Page 1
©2015LifeScan, Inc.
LifeScan Europe
Division of Cilag
GmbH International
Gubelstrasse 34
6300 Zug
Switzerland
Distributed by:
LifeScan UK & Ireland,
A division of Johnson & Johnson
Medical Limited,
50-100 Holmers Farm Way,
High Wycombe,
Bucks,
HP12 4DP
United Kingdom
Call OneTouch® Customer
Care on 0800121200 (UK)
or 1800535 676 (Ireland)
during the hours of
8:30am-6pm Monday-Friday,
9am-1pm Saturday. Or visit
www.LifeScan.co.uk.
C4
Contents covered by one or more of the following U.S. patents: 5,708,247,
5,951,836, 6,241,862, 6,284,125, 7,112,265, 7,462,265, 7,807,031, and 8,398,664. Use
of the monitoring device included herein is protected under one or more of the
following U.S. patents: 6,413,410, 6,733,655, 7,250,105, 7,468,125, 8,066,866 and
8,093,903. Purchase of this device does not act to grant a use license under these
patents. Such a license is granted only when the device is used with OneTouch®
Select® Plus Test Strip. No test strip supplier other than LifeScan is authorized to
grant such a license. The accuracy of results generated with LifeScan meters using
test strips manufactured by anyone other than LifeScan has not been evaluated
by LifeScan.
Blood Glucose Meter, Test Strips, and Control Solution
Lancing System
LifeScan self-test blood glucose monitoring devices conform to
the following EU Directives:
IVDD (98/79/EC):
MDD (93/42/EEC):
PF3130451 Rev 1
Rev. Date: 01/2015
PF3130451 Rev 1
Yellow = 5mm margin, Red = 10mm margin
Yellow = 5mm margin, Red = 10mm margin
PF3130451Rev1_OTSPF_OB_C_GB_en_zug_R2.indd 4 3/12/15 11:27 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 2

Blood Glucose Monitoring System
Instructions
for Use
Owner's
Booklet
C1
Yellow = 5mm ma
rgi
n, Red = 10mm margin
Yel low = 5mm
ma
rgi
rgi
n,
n,
n,
n,
R
Red
= 10
10m
10m
m m
arg
PF3130451Rev1_OTSPF_OB_C_GB_en_zug_R2.indd 1 3/12/15 11:27 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 3
C2
Yellow = 5mm margin, Red = 10mm margin
Yellow = 5mm margin, Red = 10mm margin
PF3130451Rev1_OTSPF_OB_C_GB_en_zug_R2.indd 2 3/12/15 11:27 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 4
C3
Yellow = 5mm margin, Red = 10mm margin
Yellow = 5mm margin, Red = 10mm margin
PF3130451Rev1_OTSPF_OB_C_GB_en_zug_R2.indd 3 3/12/15 11:27 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 5

