Page 1

Model 5505 Instruction Manual
Page 2

CervicalStim Device Patent No.
U.S. 6,024,691
U.S. 5,743,844
U.S. 6,132,362
Assembled in the United States of America
ASSEMBLED IN
USA
Page 3

The CervicalStim™ Device Instruction Manual
Table of Contents
Prescription Information ...........................................................................
• Indication ...........................................................................................
• Contraindication ................................................................................
• Warnings ............................................................................................
• Precautions .........................................................................................
• Adverse Effects Summary ...................................................................
Device Information ...................................................................................
• Device Description ..............................................................................
• How the CervicalStim Device Works ...................................................
• Device Life ..........................................................................................
Device Operation .....................................................................................
• Turning the Device On and Off .........................................................
• Treatment Instructions .......................................................................
• Timing of Treatment Sessions ...........................................................
• Charging the Battery ..........................................................................
• Visual and Audio Indicators ..............................................................
• Wearing the Device ............................................................................
Device Accessories ....................................................................................
Device Use and Care .................................................................................
• Care and Cleaning ..............................................................................
• Storage ...............................................................................................
• Travel ..................................................................................................
• Disposal ..............................................................................................
• Service ................................................................................................
Clinical Information ...................................................................................
• Clinical Data Summary .......................................................................
• Adverse Events ...................................................................................
Equipment Classification ...........................................................................
CervicalStim Device Classifications ............................................................
Compliance Statements ............................................................................
Warranty ...................................................................................................
Device Box Components
1 – CervicalStim Device
1 – Power Supply
1 – Literature Pack
Orthofix Patient Services: 800-535-4492 or 214-937-2718
To learn more about Orthofix, please visit our website at www.orthofix.com.
1
1
1
1
1
1
2
2
2
2
3
3
4
4
4
6
7
7
8
8
8
9
9
9
10
10
12
13
14
14
16
Page 4

1
Prescription Information
Indication
The CervicalStim device is a noninvasive, pulsed electromagnetic bone growth
stimulator indicated as an adjunct to cervical fusion surgery in patients at highrisk for non-fusion.
Contraindication
There are no known contraindications for the CervicalStim device as an adjunct
to cervical spine fusion surgery.
Warnings
• Do not use the CervicalStim device if you have a cardiac pacemaker
or defibrillator because it may interfere with the operation of your
pacemaker or defibrillator. If you use the CervicalStim device and it affects
your pacemaker or defibrillator, it may injure your heart. Consult your
cardiologist before using the CervicalStim device.
• Remove the CervicalStim device prior to any imaging procedures (e.g.,
CT scan, MRI, etc.). If you wear the CervicalStim device during these
procedures, you could be injured, the imaging being produced may be
ruined, and/or the CervicalStim device could be damaged.
Precautions
• Avoid using the CervicalStim device if you do not understand the
instructions your doctor has given you. If you use the CervicalStim device
incorrectly, it may harm you or may not help your healing process.
• The CervicalStim device has not been evaluated in treating patients with the
following conditions: osseous or ligamentous spinal trauma, spondylitis,
Paget’s disease, moderate to severe osteoporosis, metastatic cancer, renal
disease, rheumatoid arthritis, uncontrolled diabetes mellitus, patients prone
to vascular migraine headache, seizure, epilepsy, thyroid conditions, or
neurological diseases.
• Animal reproductive studies performed with this device did not show any
harmful effects in animals. However, the safety of this device for use on
patients who are pregnant or nursing has not been established.
Adverse Effects Summary
Adverse effects may be experienced when using the CervicalStim device. These
adverse effects may include increased pain, numbness and tingling, headache,
migraines, and nausea. These effects may or may not be directly related to
use of the CervicalStim device. Any adverse effects that are related to the
CervicalStim device should stop when you discontinue use.
See the Adverse Events Table for a list of all adverse events reported during the
clinical study.
Please refer to the Compliance Statements section of the manual for
compatibility information regarding implantable medical devices.
Page 5

2
Device Information
Device Description
The CervicalStim device is an external device that generates a pulsed
electromagnetic field (PEMF) signal as a nonsurgical, prescription treatment
to increase the chances of a successful fusion. The device is lightweight,
adjustable and portable, including a rechargeable battery that allows
freedom of movement during treatment. A Liquid Crystal Display (LCD) and
audible indicators provide important feedback during treatment. See “Device
Operation” for more information.
How the CervicalStim Device Works?
To enhance bone healing after a fusion surgery, PEMF therapy activates
and augments the body’s natural healing process that may be impaired in
some people.
The CervicalStim device contains a Control Unit and a Treatment Coil in one
integrated device. A micro-processor generates the CervicalStim device’s
electrical signal, which is a highly uniform, low-energy electromagnetic field
sent from the treatment coil. When the coil is centered over the treatment area,
the therapeutic CervicalStim PEMF signal is delivered through clothing and skin
directly to the fusion site.
To learn more about bone growth stimulation, please visit our patient website
at www.BoneGrowthTherapy.com.
Device Life
The CervicalStim device provides daily treatments for up to 365 days. The
physician determines the overall length of treatment (months/weeks) on an
individual basis according to fusion healing progress.
Model 5505
Treatment
Coil
Control
Unit
LCD
Page 6

3
Device Operation
Turning the Device On and Off
The CervicalStim device can be turned on by pressing the On/Off Button on
the Control Unit of the device.
When the device is turned on, a status screen will display the number
of days since the first use, the treatment status, and the
compliance percentage.
The LCD will show the prescribed treatment time remaining and the
battery status.
The flashing colon on the LCD screen and On/Off button indicate that the
device is on and delivering treatment.
The CervicalStim device can be turned off by pressing and holding the On/
Off Button on the Control Unit of the device until it beeps.
The On/Off Button on the Control Unit doubles as a Backlight to light up
the LCD. In low light, press the On/Off Button to light up the LCD.
1:59
Control Unit
Charging Port
LCD Treatment Indicator
On/Off Button
Page 7

4
Treatment Instructions
• The CervicalStim device should be worn for 4 hours each day as prescribed
by a physician.
• The CervicalStim device may be used at any time of day that is most
convenient for the patient.
• The device is programmed to reset daily at midnight Central Standard Time,
unless adjusted by a physician or Orthofix representative for a
different time zone or reset time.
• Hours worn before the reset time will be logged and stored in the device for
daily use compliance.
• The overall treatment duration (months/weeks) will vary based on
specific patient conditions as determined by a physician.
• Because the CervicalStim device is lightweight and portable, treatment can
be received while sitting, walking, reclining, sleeping, etc. However, since
each patient is unique, the overall activity level should be based on
physician instructions.
Timing of Treatment Sessions
• The CervicalStim device tracks the treatment time; this tracking (or timing)
begins when the device is turned on and at least one minute of treatment
is complete.
• The LCD shows a countdown of the daily treatment time remaining.
• To stop treatment at any point, simply press and hold the On/Off Button
until you hear a beep.
• To resume treatment, press the On/Off button again.
• The countdown will resume at the remaining daily treatment time.
• When daily treatment is completed, the device will automatically
turn off.
Charging the Battery
The CervicalStim device is powered by a rechargeable lithium-ion battery pack.
The battery pack will provide at least one full treatment before needing to be
recharged. A power supply to charge the battery is provided with the device.
Use only the Orthofix power supply to charge the battery (Part no. Orthofix
20110412, 20114794, WR9QA1200U23KIT(R6B), or 20123808).
To ensure that the device is functioning properly, the CervicalStim device
constantly monitors battery voltage and the electrical signal. The LCD will display
a battery capacity symbol and the device will beep to alert the patient when
the battery is low and will soon need to be recharged.
The CervicalStim device should be charged before the first use and every day
after completing treatment. Do not wear the device while charging. The device
will not deliver treatment while charging.
Page 8

