OncoTherm EHY-2000plus User Manual

USER‘S MANUAL
EHY-2000plus
OncoTherm Kft.
Both the hardware and software are
specially developed for the OncoTherm
systems.
Copyrights belong to OncoTherm Kft.
All rights reserved.
This book is sold subject to the condition that it
shall not by any way of trade or otherwise, be
lent, re-sold, hired out or otherwise circulated
without the OncoTherm prior consent in any
form of binding or cover other than that in
which it is purchased and without a similar
condition including this condition being
imposed on the subsequent purchase.

plus
UM - EHY2000plus-R24
1
User’s Manual
EHY-2000plus
Oncotherm Kft.
Date: 2018.07.27.
Version: 24

plus
UM - EHY2000plus-R24
2
Contents
CONTENTS ..................................................................................................................................................................... 2
INTRODUCTION ............................................................................................................................................................ 4
HOW TO USE THIS MANUAL............................................................................................................................................ 4
CONTACTS .................................................................................................................................................................... 5
SAFETY WARNING ........................................................................................................................................................ 6
SYMBOLS AND THEIR DEFINITIONS ........................................................................................................................... 7
INSTALLATION ........................................................................................................................................................... 8
GENERAL ..................................................................................................................................................................... 8
ELECTRICAL CONNECTION ............................................................................................................................................ 8
INSTALLATION NOTICES ............................................................................................................................................... 10
SAFETY ........................................................................................................................................................................ 11
RESIDENT RISKS:........................................................................................................................................................... 12
GENERAL DESCRIPTION ............................................................................................................................................ 13
INTENDED USE ............................................................................................................................................................. 13
TARGET GROUP .......................................................................................................................................................... 13
SIDE EFFECTS ............................................................................................................................................................... 14
AVAILABLE APPLICATORS ............................................................................................................................................ 14
CONTRA INDICATION ................................................................................................................................................. 15
IMPORTANT MEDICAL NOTICES .............................................................................................................................. 16
DEVICE CONTROL ...................................................................................................................................................... 21
Display-EHY-2000plus ...................................................................................................................................... 21
Control panel of waterbed .......................................................................................................................... 27
Tuning ................................................................................................................................................................. 28
Starting the device ......................................................................................................................................... 30
Doing a treatment .......................................................................................................................................... 32
Switch off the device ..................................................................................................................................... 34
Time-meters ....................................................................................................................................................... 35
Warning and error messages ................................................................................................ ....................... 35
THE USAGE OF THE WEB-BOX .................................................................................................................................. 38
PURPOSE .................................................................................................................................................................... 38
SYSTEM STRUCTURE...................................................................................................................................................... 38
USAGE ....................................................................................................................................................................... 39
STARTING THE WEB ..................................................................................................................................................... 39
LOGGING IN AND THE MAIN MENU ............................................................................................................................. 39
TREATMENT MENU ....................................................................................................................................................... 42
TREATMENT VIEW ........................................................................................................................................................ 46
PATIENT MANAGEMENT MENU ..................................................................................................................................... 46
IMPORTANT NOTICES ................................................................................................................................................... 52
IMPORTANT NOTES FOR THE USE OF THE SOFTWARE ...................................................................................................... 52
SUPPORTED BROWSERS ............................................................................................................................................... 52
REQUESTED TECHNICAL BACKGROUND ....................................................................................................................... 53
Client browser .................................................................................................................................................. 53
Network .............................................................................................................................................................. 53