PF No./Rev.: 3130451 Rev 1
CPS Reference No.: Refer to References Section in Enable for CPS No.
LFS Contact: Andrew Mark
Rev. Date: 12-Mar-15
Language Sequence: English
JDE Item No.: N/A
No. of Covers: 4
PMS N/A N/A
Inside No. of pages:
No. of sides: 1 2
Process Colors
Uncoated Area
Spot Colors Uncoated Area Special Instructions Dieline
N/A
Dieline
Description: Booklet, Owners, OTSPF, GB (zug)
Art Agency: ForeignExchange Translations Job No.: 28801
= CUT
= SCORE
= PERF
N/A
N/A
N/A
C M Y K
Black
N/A
Leg1
PF3130451Rev1_OTSPF_OB_C_GB_en_zug_R2.indd 1 3/12/15 11:27 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 6
1
Owner's Booklet
Blood Glucose Monitoring System
Select Plus Flex
™
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 1 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 7
2
Thanks for choosing OneTouch®!
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is one of the latest product innovations from
OneTouch®.
Your OneTouchSelect Plus Flex™ Meter is designed to
connect (sync) with a variety of devices running software
applications that let you review and graph your results,
and help identify patterns. Meter results are sent to the
compatible devices either through BLUETOOTH® SMART
(wireless) or USB cable connection.
Every OneTouch® Meter is designed to help you test your
blood glucose and manage your diabetes.
This Owner's Booklet offers a complete explanation of how
to use your new meter and testing supplies. It reviews the
do's and don'ts of testing your blood glucose level. Please
keep your Owner's Booklet in a safe place; you may want
to refer to it in the future.
We hope OneTouch® products and services will continue to
be a part of your life.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 2 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 8
3
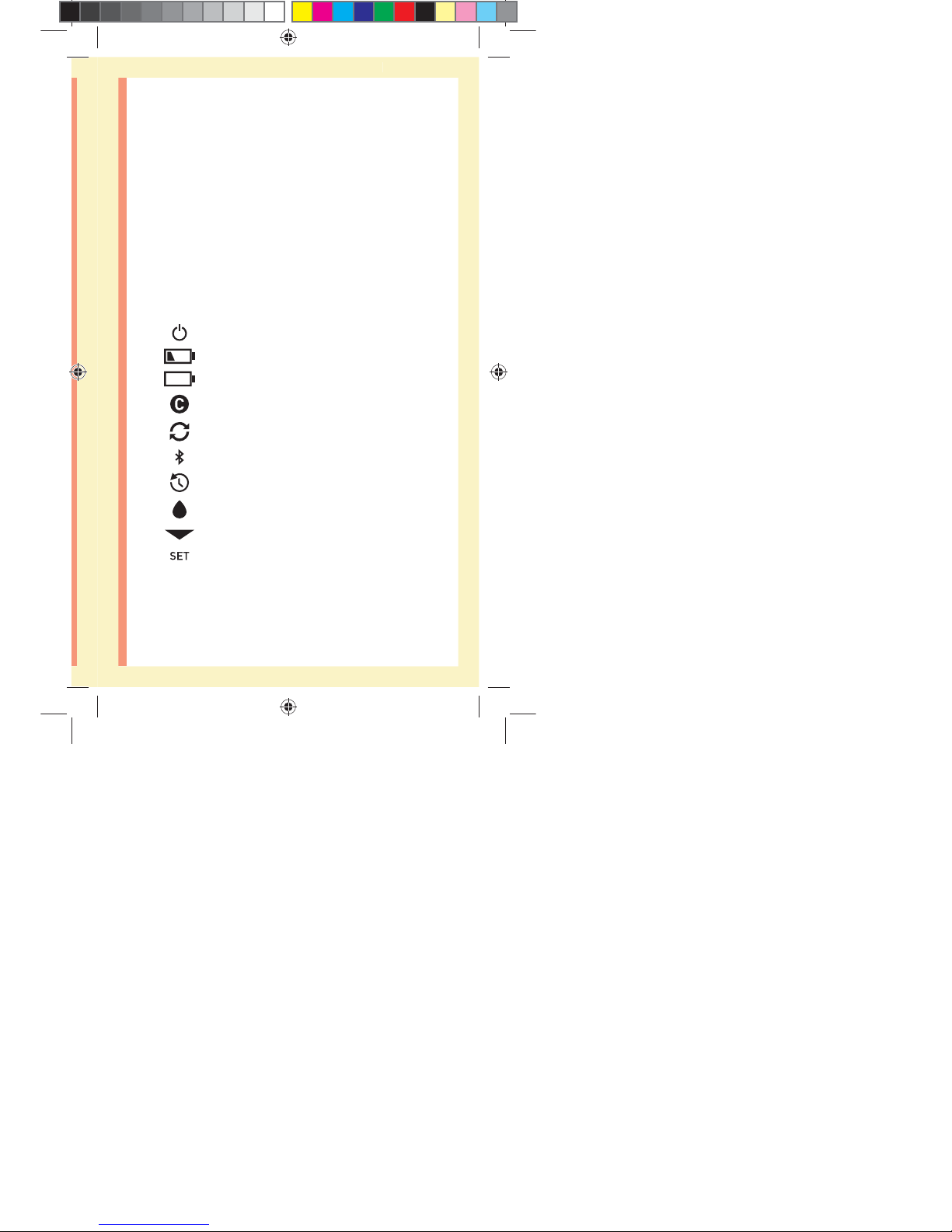
Meter symbols and icons
Meter Power
Low Battery
Battery Empty
Control Solution
Syncing
BLUETOOTH® SMART Feature On
History Mode (Past results)
Apply Sample
Range Indicator Arrow
Setting Mode
Compatible Wireless Devices
Visit www.LifeScan.co.uk for information on which wireless
devices are compatible with your OneTouchSelect Plus
Flex™ Meter, and where/how to download the software
application on your compatible wireless device.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 3 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 9
4
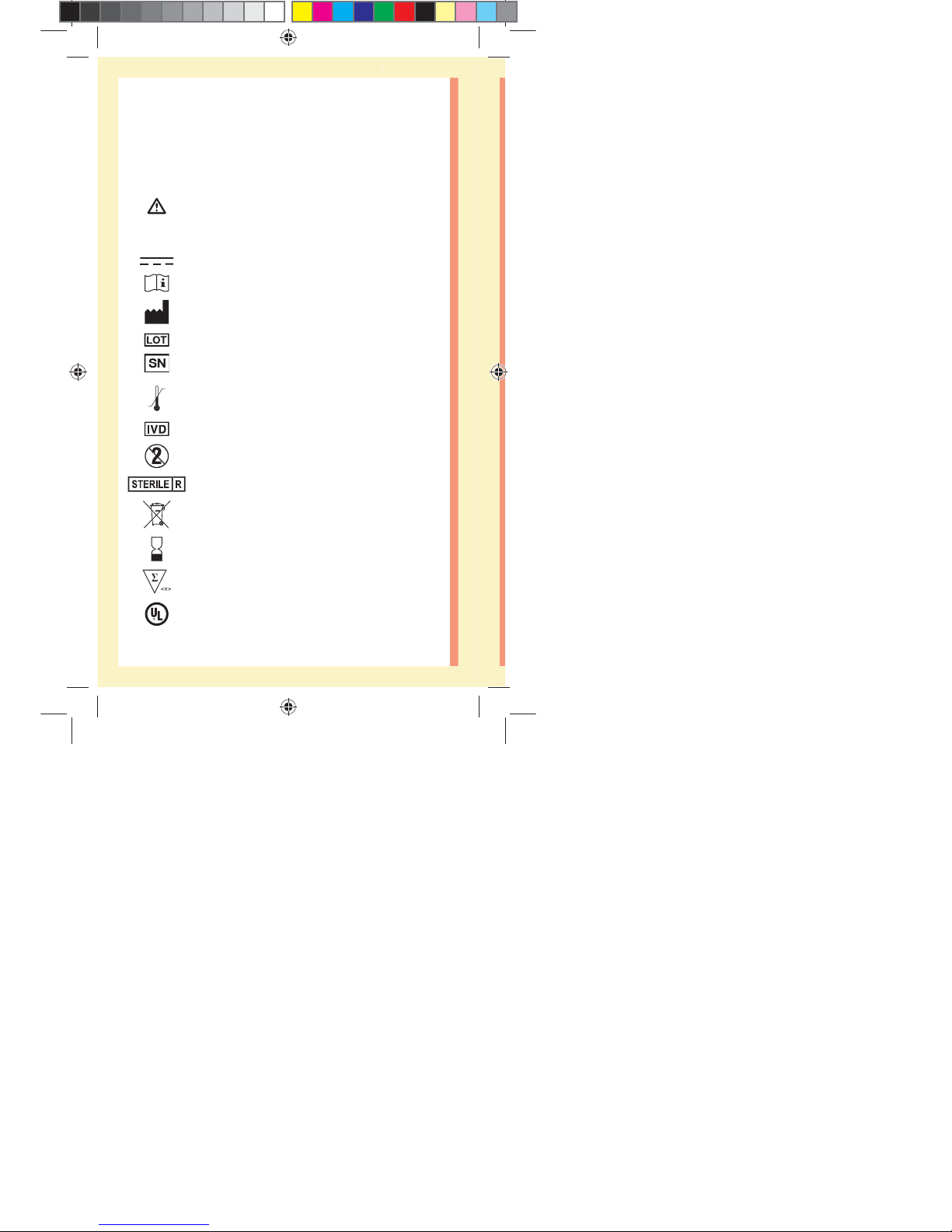
Other symbols and icons
Cautions and Warnings: Refer to the Owner's
Booklet and inserts that came with your system
for safety-related information.
Direct current
Consult Instructions for Use
Manufacturer
Lot Number
Serial Number
Storage Temperature Limits
In Vitro Diagnostic Device
Do Not Re-use
Sterilized by irradiation
Not for general waste
Use By Date
Contains sucient for n tests
Underwriters Laboratories certification
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 4 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 10
5
Before you begin
Before using this product to test your blood glucose,
carefully read this Owner's Booklet, and the inserts
that come with the OneTouchSelect® Plus Test Strips,
OneTouchSelect® Plus Control Solutions and the
OneTouch®Delica® Lancing Device.
IMPORTANT SAFETY INSTRUCTIONS:
đƫ This meter and lancing device are for single patient use
only. Do Not share them with anyone else, including
family members! Do Not use on multiple patients!
đƫ After use and exposure to blood, all parts of this kit are
considered biohazardous. A used kit may potentially
transmit infectious diseases even after you have
performed cleaning and disinfection.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 5 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 11
6
Intended use
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is intended to be used for the quantitative
measurement of glucose (sugar) in fresh capillary whole
blood samples drawn from the fingertip. The system is
intended to be used by a single patient and should not be
shared.
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is intended for self-testing outside the body (in
vitro diagnostic use) by people with diabetes at home and
with their healthcare professionals in a clinical setting as
an aid to monitor the effectiveness of diabetes control.
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is not to be used for the diagnosis of or screening
of diabetes or for neonatal use.
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is not for use on critically ill patients, patients in
shock, dehydrated patients or hyperosmolar patients.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 6 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 12
7
Test principle
Glucose in the blood sample mixes with the enzyme
Glucose Oxidase (see page103) in the test strip and
a small electric current is produced. The strength of this
current changes with the amount of glucose in the blood
sample. Your meter measures the current, calculates your
blood glucose level, displays the result, and stores it in its
memory.
Use only OneTouchSelect® Plus Control Solutions and Test
Strips with the OneTouchSelect Plus Flex™ Meter.
BLUETOOTH® SMART wireless
technology
BLUETOOTH® SMART wireless technology is used by
some smartphones and many other devices. Your
OneTouchSelect Plus Flex™ Meter uses BLUETOOTH®
SMART wireless technology to pair and to send your
glucose results to compatible wireless devices.
The OneTouchSelect Plus Flex™ Meter is designed to work
with the OneTouchReveal® Mobile App and many other
diabetes applications on compatible wireless devices.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 7 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 13
8
NOTE: Some diabetes management apps, including the
OneTouchReveal® Mobile App, may not be available in
your country. Visit www.LifeScan.co.uk to learn if the
OneTouchReveal® Mobile App is available in your country.
Visit www.LifeScan.co.uk for information on which wireless
devices are compatible with your OneTouchSelect Plus
Flex™ Meter, and where/how to download the software
application on your compatible wireless device.
When using the OneTouchSelect Plus Flex™ System, we
suggest you pair your OneTouchSelect Plus Flex™ Meter
with a compatible wireless device and track your results.
See page29 for pairing instructions.
Your meter is subject to and complies with applicable
Worldwide Radio regulation guidelines. Generally, these
rules state two conditions specific to the operation of the
device:
1. This device may not cause harmful interference.
2. This device must accept any interference received,
including interference that may cause undesirable
operation.
LifeScan Europe declares that this equipment is in
compliance with the applicable essential requirements and
other relevant provisions of Directive 1999/5/EC.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 8 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 14
9
If you experience meter interference problems, try moving
your meter away from the source of the interference.
You can also move the electronic device or its antenna to
another location to solve the problem.
These guidelines help ensure that your meter will not
affect the operation of other nearby electronic devices.
Additionally, other electronic devices should not affect the
use of your meter.
WARNING: The BLUETOOTH® SMART feature on your
meter sends test results to your compatible wireless device.
To prevent other people's results from being sent to your
compatible wireless device, Do Not let anyone else use your
meter to test their blood glucose. This meter is for single
patient use only.
WARNING: In locations where cell phone use is not
permitted, such as hospitals, some healthcare professional
oces and airplanes, you should turn the BLUETOOTH®
SMART feature o. See page27 for more information.
BLUETOOTH® SMART trademark
The BLUETOOTH® SMART word mark and logos are owned
by the Bluetooth SIG, Inc. and any use of such marks by
LifeScan Europe is under license. Other trademarks and
trade names are those of their respective owners.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 9 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 15
10
Table of Contents
1Getting to know your system ...................................12
2Setting up your system ............................................22
Setting up your meter ..................................................... 22
Connecting to a compatible wireless device ............... 27
Turning the meter off ...................................................... 33
3Taking a test .............................................................. 34
Testing your blood glucose ............................................34
Testing with control solution..........................................62
4Reviewing past results ............................................ 72
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 10 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 16
11
5Editing Your Settings ............................................... 76
Editing time and date ..................................................... 76
Editing your range limits ................................................ 77
6Caring for your system ............................................80
7Battery .......................................................................84
8Troubleshooting your system .................................88
9Detailed information about your system ...........100
10Index ......................................................................... 114
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 11 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 17
12
1Getting to know your
system
Your OneTouchSelect Plus Flex™ Blood
Glucose Monitoring System
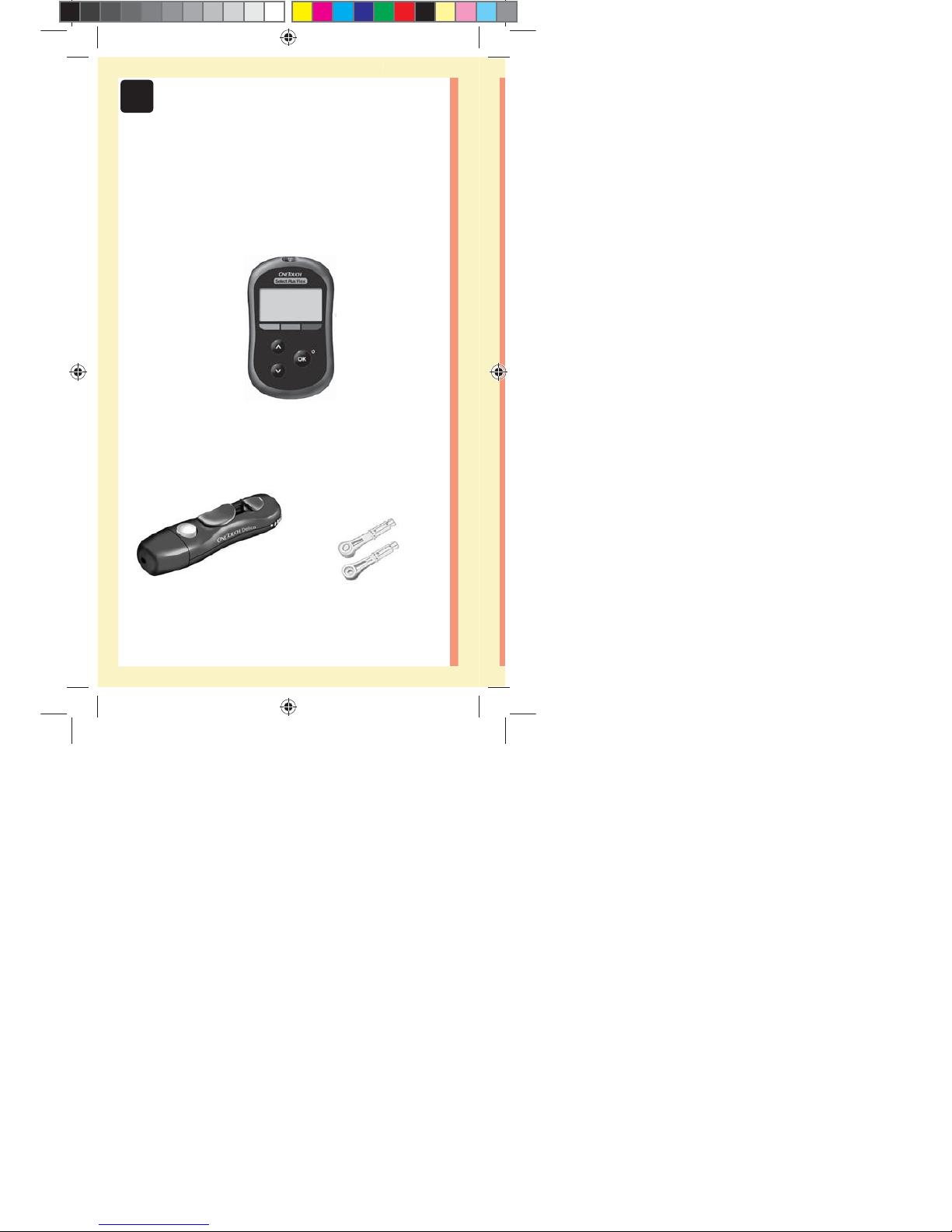
Included with your kit:
OneTouchSelect Plus Flex™
Meter (CR2032 lithium coin
cell battery included)
Lancing device Lancets
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 12 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 18
13
1
Getting to know your system
NOTE: If any item is missing or defective in your kit,
contact Customer Service. Call OneTouch® Customer Care
on 0800121200 (UK) or 1800535 676 (Ireland) during the
hours of 8:30am-6pm Monday-Friday, 9am-1pm Saturday.
Or visit www.LifeScan.co.uk.
NOTE: If another type of lancing device was included, see
the separate instructions for that lancing device.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 13 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 19
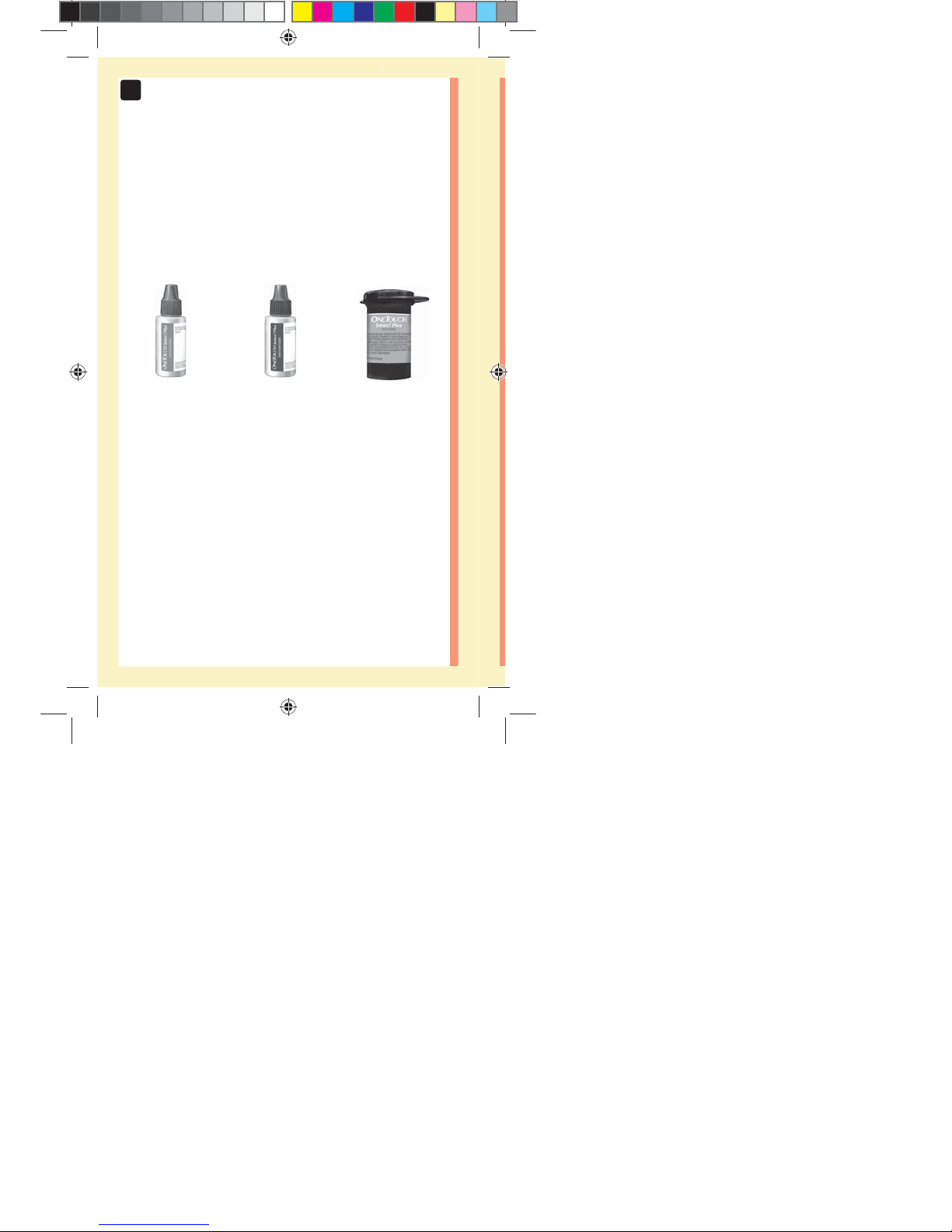
14
1
Getting to know your system
OneTouchSelect®
Plus Mid Control
Solution*
(vial with blue cap)
OneTouchSelect®
Plus High Control
Solution*
(vial with red cap)
*OneTouchSelect® Plus Control Solution and Test Strips
are available separately. For availability of test strips
and control solution, contact customer service or your
healthcare professional.
Available separately:
Items listed below are required, but may not be included
in your kit:
They are sold separately. Refer to your meter carton for a
list of included items.
OneTouchSelect®
Plus Test Strips*
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 14 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 20
15
1
Getting to know your system
OneTouchSelect® Plus High Control Solution (vial with
red cap) can be used in addition to OneTouchSelect®
Plus Mid Control Solution (vial with blue cap). If you test
with OneTouchSelect® Plus High Control Solution, you
should always test with OneTouchSelect® Plus Mid Control
Solution first.
WARNING: Keep the meter and testing supplies away
from young children. Small items such as the battery door,
batteries, test strips, lancets, protective covers on the
lancets, and control solution vial cap are choking hazards.
Do Not ingest or swallow any items.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 15 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 21