Follow these steps to recharge the battery:
1. Open the Charging Port Cover.
2. Plug the Charging Connector into the Charging Port located on the
Control Unit.
3. Plug the power supply into any standard AC Wall Outlet. Do not plug in
the power supply for the CervicalStim device where it will be difficult to
unplug.
4. The Control Unit LCD will display a battery symbol filling to verify that
the device is charging. When the battery reaches a complete charge,
a check mark symbol will be displayed next to the battery symbol. In
addition, the device will beep once to alert the patient.
5. If the battery is fully depleted, it may require up to 4 hours to charge
completely.
6. After charging is complete, remove the Charging Connector and replace
the Charging Port Cover.
Angled Corners
AC Power Supply
AC Charging Connector
Charging Port Cover
Page 9
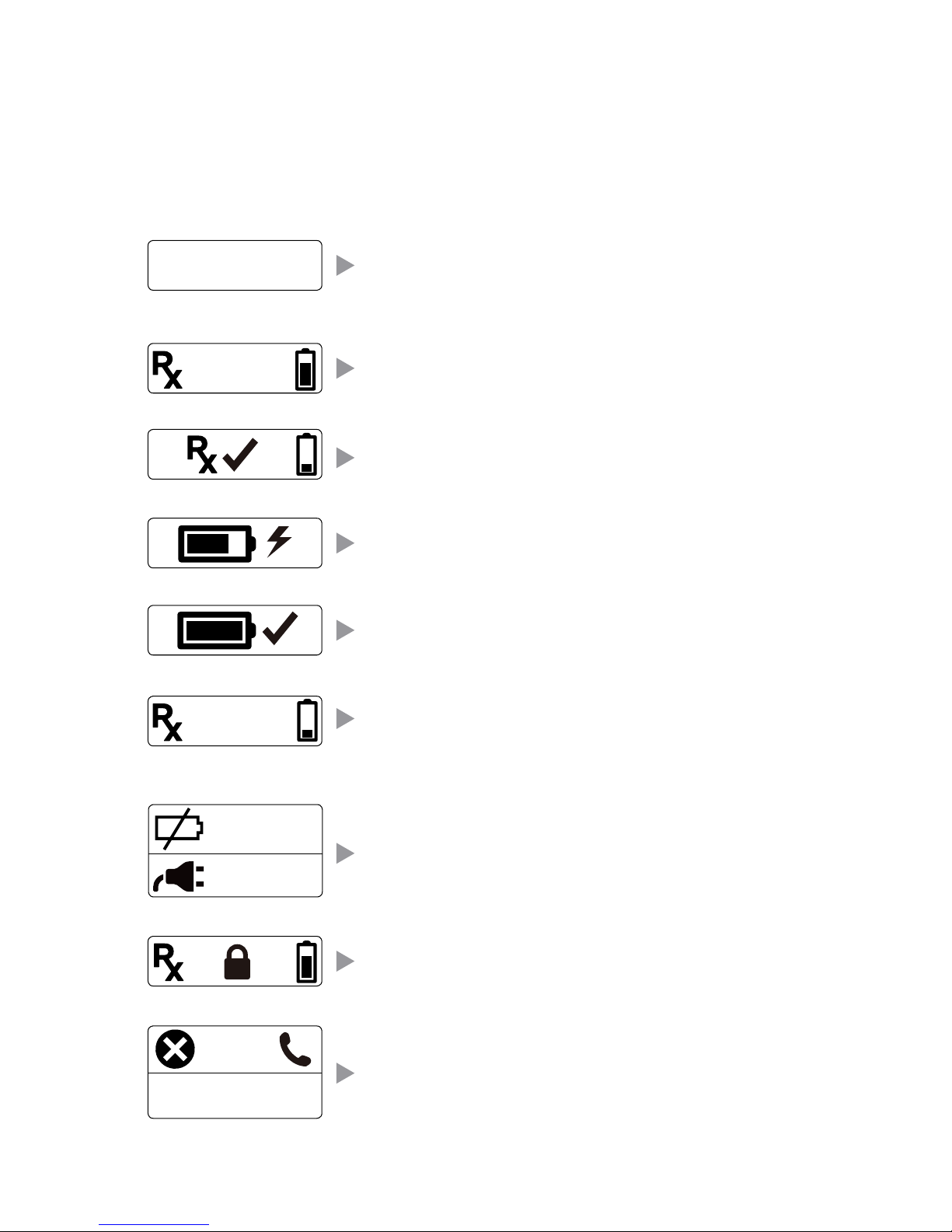
6
Visual and Audio Indicators
The LCD and audible beeps are designed to provide helpful information
to the user. The screens, symbols, and beeps are explained below.
Compliance Screen
170/185 = 91.9%
Treatment Screen
1:59
Treatment Screen – displays the treatment time remaining
in hours and minutes. The timer counts down to zero until
daily treatment is complete.
Exception Screen
E123
Exception Codes – Display of ERROR, any E codes
(e.g., E01, E02), along with three slow beeps. Contact
Patient Services at 800-535-4492 or 214-937-2718.
Low Battery Warning Screen
1:59
Low Battery – Displays along with three fast beeps when
recharging is recommended.
Charging Complete
Charging Complete – Indicates when the battery is
fully charged.
Battery must be charged
to turn on
E12345678
E12345678
Battery Empty – Indicates that the battery must be
charged before treatment may continue.
Device Expired
Device Expired – Display of a closed lock indicates the
device has been available for treatment for 365 days
and will no longer provide a treatment..
Compliance Screen – Displays a compliance percentage
which is calculated by the number of full treatments days
completed over the number of available treatment days.
The treatments days available begin once the device has
been delivered to the patient and a minute of treatment
time has been established.
Charging Screen
Battery Charging – Battery symbol filling repeatedly
verifies that the device is charging.
Treatment Complete
Daily Prescribed Treatment complete
1-800-535-4492
Page 10

7
Wearing the Device
The CervicalStim device may be worn over a brace, cervical collar, halo, or
clothing. Proper treatment does not require direct contact with the body.
However, the coil must be centered around the fusion site to be effective. Users
can gently bend and shape the treatment coil to fit more comfortably around
the neck.
The following is the suggested method for wearing the CervicalStim device:
1. To put on the CervicalStim device, simply slip the device over your head.
2. For a wider opening, detach the Velcro
®
Tab near the control unit
and place over your head.
3. The device does not need to be tight against the back of the neck;
it should rest comfortably on your shoulders.
Device Accessories
An accessory available to the patient is a user friendly mobile application which
allows the patient to easily monitor their device use. This may be downloaded to
the patient’s smartphone.
For additional comfort, a Comfort Collar is available as an accessory. Please contact
Patient Services at 800-535-4492 or 214-937-2718 to order.
Velcro
Tabs
1
2
3
Page 11

Device Use and Care
• The CervicalStim device is for single patient use.
• The CervicalStim device is a technologically advanced electronic device
and should be handled with care. Dropping or other mishandling of the
CervicalStim device may damage the device and it may stop working.
• For safe usage, follow manufacturer instructions when using the
CervicalStim device. You (the patient) are the intended operator of this
device.
• Use of the device in any other manner could have harmful effects and/or
void the warranty.
• The use of accessories other than those specified may result in increased
emissions or decreased immunity of the device.
• Inspect the device prior to each use for wear, deterioration or damage.
• Do not use or charge the device if it does not appear to be in suitable
condition, displays an error or stops working. Contact Patient Services if
any of these occur.
• WARNING: Do not modify this equipment as this could make it unsafe to
use. Do not attempt to open or disassemble the CervicalStim device as
there are no user serviceable parts inside.
• CAUTION: STRANGULATION HAZARD – Keep the Power Supply cord out
of the reach of children.
Care and Cleaning
When cleaning the CervicalStim device, follow these instructions:
• WARNING: Do not clean the device during treatment or charging.
• Clean the device by wiping surfaces with a damp, soft cloth
(wet with water only).
• DO NOT sterilize the CervicalStim device.
• DO NOT expose the CervicalStim device to excessive moisture.
• DO NOT use solvents or alcohol-based liquids (anti-bacterial
cleaners, hand sanitizers, perfume, etc.) to clean the CervicalStim device.
Storage
When moving the CervicalStim device from very cold or very hot storage areas
(like your car), wait at least an hour to use or charge the device. The device
requires time to return to operating temperature.
Unpacked Storage:
Temperature Range:
• -25°C to 5°C
• 5°C to 35°C at up to 90% relative humidity, non-condensing
• 35°C to 60°C at a water vapor pressure up to 50 hPa
8
Page 12

