Page 1

CARE AND HANDLING INSTRUCTIONS
Orthofix Inc.
3451 Plano Park way
Lewisville, Texas 75056-9453 U.S.A .
1-214-937-3199
1-888-298-5700
www.orthofix.com
OSI-CustomerService@Orthofix.com
Spinal Kinetics LLC, an Orthofix Company
501 Mercury Drive
Sunnyvale, CA 94085 USA
1-888-298-5700
M6-C™ Artificial Cervical Disc Surgical Instruments
English 2-7
EN
PK 0248 Rev 02
Page 2

M6-C™ Artificial Cervical Disc Surgical Instruments Care and Handling Instructions
DEVICE DESCRIPTION
The surgical implantation of the M6-C Articial Cervical Disc requires specic surgical instruments including: a
footprint template and a trial to determine the appropriate size and position of the implant; a n cutter to create
tracks in the superior and inferior vertebral endplates; and an implant inserter to place the disc into the desired
position to aid in and ensure correct placement within the intervertebral space. Additionally, there is a tamp to
independently adjust the posterior position of the M6-C endplates, a removal tool to remove the disc from the
disc space, and general surgical instruments to assist in the distraction and mobilization of the disc space. The
instruments are composed primarily of surgical stainless steel coated with ME-92 coating to increase corrosion
resistance, with some instrument handles also featuring aluminum and Radel materials. Surgical instruments
are provided non-sterile and are intended to be reusable.
The instruments provided in the kit include Footprint Templates, Trials, Inserters, Fin Cutters, a Tamp, Removal
Tools, an Intervertebral Distractor, a Paddle Distractor, a Distractor Spacer, a Slide Hammer, a Retainer, Retainer
Pins and Locking Nuts. All instruments are made of surgical stainless steels per ASTM F899-12b, aluminum
(mallet handle), and Radel (pin driver handle).
The M6-C Instrument Tray is composed of stainless steel, Nylon, Radel Plastic, and Silicone. The tray is multilayered with various inserts to hold surgical instrumentation in place during handling and storage. The
instrument tray is perforated to allow steam to penetrate which will allow sterilization of the contents to occur
in a steam autoclave.
Page 2 of 8
Page 3

Care and Handling Instructions M6-C™ Artificial Cervical Disc Surgical Instruments
INDICATIONS FOR USE
For M6-C™ Articial Cervical Disc Indications, Contraindications, Warnings and Precautions, and other important
medical information, please refer to the M6-C Instructions for Use available at www.orthox.com/ifu, and the
M6-C Operative Technique Manual.
DISCLAIMER
The M6-C Instrument Tray is intended to protect the M6-C Surgical Instruments and facilitate the sterilization
process by allowing steam penetration and drying. Laboratory testing has veried that the instrument tray is
suitable for the specic sterilization methods and cycles for which it has been tested. Health care personnel
bear the ultimate responsibility for ensuring that any packaging method or material, including a reusable
rigid container system, is suitable for use in sterilization processing and sterility maintenance in a particular
health care facility. Testing should be conducted in the health care facility to ensure that conditions essential to
sterilization can be achieved. Orthox does not accept responsibility or liability arising from a lack of cleanliness
or sterility of any medical devices supplied by Orthox that should have been properly cleaned and/or sterilized
by the end user prior to use.
RECOMMENDATIONS
• The instruments are provided non-sterile. Clean instruments before each use. After cleaning, sterilize as
directed prior to use.
• As soon as possible after surgery, instruments should be cleaned and sterilized.
• Clean the instruments per the instructions below. Strictly follow the dosage, temperature, exposure time,
and material compatibility specications for the cleaning agent.
• Sterilize the instruments using the steam sterilizer operating conditions below.
• Stainless steel instruments can become stained or corroded if the cleaning and sterilization instructions
are not followed. If excessive staining is observed use demineralized water for cleaning and sterilization.
Staining may be eliminated by scrubbing the device following standard cleaning procedures. Instruments
with corrosion should be removed from use.
• Repeated reprocessing of the instruments has minimal eect. End of life is normally determined by wear
and damage due to use.
• After each use inspect all instruments for any damage. Discard and replace instruments as necessary.
• After cleaning and sterilization, verify functionality prior to use.
CAUTION:
• If sterilizing for the rst time, remove all vinyl caps and packing foam prior to autoclaving.
• Soak or rinse instruments immediately after use. To avoid risk of corrosion or staining, DO NOT exceed
15 minutes soaking time.
• Do not use Glutaraldehyde, Chlorine, or Ammonium for soaking; this may cause damage to the
instrument nish.
• Do not use dry heat sterilization, as this may damage the instrument nish.
• Only sterilize clean instruments; sterilization is only eective on clean items.
• Store instruments in a clean, dry area.
Page 3 of 8
Page 4