plus
UM - EHY2000plus-R24
3
Alternative configuration ................................................................ ................................ .............................. 53
TECHNICAL DESCRIPTION ........................................................................................................................................ 54
THE MAIN RF-UNIT ....................................................................................................................................................... 54
TECHNICAL DATA ....................................................................................................................................................... 54
TECHNICAL DATA OF THE WATERBED ........................................................................................................................... 55
CLEANING ................................................................................................................................................................. 56
SERVICING/MAINTENANCE ........................................................................................................................................ 57
DISPOSAL ................................ ................................................................................................ ................................... 57
ACCESSORIES ............................................................................................................................................................. 58
APPENDIX 1 – GUARANTEE ...................................................................................................................................... 60
APPENDIX 2 – DECLARATION OF CONFORMITY SAMPLE ................................................................................. 61
APPENDIX 3 – TABLE OF PROPOSED ELECTRODES .............................................................................................. 62
APPENDIX 4 – REFILLING OF THE BOLUS ELECTRODE ......................................................................................... 63
APPENDIX 5 - ELECTROMAGNETIC COMPATIBILITY INFORMATION .............................................................. 69
APPENDIX 6 - TREATMENT IN 9 STEPS .................................................................................................................... 77
APPENDIX 7 - PATIENT CONSENT ........................................................................................................................... 78
APPENDIX 8 - BRAIN TREATMENT ........................................................................................................................... 79
APPENDIX 9 - ROLLICEL DATASHEET ...................................................................................................................... 81
APPENDIX 10 - MEMORY FOAM PILLOW .............................................................................................................. 82

plus
UM - EHY2000plus-R24
4
Introduction
Congratulation on your excellent choice!
You are the owner of a high-tech medical product, developed and produced by
Oncotherm Kft.
on the basis of the latest bio-engineering and medical knowledge.
The design and production of EHY-2000plus is controlled by the rigorous EU standards, certified for
ISO 13485 certified also by the German TÜV SÜD Product Service GmbH (Munich). The product is
completely manufactured in the European Union.
How to use this manual
The user’s manual of EHY-2000plus explains the proper use and maintenance of
the device. We recommend you to follow the content order first time you study
the manual. After you are familiar with the safe operation of the EHY-2000plus,
you can continue with the technical and theoretical background. On the base
of this knowledge, you can learn the treatment process with EHY-2000plus. The
device control part should be used as a guideline for treatments. We
recommend to keep this manual close at hand since you may need to refer to it
in the future.
You can find the latest valid version of the User’s Manual on our website
(www.oncotherm.org/for Specialists (please login)/User’s Manuals).
plus
UM - EHY2000plus-R24
5
Contacts
Please do not hesitate to contact us if you have any questions:
Questions in connection with production and management:
Oncotherm Group
Dr. Olivér Szász
CEO Hungary/Germany
Belgische Allee 9, D-53842 Troisdorf, Germany
Phone: +49-2241-319-920
Fax: +49-2241-319-9211
Email: info@oncotherm.de
and
Gyár utca 2., H-2040 Budaörs, Hungary
Phone: +36-23-555-510
Fax: +36-23-555-515
E-mail: info@oncotherm.org
Questions about research and development:
Prof. Dr. András Szász
Chief Scientific Officer
E-mail: ProfSzasz@oncotherm.org
Your local distributor:

plus
UM - EHY2000plus-R24
6
Safety Warning
Please read these installation- and operating-instructions carefully before using your
device. These instructions contain important notes regarding safe installation, use
and maintenance of your appliance.
Please keep these instructions in a safe place you can always access and, if you sell
the appliance, hand them over to the new owner.
The manufacturer cannot accept liability if these instructions are not adhered to.
A technical training is required to operate the equipment! For this procedure,
please ask the manufacturer or the distributor.
To reduce the risk of fire or electric shock, do not expose this appliance to rain or
moisture. Due to dangerous high voltage, do not open the cabinet. For technical
support please contact the qualified personnel of Oncotherm.