9
Packed Storage, Shipping and Transport:
Temperature Range: within -40°C to 60°C
• Between 10-100% relative humidity
• Including condensation at pressures between 500 hPa and 1060 hPa
Operating Environment:
Temperature Range: within 5°C to 40°C
• 15-90% relative humidity, non-condensing but not requiring a water
vapor pressure greater than 50 hPa
• 700-1060 hPa
The CervicalStim device is designed for a storage life of twelve months plus
one year of usage.
Travel
When traveling by air, it is recommended to pack the CervicalStim device
with checked luggage. If taken onboard the airplane, it should be turned off
when passing through security screening equipment, as the device could be
damaged. The CervicalStim device instruction manual should be taken with
you to quickly and easily identify the device for security personnel. Do not
wear or operate the CervicalStim device while onboard the airplane.
Disposal
After treatment is complete and a physician advises you to discontinue use,
you may dispose of the device according to your local governing ordinances
or recycling plans. Contact your local authorities to determine the proper
method for disposal since this is electronic equipment containing a lithium-ion
battery. You may also contact Orthofix Patient Services regarding recycling.
The CervicalStim device is for single patient use.
The CervicalStim device is a Class III medical device (prescription only) that
cannot be sanitized or used by another person.
Dispose of the device properly to prevent injury.
DO NOT dispose of the CervicalStim device in an incinerator. This
device contains lithium batteries.
Service
If you have questions concerning the device or require any assistance, please
call 800-535-4492 (U.S. only) or 214-937-2718. There are no user serviceable
parts. Notify Orthofix for any servicing needs.
Page 13

10
Clinical Information
Clinical Data Summary
The CervicalStim device was studied in humans to evaluate its safety and
effectiveness as a therapy added to routine care (adjunct therapy) for high-risk
patients having a cervical fusion surgery for degenerative conditions. Patients
were high-risk if they were a smoker (one pack per day or more) and/or had a
multi-level fusion surgery (more than one level).
The 323 patients were randomly assigned, to one of two groups: either the
control group (routine care only) or the treatment group (CervicalStim device +
routine care). One hundred and sixty (160) patients were assigned to the control
group and 163 patients were assigned to the CervicalStim device group. Patients
wore the CervicalStim unit for 4 hours each day either for 4 continuous hours or
in one hour sessions.
Safety and effectiveness was evaluated by measuring the following:
• rate and severity of adverse events
• rate of cervical fusion by six months after surgery as determined by x-ray
Eighty-four percent (84%) of the CervicalStim device group were fused by
six months (102/122 patients) versus only 69% of the control group (81/118
patients). This is a 15% difference between these two groups and is statistically
significant (meaningful); p=0.0065. That is, more patients fused in the
CervicalStim device group than in the control group.
Clinical Trial: Overall Success Rate
100
80
60
40
20
0
84%
69%
% Patients Fused
Treated Untreated
Page 14

11
The rate of patients who came back for their six month examinations and
x-rays was 74% for the CervicalStim device group and 73% for the control
group. Patients who did not come back for scheduled examinations could not
be evaluated; thus their success or failure is not known. These unavailable
data could have a positive or negative effect on the overall success of this
study.
One hundred and twelve (112) patients reported a total of 157 adverse
(negative) effects for both groups combined at six months after surgery. There
was no significant (meaningful) difference in the total number of adverse
events or the number of patients reporting effects in the control group
and the CervicalStim device group nor in the numbers of patients in each
group who experienced an adverse event. The adverse effects that may be
experienced include: increased pain, numbness and tingling, headache,
migraines and nausea. These effects may or may not be directly related to the
use of the CervicalStim device.
Clinical success with regard to symptoms was evaluated by the following:
• no worsening in neurological function
• an improvement in pain
• no worsening in Neck Disability Index
Based on the criteria above, there was no major difference between the
control group and the CervicalStim device group in clinical success. An equal
number of patients in both groups showed an improvement in their clinical
condition after surgery, regardless of treatment.
Long-term x-ray information collected at 11 months after surgery or later
showed no meaningful difference in fusion rate between the CervicalStim
device treatment group and the control group who received routine care
alone.
The results of this study show that the use of the CervicalStim device is both
safe and effective in increasing the frequency of fusion by six months after
surgery in high-risk subjects having cervical fusion.
Page 15

12
Adverse Events Reported at 6 Months by Treatment Group
Control Group (n=160) CervicalStim Device Group (n=163)
Increased Neck Pain
Shoulder/Arm Pain
Re-Injury to Cervical Spine
Adjacent level pathology
Surgical Complications
LBP/Lumbar pathology
Trauma/Injury(not cervical)
Numbness/Tingling
Headache/Migraine
Nonspecific/Unrelated Pain
Nausea
Dizziness/Vertigo
Rash/Discoloration
Rapid/Irregular Heartbeat
Shortness of Breath
Ringing in Ears
Neurologic Symptom/Stroke
Lump in Throat
Diagnosis of Diabetes
Diagnosis of Breast Cancer
Seizure
Death, Unrelated
Tenderness
Screw Broken
Graft Collapse
Carpal Tunnel Syndrome
Choking Sensation
Cardiac Symptoms
Nephrotic Syndrome
Suicide Attempt
TOTAL
Adverse Events
# (%) of
Events
10 (14.9)
10(14.9)
10(14.9)
3(4.5)
2(3.0)
8(11.9)
2(3.0)
6(8.9)
2(3.0)
2(3.0)
0
2(3.0)
0
0
0
0
1(1.5)
0
0
0
0
0
1(1.5)
1(1.5)
1(1.5)
2(3.0)
1(1.5)
1(1.5)
1(1.5)
1(1.5)
67
# (%)
1
of
Patients
Experiencing
the Event
9(5.6)
9(5.6)
8(5,0)
3(1.9)
2(1.3)
8(5.0)
2(1.3)
6(3.8)
2(1.3)
2(1.3)
0
2(1.3)
0
0
0
0
1(0.6)
0
0
0
0
0
1(0.6)
1(0.6)
1(0.6)
2(1.3)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
47
2
#*(%) of
Events
16(17.8)
16(17.8)
9(10.0)
8(8.8)
7(7.7)
5(5.5)
5(5.5)
4(4.4)
4(4.4)
3(3.3)
2(2.2)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
1(1.1)
0
0
0
0
0
0
0
0
90
# (%)
1
of
Patients
Experiencing
the Event
15(9.2)
16(9.8)
9(5.5)
8(4.9)
5(3.1)
5(3.1)
4(2.5)
4(2.5)
4(2.5)
3(1.8)
2(1.2)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
1(0.6)
0
0
0
0
0
0
0
0
58
2
Control Group (n=160)
CervicalStim Device Group
(n=163)
1
% expressed as number of patients experiencing the event / total number of patients in the group
2
Some patients experienced multiple adverse events
*
There were several adverse events that were more frequently observed in the CervicalStim device group than in
the control group. Given the types of events, it is unlikely that these adverse events are related to the treatment.
Page 16

Equipment Classification
Device Symbol Descriptions
13
Symbol Meaning Symbol Location
Attention – Refer to Instruction Manual Device and Device Box
Type BF Applied Part Device and Device Box
On/Off Device
Prescription Only Device
Storage Temperature Range Device Box
Year of Manufacture for Active Device Device and Device Box
Manufacturer Instruction Manual
Not for General Waste Device and Device Box
Keep Dry Device and Device Box
FCC Mark Device and Device Box
CE Mark Device and Device Box
Storage Humidity Limits Device and Device Box
Atmospheric Pressure Limitations Device Box
EU Authorized Representative Instruction Manual
Catalog Number Device and Device Box
Serial Number Device and Device Box
REF
SN
RCM - Regulatory Compliance Mark
(Australia)
Device
Page 17