16
1
Getting to know your system
Meter
Getting to know your OneTouchSelect
Plus Flex™ Blood Glucose Monitoring
System
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 16 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 22

17
1
Getting to know your system
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 17 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 23
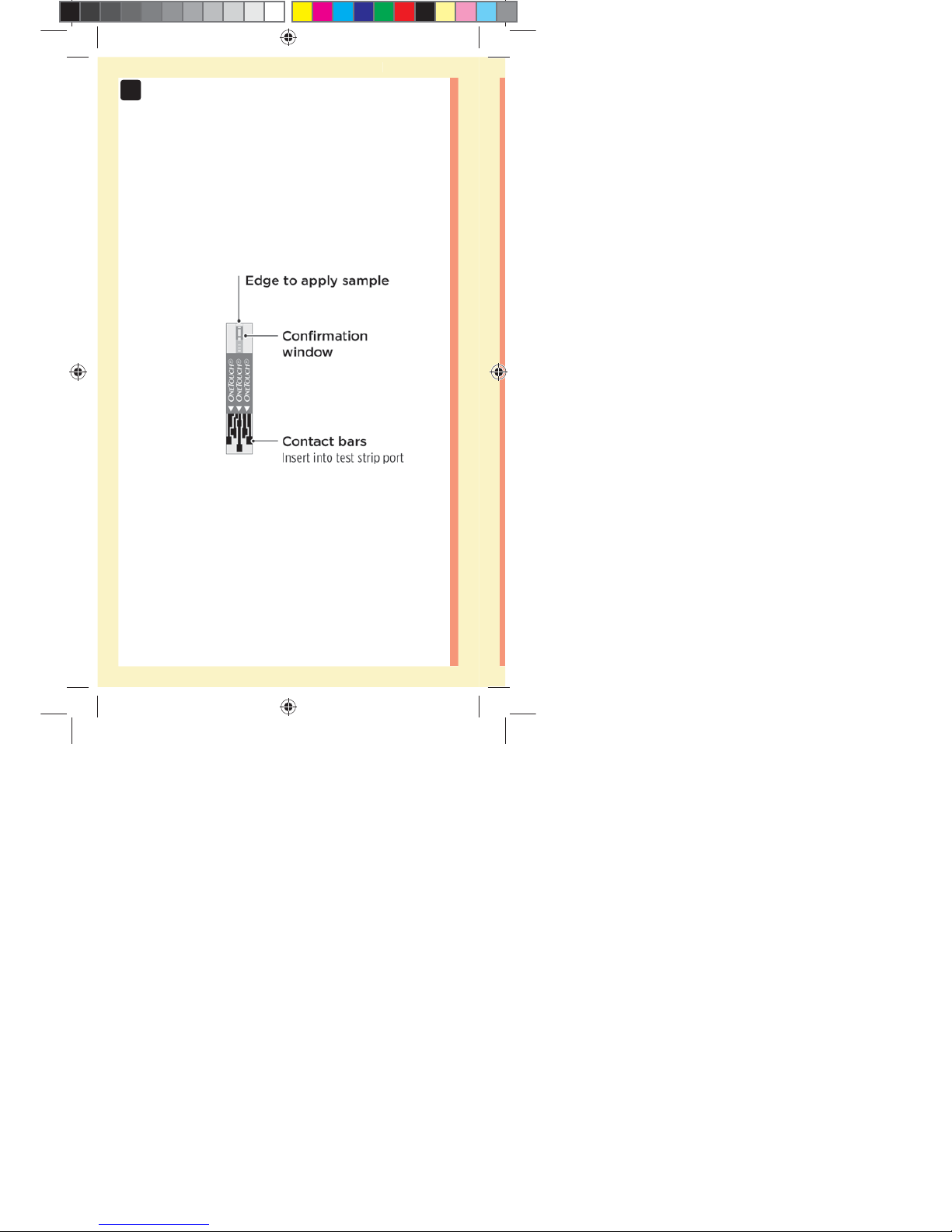
18
1
Getting to know your system
Test strip
Getting to know your OneTouchSelect®
Plus Test Strip
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 18 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 24

19
1
Getting to know your system
The Range Indicator feature
The OneTouchSelect Plus Flex™ Meter automatically
lets you know if your current result is below, above or
within your range limits. It does this by displaying your
current result with a Range Indicator Arrow, pointing to a
corresponding Range Indicator Color Bar below the meter
display. Use the Range Indicator Arrow and Color Bar
together to interpret your results.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 19 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 25
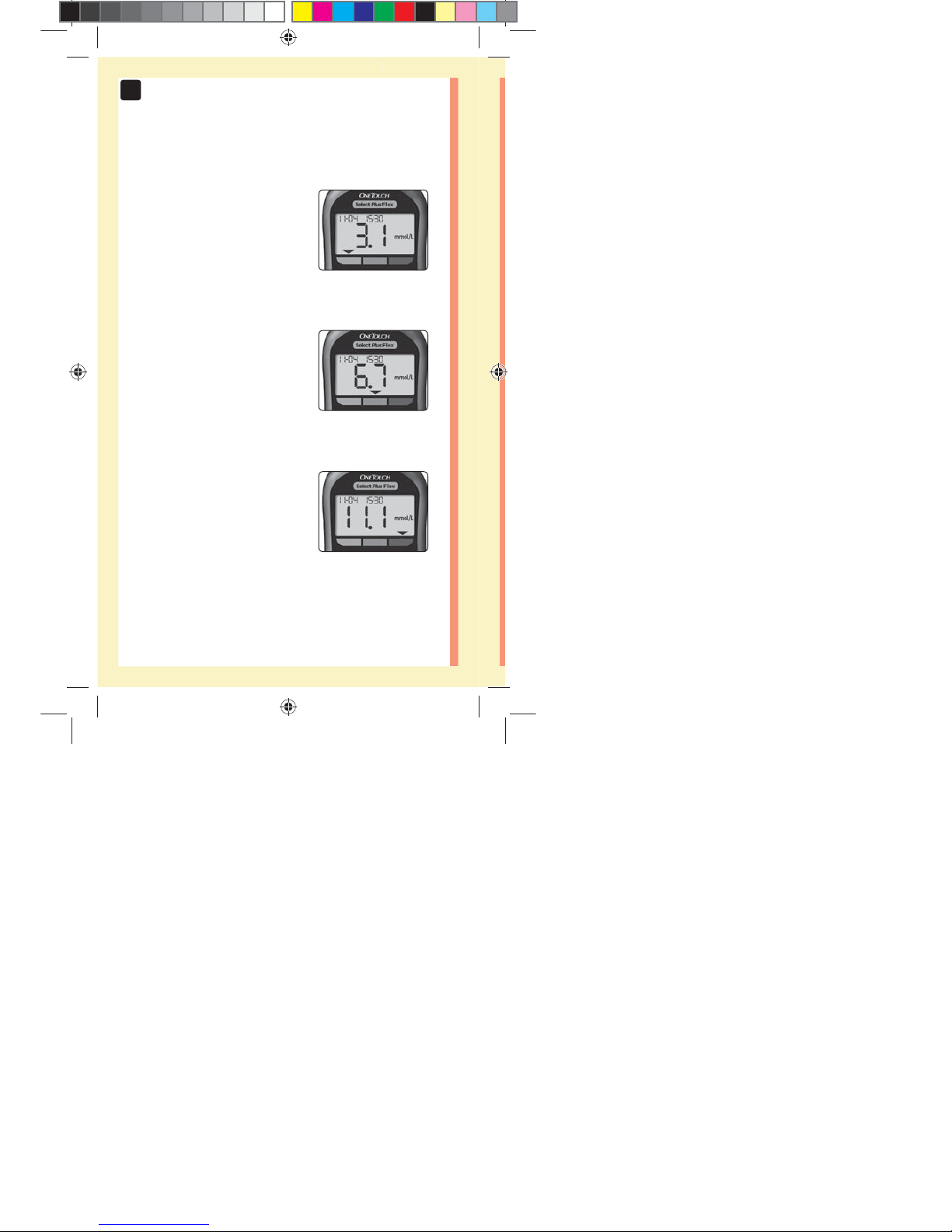
20
1
Getting to know your system
3 Possible Range Indicator Displays
A Range Indicator Arrow will
appear just below your result
after each test depending upon
how you set your low and high
range limits in the meter.
Things you should know before
using the Range Indicator feature:
đƫ The meter comes with pre-set
range limits. The pre-set low
range limit is 3.9mmol/L and
the pre-set high range limit is
10.0mmol/L. You can change
these limits as needed to meet
your needs. See page77 for
details on the pre-set range
limits and on editing your
range limits.
đƫ If you decide to change
your range limits, the Range
Indicator Arrows stored with
previous results in meter
memory will not change.
However, any new tests will
display Range Indicator Arrows
which reflect your changes.
Example
Below Range Result
Example
In Range Result
Example
Above Range Result
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 20 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 26
21
1
Getting to know your system
This page left blank intentionally.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 21 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 27

22
2Setting up your system
Setting up your meter
Turn your meter on
To turn your meter on, press
and hold until the start-up
test screen appears. Once the
device is on, release
. You
can also turn the meter on by
inserting a test strip.
CAUTION:
If you see any missing segments within the start-up
screen, there may be a problem with the meter. Contact
Customer Service. Contact OneTouch® Customer Care on
0800121200 (UK) or 1800535 676 (Ireland).
NOTE: If you turned the meter on for the first time by
inserting a test strip instead of pressing
, you will not
be able to perform a glucose test until you complete the
first time setup.
Every time you turn your meter on, a start-up screen will
appear for a few seconds. All segments of the display
should appear briefly, indicating your meter is working
properly. If the meter does not power on, check the
battery.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 22 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 28
23
2
Setting up your system
First time setup
To turn your meter on, press and hold
until the start-
up screen appears. Once the device is on, release
.
The meter will now automatically prompt you to set the
time and date. The SET icon will appear on the screen to
indicate that the meter has entered setup mode.
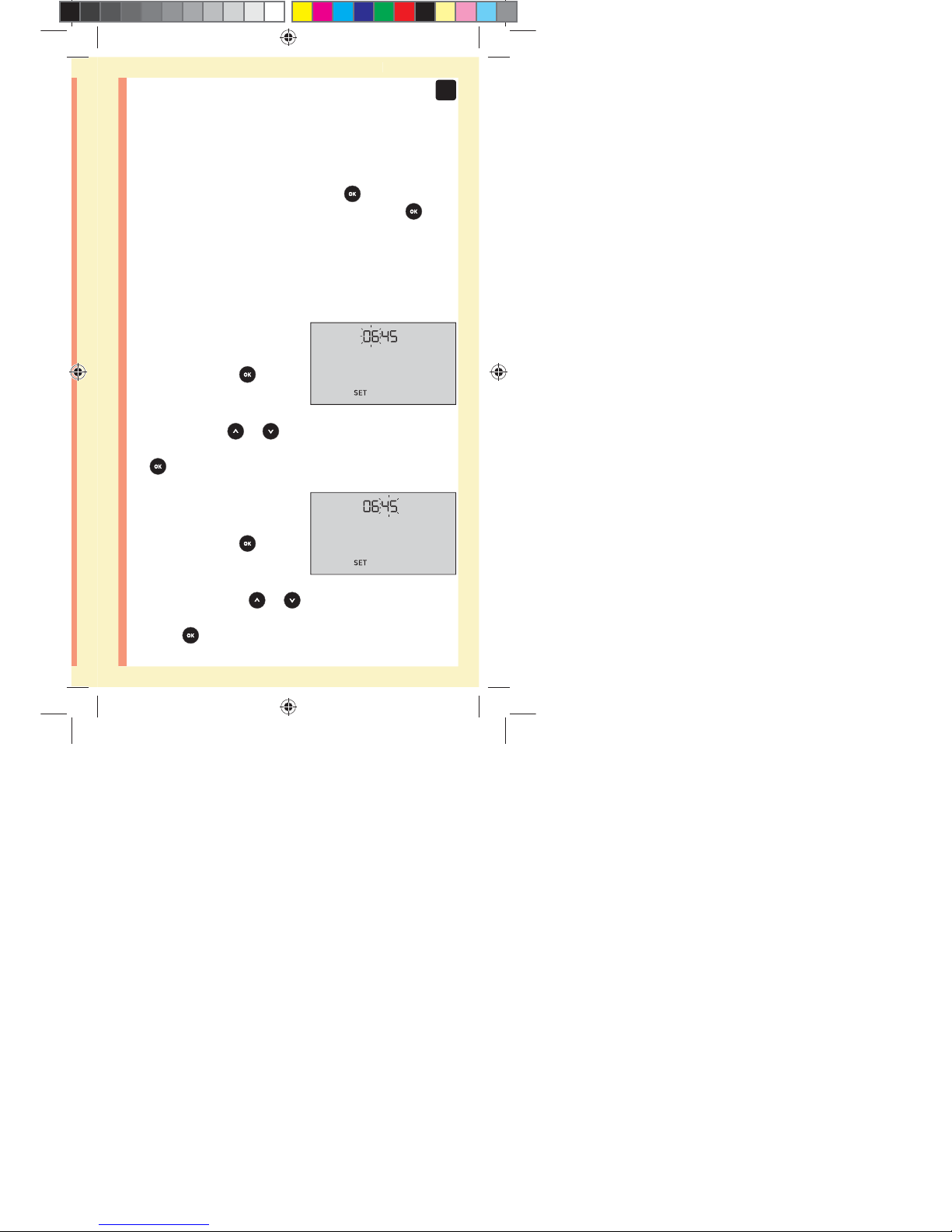
Setting the time
Hour will flash
đƫ If the hour displayed
is correct, press
to
confirm.
đƫ If the hour displayed is not
correct, press
or to
change the hour and press
to confirm.
Minutes will flash
đƫ If the minutes displayed
is correct, press
to
confirm.
đƫ If the minutes displayed is
not correct, press
or
to change the minutes and
press
to confirm.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 23 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 29