M6-C™ Artificial Cervical Disc Surgical Instruments Care and Handling Instructions
WARNING:
• Only trained personnel should clean and sterilize instruments as described in these Instructions for
Use. Use appropriate personal protection equipment when handling contaminated instruments.
• Instruments should be dried after cleaning and after any exposure to water.
• Only sterile instruments may be used for surgery.
• Do not use these instruments for purposes other than those for which they are intended.
• Do not bend, pry, or use excessive force; breakage or failure of the instrument could occur resulting in
possible harm to the patient or user.
• Use extreme care during handling and cleaning of delicate or sharp instruments as injury or damage
could occur.
RESPONSIBILITIES OF THE USER
General. Health care personnel bear the ultimate responsibility for ensuring that any packaging method or
material is suitable for use in sterilization processing and sterility maintenance.
Cleaning/Decontamination. The health care facility is responsible to ensure that conditions essential to safe
handling and decontamination can be achieved. ANSI/A AMI ST35 Safe Handling and Biolo gical Decontamination
of Reusable Medical Devices in Health Care Facilities and in Nonclinical Settings and ISO 15883-1 Washerdisinfectors – Part 1: General requirements, terms and denitions and tests provide relevant guidelines.
Sterility. Users should conduct testing in the health care facility to ensure that conditions essential to
sterilization can be achieved and that specic conguration of the container contents is acceptable for the
sterilization process and for the requirements at the point of use. ANSI/AAMI ST33 Guidelines for the Selection
and Use of Reusable Rigid Container Systems for Ethylene Oxide Sterilization and Steam Sterilization in Health
Care Facilities and ISO 17665-1 Sterilization of health care products – Moist heat – Part 1: Requirements for
the development, validation and routine control of a sterilization process for medical devices provide relevant
guidelines.
PRECAUTIONS:
• When handling sharp instruments use extreme caution to avoid injury.
• Consult with an infection control practitioner to develop and verify safety procedures appropriate for all
levels of direct instrument contact.
• Unless otherwise indicated, instruments and tray are NOT Sterile and must be thoroughly cleaned and
sterilized prior to use.
• An unwrapped instrument tray DOES NOT maintain sterility.
• Instruments must be removed from tray for manual or automated cleaning procedures. Instrument trays
and lids must be cleaned separately from the instruments. Instrument trays must be thoroughly cleaned
until visually clean prior to sterilization. If tray is not visually clean, then repeat the entire cleaning process.
Page 4 of 8
Page 5