plus
UM - EHY2000plus-R24
7
Symbols and their definitions
Please note the symbols below for correct usage of the equipment:
This symbol is intended to inform the user about the ground independent (body
floating) construction. Do not rearrange the professional installation.
This symbol is intended to alert the user to the presence of important operation
and maintenance instructions in the literature accompanying this product.
This symbol warns the user to read the relevant part in the User’s manual.
This symbol informs the user that the device is intended to emit non-ionizing
radiation.
This symbol indicates that the waste of electrical and electronic equipment
must not be disposed as unsorted municipal waste but has to be collected
separately. Please contact the authorized representative of Oncotherm Group
for information concerning the decommissioning of your equipment.
CLASS I
Conductive-enclosed CLASS I EQUIPMENT:
EQUIPMENT having a durable and substantially continuous ENCLOSURE of
conductive material which envelops all conductive parts with the exception of
small parts, such as nameplates, screws and rivets, which are isolated from LIVE
parts by insulation at least equivalent to REINFORCED INSULATION. The
ENCLOSURE of insulation-enclosed CLASS II EQUIPMENT may form a part or the
whole of the SUPPLEMENTARY INSULATION.
plus
UM - EHY2000plus-R24
8
Installation
General
The device must be installed by a qualified technician/engineer on behalf of the
Oncotherm Group in compliance with the instructions provided. The
manufacturer declines all responsibility for improper installation which may harm
people and damage property.
When the packing is removed, check that the appliance is not damaged. If you
have any doubts, do not use the appliance, but call for a qualified technician.
The packaging items (plastic bags, foamed polystyrene, nails, etc.) are potential
sources of danger. Never leave them within the reach of children.
This device shall be used for the purpose it was expressly designed for. Any other
use is considered improper and consequently dangerous. The manufacturer
declines all responsibility for damage resulting from improper and irresponsible
use.
Electrical connection
1. Connect the equipment to 230/110V A/C socket with ground only.
Ensure that the socket is properly installed.
2. Observe that a min. 16 Ampere fuse protects the socket.
3. Make sure that the device uses the single-phase independently from
other appliances. Use an independent phase for the device and others
for the other applied electric appliances (for example: air-conditioning,
diagnostics systems, computers, sterilization equipment, etc.) in the
room.
(Our service team controls these conditions, and we will be taken into
consideration during installation to satisfy these requests.)

plus
UM - EHY2000plus-R24
9
Pre-installation notices
1. Put the device into a quiet, independent room, devoted only to the
Oncotherm device and treatment procedure.
2. The room must have normal conditions (e.g. temperature humidity,
pollution, etc.) at all times.
• temperature range: 15 – 23 °C
• humidity range: 20 – 60 %
• No aggressive pollution (e.g. chemicals, fibers, dust, smoke,
etc.) is allowed in the room where the device is installed
3. Let the room have enough natural and/or artificial light for the proper
handling of the treatment.
4. Do not install the device on textile carpet. Avoid using the equipment
on soft surfaces.
5. Do not use the equipment where it may be exposed to vibrations.
6. Avoid using the equipment near appliances generating strong electro-
magnetic fields (e.g. motors, transformers, etc.).
7. There has to be a safe place for treatment accessories in the room.
8. On delivery, observe the device carefully and wait for the authorized
Service for the installation.
9. If your device looks damaged, do not use it. In case of any questions
ask the Oncotherm service team for advice.
10. To move the device into another room and/or into any other place
from the place where it was installed originally please ask for the
assistance of the Oncotherm service. The unauthorized relocation is
strictly prohibited in order to avoid the mains and grounding
discrepancies and the possible electric shock.
11. Avoid using your equipment immediately after sudden changes of the
outside temperature, due to the moisture damage in the electronics.
12. Ensure that no objects fall or no fluid passes through the ventilation
openings. If liquid is spilt into the equipment, disconnect it from the
mains and consult a qualified service technician.
13. Dangerous voltage inside. Do not open the cabinet. There are no user
serviceable parts inside. Only qualified service personnel should carry
out repairs.