CervicalStim Device Classifications
• Product Family Name: Orthofix PEMF Device
• Internally powered equipment. The service life of the non-replaceable
lithium-ion battery is 2.5 years.
• This device generates a non-ionizing pulsed electromagnetic field with an
intensity of approximately 2 Gauss and frequency components in the
1Hz-50KHz range. This field is distributed within and near the treatment coil.
• Type BF applied part. The applied part is the treatment coil with integrated
control unit.
• IEC 60529 enclosure rating: IP22. IP22 means the enclosure provides
protection from solid objects greater than 12.5 mm and dripping liquids when
tilted 15° from normal use. It is recommended you keep the unit dry.
• Mode of operation: intermittent operation
• This device is non-sterile. It does not require sterilization.
• Shelf life for equipment: 1 year
• Equipment not suitable for use in the presence of a flammable
anesthetic mixture with air or nitrous oxide.
• The power supply is considered double insulated with Class II
construction throughout.
• Power supply ratings:
Orthofix # 20110412:
Orthofix # 20114794:
Input: 100-240VAC, 50-60Hz, 200mA Input: 100-240VAC, 50-60Hz, 150-350mA
Output Voltage: 5VDC, 1.3A
Output Voltage: 5VDC, 2.4A
Orthofix#: 20123808: Orthofix#: WR9QA1200U23KIT(R6B):
Input: 100-240VAC, 50-60Hz, 0.6-0.3A Input: 100-240VAC, 50-60Hz, 0.6A
Output Voltage: 5VDC, 1.2A Output Voltage: 5VDC, 1.2A
14
Compliance Statements
This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference,and (2)
this device must accept any interference received, including interference that may
cause undesired operation.
IMPORTANT! Changes or modifications not expressly approved by Orthofix, Inc. could
void the user’s authority to operate the equipment.
NOTE: This equipment has been tested and found to comply with the limits for a
Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed
to provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate radio frequency energy
and, if not installed and used in accordance with the instructions, may cause
harmful interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation.
Page 18

15
If this equipment does cause harmful interference to radio or television reception,
which can be determined by turning the equipment off and on, the user is encouraged
to try to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which
the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
Information regarding Electromagnetic Compatibility and Immunity
The CervicalStim device complies with IEC 60601-1-2 for electromagnetic
compatibility (EMC).
The CervicalStim device needs special precautions regarding
EMC and needs to be used in accordance with the EMC information provided
in this manual. Wireless communications equipment such as home network
devices, mobile phones, cordless telephones and their base stations, and walkietalkies can affect the CervicalStim device. These types of equipment should be
kept at least 0.198 m (7.8 in) away from the CervicalStim device.
The CervicalStim device has not been evaluated with regard to use with specific
implantable electronic medical devices. Please consult your physician prior to use
of the CervicalStim device with implantable electronic medical devices.
Page 19

16
Warranty
Orthofix Inc. warrants the CervicalStim device to be free from defects in
materials and workmanship for one year from the date of first use. Provided
that all
terms and conditions of this Limited Warranty are complied with,
Orthofix Inc.
will replace defective components.
This Limited Warranty applies to the product only under normal use and does
not cover any damage or defect caused by accident, misuse, abuse, fire, flood,
and acts of God, or by any alteration, tampering, repair, or attempted repair
by anyone other than Orthofix Inc. This warranty only applies to the patient
for whom the product is prescribed and is not assignable or transferable.
Defective products covered by this Limited Warranty must be returned to
Orthofix Inc., Attention: Orthofix Returns. You must call a Patient Services
Representative or your local distributor to obtain the Return Authorization
number and address prior to returning the product.
Except as specifically required by applicable law, the foregoing warranty is in
lieu of all other warranties, expressed or implied, and Orthofix Inc. specifically
disclaims any and all warranties of merchantability or fitness for a particular
purpose. Under no circumstances shall Orthofix Inc., its authorized
representative, affiliated, or subsidiary companies be liable for special,
consequential, or incidental damages. The sole remedy with respect to any
defective product shall be limited to replacement.
This Limited Warranty may not be extended or modified except in writing by
Orthofix Inc. No sales person, representative, distributor or physician is
authorized to make or consent to any extension or modification of the terms
of this Limited Warranty.
For additional information and/or device assistance, contact Orthofix
Patient Services at 800-535-4492 or 214-937-2718.
Page 20

Page 21

Modelo 5505 Manual De Instrucciones
Spanish/Español
Page 22

CervicalStim, patente de dispositivo n.º
U.S. 6,024,691
U.S. 5,743,844
U.S. 6,132,362
Ensamblado en los Estados Unidos de América
ASSEMBLED IN
USA
Page 23

1
1
1
1
1
1
2
2
2
2
3
3
4
4
4
6
7
7
8
8
8
9
9
9
10
10
12
13
14
14
16
Manual de instrucciones del dispositivo CervicalStim
™
Índice
Información sobre la prescripción .............................................................
• Indicación ...........................................................................................
• Contraindicaciones ............................................................................
• Advertencias .......................................................................................
• Precauciones .......................................................................................
• Resumen de eventos adversos ............................................................
Información del dispositivo ......................................................................
• Descripción del dispositivo .................................................................
• Funcionamiento del dispositivo CervicalStim ......................................
• Vida útil del dispositivo ......................................................................
Funcionamiento del dispositivo ...............................................................
• Encendido y apagado del dispositivo ................................................
• Instrucciones del tratamiento .............................................................
• Control del tiempo de las sesiones de tratamiento ...........................
• Carga de la batería .............................................................................
• Indicadores visuales y audibles ..........................................................
• Transporte del dispositivo ..................................................................
Accesorios del dispositivo .........................................................................
Utilización y cuidado del dispositivo .........................................................
• Cuidado y limpieza .............................................................................
• Almacenamiento ................................................................................
• Viajes ..................................................................................................
• Eliminación .........................................................................................
• Mantenimiento ...................................................................................
Información clínica ....................................................................................
• Resumen de los datos clínicos ............................................................
• Eventos adversos ................................................................................
Clasificación del equipo ............................................................................
Clasificaciones del dispositivo CervicalStim ...............................................
Declaraciones de cumplimiento ................................................................
Garantía ....................................................................................................
Componentes de la caja del dispositivo
1 – Dispositivo CervicalStim
1 – Fuente de alimentación
1 – Paquete de documentación
Atención al paciente de Orthofix: +1 800-535-4492 o +1 214-937-2718
Para obtener más información sobre Orthofix, visite nuestro sitio web en
www.orthofix.com.
Spanish/Español
Page 24

1
Información sobre la prescripción
Indicación
El dispositivo CervicalStim es un estimulador electromagnético por pulsos para
el crecimiento óseo no invasivo indicado como coadyuvante de la cirugía de
fusión cervical en pacientes con un alto riesgo de ausencia de fusión.
Contraindicaciones
El dispositivo CervicalStim como coadyuvante de las intervenciones de fusión
de la columna cervical no tiene contraindicaciones conocidas.
Advertencias
• No utilice el dispositivo CervicalStim si tiene un marcapasos o un
desfibrilador, ya que este podría interferir en el funcionamiento de
dichos dispositivos. Si utiliza el dispositivo CervicalStim y este le afecta el
marcapasos o desfibrilador, podría lesionarle el corazón. Consulte a su
cardiólogo antes de utilizar del dispositivo CervicalStim.
• Retire el dispositivo CervicalStim antes de cualquier procedimiento de
obtención de imágenes (por ejemplo, TAC, IRM, etc.). Si lleva puesto el
dispositivo CervicalStim durante estos procedimientos, podría resultar
lesionado, la imagen capturada podría arruinarse o el dispositivo
CervicalStim podría resultar dañado.
Precauciones
• Evite utilizar el dispositivo CervicalStim si no entiende las instrucciones que
le dio el médico. Si utiliza el dispositivo CervicalStim de forma incorrecta,
este podría causarle lesiones o no ayudarle en su proceso de curación.
• El dispositivo CervicalStim no se ha evaluado en el tratamiento de pacientes
con las siguientes enfermedades: traumatismo espinal ligamentoso u
óseo, espondilitis, enfermedad de Paget, osteoporosis de moderada a
grave, cáncer metastásico, enfermedad renal, artritis reumatoide, diabetes
mellitus no controlada, tendencia a migrañas vasculares, convulsiones,
epilepsia, enfermedades tiroideas o enfermedades neurológicas.
• Los estudios reproductivos realizados en animales con este dispositivo no
mostraron efectos dañinos en animales. Sin embargo, no se ha establecido la
seguridad de este dispositivo en pacientes embarazadas o durante la lactancia.
Resumen de eventos adversos
Durante el uso del dispositivo CervicalStim, podrían experimentarse eventos
adversos. Estos eventos adversos podrían incluir un aumento en el dolor,
entumecimiento y hormigueo, dolor de cabeza, migrañas y náuseas. Estos
efectos podrían estar o no directamente relacionados con el uso del dispositivo
CervicalStim. Cualquier evento adverso relacionado con el dispositivo
CervicalStim deberá desaparecer al interrumpir el uso del dispositivo.
Consulte la tabla de eventos adversos para obtener una lista de todos los
eventos adversos notificados durante el estudio clínico.
Consulte la sección Declaraciones de cumplimiento del manual para
obtener información de la compatibilidad con respecto a dispositivos
médicos implantables.
Page 25