24
2
Setting up your system
Setting the date
After completing the time setup, the meter will
automatically move to date setup.
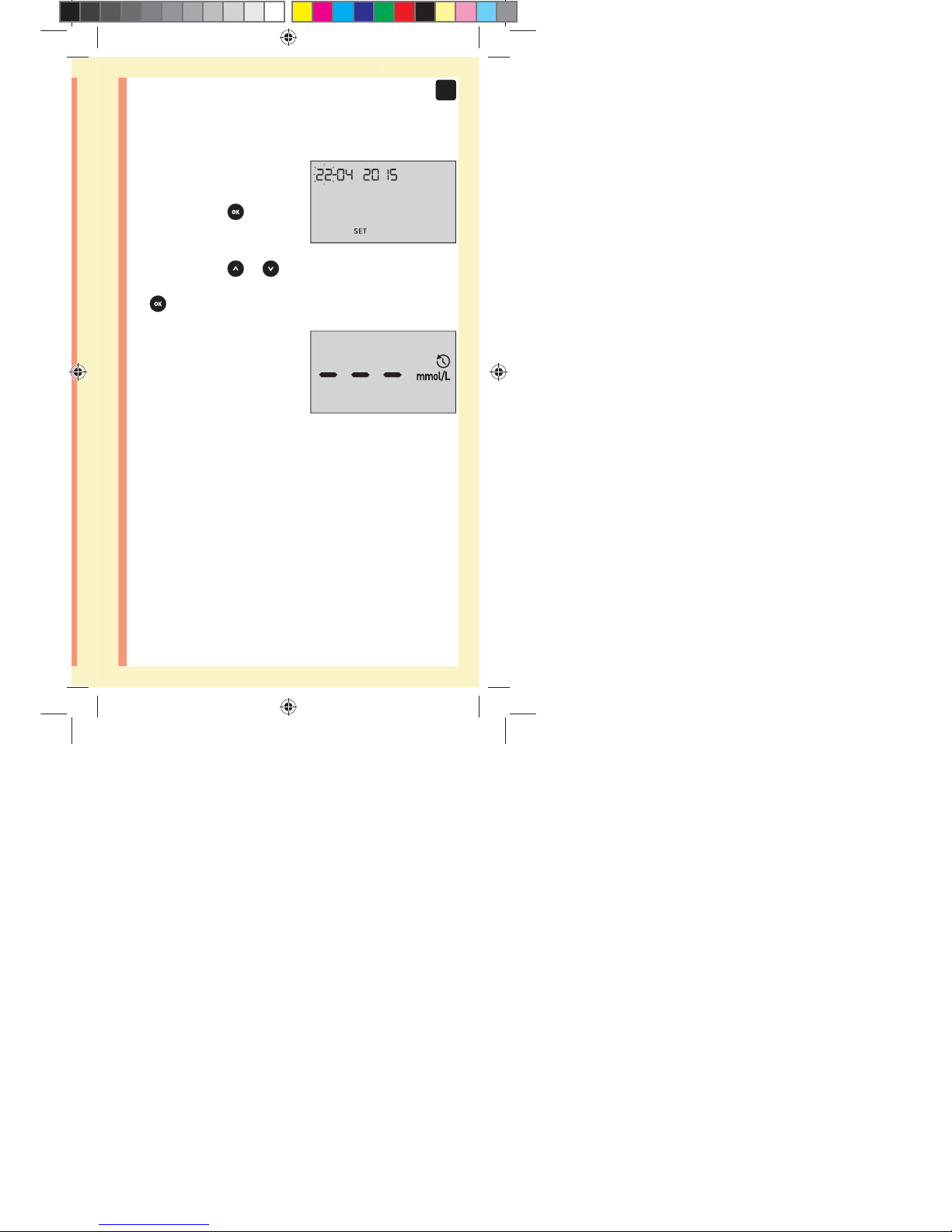
Year will flash
đƫ If the year displayed
is correct, press
to
confirm.
đƫ If the year displayed is not
correct, press
or to
change the year and press
to confirm.
Month will flash
đƫ If the month displayed
is correct, press
to
confirm.
đƫ If the month displayed is
not correct, press
or
to change the month
and press to confirm.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 24 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 30
25
2
Setting up your system
Day will flash
đƫ If the day displayed is
correct, press
to
confirm.
đƫ If the day displayed is not
correct, press
or to
change the day and press
to confirm.
You are now ready to take
a test. See the section
Testing your blood glucose
in Chapter 3.
NOTE: After completing first time setup, a screen with
three dashes will appear. Once you begin testing, your last
result will appear in place of the three dashes, along with
the date and time the test was taken.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 25 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 31

26
2
Setting up your system
Adjusting the time and date settings after
first time setup
You can adjust the meter's time and date settings after
first time setup. Press and hold
to turn the meter on,
then press and hold and at the same time. The SET
screen will appear. See page76.
After adjusting the settings, your meter will exit settings
mode and your last result screen will appear.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 26 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 32

27
2
Setting up your system
Connecting to a compatible wireless
device
Turning the BLUETOOTH® SMART feature
on or o
In order to connect your meter with your compatible
wireless device, the BLUETOOTH® SMART feature will
need to be turned on. The
symbol will appear on the
meter screen when the BLUETOOTH® SMART feature is
on. When the symbol is not present on the screen the
BLUETOOTH® SMART feature is off.
đƫ To turn the BLUETOOTH® SMART feature on press
and at the same time.
đƫ To turn the BLUETOOTH® SMART feature off press
and at the same time.
The
symbol indicates
the BLUETOOTH® SMART
feature is on
NOTE: The BLUETOOTH® SMART feature will turn OFF
during a blood glucose test.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 27 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 33

28
2
Setting up your system
Pairing Overview
Pairing allows your OneTouchSelect Plus Flex™ Meter
to communicate with compatible wireless devices. The
devices must be within 8meters of each other to pair and
sync. Download the OneTouchReveal® Mobile App from
the appropriate app store before pairing your meter and
compatible wireless device.
NOTE: Some diabetes management apps, including the
OneTouchReveal® Mobile App, may not be available in
your country. Visit www.LifeScan.co.uk to learn if the
OneTouchReveal® Mobile App is available in your country.
Multiple OneTouchSelect Plus Flex™ Meters can be paired
with your compatible wireless device. For example, your
compatible wireless device can be paired with a meter at
home and another at work. To pair multiple meters, repeat
the pairing instructions for each meter. See page29 for
pairing instructions.
Your OneTouchSelect Plus Flex™ Meter can be paired
with multiple compatible wireless devices. To pair
multiple compatible wireless devices, repeat the pairing
instructions for each compatible wireless device.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 28 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 34

29
2
Setting up your system
Pairing Instructions
1. Start by turning your meter on using the
button
2. The BLUETOOTH® SMART feature is turned on by
pressing
and together
The
symbol will appear to
indicate that the BLUETOOTH®
SMART feature is on.
3. Open the OneTouchReveal® Mobile App and follow
instructions to pair meter with your compatible
wireless device
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 29 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 35

30
2
Setting up your system
4. Look for "OneTouch" and the last 4 characters of the
meter serial number on the compatible wireless device
display to correctly identify your meter
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 30 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 36

31
2
Setting up your system
5. When prompted by the OneTouchReveal® Mobile App,
the meter will display a six digit PIN number
Enter the PIN number into your wireless compatible device
using the keypad on your compatible wireless device.
Example of PIN number
display on meter
CAUTION:
Make sure the PIN you enter on your compatible device
matches the PIN on your meter display. If a PIN number
unexpectedly appears on your meter display, cancel the
PIN request by either inserting a test strip to take a test or
press the
button to enter History Mode.
6. Wait for your compatible wireless device to indicate
that your meter and compatible wireless device are
paired
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 31 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 37

32
2
Setting up your system
Syncing to send results wirelessly to the
OneTouchReveal® Mobile App
After pairing the meter with your compatible
wireless device, you are ready to send results to the
OneTouchReveal® Mobile App.
1. Open the OneTouchReveal® Mobile App on your
compatible wireless device
2. Press and hold
to turn the meter on and make sure
the BLUETOOTH® SMART feature is ON as indicated by ( )
If needed, press
and at the same time to turn the
BLUETOOTH® SMART feature on.
The Sync symbol (
)
flashes on the meter display.
"Syncing Data" will appear
on the app to notify you that
the meter is communicating
with the app.
Syncing Data
After syncing, the Sync symbol will disappear, the
"Syncing Data" message will disappear on the app, and
the app will display a list of any new results sent from the
meter.
NOTE: Inserting a test strip during the transmission will
cancel the transfer of all results. The flashing
symbol
appears on the screen and you can proceed with testing.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 32 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 38

33
2
Setting up your system
Turning the meter o
There are three ways to turn your meter off:
đƫ Press and hold
for several seconds until the meter
turns off.
đƫ Remove the test strip.
đƫ Your meter will turn off by itself if left alone for two
minutes.
NOTE: Following a glucose test, the meter will still be
available for BLUETOOTH® SMART connection for up to
4 hours. See page57 for more details.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 33 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 39

34
Testing your blood glucose
Test your blood glucose
NOTE: Many people find it helpful to practice testing with
control solution before testing with blood for the first
time. See page62.
Preparing for a test
Have these things ready when you test:
OneTouchSelect Plus Flex™ Meter
OneTouchSelect® Plus Test Strips
Lancing device
Sterile lancets
3Taking a test
NOTE:
đƫ Use only OneTouchSelect® Plus Test Strips.
đƫ Make sure your meter and test strips are about the
same temperature before you test.
đƫ Do Not test if there is condensation (water build-up) on
your meter. Move your meter and test strips to a cool,
dry spot and wait for the meter surface to dry before
testing.
đƫ Keep test strips in a cool, dry place between 5°C and
30°C.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 34 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 40

35
3
Taking a test
đƫ Do Not open the test strip vial until you are ready to
remove a test strip and perform a test. Use the test
strip immediately after removing it from the vial,
especially in high humidity environments.
đƫ Tightly close the cap on the vial immediately after use
to avoid contamination and damage.
đƫ Store unused test strips only in their original vial.
đƫ Do Not return the used test strip to the vial after
performing a test.
đƫ Do Not re-use a test strip that had blood or control
solution applied to it. Test strips are for single use only.
đƫ Do Not test with a test strip that is bent or damaged.
đƫ With clean, dry hands, you may touch the test strip
anywhere on its surface. Do Not bend, cut or modify
the test strip in any way.
IMPORTANT: If another person assists you with testing,
the meter, lancing device and cap should always be
cleaned and disinfected prior to use by that person. See
page80.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 35 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 41

36
3
Taking a test
NOTE: Comparing your blood glucose test results taken
with this meter to your results taken from a different
meter is not recommended. Results may differ between
meters and are not a useful measure of whether your
meter is working properly. To check your meter accuracy,
you should periodically compare your meter results to
those obtained from a lab. See page100 for more
information.
CAUTION:
đƫ Do Not use the OneTouchSelect Plus Flex™ System
when PAM (Pralidoxime) is known or suspected to be
in the patient's whole blood sample, as it may cause
inaccurate results.
đƫ Do Not use your test strips if your vial is damaged
or left open to air. This could lead to error messages
or inaccurate results. Contact Customer Service
immediately if the test strip vial is damaged. Contact
OneTouch® Customer Care on 0800121200 (UK) or
1800535 676 (Ireland).
đƫ If you cannot test due to a problem with your testing
supplies, contact your healthcare professional. Failure
to test could delay treatment decisions and lead to a
serious medical condition.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 36 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 42

37
3
Taking a test
đƫ The test strip vial contains drying agents that are
harmful if inhaled or swallowed and may cause skin or
eye irritation.
đƫ Do Not use test strips after the expiration date printed
on the vial.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 37 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 43

38
3
Taking a test
Getting to know your OneTouch®Delica®
Lancing Device
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 38 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 44

39
3
Taking a test
NOTE:
đƫ The OneTouch®Delica® Lancing Device uses ONLY
OneTouch®Delica® Lancets.
đƫ If another type of lancing device was included, see the
separate instructions for that lancing device.
đƫ The OneTouchSelect Plus Flex™ Blood Glucose
Monitoring System has not been evaluated for Alternate
Site Testing (AST). Use only fingertips when testing
with the system.
đƫ The OneTouch®Delica® Lancing System does not
include the materials needed to perform Alternate Site
Testing (AST). The OneTouch®Delica® Lancing System
should not be used on the forearm or palm with the
OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 39 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 45