plus
UM - EHY2000plus-R24
10
Installation notices
1. This unit should be kept away from heat registers, radiators, stoves or
other appliances that produce heat. Windy places or the vicinity of
windows should also be avoided.
2. For the best performance and safety, please place this unit in the
middle of the room. Make it possible for the operator to access the
device from any direction. Any walls and/or grounded surface have to
be minimum 1.5 meter away from the treatment bed, so that the
patient cannot reach any surfaces independent from the device, which
could cause an electric shock for them.
3. The EHY unit must be located in a suitable place, where the emergency
switches of the device are accessible for everybody in case of a
dangerous situation. This emergency switch cuts off the mains voltage of
EHY device itself, but this button does not affect the waterbed and the
PC.
4. Good air circulation around the device is essential to prevent internal
overheat of the electronic parts.
5. Choose a dust-free, well-ventilated position. Do the everyday cleaning
by washing up the floor, as it is usual in medical institutions.
6. Take care of the intactness of cables and water pipes. Do not break
them, or obstruct the free flow of the cooling water.
7. Electrical safety of the appliance is only guaranteed if the grounding
system of the building is in accordance with the local electricity board
regulations. The OncoTherm service team controls it at the regular
services. The proper electric conditions are the liability of the customer.
8. Only authorized service personnel should carry out repairs and any
other work on the device. The approval must be in written form from the
Oncotherm Group. The relocation and/or using mains-socket other than
the originally installed, must be done only by the Oncotherm or its
authorized service-representative. Only the qualified and authorised
Oncotherm personnel should service the unit when it does not operate
normally or shows marked change in its performance.
9. Devices, which are to be discarded, must be made unusable. Pull out
the plug and remove the cable.

plus
UM - EHY2000plus-R24
11
Safety
1. The device is only suitable for normal treatment use and for the
purposes and intended uses stated in the operating instructions.
2. Do not use any extension- and radio-frequency-cables, only those
which are provided by the authorized service and/or by the
Oncotherm.
3. Before starting any cleaning work on the device it must be
disconnected from the electric supply by removing the plug from the
socket. Do not pull the cable!
4. The mains lead of the unit should be unplugged when the unit is not in
use.
5. Do not plug in or unplug the mains lead with wet hands.
6. Do not use the device when you are barefoot.
7. Make sure that objects do not fall and liquids are not spilled into the
interior of the device through openings.
8. Do not allow children to operate and/or control the equipment.
9. Do not allow people who are not experienced nor trained in operating
the equipment to use it without supervision.
10. Never leave the device exposed to environmental effects (rain, sun
etc.).
11. Every six months the service team has to check the device according to
the ‘regular safety testing instruction’.
12. Never use the waterbed without the mattress. Touching the heating
surface directly can cause burn!
13. Never use the mattress without water (only distilled water can be used.)
as the patient can compress it and can touch heating surface almost
directly! (Check of filling up: Push the middle of one half of the mattress
with one hand till reaching bottom. Then push the middle of the other
half with the other hand - minimum ½ meter from the first point. It should
not reach the bottom.)
14. Water leakage is shown on display (sensed electronically in wastewater
tank). In case of water-loss failure, treatment should be stopped
immediately and the service team should be called for help. If water
flows quickly, treatment must be stopped, switch off the bed and pull
out the mains plug.

plus
UM - EHY2000plus-R24
12
15. In case of noticing water in the plastic tray or on the surface of the
mattress, please check the water level in mattress and presence of
possible mechanical damage. Do not use damaged mattress in order
to avoid an electric shock.
16. The patient’s surface must not be damp. After cleaning the surface the
user has to wait until it is dry.
17. Using EHY-2000plus without a heated waterbed may decrease the
temperature of the body of the patient. In case of everyday use, keep
the bed switched on permanently. (Heating up the water from room
temperature to treating temperature level requires about 12 -16 hours.)
18. Check the temperature settings before each treatment!
Recommended temperature setting is 30-32°C, because the treatment
itself also heats and the patient will have sweaty skin which is not
suitable for correct EHY-2000plus treatment.
19. Check the alarm system daily during continuous use. Switch the device
off for a short time. When you switch on the waterbed, a self-test is
implemented, and a warning is shown until the user sets the desired
temperature again.
20. Do not use other surgical or endocardial devices while the patient is on
the waterbed.
Resident risks:
The user has to follow the instructions mentioned above, otherwise burning or
overheating of the tissue can occur.