2
Información del dispositivo
Descripción del dispositivo
El dispositivo CervicalStim es un dispositivo externo que genera una señal de
campo electromagnético pulsado (CEMP) como tratamiento no quirúrgico que
se receta para aumentar las probabilidades de una fusión exitosa. El dispositivo
es ligero, ajustable y portátil, e incluye una batería recargable que permite
libertad de movimiento durante el tratamiento. Una pantalla de cristal líquido
(LCD) y sus indicadores audibles proporcionan información importante durante
el tratamiento. Consulte “Funcionamiento del dispositivo” para obtener más
información.
Funcionamiento del dispositivo CervicalStim
Para mejorar la curación del hueso después de la cirugía de fusión, el
tratamiento con CEMP activa y acelera el proceso natural de curación del
cuerpo que puede estar afectado en algunas personas.
El dispositivo CervicalStim incluye una unidad de control y un transductor
de tratamiento en un dispositivo integrado. Un microprocesador genera la
señal eléctrica del dispositivo CervicalStim, que es un campo electromagnético
de baja energía altamente uniforme que se envía desde el transductor de
tratamiento. Cuando el transductor está centrado sobre el área de tratamiento,
la señal terapéutica del CEMP del dispositivo CervicalStim se aplica a través de
la ropa y la piel directamente al lugar de la fusión.
Para obtener más información sobre la estimulación del crecimiento óseo, visite
nuestro sitio web para pacientes en www.BoneGrowthTherapy.com.
Vida útil del dispositivo
El dispositivo CervicalStim proporciona tratamientos diarios durante hasta
365 días. El médico determinará la duración total del tratamiento (meses o
semanas) de forma individual dependiendo del proceso de curación de la fusión.
Transductor de
tratamiento
Unidad de
control
Modelo 5505
Spanish/Español
LCD
Page 26

3
Funcionamiento del dispositivo
Encendido y apagado del dispositivo
El dispositivo CervicalStim puede encenderse manteniendo pulsado el
botón de Encendido/Apagado de la unidad de control del dispositivo.
Cuando el dispositivo se encienda, se mostrará una pantalla de estado
con el número de días desde el primer uso, el estado del tratamiento y el
porcentaje de cumplimiento.
La LCD mostrará el tiempo de tratamiento prescrito restante y el estado de
la batería.
Los dos puntos parpadeantes de la pantalla LCD y del botón de Encendido/
Apagado indican que el dispositivo está encendido y aplicando el
tratamiento.
El dispositivo CervicalStim puede apagarse manteniendo pulsado el botón
de Encendido/Apagado de la unidad de control del dispositivo hasta que
emita un pitido.
El botón de Encendido/Apagado de la unidad de control también sirve
como retroiluminación para iluminar la LCD. Cuando la luz esté baja,
oprima el botón de Encendido/Apagado para encender la LCD.
1:59
Unidad de control
Puerto de carga
Indicador de tratamiento
Botón de Encendido/Apagado
Page 27

4
Instrucciones del tratamiento
• El dispositivo CervicalStim debe llevarse durante 4 horas cada día, según
lo que recete el médico.
• El dispositivo CervicalStim podrá utilizarse en el momento del día que sea
más cómodo para el paciente.
• El dispositivo está programado para reiniciarse diariamente a media noche
en el horario estándar del centro de EE. UU., a menos que un médico o
un representante de Orthofix lo ajuste para una zona horaria o una hora
de reinicio diferente.
• Las horas que lo haya llevado puesto antes de la hora de reinicio
quedarán registradas y almacenadas en el dispositivo para el
cumplimiento del uso diario.
• La duración total del tratamiento (meses o semanas) variará dependiendo
de las enfermedades específicas del paciente según lo determine el médico.
• El dispositivo CervicalStim es ligero y portátil, de modo que puede recibir
el tratamiento mientras está sentado, andando, recostado, dormido, etc.
Sin embargo, debido a que cada paciente es único, la actividad total
deberá basarse en las instrucciones del médico.
Control del tiempo de las sesiones de tratamiento
• El dispositivo CervicalStim realiza un seguimiento del tiempo de tratamiento;
este seguimiento (o control del tiempo) comienza cuando el dispositivo se
enciende y se completa por lo menos un minuto de tratamiento.
• La LCD muestra una cuenta regresiva del tiempo restante de tratamiento
diario.
• Para detener el tratamiento en cualquier momento, solo tiene que mantener
oprimido el botón de Encendido/Apagado hasta que escuche un pitido.
• Para reanudar el tratamiento, oprima de nuevo el botón de Encendido/
Apagado.
• La cuenta regresiva se reanudará con el tiempo restante de tratamiento
diario.
• Cuando haya completado el tratamiento diario, el dispositivo se apagará
automáticamente.
Carga de la batería
El dispositivo CervicalStim recibe alimentación de una batería recargable de ion
de litio. La batería suministrará un mínimo de un tratamiento completo antes de
necesitar recargarse. Junto con el dispositivo, se incluye una fuente de alimentación
para cargar la batería. Utilice únicamente la fuente de alimentación de Orthofix
para cargar la batería (n.º de referencia Orthofix 20110412, 20114794,
WR9QA1200U23KIT(R6B), o 20123808).
Para asegurarse de que el dispositivo funciona correctamente, el dispositivo
CervicalStim monitorea constantemente el voltaje de la batería y la señal eléctrica.
La LCD mostrará un símbolo de capacidad de la batería y el dispositivo pitará para
avisarle al paciente cuando la batería esté baja y próxima a necesitar una recarga.
El dispositivo CervicalStim deberá cargarse antes del primer uso y cada día
después de completar el tratamiento. No lleve el dispositivo mientras se carga.
El dispositivo no aplicará el tratamiento mientras esté cargándose.
Spanish/Español
Page 28

5
Siga estos pasos para recargar la batería:
1. Abra la cubierta del puerto de carga.
2. Enchufe el conector de carga en el puerto de carga de la unidad de control.
3. Enchufe la fuente de alimentación a cualquier tomacorriente de pared
de CA estándar. No enchufe la fuente de alimentación del dispositivo
CervicalStim en sitios en los que sea difícil desenchufarla.
4. La LCD de la unidad de control mostrará un símbolo de una batería
llenándose para verificar que el dispositivo se está cargando. Cuando la
batería alcance la carga completa, se mostrará un símbolo de marca de
comprobación junto al símbolo de la batería. Además, el dispositivo pitará
una vez para avisarle al paciente.
5. Si la batería está completamente descargada, es posible que necesite
hasta 4 horas para cargarse por completo.
6. Una vez completada la carga, retire el conector de carga y vuelva a
colocar la cubierta del puerto de carga.
Esquinas en
ángulo
Fuente de alimentación de CA
Conector de carga de CA
Cubierta del puerto
de carga
Page 29