40
3
Taking a test
Lancing precautions
CAUTION:
To reduce the chance of infection and disease spread by
blood:
đƫ Make sure to wash the sample site with soap and warm
water, rinse and dry before sampling.
đƫ The lancing device is intended for a single user. Never
share a lancet or lancing device with anyone.
đƫ Always use a new, sterile lancet each time you test.
đƫ Always keep your meter and lancing device clean (See
page80).
đƫ The meter and lancing device are for single patient use
only. Do Not share them with anyone, including family
members! Do Not use on multiple patients!
đƫ After use and exposure to blood, all parts of this kit
are considered biohazardous. A used kit may transmit
infectious diseases even after you have performed
cleaning and disinfection.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 40 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 46

41
3
Taking a test
Preparing the lancing device
1. Remove the lancing device cap
Remove the cap by turning
it counterclockwise and then
pulling it straight off of the
device.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 41 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 47

42
3
Taking a test
2. Insert a sterile lancet into the lancing device
Align the lancet as shown
here, so that the lancet fits
into the lancet holder. Push
the lancet into the device
until it snaps into place and is
fully seated in the holder.
Twist the protective
cover one full turn until it
separates from the lancet.
Save the protective cover
for lancet removal and
disposal. See page59.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 42 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 48

43
3
Taking a test
3. Replace the lancing device cap
Place the cap back onto the device; turn clockwise to
secure the cap.
Do Not overtighten.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 43 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 49

44
3
Taking a test
4. Adjust the depth setting
The lancing device has seven
puncture depth settings,
numbered 1 through 7.
Smaller numbers are for a
shallower puncture and the
larger numbers are for a
deeper puncture. Shallower
punctures work for children
and most adults. Deeper
punctures work well for people with thick or callused skin.
Turn the depth wheel to choose the setting.
NOTE: A shallower fingertip puncture may be less painful.
Try a shallower setting first and increase the depth until
you find the one deep enough to get a blood sample of
the proper size.
5. Cock the lancing device
Slide the cocking control
back until it clicks. If it does
not click, it may already
have been cocked when you
inserted the lancet.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 44 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 50

45
3
Taking a test
Preparing the meter
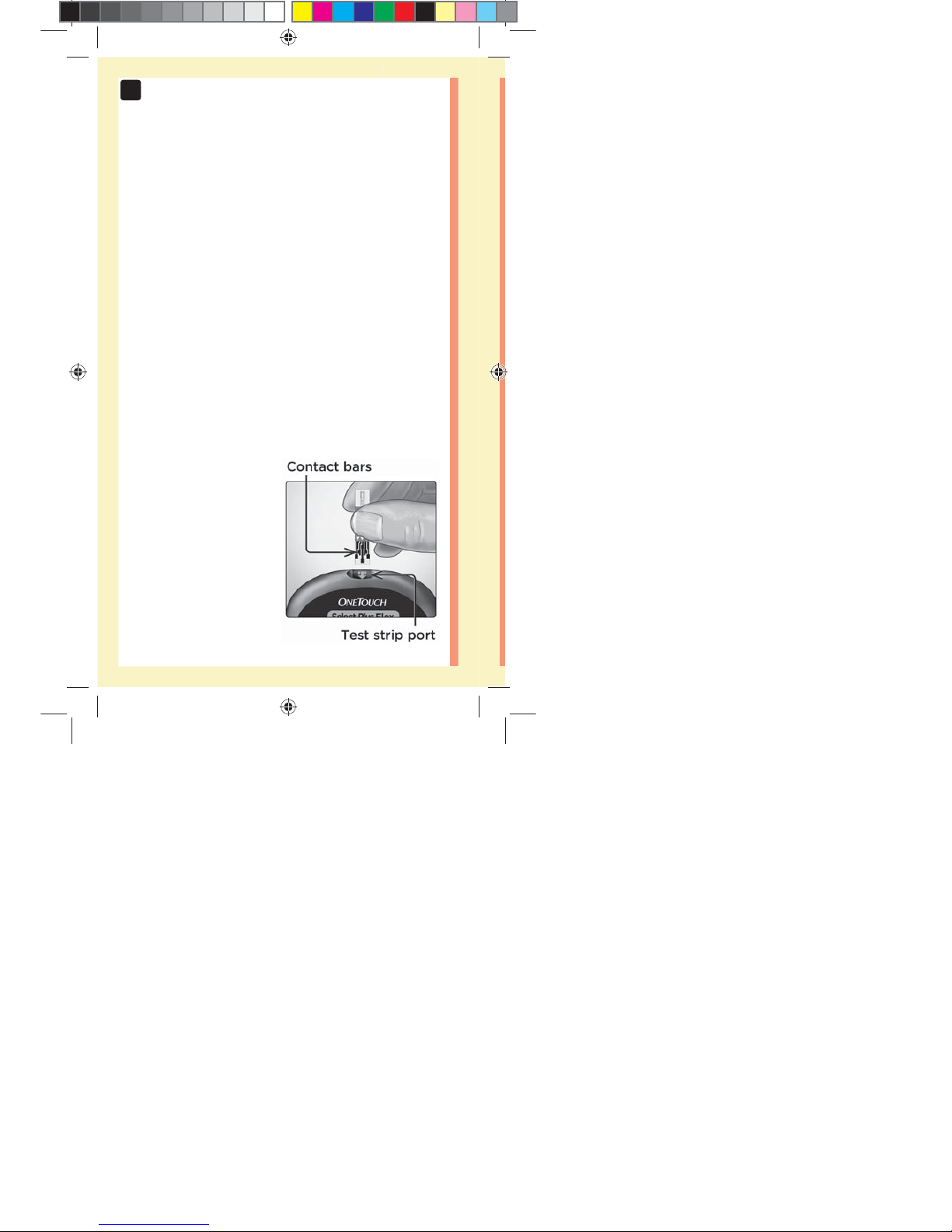
1. Insert a test strip to turn the meter on
Insert a test strip into the test strip port with the contact
bars facing you.
NOTE: No separate step to
code the meter is required.
The flashing blood drop icon
(
) appears on the display.
You can now apply your
blood sample to the test
strip.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 45 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 51

46
3
Taking a test
Getting a blood sample from the fingertip
Choose a different puncture site each time you test.
Repeated punctures in the same spot may cause soreness
and calluses.
Before testing, wash your hands thoroughly with warm,
soapy water. Rinse and dry completely.
1. Puncture your finger
Hold the lancing device
firmly against the side of
your finger. Press the release
button. Remove the lancing
device from your finger.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 46 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 52

47
3
Taking a test
2. Get a round drop of blood
Gently squeeze and/or
massage your fingertip until
a round drop of blood forms
on your fingertip.
NOTE: If the blood smears or
runs, Do Not use that sample.
Dry the area and gently squeeze
another drop of blood or
puncture a new site.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 47 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 53
48
3
Taking a test
Applying blood and reading results
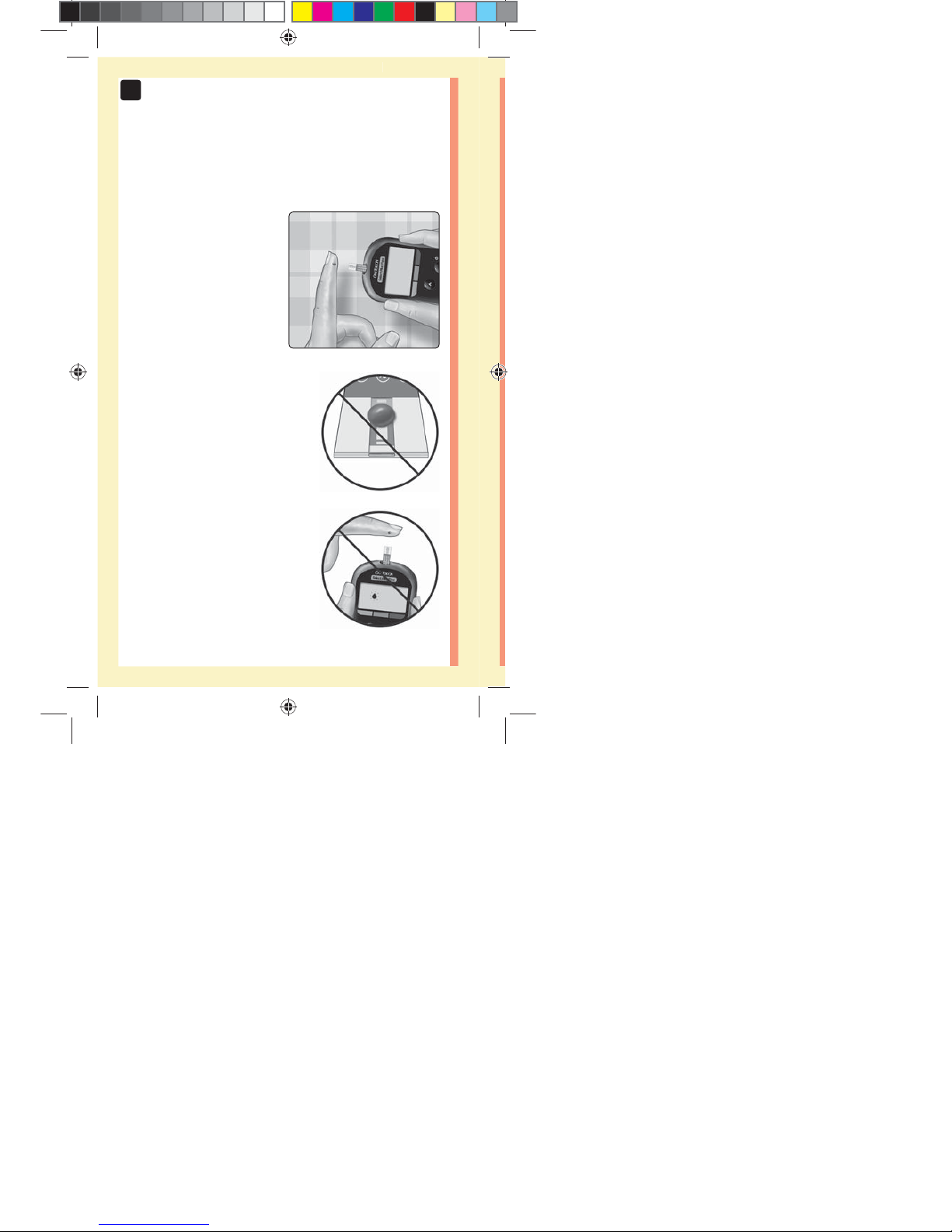
Prepare to apply the sample
Keeping your finger
extended and steady, move
the meter and test strip
toward the blood drop.
Do Not apply blood on the top
of the test strip.
Do Not hold the meter and test
strip underneath the blood drop.
This may cause blood to run into
the test strip port and damage the
meter.
Do Not allow blood to enter the
Data Port.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 48 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 54
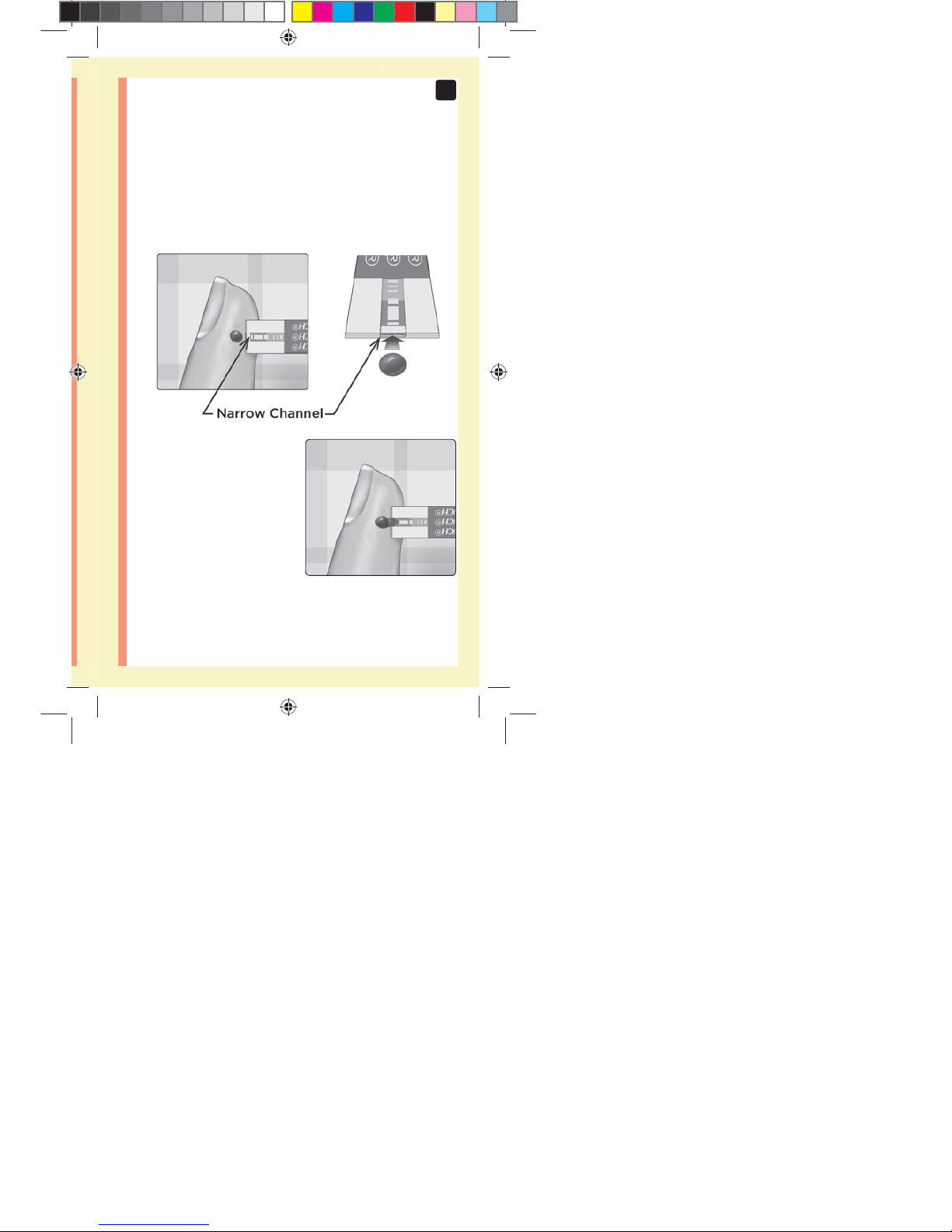
49
3
Taking a test
Applying the sample
Line up the test strip with the blood drop so that the
narrow channel on the edge of the test strip is almost
touching the edge of the blood drop.
Gently touch the channel to
the edge of the blood drop.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 49 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 55