plus
UM - EHY2000plus-R24
13
General description
Intended use
The EHY-2000plus device is intended for the adjuvant treatment of deepseated primer and metastasized solid malignant tumors in combination regimen
with chemotherapy and/or radiation therapy. The performance of the EHY2000plus device is supported with clinical evidences in the following indications:
▪ Glioblastoma,
▪ Esophageal cancer,
▪ Gastric cancer,
▪ Liver cancer,
▪ Cancer of kidney and renal pelvis,
▪ Cervical cancer,
▪ Pancreatic cancer,
▪ Breast cancer,
▪ Lung cancer.
The performance of the EHY-2000plus device is also supported with clinical
experience in the following indications:
▪ Bladder cancer,
▪ Prostate cancer,
▪ Colorectal cancer,
The collection of additional clinical data to establish clinical evidence
concerning the performance of the EHY-2000plus device in the latter indications
is in progress.
Target group
Patients who suffer from solid malignant tumors with age over 6 years.
plus
UM - EHY2000plus-R24
14
Side effects
Short-term and long-term unwanted side-effects may be associated with the
use of the EHY-2000plus device:
Short-term side-effects:
Moderate pain during the procedure,
Skin effects, typically the local skin hyperemia in the area of exposure,
Fatigue, short-term asthenia,
Headache (mainly in brain applications),
Short-term euphoria,
Subfebrile temperature for several days after treatment.
Long-term side-effects:
Burns of the skin;
Burns of subcutaneous tissue,
Subcutaneous fat-burn,
Subcutaneous fatty fibrosis.
The occurrence of side effects is rare, they are transient and do not require
interruption or termination of treatment. In some cases, symptomatic therapy
is necessary.
Available applicators
• Waterbed electrode (permanent part of the device)
• Bolus electrode, standard (20 cm diameter)
• Bolus electrode, small (10 cm diameter)
• Bolus electrode, large (30 cm diameter)

plus
UM - EHY2000plus-R24
15
Contra indication
• Cannot be used when the patient is under deep-sedation or anesthesia
(missing thermal sensitivity). Application of analgesics in the treated
area is prohibited.
• Cannot be used when the patient is unconscious.
• Cannot be used when the patient is not able to communicate with the
physician.
• Do not use the electrodes in the vicinity of the patient’s
metallic/prosthesis (bone-replacement, joint support, etc.). The distance
between the implanted metal and the circumflex of the upper
electrode shall be more than the radius of the electrode.
• Do not use the electrodes in the vicinity of the patient’s silicone
prosthesis (breast implant.). The distance between the implanted
prosthesis and the circumflex of the upper electrode shall be more than
the radius of the electrode.
• Before the treatment all metallic pieces (necklaces, rings, jewels,
watches, pipes, coins, phones, hairpins, pens, etc.) have to be left far
away from the treatment bed. Do not treat patients who have
earphones, hearing-aid, music devices (Walkman, walk-watch, etc.)
and or/any wire-connected instruments.
• Cannot be used for treating patients who have pacemaker or any
other type of electrical implants (e.g. implanted. deep brain stimulator
(DBS), implanted hearing-aids, implanted erectile function stimulator,
etc.).
• Must not be used in case of tendency to hemorrhage, including
menstruation or open wound (e.g. newly operated patients).
• Do not apply on person with organ-transplants.

plus
UM - EHY2000plus-R24
16
Important medical notices
Please remember:
1. The user of this device has to be a physician and/or a trained clinical
staff under the physician’s control.
2. The treatment has to be permanently monitored by the staff.
3. The treatment needs extra care, when the patient has reduced thermal
sensitivity on the treated area.
4. Check the position of the electrode to keep it as parallel to the bed
surface as possible (try to avoid the electrode being placed on an
inclined angle). The applicator (electrode) has a flexible water-bolus to
enable the best interface with the patient. Please note that positioning
of the electrode is to be carefully controlled because some
temperature increase can occur at the skin’s surface. This will not be a
sudden effect and is can be controlled with patient feedback during
the treatment. In addition, users are advised to place medical hygienic
paper (see detailed description in Accessories and Appendix 9 for
datasheet) between the electrode and the patient to avoid direct skin
contact. Also, when positioning the electrode, avoid direct patient
contact with the black plastic border of the electrode during the
treatment to eliminate the risk of burning.
5. Do not use the equipment improperly: it can be dangerous. Always
check the user’s manual.
6. The EHY device should not operated by staff during pregnancy and also
not advised to treat pregnant patients. It can cause abortion.
7. It is suggested to remove extra fluid (e.g. ascites, pleural liquid, etc.)
from the treated area before the treatment. Furthermore, it is suggested
to empty the urinary bladder, stomach, rectum before treatments in the
area.
8. Extra care is necessary when patient has surgery clips in the treated
volume.
9. Extra care is necessary when the patient has a diathesis of convulsion
(epileptic).
10. Attention is necessary when the patient is allergic to the electric field or
electromagnetic effects.