Care and Handling Instructions M6-C™ Artificial Cervical Disc Surgical Instruments
Manual Cleaning Process:
• Prior to cleaning, disassemble any instrument capable of being disassembled.
• Rinse the instruments under running tap water. Use a syringe to ush lumens and hard to reach areas. A
wire guide and/or pipe cleaner should be used to aid in cleaning lumens and other hard to reach areas.
Actuate any knobs, clamps, or joints while performing this process.
• Prepare an enzymatic cleaning solution, such as Enzol®, per the manufacturer’s instructions.
• Fully immerse the instruments in the prepared enzymatic cleaning solution and allow them to soak for a
minimum of one minute.
• After the soak, use a soft bristle brush to thoroughly clean any crevices or other dicult to clean areas of
the instruments. Flush lumens and small spaces with a syringe. A wire guide and/or pipe cleaner should be
used to aid in cleaning lumens and other hard to reach areas. Actuate any knobs, clamps, or joints while
performing this process.
• Remove the instruments from the enzymatic cleaning solution and rinse them with reverse osmosis or de-
ionized (RO/DI) water for a minimum of one minute. Use a syringe to ush lumens and hard to reach areas.
Actuate any knobs clamps or joints while performing this process.
• Prepare a neutral detergent, such as ValSure® Neutral Detergent, according to the manufacturer’s
recommendations using warm tap water in an ultrasonic bath.
• Fully immerse the instruments into the sonication bath and sonicate for two minutes.
• Remove the instruments from the neutral detergent solution and rinse them with RO/DI water for a
minimum of one minute. Use a syringe to ush lumens and hard to reach areas. Actuate any knobs, clamps,
or joints while performing this process.
• Dry the instruments using a clean, lint-free cloth to avoid damage to the surface. Pressurized air (20psi) may
be used to assist in drying.
• Visually inspect the surgical instruments for cleanliness. If instruments are not visually clean, then repeat the
entire cleaning process.
Automated Cleaning Process:
• Prior to cleaning, disassemble any instrument capable of being disassembled.
• Rinse the instruments under running tap water. Use a syringe to ush lumens and hard to reach areas. A
wire guide and/or pipe cleaner should be used to aid in cleaning lumens and other hard to reach areas.
Actuate any knobs, clamps, or joints while performing this process.
• Prepare an enzymatic cleaning solution, such as Enzol®, per the manufacturer’s instructions.
• Fully immerse the instruments in the prepared enzymatic cleaning solution and allow them to soak for a
minimum of one minute.
• After the soak, use a soft bristle brush to thoroughly clean any crevices or other dicult to clean areas of
the instruments. Flush lumens and small spaces with a syringe. A wire guide and/or pipe cleaner should be
used to aid in cleaning lumens and other hard to reach areas. Actuate any knobs, clamps, or joints while
performing this process.
• Remove the instruments from the enzymatic cleaning solution and rinse them with reverse osmosis or de-
ionized (RO/DI) water for a minimum of one minute. Flush lumens and small spaces with a syringe. Actuate
any knobs, clamps, or joints while performing this process.
• Place the instruments into the washer for processing.
• Run the validated cycle parameters below.
Page 5 of 8
Page 6
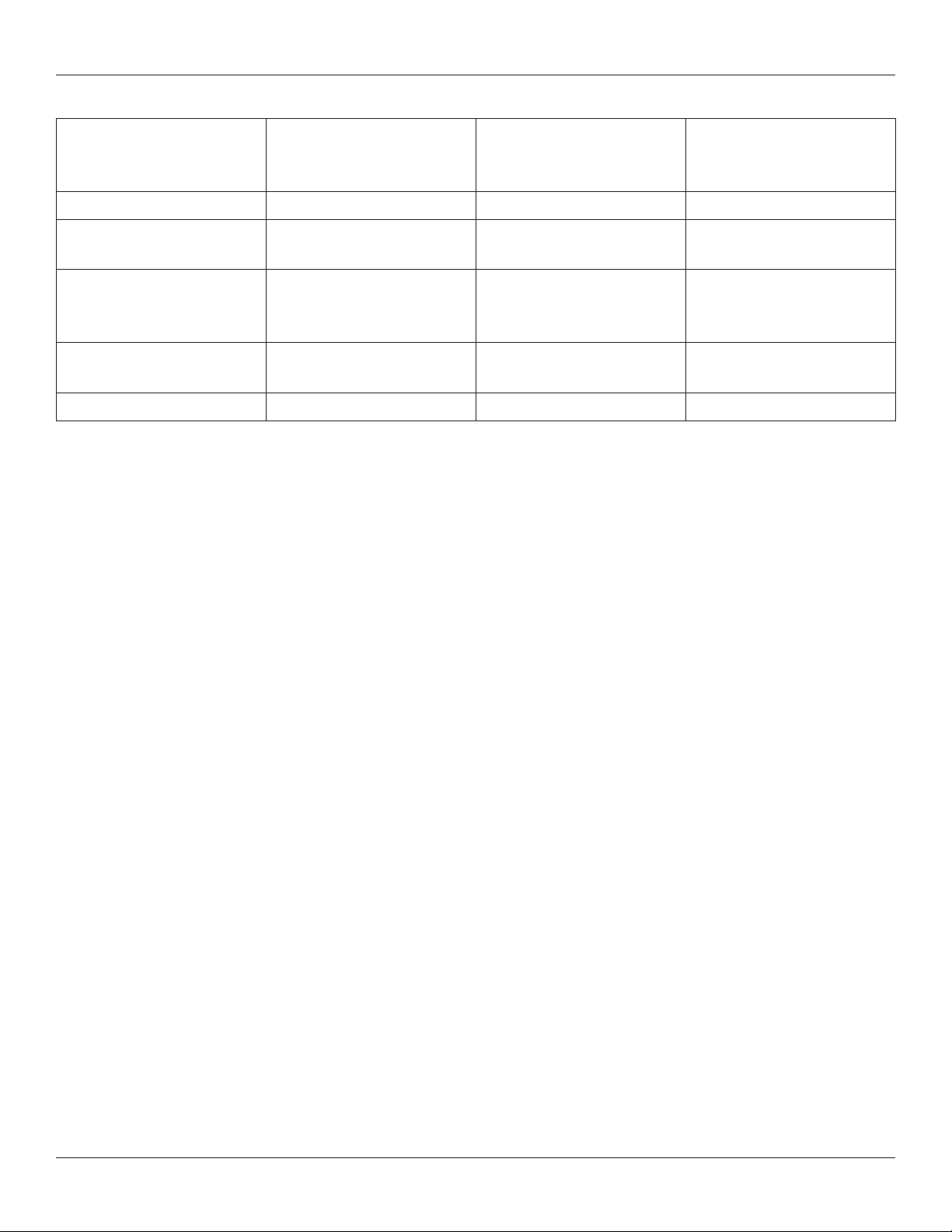
M6-C™ Artificial Cervical Disc Surgical Instruments Care and Handling Instructions
Validated Automated Cleaning Cycle