50
3
Taking a test
đƫ Do Not press the test strip too firmly against the
puncture site or the channel may be blocked from filling
properly.
đƫ Do Not smear or scrape the drop
of blood with the test strip.
đƫ Do Not apply more blood to the
test strip after you have moved
the drop of blood away.
đƫ Do Not move the test strip in
the meter during a test or you
may get an error message or the
meter may turn off.
đƫ Do Not remove the test strip until the result is displayed
or the meter will turn off.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 50 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 56

51
3
Taking a test
Wait for the confirmation window to fill completely.
The blood drop will be drawn into the narrow channel and
the confirmation window should fill completely.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 51 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 57
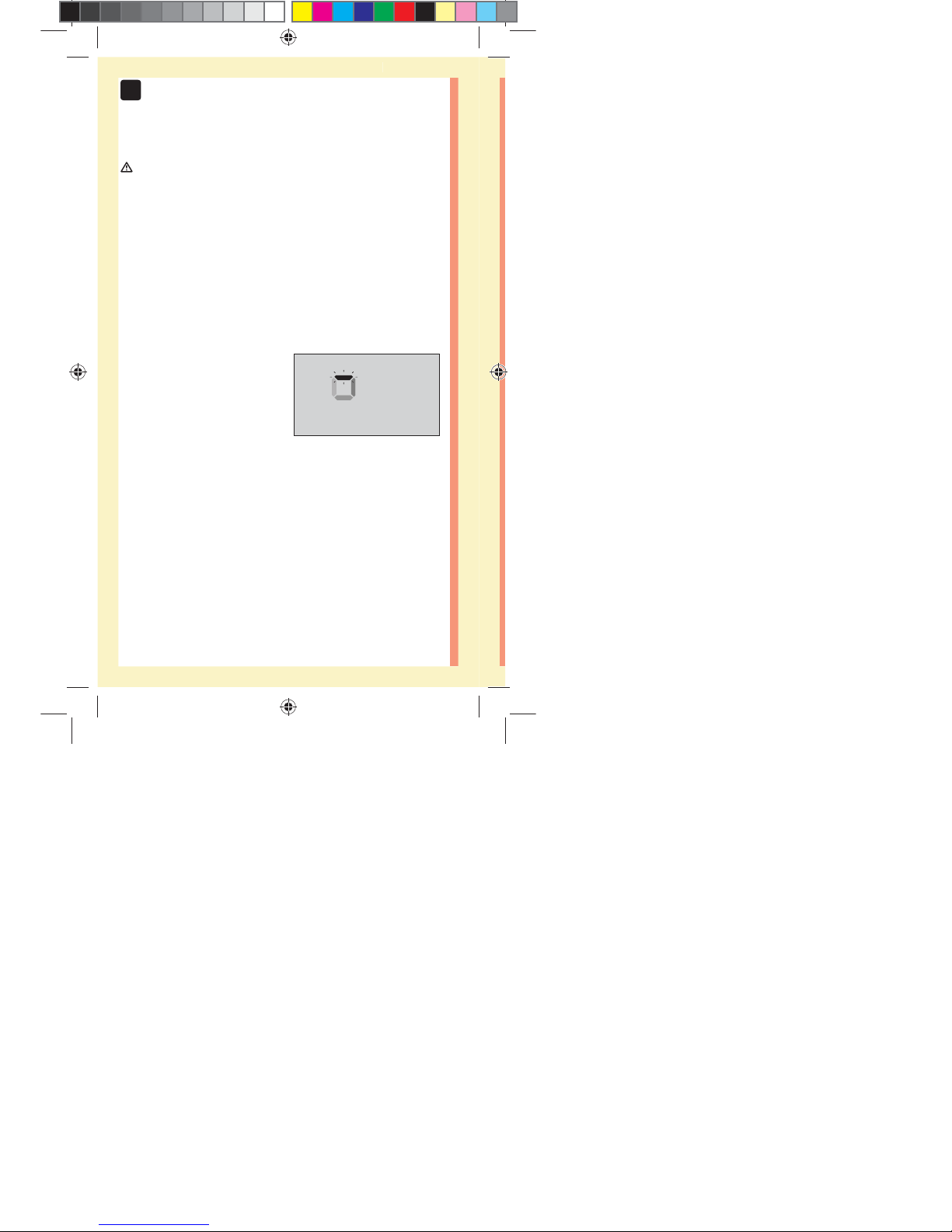
52
3
Taking a test
CAUTION:
You may get an error message or an inaccurate result if
the blood sample does not fill the confirmation window
completely. Discard the used strip and re-start the test
process with a new test strip.
When the confirmation window is full, this means you
have applied enough blood. The Countdown screen will
appear. Now you can move the test strip away from the
drop of blood and wait for the meter to count down
(about 5 seconds).
Countdown Screen
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 52 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 58

53
3
Taking a test
Viewing your result
Your result appears on the display,
along with the unit of measure,
and the date and time of the test.
After your glucose result appears,
the meter will also display a Range
Indicator Arrow below your glucose
result to indicate if your result is
below, above or within your range
limits (see page19). The arrow
will point to the appropriate Range
Indicator Color Bar on the meter as
a visual reminder.
Example
Below Range Result
Example
In Range Result
Example
Above Range Result
CAUTION:
Do Not make immediate treatment
decisions based on the Range
Indicator feature. Treatment
decisions should be based on the
numerical result and healthcare
professional recommendation and
not solely on where your result
falls within your range limits.
WARNING: Confirm that the unit of measure mmol/L is
displayed. If your display shows mg/dL rather than mmol/L,
stop using the meter and contact Customer Service.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 53 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 59

54
3
Taking a test
Interpreting unexpected results
Refer to the following cautions when your results are
higher or lower than what you expect.
CAUTION:
Low results
If your result is below
3.9mmol/L or is shown as
LO (meaning the result is less
than 1.1mmol/L), it may mean
hypoglycemia (low blood
glucose). This may require immediate treatment according
to your healthcare professional's recommendations.
Although this result could be due to a test error, it is safer
to treat first, then do another test.
NOTE: When your glucose result is below 1.1mmol/L, both
the LO and the Range Indicator Arrow will flash on the
meter screen.
CAUTION:
Dehydration and low results
You may get false low results if you are severely
dehydrated. If you think you are severely dehydrated,
contact your healthcare professional immediately.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 54 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 60

55
3
Taking a test
CAUTION:
High results
đƫ If your result is above 10.0mmol/L, it may mean
hyperglycemia (high blood glucose) and you should
consider re-testing. Talk to your healthcare professional
if you are concerned about hyperglycemia.
đƫ HI is displayed when your
result is over 33.3mmol/L.
You may have severe
hyperglycemia (very high
blood glucose). Re-
test your blood glucose
level. If the result is HI again, this indicates a severe
problem with your blood glucose control. Obtain and
follow instructions from your healthcare professional
immediately.
NOTE: When your glucose result is above 33.3mmol/L,
both the HI and the Range Indicator Arrow will flash on
the meter screen.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 55 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 61

56
3
Taking a test
CAUTION:
Repeated unexpected results
đƫ If you continue to get unexpected results, check your
system with control solution.
đƫ If you are experiencing symptoms that are not
consistent with your results and you have followed
all instructions in this Owner's Booklet, call your
healthcare professional. Never ignore symptoms or
make significant changes to your diabetes management
program without speaking to your healthcare
professional.
Unusual red blood cell count
A hematocrit (percentage of your blood that is red blood
cells) that is either very high (above 55%) or very low
(below 30%) can cause false results.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 56 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 62

57
3
Taking a test
Sending your results to the app
If the BLUETOOTH® SMART feature on the meter is turned
on, indicated by the BLUETOOTH® SMART symbol (
),
the meter will automatically send the latest result to
any paired wireless compatible device. The compatible
wireless device must have the app running and have
already been paired to the meter before sending a result.
NOTE: The compatible wireless device must have the app
open and have already been paired to the meter before
sending a result. See page28.
NOTE: If the BLUETOOTH® SMART feature on the meter is
turned off, or the meter is out of range, the result is not
sent to the compatible wireless device. The result is saved
in the meter memory with the current date and time, and
will be sent to the app the next time you sync. The sent
results are also stored in the meter. To sync, the app must
be open and running on your compatible wireless device.
To ensure that glucose test results are successfully sent
to the app, turn on the BLUETOOTH® SMART feature and
check the following:
đƫ The compatible wireless device and meter are both
turned on, and the app is running.
đƫ The meter is correctly paired with your compatible
wireless device.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 57 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 63

58
3
Taking a test
đƫ The Bluetooth feature on both devices is running
(indicated by
) and the devices are within 8meters of
each other.
đƫ The meter will attempt to transmit results up to 4 hours
after a test, even if the meter appears to be off. This
is indicated by the BLUETOOTH® SMART symbol (
)
remaining on the meter screen.
Example
If you are still unable to send results to the compatible
wireless device, please call Customer Service. Contact
OneTouch® Customer Care on 0800121200 (UK) or
1800535 676 (Ireland).
NOTE: Inserting a test strip during the transmission will
cancel the transfer of all results. The
symbol appears on
the screen and you can proceed with testing.
Using the meter without syncing to an app
The meter can be used without a compatible wireless
device or the app. You can still test your blood glucose and
review up to 500 results on the meter.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 58 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 64

59
3
Taking a test
Removing the used lancet
NOTE: This lancing device has an ejection feature, so you
do not have to pull out the used lancet.
1. Remove the lancing device cap
Remove the cap by turning
it counterclockwise and then
pulling it straight off of the
device.
2. Cover the exposed lancet tip
Before removing the lancet, place the lancet protective
cover on a hard surface then push the lancet tip into the
cupped side of the cover.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 59 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 65
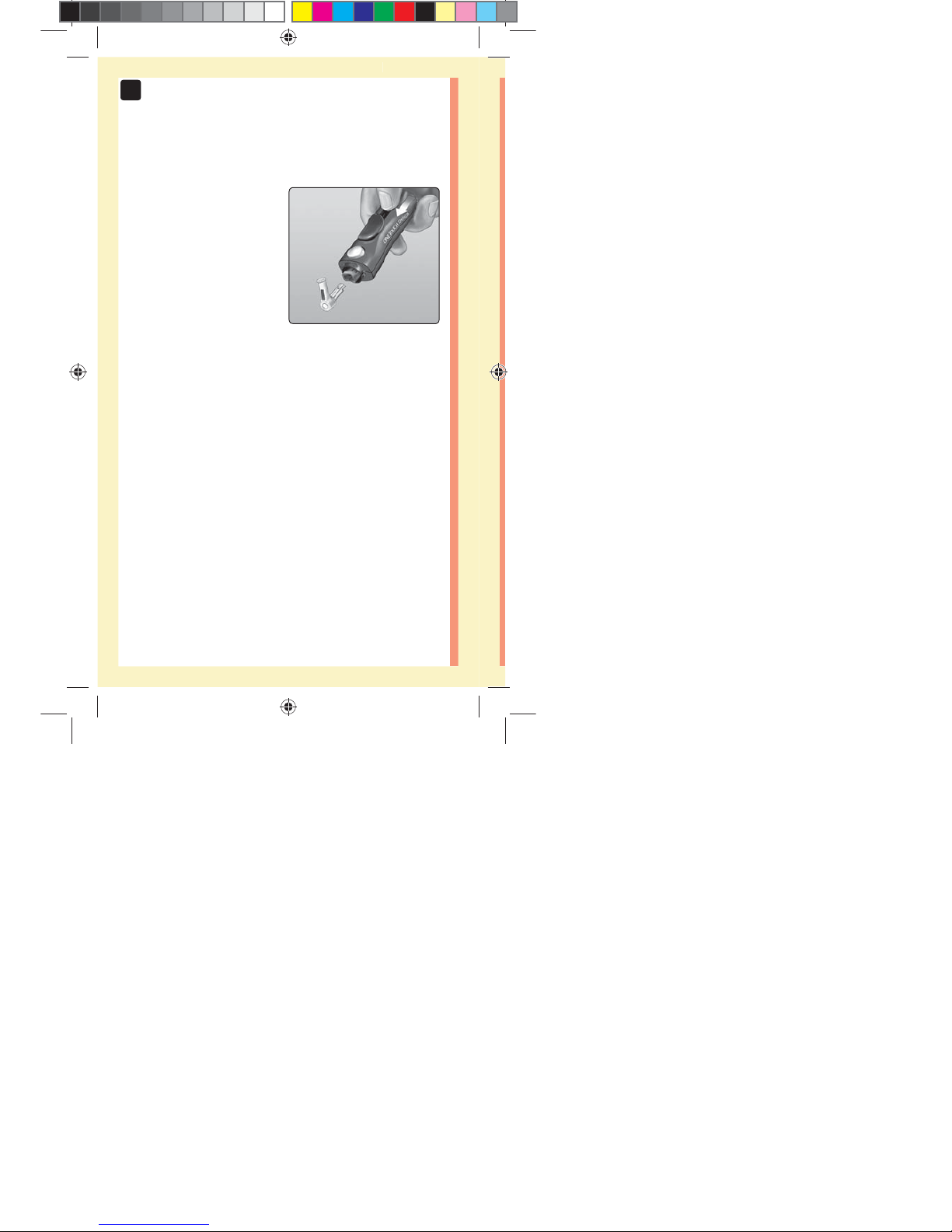
60
3
Taking a test
3. Eject the lancet
Slide the ejection control
forward until the lancet
comes out of the lancing
device. Return the ejection
control to its back position.
If the lancet fails to eject
properly, cock the device
again and then slide the
ejection control forward until
the lancet comes out.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 60 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 66

