Fused
20
19%
0
Consistent Users Inconsistent Users
The four year success rates for these patients in the open trial, non-operative
salvage phase for all subjects (consistent and inconsistent users combined)
was 39% (n=119) as compared with 57% in this phase of the original clinical
trial (i.e., one year postoperative). Consistent users (n=93) of the device in this
phase had a success rate of 44% after four years. Inconsistent users (n=26) of
the device in this phase had a success rate of 19% after four years.
The reduction in success rates from the time of commercial marketing
compared with those at four years showed a similar percentage decrease
(31%) to those in the randomized double-masked trial. During this four year
period, 6% of the original patients in the open phase were lost to follow-up
and are not reflected in these success rates.
1
Mooney, V., “A Randomized Double-Blind Prospective Study of the Efficacy of Pulsed Electromagnetic
Field for Interbody Lumbar Fusions”, SPINE, Vol. 15, No. 7, P708, 1990.
2
Simmons, JW, Hayes, MA, Christensen, KD, Dwyer, AP, Koulisis, CW, Kimmich, SJ: “The Effect of
Postoperative Pulsed Electromagnetic Fields on Lumbar Fusion: Open Trial Study”. Presented at the
Annual Meeting of the North American Spine Society, Quebec City, Canada, 2 July 1989.
15
Page 19

Equipment Classification
REF
SN
Device Symbol Descriptions
Symbol Meaning Symbol Location
Attention – Refer to Instruction Manual Device and Device Box
Type BF Applied Part Device and Device Box
On/Off Device
Prescription Only Device
Storage Temperature Range Device Box
Year of Manufacture for Active Device Device and Device Box
Manufacturer Instruction Manual
Not for General Waste Device and Device Box
Keep Dry Device and Device Box
FCC Mark Device and Device Box
CE Mark Device and Device Box
Storage Humidity Limits Device and Device Box
EU Authorized Representative Instruction Manual
100%
10%
Catalog Number Device and Device Box
Serial Number Device and Device Box
16
Page 20

Spinal-Stim Classifications
• Product Family Name: Orthofix PEMF Device
• Internally powered equipment
• Type BF applied part
• IEC 60529 enclosure rating: IP22
• Mode of operation: intermittent operation
• This device is non-sterile. It does not require sterilization.
• Equipment not suitable for use in the presence of a flammable
anesthetic mixture with air or nitrous oxide.
• The battery charger is considered double insulated with Class II
construction throughout.
Compliance Statements
This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference,
and (2) this device must accept any interference received, including interference
that may cause undesired operation.
This device complies with Industry Canada licence-exempt RSS standard(s). Operation
is subject to the following two conditions: (1) this device may not cause interference,
and (2) this device must accept any interference, including interference that may
cause undesired operation of the device.
IMPORTANT! Changes or modifications not expressly approved by Orthofix, Inc.
could void the user’s authority to operate the equipment.
NOTE: This equipment has been tested and found to comply with the limits for a
Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed
to provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate radio frequency energy
and, if not installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this equipment does cause
harmful interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which
the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
CAN ICES-3 (B)/NMB-3(B)
This equipment complies with radiation exposure limits set forth for uncontrolled
environment.
17
Page 21

Information regarding Electromagnetic Compatibility and Immunity
Spinal-Stim complies with IEC 60601-1-2 for electromagnetic compatibility (EMC).
Spinal-Stim needs special precautions regarding EMC and needs to be used
in accordance with the EMC information provided in this manual. Wireless
communications equipment such as home network devices, mobile phones,
cordless telephones and their base stations, and walkie-talkies can affect
Spinal-Stim. These types of equipment should be kept at least 0.198 m (7.8 in)
away from Spinal-Stim.
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) this device may not cause harmful interference,
and (2) this device must accept any interference received, including interference
that may cause undesired operation.
18
Page 22

Warranty
Orthofix Inc. warrants the Spinal-Stim Osteogenesis Stimulator to be free from
defects in materials and workmanship for one year from the date of first use.
Provided that all terms and conditions of this Limited Warranty are complied
with, Orthofix Inc. will replace defective components.
This Limited Warranty applies to the product only under normal use and does
not cover any damage or defect caused by accident, misuse, abuse, fire, flood,
and acts of God, or by any alteration, tampering, repair, or attempted repair
by anyone other than Orthofix Inc. This warranty only applies to the patient
for whom the product is prescribed and is not assignable or transferable.
Defective products covered by this Limited Warranty must be returned to
Orthofix Inc., Attention: Orthofix Returns. You must call a Patient Services
representative or your local distributor to obtain the Return Authorization
number and address prior to returning the product.
Except as specifically required by applicable law, the foregoing warranty is in
lieu of all other warranties, expressed or implied, and Orthofix Inc. specifically
disclaims any and all warranties of merchantability or fitness for a particular
purpose. Under no circumstances shall Orthofix Inc., its authorized
representative, affiliated, or subsidiary companies be liable for special,
consequential, or incidental damages. The sole remedy with respect to any
defective product shall be limited to replacement.
This Limited Warranty may not be extended or modified except in writing by
Orthofix Inc. No sales person, representative, distributor or physician is
authorized to make or consent to any extension or modification of the terms
of this Limited Warranty.
For additional information and/or device assistance, contact Orthofix
Patient Services at 800-535-4492 or 214-937-2718.
19
Page 23

20
Page 24

Caution: Federal law (USA) restricts this device to sale
by or on the order of a physician.
Orthofix Inc.
3451 Plano Parkway
Lewisville, Texas 75056
Tel 214-937-2718
Patient Services
800-535-4492 toll free
www.bonestimulation.com
www.orthofix.com
P/N 20115189 Rev.03 05/2016
SS-1602-US © Orthofix Holdings, Inc.
 Loading...
Loading...