6
Indicadores visuales y audibles
La LCD y los pitidos están diseñados para proporcionarle información útil al
usuario. A continuación se explican las pantallas, los símbolos y los pitidos.
Pantalla de cumplimiento
170/185 = 91.9%
Pantalla de tratamiento
1:59
Pantalla de tratamiento: muestra el tiempo restante de
tratamiento en horas y minutos. El temporizador lleva
una cuenta regresiva a cero hasta que se completa el
tratamiento diario.
Pantalla de excepciones
E123
Códigos de excepción: visualización de ERROR, cualquier
código E (por ejemplo, E01, E02), junto con tres pitidos
lentos. Comuníquese con Atención a pacientes al
+1 800-535-4492 o al +1 214-937-2718.
Pantalla de advertencia
de batería baja
1:59
Batería baja: se muestra junto con tres pitidos rápidos
cuando se recomienda la recarga.
Carga completa
Carga completa: indica cuando la batería está
completamente cargada.
La batería debe estar
cargada para encender
el dispositivo
E12345678
E12345678
Batería vacía: indica que la batería debe cargarse antes de
que el tratamiento pueda continuar.
Dispositivo vencido
Dispositivo vencido: se muestra un candado cerrado
que indica que el dispositivo ha estado disponible para
el tratamiento durante 365 días y ya no administrará
tratamiento.
Pantalla de cumplimiento: muestra un porcentaje de
cumplimiento que se calcula con el número de días completos
de tratamientos finalizados entre la cantidad de días de
tratamiento disponibles. Los días de tratamiento disponibles
comienzan una vez que el dispositivo se le entrega al paciente
y se establece un minuto de tiempo de tratamiento.
Pantalla de carga
Batería cargando: el símbolo de una batería llenándose de
forma repetitiva indica que el dispositivo se está cargando.
Tratamiento completo
Tratamiento diario prescrito completado
Spanish/Español
1-800-535-4492
Page 30

7
Transporte del dispositivo
El dispositivo CervicalStim puede llevarse puesto sobre un aparato ortopédico,
collarín cervical, halo o ropa. Un tratamiento adecuado no requiere un contacto
directo con el cuerpo. Sin embargo, para ser efectivo, el transductor debe
centrarse alrededor del lugar de la fusión. Los usuarios pueden doblar y darle
forma con cuidado el transductor de tratamiento para ajustarlo con mayor
comodidad alrededor del cuello.
El siguiente es el método sugerido para llevar el dispositivo CervicalStim:
1. Para ponerse el dispositivo CervicalStim, solo tiene que colocarse el
dispositivo por encima de la cabeza.
2. Para una mayor abertura, desprenda la pestaña de Velcro
®
situada cerca
de la unidad de control y colóquesela por encima de la cabeza.
3. Este dispositivo no necesita apretarse contra la parte trasera del cuello;
debe apoyarse cómodamente sobre los hombros.
Accesorios del dispositivo
El paciente dispone de un accesorio que es una aplicación móvil fácil de usar,
que permite al paciente controlar fácilmente el uso del dispositivo. Esta puede
descargarse en el smartphone del paciente.
Para mayor comodidad, está disponible un Comfort Collar como accesorio. Para
pedidos, póngase en contacto con Servicios al paciente al 800-535-4492 o 214937-2718.
1
2
3
Pestañas
de Velcro
Page 31

8
Utilización y cuidado del dispositivo
• El dispositivo CervicalStim debe utilizarse en un único paciente.
• El dispositivo CervicalStim es un dispositivo electrónico tecnológicamente
avanzado y deberá manipularse con cuidado. Si el dispositivo CervicalStim
se le cae o lo maneja de forma incorrecta, esto podría dañar el dispositivo,
y podría dejar de trabajar.
• Para un uso seguro, siga las instrucciones del fabricante cuando utilice el
dispositivo CervicalStim. Usted (el paciente) es el usuario previsto de este
dispositivo.
• El uso del dispositivo de cualquier otra forma podría tener efectos dañinos
o anular la garantía.
• El uso de accesorios que no sean los especificados puede aumentar las
emisiones o disminuir la inmunidad del dispositivo.
• Inspeccione el dispositivo antes de cada uso en busca de signos de
desgaste o deterioro.
• No utilice ni cargue el dispositivo si no parece estar en una situación apta,
muestra un error o deja de funcionar. Póngase en contacto con Atención al
paciente si se da alguno de estos casos.
• ADVERTENCIA: No modifique este equipo, ya que su uso podría dejar de
ser seguro. No intente abrir ni desarmar el dispositivo CervicalStim, ya que
no contiene ninguna pieza que el usuario pueda reparar.
• PRECAUCIÓN: PELIGRO DE ESTRANGULAMIENTO: mantenga el cable de la
fuente de alimentación alejado del alcance de los niños.
Cuidado y limpieza
Cuando limpie el dispositivo CervicalStim, siga estas instrucciones:
• ADVERTENCIA: No limpie el dispositivo durante el tratamiento o la carga.
• Limpie las superficies del dispositivo con un paño suave y húmedo
(humedézcalo solo con agua).
• NO esterilice el dispositivo CervicalStim.
• NO exponga el dispositivo CervicalStim a una humedad excesiva.
• NO utilice disolventes ni líquidos basados en alcohol (limpiadores
antibacterianos, geles antisépticos de manos, perfumes, etc.) para limpiar
el dispositivo CervicalStim.
Almacenamiento
Cuando mueva el dispositivo CervicalStim de un área de almacenamiento muy
fría a una muy caliente (como el coche), espere al menos una hora para usar o
cargar el dispositivo. El dispositivo necesita tiempo para volver a la temperatura
de funcionamiento.
Almacenamiento desempacado:
Intervalo de temperatura:
• De -25 a 5 °C.
• De 5 a 35 °C con una humedad relativa de hasta el 90 %, sin condensación.
• De 35 a 60 °C con una presión de vapor de agua de hasta 50 hPa.
Spanish/Español
Page 32

9
Almacenamiento, envío y transporte empacado:
Intervalo de temperatura: entre -40 y 60 °C.
• De 10 a 100 % de humedad relativa.
• Incluye condensación a presiones de entre 500 hPa y 1060 hPa.
Ambiente de funcionamiento:
Intervalo de temperatura: entre 5 y 40 °C.
• Del 15 al 90 % de humedad relativa sin condensación, pero sin requerir
una presión de vapor de agua mayor que 50 hPa.
• 700 a 1060 hPa.
El dispositivo CervicalStim está diseñado para una vida de almacenamiento de
doce meses más un año de uso.
Viajes
Cuando viaje por aire, le recomendamos que empaque el dispositivo
CervicalStim en el equipaje facturado. Si lo lleva en cabina, deberá apagarlo al
pasar por el equipo de control de seguridad, ya que podría resultar dañado.
Deberá llevar el manual de instrucciones del dispositivo CervicalStim con usted
para que el personal de seguridad identifique el dispositivo de forma rápida y
sencilla. No lleve ni utilice el dispositivo CervicalStim a bordo del avión.
Eliminación
Una vez que complete el tratamiento y un médico le indique que deje de
usarlo, deberá desechar el dispositivo de acuerdo con las ordenanzas o planes
de reciclaje locales. Póngase en contacto con las autoridades locales con el
fin de determinar el método adecuado para desecharlo, ya que este equipo
electrónico contiene una batería de ion de litio. También puede comunicarse
con Atención a pacientes de Orthofix para obtener información sobre el
reciclaje. El dispositivo CervicalStim debe utilizarse en un único paciente.
El dispositivo CervicalStim es un dispositivo médico de clase III (solo bajo
prescripción) que no se puede esterilizar ni utilizar para otra persona.
Deseche el dispositivo de forma adecuada para evitar lesiones.
NO deseche el dispositivo CervicalStim en un incinerador. Este
dispositivo contiene baterías de litio.
Mantenimiento
Si tiene preguntas sobre el dispositivo o necesita ayuda, llame al
+1 800-535-4492 (solo EE. UU.) o al +1 214-937-2718. No contiene piezas
que el usuario pueda reparar. Notifique a Orthofix sobre cualquier necesidad
de mantenimiento.
Page 33