61
3
Taking a test
4. Replace the lancing device cap
Place the cap back onto the device; turn clockwise to
secure the cap.
Do Not overtighten.
It is important to use a new lancet each time you obtain a
blood sample. Do Not leave a lancet in the lancing device.
This will help prevent infection and sore fingertips.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 61 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 67

62
3
Taking a test
Disposing of the used lancet and test strip
Discard the used lancet carefully after each use to avoid
unintended lancet stick injuries. Used lancets and test
strips may be considered biohazardous waste in your
area. Be sure to follow your healthcare professional's
recommendations or local regulations for proper disposal.
Wash hands thoroughly with soap and water after
handling the meter, test strips, lancing device and cap.
Testing with control solution
Control solution testing precautions
OneTouchSelect® Plus Control Solution is used to check
that the meter and test strips are working together
properly and that the test is performing correctly.
(Control solution is available separately.)
NOTE:
đƫ OneTouchSelect® Plus High Control Solution (vial with
red cap) can be used in addition to OneTouchSelect®
Plus Mid Control Solution (vial with blue cap). If you
test with OneTouchSelect® Plus High Control Solution,
you should always test with OneTouchSelect® Plus
Mid Control Solution first.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 62 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 68
63
3
Taking a test
đƫ When you first open a new vial of control solution,
record the discard date on the vial label. Refer to the
control solution insert or vial label for instructions on
determining the discard date.
đƫ Tightly close the cap on the control solution vial
immediately after use to avoid contamination or
damage.
đƫ Do Not open the test strip vial until you are ready to
remove a test strip and perform a test. Use the test
strip immediately after removing it from the vial,
especially in high humidity environments.
đƫ Control solution tests must be done at room
temperature (20-25°C). Make sure your meter, test
strips and control solutions are at room temperature
before testing.
CAUTION:
đƫ Do Not swallow or ingest control solution.
đƫ Do Not apply control solution to the skin or eyes as it
may cause irritation.
đƫ Do Not use control solution after the expiration
date (printed on the vial label) or the discard date,
whichever comes first, or your results may be
inaccurate.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 63 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 69
64
3
Taking a test
Do a control solution test
đƫ Whenever you open a new vial of test strips.
đƫ If you suspect that the meter or test strips are not
working properly.
đƫ If you have had repeated unexpected blood glucose
results.
đƫ If you drop or damage the meter.
Preparing your meter for a control solution
test
1. Insert a test strip to turn the meter on
Insert the test strip with the test strip port and contact
bars facing you.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 64 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 70

65
3
Taking a test
Preparing the control solution
1. Before removing the cap, shake the vial gently
2. Remove the vial cap and place it on a flat surface with
the top of the cap pointing up
3. Squeeze the vial to discard
the first drop
2. Wait for the flashing blood
drop icon (
) to appear on
the display
3. Press
or until the
control solution icon (
)
appears on the display
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 65 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 71

66
3
Taking a test
4. Wipe both the tip of the
control solution vial and the
top of the cap with a clean,
damp tissue or cloth
5. Squeeze a drop into the
small well on the top of the
cap or onto another clean,
non-absorbent surface
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 66 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 72

67
3
Taking a test
Applying the control solution
1. Hold the meter so that the
narrow channel at the top
edge of the test strip is at a
slight angle to the drop of
control solution
3. Wait for the channel to fill
completely
2. Touch the channel on the top edge of the test strip to
the control solution
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 67 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 73
68
3
Taking a test
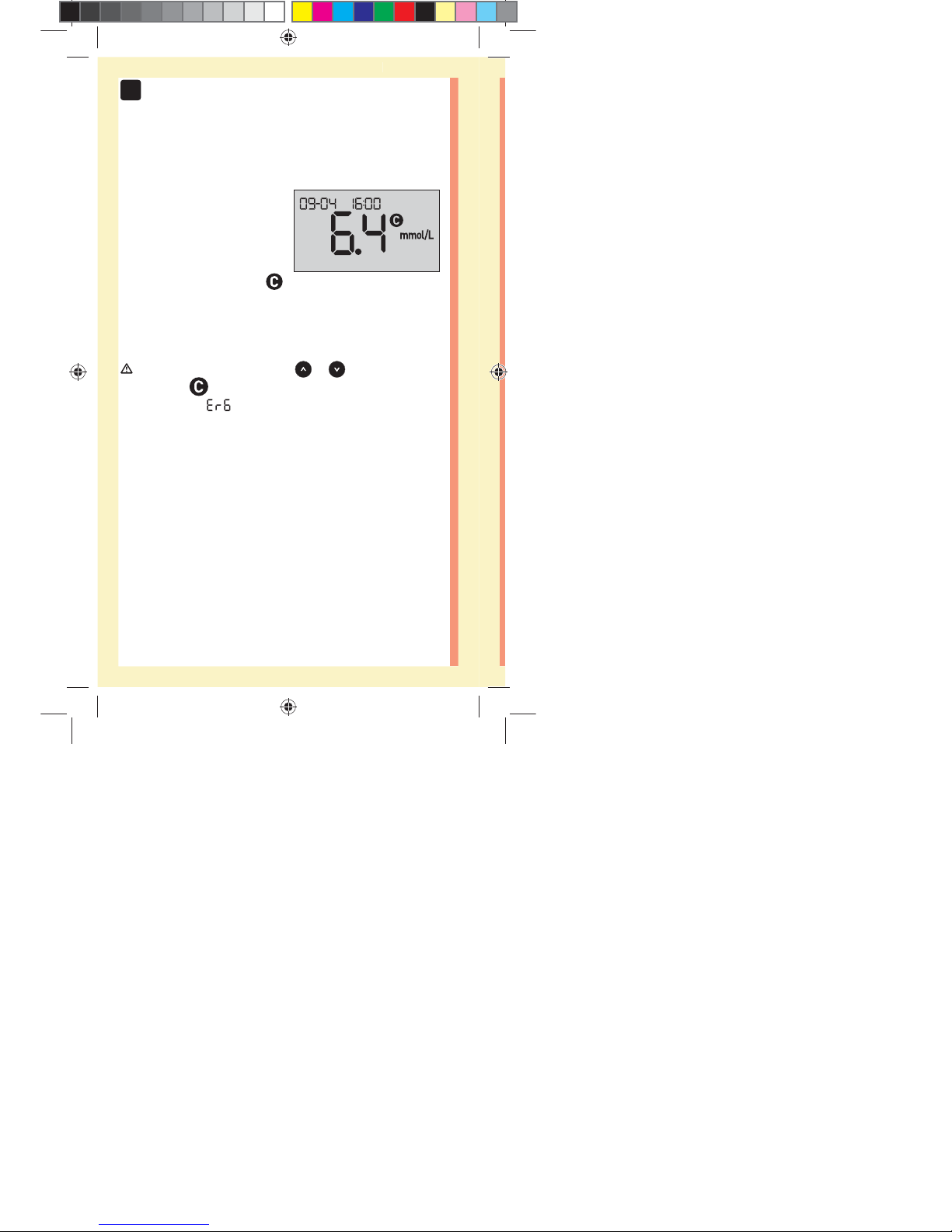
Viewing your control solution result
After the control solution
is applied, the meter will
count down until the test
is complete. Your result is
displayed along with the date,
time, unit of measure, and
(for control solution) and stored in the meter.
Control solution results can be seen when reviewing past
results on the meter.
CAUTION: Make sure you press or until the control
solution icon
appears before you begin a control
solution test. An screen may appear if you applied
control solution to the test strip without following the
steps beginning on page64. See page97 for more
information.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 68 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 74

69
3
Taking a test
Checking if the result is in range
Example Range
OneTouchSelect® Plus Mid
Control Solution Control
Range 5.7-7.7mmol/L
OneTouchSelect® Plus High
Control Solution Control
Range 16.5-22.4mmol/L
Compare the result
displayed on the meter to
the range printed on your
OneTouchSelect® Plus
Control Solution vial.
If your control solution
result falls outside the
expected range, repeat
the test with a new test
strip.
CAUTION:
The control solution ranges printed on the control
solution vial are for control solution tests only and are not
recommended ranges for your blood glucose level.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 69 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 75

70
3
Taking a test
Causes of out-of-range results
Out-of-range results may be due to:
đƫ Not following the instructions for performing a control
solution test.
đƫ Control solution is contaminated, expired, or past its
discard date.
đƫ Test strip or test strip vial is damaged, expired, or past
its discard date.
đƫ Meter, test strips and/or control solution were not all at
the same temperature when the control solution test
was performed.
đƫ A problem with the meter.
đƫ Dirt or contamination in the small well on the top of the
control solution cap.
CAUTION:
If you continue to get control solution results that fall
outside the range printed on the control solution vial, Do
Not use the meter, test strips, or control solution. Contact
Customer Service. Contact OneTouch® Customer Care on
0800121200 (UK) or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 70 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 76

71
3
Taking a test
Cleaning the control solution cap
Clean the top of the control solution cap with a clean,
damp tissue or cloth.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 71 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 77

72
4Reviewing past results
2. Scroll through your results by pressing to move
backwards and to move forward through your results
Reviewing past results on your meter
Your meter stores your most recent 500 blood glucose and
control solution test results and displays them in the order
the tests were taken. The ( ) symbol will appear on your
screen when in History Mode.
1. When the meter is o, press and hold to turn
History Mode on
The ( ) symbol indicates you are viewing your past
results.
The (
) symbol indicates if the result was below,
above or within range at the time of the test, by pointing
to the appropriate color bar.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 72 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 78

73
4
Reviewing past results
Downloading results to a computer
Your meter can work with diabetes management software,
which provides a visual way to track key factors that
affect your blood sugar. To learn more about diabetes
management tools available to you, contact Customer
Service. Call OneTouch® Customer Care on 0800121200
(UK) or 1800535 676 (Ireland) during the hours of
8:30am-6pm Monday-Friday, 9am-1pm Saturday. Or visit
www.LifeScan.co.uk.
Connect only to a computer certified to UL 60950-1 (
).
To transfer meter data, follow the instructions provided
with the diabetes management software product to
download the results from the meter. You will need a
standard micro USB interface cable to connect your
OneTouchSelect Plus Flex™ Meter to a computer to
download results (not included).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 73 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 79

74
4
Reviewing past results
Once the command to start
the download is sent from the
computer to the meter, the
meter display will show the
flashing Sync symbol (
)
indicating that the meter is in
communication mode.
Do Not insert a test strip while the meter is connected to
a computer.
If you are unable to download your results to a computer,
please call Customer Service. Contact OneTouch® Customer
Care on 0800121200 (UK) or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 74 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 80

75
4
Reviewing past results
This page left blank intentionally.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 75 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 81

76
5Editing Your Settings
Editing time and date
You can adjust the meter's time and date settings after
first time setup. Press and hold to turn the meter on,
then press and hold and at the same time. The SET
screen will appear, and the hour will flash.
For instructions on adjusting the time and date, see
page23.
After adjusting the settings, you will exit settings mode
and your last glucose result screen will appear. The
adjusted time and date will be displayed once a new
glucose test has been completed and the result appears on
the screen.
NOTE: You will not be able to perform a blood glucose test
until you finish editing the time and date.
NOTE: The OneTouchReveal® Mobile App on your
compatible wireless device checks and updates the time
and date in your meter each time you sync. Check the time
and date often on your compatible wireless device to be
sure they are correct. See the App instructions for more
information.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 76 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 82
77
5
Editing Your Settings
Editing your range limits
Your meter uses low and high range limits to tell you
when your result is below, above or within your set range.
The meter comes with pre-set range limits that can be
changed. The pre-set low range limit is 3.9mmol/L and
the pre-set high range limit is 10.0mmol/L. To edit the
pre-set range limits press and hold
and at the
same time. The SET screen will appear with the current
low range limit displayed, and the number and range
indicator arrow will flash.
NOTE: The low and high range limits you set apply to
all glucose test results. This includes tests taken before
or after mealtimes, medications and around any other
activities that may affect your blood glucose.
CAUTION:
Be sure to talk to your healthcare professional about the
low and high range limits that are right for you. When
selecting or changing your limits, you should consider
factors such as your lifestyle and diabetes therapy. Never
make significant changes to your diabetes care plan
without consulting your healthcare professional.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 77 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 83