Phase: Recirculation Time:
(minutes)
Pre-wash 1 2:00 Cold Tap Water N/A
Enzyme Wash 2:00 Hot Tap Water
Wash 1 2:00 65.5 °C
Thermal Rinse
(PURW Rinse)
Drying 7:00 90 °C N/A
1:00 90 °C N/A
Water Temperature: Detergent Type and
Concentration:
(if applicable)
Enzol®
1 oz/gallon
ValSure® Neutral
Detergent
¼ oz/gallon
• Remove the instruments from the washer following the cycle.
• Visually inspect the instruments for cleanliness. If instruments are not visually clean, then repeat the entire
cleaning process.
STERILIZATION PROCESS
Since Orthox is not familiar with individual hospital handling procedures, cleaning methods, bioburden levels,
and other conditions, Orthox assumes no responsibility for sterilization of product by a hospital even if the
specied guidelines are followed.
When sterilizing multiple instruments in an autoclave cycle, ensure that the sterilizer’s maximum load is
notexceeded.
Do not stack another instrument tray on top of the M6-C Instrument Tray during the sterilization process.
The following sterilization cycle parameters were validated under laboratory conditions; however, these cycles
must be re-validated by the end user to ensure that sterility can be achieved on site.
• Instruments should be placed in the designated location for each instrument in the instrument tray. The lock
nut of the intervertebral distractor should be used to slightly open the jaws of the intervertebral distractor.
• Wrap the M6-C Surgical Instrument Tray in two layers of an FDA-cleared sterilization wrap such as Halyard
Health H600.
• Sterilizer Type: Pre-Vacuum
• Temperature: 132°C (270°F)
• Full Exposure Time: 4 minutes
• Dry Time: 60 minutes (45 minute cycle time followed by a 15 minutes dwell in the sterilizer after cycle
completion with the sterilizer door opened for cooling)
STORAGE AND SHELF LIFE
Instrument trays that have been processed and wrapped to maintain sterility should be stored in a manner to
avoid extremes in temperature and moisture. Care must be exercised in handling of wrapped cases to prevent
damage to the sterile barrier. The health care facility should establish a shelf life for wrapped instrument cases
based upon the type of sterile wrap used and the recommendations of the sterile wrap manufacturer. The user
must be aware that maintenance of sterility is event-related. Handling over time increases the probability of a
contaminating event.
Page 6 of 8
Page 7

Care and Handling Instructions M6-C™ Artificial Cervical Disc Surgical Instruments
PRODUCT COMPLAINTS
Any health care professional (e.g., customer or user of this system), who has complaints or who has experienced
any dissatisfaction in the product quality, identity, durability, reliability, safety, eectiveness and/or performance,
should notify Orthox. Further, if the device (implant or instruments) ever “malfunctions,” (i.e. does not meet
any of its performance specications or otherwise does not perform as intended) or may have caused or
contributed to the death or serious injury of a patient, Orthox should be notied immediately by telephone
or written correspondence. When ling a complaint, please provide the device name and serial number, lot
number, your name and address, and the nature of the complaint. Complaints may also be reported directly to
Medwatch at http://www.fda.gov/medwatch.
Page 7 of 8
Page 8

SPINAL KINETICS, MOTION FOR LIFE, ORTHOFIX, M6 and associated logos are trademarks or registered
trademarks of Orthox Medical Inc. or its aliates in the U.S. and other countries. Patents: Orthox.com
PK 0248 Rev 02
 Loading...
Loading...