10
Información clínica
Resumen de los datos clínicos
El dispositivo CervicalStim se estudió en humanos para evaluar su seguridad y
efectividad como tratamiento añadido a los cuidados rutinarios (tratamiento
coadyuvante) en pacientes de alto riesgo con una cirugía de fusión cervical
para enfermedades degenerativas. Los pacientes eran de alto riesgo si eran
fumadores (un paquete al día o más) o si presentaban una cirugía de fusión
multinivel (más de un nivel).
Los 323 pacientes se asignaron aleatoriamente a uno de dos grupos: el
grupo de control (solo cuidados rutinarios) o el grupo de tratamiento
(dispositivo CervicalStim + cuidados rutinarios). Se asignaron ciento sesenta
(160) pacientes al grupo de control y 163 pacientes al grupo del dispositivo
CervicalStim. Los pacientes llevaron puesto el dispositivo CervicalStim durante
4 horas al día en sesiones de 4 horas continuas o de una hora.
La seguridad y la efectividad se evaluaron midiendo lo siguiente:
• tasa y gravedad de los eventos adversos
• tasa de fusión cervical a los seis meses de la cirugía según se determinó
por medio de rayos X
El ochenta y cuatro por ciento (84 %) del grupo del dispositivo CervicalStim
presentó fusión a los seis meses (102/122 pacientes) frente a tan solo el 69 %
del grupo de control (81/118 pacientes). Esto supone una diferencia del 15 %
entre estos dos grupos y es estadísticamente significativo; p = 0.0065. Esto
significa que existen más pacientes fusionados en el grupo del dispositivo
CervicalStim que en el grupo de control.
Ensayo clínico: Tasa total de éxito
84 %
69 %
% de pacientes fusionados
Tratado Sin tratar
Spanish/Español
Page 34

11
La tasa de pacientes que volvieron para las exploraciones y las radiografías a
los seis meses fue del 74 % en el grupo del dispositivo CervicalStim y del 73 %
en el grupo de control. Los pacientes que no volvieron para exploraciones
programadas no se pudieron evaluar, por lo que se desconoce su éxito
o fracaso. Estos datos no disponibles podrían tener un efecto positivo o
negativo en el éxito general de este estudio.
Ciento doce (112) pacientes reportaron un total de 157 eventos adversos
(negativos) en ambos grupos combinados a los seis meses de la intervención.
No hubo una diferencia significativa en la cantidad total de eventos adversos
o en la cantidad de pacientes que reportaron efectos en el grupo de grupo
de control y en el grupo del dispositivo CervicalStim, ni en la cantidad
de pacientes de cada grupo que experimentaron eventos adversos. Los
eventos adversos que podrían experimentarse incluyen un aumento en el
dolor, entumecimiento y hormigueo, dolor de cabeza, migrañas y náuseas.
Estos efectos podrían estar o no directamente relacionados con el uso del
dispositivo CervicalStim.
El éxito clínico con respecto a los síntomas se evaluó mediante lo siguiente:
• ausencia de empeoramiento de la función neurológica
• mejoría en el dolor
• ausencia de empeoramiento del índice de discapacidad del cuello
De acuerdo con los criterios anteriores, no se produjo una diferencia
importante en el éxito clínico entre el grupo de control y el grupo del
dispositivo CervicalStim. Una cantidad igual de pacientes en ambos grupos
mostró una mejoría en su situación clínica después de la intervención, sin
importar el tratamiento.
La información de las radiografías a largo plazo recolectadas a los 11 meses
de la cirugía o después no mostró una diferencia significativa en la tasa de
fusión entre el grupo de tratamiento del dispositivo CervicalStim y el grupo de
control, que recibió solo cuidados rutinarios.
Los resultados de este estudio muestran que el uso del dispositivo CervicalStim
es seguro y eficaz para aumentar la frecuencia de la fusión a los seis meses de
la cirugía en sujetos de alto riesgo con una fusión cervical.
Page 35

12
Eventos adversos comunicados a los 6 meses por grupo de tratamiento
Grupo de control (n = 160) Grupo del dispositivo CervicalStim (n = 163)
1
% expresado como la cantidad de pacientes que experimentaron el evento / la cantidad total de pacientes del grupo.
2
Algunos pacientes experimentaron múltiples eventos adversos.
*
Se produjeron varios eventos adversos que se observaron con mayor frecuencia en el grupo del dispositivo
CervicalStim que en el grupo de control. Debido a los tipos de eventos, es poco probable que estos eventos
adversos estén relacionados con el tratamiento.
Grupo de control
(n = 160)
Grupo del dispositivo
CervicalStim
(n = 163)
Eventos adversos Cantidad
(%) de
eventos
Cantidad
(%)
1
de
pacientes que
experimentan
el evento
Cantidad
*
(%) de
eventos
Cantidad
(%) 1 de
pacientes que
experimentó
el evento
Aumento del dolor de cuello 10 (14.9) 9(5.6) 16(17.8) 15(9.2)
Dolor de hombros o brazos 10(14.9) 9(5.6) 16(17.8) 16(9.8)
Lesión reincidente de la columna cervical 10(14.9) 8(5.0) 9(10.0) 9(5.5)
Patología de nivel adyacente 3(4.5) 3(1.9) 8(8.8) 8(4.9)
Complicaciones quirúrgicas 2(3.0) 2(1.3) 7(7.7) 5(3.1)
DEB/patología lumbar 8(11.9) 8(5.0) 5(5.5) 5(3.1)
Traumatismo o lesión (no cervical) 2(3.0) 2(1.3) 5(5.5) 4(2.5)
Entumecimiento u hormigueo 6(8.9) 6(3.8) 4(4.4) 4(2.5)
Dolor de cabeza o migraña 2(3.0) 2(1.3) 4(4.4) 4(2.5)
Dolor no específico o no relacionado 2(3.0) 2(1.3) 3(3.3) 3(1.8)
Náuseas 0 0 2(2.2) 2(1.2)
Mareo o vértigo 2(3.0) 2(1.3) 1(1.1) 1(0.6)
Exantema o decoloración 0 0 1(1.1) 1(0.6)
Latidos rápidos o irregulares 0 0 1(1.1) 1(0.6)
Falta de aliento 0 0 1(1.1) 1(0.6)
Acúfenos 0 0 1(1.1) 1(0.6)
Síntoma neurológico o accidente
cerebrovascular
1(1.5) 1(0.6) 1(1.1) 1(0.6)
Nódulo en la garganta 0 0 1(1.1) 1(0.6)
Diagnóstico de diabetes 0 0 1(1.1) 1(0.6)
Diagnóstico de cáncer de mama 0 0 1(1.1) 1(0.6)
Convulsión 0 0 1(1.1) 1(0.6)
Muerte, no relacionada 0 0 1(1.1) 1(0.6)
Sensibilidad 1(1.5) 1(0.6) 0 0
Tornillo roto 1(1.5) 1(0.6) 0 0
Colapso del injerto 1(1.5) 1(0.6) 0 0
Síndrome del túnel carpiano 2(3.0) 2(1.3) 0 0
Sensación de asfixia 1(1.5) 1(0.6) 0 0
Síntomas cardíacos 1(1.5) 1(0.6) 0 0
Síndrome nefrótico 1(1.5) 1(0.6) 0 0
Intento de suicidio 1(1.5) 1(0.6) 0 0
TOTAL 67 47
2
90 58
2
Spanish/Español
Page 36