78
5
Editing Your Settings
1. Review the pre-set low range limit displayed
đƫ To accept the pre-set low
range limit, press
.
đƫ To edit the pre-set
low range limit, press
or to change
the value between
3.3mmol/L-6.1mmol/L,
and then press .
2. Review the pre-set high range limit displayed
đƫ To accept the pre-set high
range limit, press
.
đƫ To edit the pre-set
high range limit, press
or to change
the value between
5.0mmol/L-16.7mmol/L,
and then press
.
Your meter will exit settings mode and your last result
screen will appear.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 78 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 84

79
5
Editing Your Settings
NOTE: If you change your range limits, this will only affect
whether future test results are displayed as below, above
or within your range limits. Changing your range limits
does not affect how past results are displayed.
NOTE: You will not be able to perform a glucose test until
you finish editing the range limits.
NOTE: You can use the OneTouchReveal® Mobile App on
your compatible wireless device to change the range limits
stored in your meter. See the instructions that came with
the app for more information.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 79 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 85

80
6Caring for your system
Cleaning and disinfection
Cleaning and disinfection are different and both should
be performed. Cleaning is part of your normal care
and maintenance and should be performed prior to
disinfection, but cleaning does not kill germs. Disinfection
is the only way to reduce your exposure to disease. For
cleaning information, see page80 and for disinfecting
information, see page82.
Storing your system
Store your meter, test strips, control solution and other
items in your carrying case. Keep in a cool, dry place
between 5°C and 30°C. Do Not refrigerate. Keep all items
away from direct sunlight and heat.
Cleaning your meter, lancing device
and cap
The meter, lancing device and cap should be cleaned
whenever they are visibly dirty and before disinfection.
Clean your meter at least once per week. For cleaning
obtain regular strength liquid dish soap and a soft cloth.
Prepare a mild detergent solution by stirring 2.5mL of
regular strength liquid dish soap into 250mL of water.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 80 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 86

81
6
Caring for your system
đƫ Do Not use alcohol or any other solvent.
đƫ Do Not allow liquids, dirt, dust, blood or control
solution to enter the test strip port or the data port.
(See page16.)
đƫ Do Not spray cleaning solution
on the meter or immerse it in
any liquid.
1. Holding the meter with the test strip port pointed
down, use a soft cloth dampened with water and mild
detergent to wipe the outside of the meter and lancing
device
Be sure to squeeze out any
excess liquid before you wipe
the meter. Wipe the outside of
the cap.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 81 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 87
82
6
Caring for your system
Disinfecting your meter, lancing device
and cap
The meter, lancing device and cap should be disinfected
periodically. Clean your meter, lancing device and cap
prior to disinfecting. For disinfecting, obtain regular
household bleach (containing a minimum of 5.5% sodium
hypochlorite as the active ingredient)*. Prepare a solution
of 1 part household bleach and 9 parts water.
*Follow manufacturer’s instruction for handling and
storage of bleach.
2. Wipe dry with a clean,
soft cloth
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 82 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 88
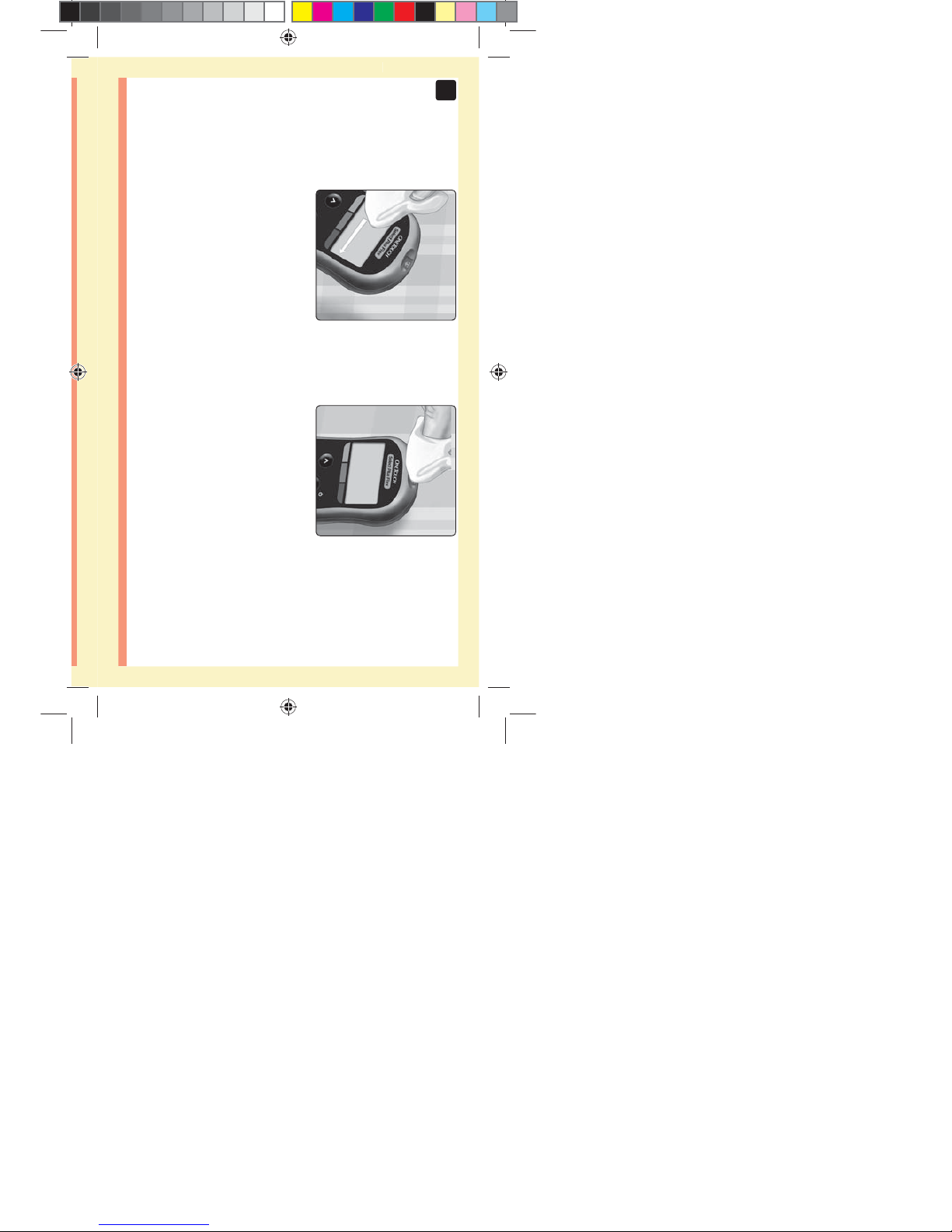
83
6
Caring for your system
1. Hold the meter with the test strip port pointed down
Use a soft cloth dampened
with this solution to wipe
the outside of the meter and
lancing device until the surface
is damp. Be sure to squeeze
out any excess liquid before
you wipe the meter.
2. After wiping, cover the surface you are disinfecting
with the soft cloth dampened with the bleach solution
for 1 minute
Then wipe with a clean, damp,
soft cloth.
Wash hands thoroughly with
soap and water after handling
the meter, lancing device and
cap.
If you see signs of wear, please contact Customer Service.
Contact OneTouch® Customer Care on 0800121200 (UK)
or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 83 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 89

84
7Battery
Replacing the battery
Your OneTouchSelect Plus Flex™ Meter uses one CR2032
lithium coin cell battery.
IMPORTANT: Use only one CR2032 lithium coin cell battery
with your meter. Do Not use rechargeable batteries. Use
of an incorrect battery type may result in your meter
providing fewer tests than normal.
If the meter does not turn on, you may need to replace the
battery. See below for instructions.
WARNING: Certain batteries may cause leaking which can
damage the meter or cause the battery to lose power sooner
than normal. Replace leaking battery immediately.
NOTE: After replacing the battery, you will be prompted
to set time and date, as if you are turning the meter on for
the first time.
1. Remove the old battery
Start with the meter turned off.
Remove the battery cover by
sliding it downward.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 84 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 90

85
7
Battery
Pull up on the battery ribbon
to lift the battery out of the
compartment.
Do Not remove the battery while
the meter is connected to a
computer.
2. Insert the new battery
Insert one CR2032 lithium coin
cell battery on top of the battery
ribbon, with the plus (+) side up.
If the meter does not power on after you have replaced
the meter battery, check that the battery is correctly
installed. If the meter still does not power on, contact
Customer Service. Contact OneTouch® Customer Care on
0800121200 (UK) or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 85 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 91

86
7
Battery
3. Replace battery cover by
sliding it upwards onto the meter
5. Dispose of battery
Dispose of the battery according to your local
environmental regulations.
4. Check your meter settings
Removing the meter battery will not affect your stored
results. However, you will need to check your date and
time settings.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 86 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 92

87
7
Battery
This page left blank intentionally.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 87 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 93

88
8Troubleshooting your
system
Error and other messages
The OneTouchSelect Plus Flex™ Meter displays messages
when there are problems with the test strip, with the
meter or when your glucose levels are above 33.3mmol/L
or below 1.1mmol/L. Improper use may cause an
inaccurate result without producing an error message.
NOTE: If the meter is on but does not operate (locks-up),
contact Customer Service. Contact OneTouch® Customer
Care on 0800121200 (UK) or 1800535 676 (Ireland).
What it means
You may have a very
low blood glucose level
(severe hypoglycemia),
below 1.1mmol/L.
NOTE: When your glucose result is below 1.1mmol/L, both
the LO and the Range Indicator Arrow will flash on the
meter screen.
What to do
This may require immediate treatment. Although this
message could be due to a test error, it is safer to treat
first and then do another test. Always treat according to
your healthcare professional’s recommendations.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 88 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 94

89
Troubleshooting your system
8
What it means
You may have a very
high blood glucose level
(severe hyperglycemia),
over 33.3mmol/L.
NOTE: When your glucose result is above 33.3mmol/L,
both the HI and the Range Indicator Arrow will flash on
the meter screen.
What to do
Re-test your blood glucose level. If the result is HI
again, obtain and follow instructions from your healthcare
professional right away.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 89 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 95

90
Troubleshooting your system
8
What it means
Meter is too hot (above 44°C)
to perform a test.
What to do
Move the meter and test strips to a cooler area. Insert a
new test strip when the meter and test strips are within
the operating range (10-44°C). If you do not get another
HI .t message, you can proceed with testing.
If this message continues to appear, contact Customer
Service. Contact OneTouch® Customer Care on
0800121200 (UK) or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 90 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 96

91
Troubleshooting your system
8
What it means
Meter is too cold (below 10°C)
to perform a test.
What to do
Move the meter and test strips to a warmer area. Insert a
new test strip when the meter and test strips are within
the operating range (10-44°C). If you do not get another
LO.t message, you can proceed with testing.
If this message continues to appear, contact Customer
Service. Contact OneTouch® Customer Care on
0800121200 (UK) or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 91 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 97

92
Troubleshooting your system
8
Example Error
Screen Code
Error Screens
If there is a problem with your meter, there are six possible
error screens that may appear. Along with an error
number, there is also an error code in the upper left corner
of your meter screen. If you cannot resolve the error with
your meter, contact Customer Service. Contact OneTouch®
Customer Care on 0800121200 (UK) or 1800535 676
(Ireland). They will refer to the error number and code to
help troubleshoot the problem.
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 92 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 98

93
Troubleshooting your system
8
What it means
There is a problem with
the meter.
What it means
Error message could be
caused either by a used test
strip or a problem with the
meter or test strip.
What to do
Do Not use the meter. Contact Customer Service. Contact
OneTouch® Customer Care on 0800121200 (UK) or
1800535 676 (Ireland).
What to do
Repeat the test with a new test strip; see page49 or
page67. If this message continues to appear, contact
Customer Service. Contact OneTouch® Customer Care on
0800121200 (UK) or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 93 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 99

94
Troubleshooting your system
8
What it means
The sample was applied
before the meter was ready.
What to do
Repeat the test with a new test strip. Apply a blood or
control solution sample only after the flashing
symbol
appears on the display. If this message continues to
appear, contact Customer Service. Contact OneTouch®
Customer Care on 0800121200 (UK) or 1800535 676
(Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 94 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
Page 100

95
Troubleshooting your system
8
What it means
The meter has detected a
problem with the test strip.
Possible cause is test strip
damage.
What to do
Repeat the test with a new test strip. See page34 for
taking a blood glucose test, or page64 for taking a
control solution test. If the error message appears again,
contact Customer Service. Contact OneTouch® Customer
Care on 0800121200 (UK) or 1800535 676 (Ireland).
ellow= 5mm Red=7mm margin
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 95 3/12/15 11:36 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
 Loading...
Loading...