13
Clasificación del equipo
Descripciones de los símbolos del dispositivo
Símbolo Significado
Ubicación del
símbolo
Atención: consulte el manual de
instrucciones
Dispositivo y caja del
dispositivo
Parte aplicada tipo BF
Dispositivo y caja del
dispositivo
Encendido/Apagado Dispositivo
Solo con receta médica Dispositivo
Intervalo de temperatura de
almacenamiento
Caja del dispositivo
Año de fabricación del dispositivo activo
Dispositivo y caja del
dispositivo
Fabricante Manual de instrucciones
No debe desecharse en la basura general
Dispositivo y caja del
dispositivo
Manténgase seco
Dispositivo y caja del
dispositivo
Marca FCC
Dispositivo y caja del
dispositivo
Marca CE
Dispositivo y caja del
dispositivo
10%
100%
Límites de humedad de almacenamiento
Dispositivo y caja del
dispositivo
1060
500
Límites de presión atmosférica Caja del dispositivo
Representante autorizado en la UE Manual de instrucciones
REF
Número de catálogo
Dispositivo y caja del
dispositivo
SN
Número de serie
Dispositivo y caja del
dispositivo
RCM - Marca de cumplimiento
normativo (Australia)
Dispositivo
Page 37

14
Clasificaciones del dispositivo CervicalStim
• Nombre de la familia de productos: Dispositivo de CEMP Orthofix
• Equipo alimentado internamente. La vida útil de la batería de ion de litio no
reemplazable es de 2.5 años.
• Este dispositivo genera un campo electromagnético pulsado no ionizante
con una intensidad de aproximadamente 2 gauss y componentes de
frecuencia en el intervalo 1 Hz-50 KHz. Este campo se distribuye dentro y
cerca del transductor de tratamiento.
• Pieza aplicada tipo BF. La parte aplicada es el transductor de tratamiento
con la unidad de control integrada.
• Grado de protección de la IEC 60529: IP22. IP22 significa que la carcasa
proporciona una protección frente a objetos sólidos de más de 12.5 mm y
el goteo de líquidos cuando se inclina 15° con respecto al uso normal. Se
recomienda mantener seca la unidad.
• Modo de funcionamiento: funcionamiento intermitente
• Este dispositivo no es estéril. No requiere esterilización.
• Vida útil del equipo: 1 año
• El equipo no debe utilizarse en presencia de una mezcla anestésica
inflamable con aire u óxido nitroso.
• La fuente de alimentación se considera doblemente aislada con una
construcción clase II en toda la fuente.
• Clasificaciones de la fuente de alimentación:
Orthofix n.º 20110412:
Entrada: 100-240 V CA, 50-60 Hz,
200 mA
Voltaje de salida: 5 V CC, 1.3 A
Orthofix n.º 20123808: Orthofix n.º WR9QA1200U23KIT(R6B):
Entrada: 100-240VAC, 50-60Hz, Entrada: 100-240VAC, 50-60Hz, 0.6A
0.6-0.3A
Voltaje de salida: 5VDC, 1.2A Voltaje de salida: 5VDC, 1.2A
Declaraciones de cumplimiento
Este dispositivo cumple con la sección 15 de las normas de la FCC. El
funcionamiento está sujeto a las dos condiciones siguientes: (1) Este dispositivo
no provocará interferencias perjudiciales y (2) este dispositivo debe aceptar
cualquier interferencia recibida, incluidas las interferencias que podrían provocar
un funcionamiento indeseable.
¡IMPORTANTE! Los cambios o las modificaciones no aprobadas expresamente
por Orthofix, Inc. podrían anular la autoridad del usuario para utilizar el equipo.
Orthofix n.º 20114794:
Entrada: 100-240 V CA, 50-60 Hz,
150-350 mA
Voltaje de salida: 5 V CC, 2.4 A
Spanish/Español
Page 38

15
NOTA: este equipo fue probado y se declaró que cumple con los límites de
los dispositivos digitales de clase B, de conformidad con la sección 15 de
las normas de la FCC. Estos límites están diseñados para proporcionar una
protección razonable contra las interferencias perjudiciales en instalaciones
residenciales. Este equipo genera, utiliza y puede irradiar energía de RF y,
si no se instala y utiliza de acuerdo con las instrucciones, podría producir
interferencias perjudiciales en las comunicaciones por radio. Sin embargo, no
existen garantías de que no se produzcan interferencias en una instalación
específica.
Si este equipo causa interferencias perjudiciales en la recepción de radio o
televisión, las cuales pueden determinarse apagando y encendiendo el equipo,
el usuario deberá intentar corregir la interferencia mediante una o más de las
siguientes medidas:
• Reoriente o reubique la antena receptora.
• Aumente la separación entre el equipo y el receptor.
• Conecte el equipo a un tomacorriente de un circuito diferente al circuito
donde está conectado el receptor.
• Consulte al proveedor o a un técnico de radio/TV con experiencia para
obtener ayuda.
Información sobre inmunidad y compatibilidad electromagnéticas
El dispositivo CervicalStim cumple con la IEC 60601-1-2 sobre compatibilidad
electromagnética (CEM). El dispositivo CervicalStim
necesita la aplicación de
precauciones especiales con respecto a la CEM y debe utilizarse de acuerdo
con la información de CEM proporcionada en este manual. Los equipos de
comunicaciones inalámbricas, como los dispositivos de redes residenciales,
los teléfonos celulares, los teléfonos inalámbricos y sus estaciones base, y los
walkie-talkies pueden afectar al dispositivo CervicalStim. Estos tipos de equipos
deberán mantenerse a un mínimo de 0.198 m (7.8 pulg.) del dispositivo
CervicalStim.
El uso del dispositivo CervicalStim con dispositivos médicos electrónicos
implantables específicos no ha sido evaluado. Consulte a su médico antes
de utilizar el dispositivo CervicalStim con dispositivos médicos electrónicos
implantables.
Page 39

16
Garantía
Orthofix Inc. garantiza que el dispositivo CervicalStim no presentará defectos
en los materiales ni en la mano de obra durante un año a partir de la
fecha del primer uso. Siempre y cuando se cumplan todos los términos y
condiciones de esta garantía limitada, Orthofix Inc. sustituirá los componentes
defectuosos.
Esta garantía limitada se aplica al producto solo en caso de un uso normal y
no cubre ningún daño o defecto provocado por accidentes, uso incorrecto,
uso indebido, incendios, inundaciones y fuerza mayor, ni ninguna alteración,
falsificación, reparación o intento de reparación por parte de alguien ajeno a
Orthofix Inc. Esta garantía solo se aplica al paciente para el que se recetó el
producto y no puede asignarse ni transferirse.
Los productos defectuosos cubiertos por esta garantía limitada deberán
devolverse a Orthofix Inc. a la atención de: Orthofix Returns. Deberá llamar
a un representante de Atención a pacientes o a su distribuidor local para
obtener el número de autorización de la devolución y la dirección antes de
devolver el producto.
Excepto que las leyes correspondientes lo requieran específicamente, la
garantía anterior sustituye cualquier otra garantía, expresa o implícita,
y Orthofix Inc. renuncia específicamente a todas las garantías de
comerciabilidad o adecuación para fines específicos. En ningún caso, Orthofix
Inc., su representante autorizado, filiales o empresas subsidiarias serán
responsables de los daños especiales, consecuentes o incidentales. El único
remedio con respecto a cualquier producto defectuoso estará limitado
al reemplazo.
Esta garantía limitada no se ampliará ni modificará, excepto mediante
un escrito de Orthofix Inc. Ningún vendedor, representante, distribuidor
ni médico está autorizado a realizar o aceptar ninguna ampliación o
modificación de los términos de esta garantía limitada.
Para obtener información adicional o ayuda con el dispositivo, comuníquese
con Atención a pacientes de Orthofix en el +1 800-535-4492 o al
+1 214-937-2718.
Spanish/Español
Page 40

Orthofix Inc.
3451 Plano Parkway
Lewisville, Texas 75056
Tel 214-937-2718
Patient Services
800-535-4492 toll free
orthofix.com
BoneGrowthTherapy.com
P/N 20115188 Rev.AD 2018-09-05
CS-1804 PL-US © Orthofix Holdings, Inc.
Caution: Federal law (USA) restricts this device to sale
by or on the order of a physician.
 Loading...
Loading...