plus
UM - EHY2000plus-R24
17
11. Special care is necessary concerning the patient’s hair in the treated
area if the patient has hair (e.g. pubic hair or at head-hair or hair on
breast (for men)), because the burning and the mistreating is very likely.
Please shave the treated area before treatment if necessary, or at least
please make very tight control of the treatment, using small power for a
longer time. If you are not able to shave the treated area, please put
ultrasound/ECG gel on the hair for better contact. Please ask the
patient about their cavities (bladder, stomach, pleural cavity, etc.)
sensing. Stop the treatment immediately if anything unusual happens
near the cavities, and continue it only when the hair is removed.
12. Check the patient and ask about their feelings frequently.
13. Check the water-cooling of the electrode before positioning it.
14. At rearranging and/or positioning the applicator, please pause/stop the
treatment.
15. In case of any necessary medical aid (injection, infusion, etc.) please
pause/stop the treatment.
16. Stop the treatment immediately if anything unusual happens (eg.
eritema, burning, etc.) and ask help of a trained doctor if it is needed.
17. Be careful with temperature measurements and other controlling units.
Any metallic part could be an antenna. Using any non-Oncotherm
product to control is prohibited. Do not use any system-independent
electric device during the treatment. It can cause electric shock due to
the broken safety isolation.
18. Credit-cards and/or any other magnetically sensitive products
(diskettes, tapes, etc.) are recommended not to be kept near the
treatment. No guarantee of not losing the information from the datacarriers.
19. Do not treat near the eyes of the patient. The direct RF-radiation can
cause temporary or permanent blindness. The treatment of the head
requires special training at one of the OncoTherm reference clinics. For
brain treatment, please follow the protocol detailed in Appendix 8 of
the User’s manual.
20. Do not clean the electrodes while the equipment is on! Do not use such
wet textile-tissue that could release water which might penetrate into
any part of the equipment!

plus
UM - EHY2000plus-R24
18
21. The optimal placement of the applicators is parallel to each other. Such
an arrangement gives the most effective heating power. Note that in
many cases only a small power is required for the treatment (for
example when treating a brain tumour) which can be regulated by the
output power control or by placing the electrodes in a non-parallel
arrangement. The patient must be between the electrode and counter
electrode placed into the waterbed.
22. Do not wrap the electrode into any textile material for the treatment.
Reduce the thickness of any textile material to minimum between the
electrode and the patient’s skin. Textile quality has an effect on the
tuning frequency which can lead to system error in tuning under the
given circumstances. Ideally, isolate the patient’s skin from the
electrode surface using medical hygienic paper (see detailed
description in Accessories and Appendix 9 for datasheet) – or other
medical hygienic paper with CE mark, between the bare skin and the
electrodes. Any other material can have an impact on the device
tuning.
23. During treatment, relaxing (so called 'alpha') music is suggested for
psychological reasons (faster recovery is possible).
23. This kind of radio-frequency treatment has an effect on the
surroundings. This is why it is important to pay attention to setting up the
treatment system and the furnishing of the treatment room - in which
treatments will take place. Do not install the machine in the vicinity of
any sensitive equipment (ECG, EEG, intensive-care control-monitor,
ultra-sound, video-rectoscopy and/or other sensitive imaging systems,
etc.) without shielding. Shielding is also required to protect the EHY2000plus in the vicinity of large electro-magnetic sources and/or highpower machines (power transformer, X-ray units, NMR, CT, etc. It should
be noted that microwaves could influence the Oncotherm device in
the treatment room and vice-versa. Make sure that these machines are
well shielded.
24. It is optimal if the device is separated from the control-computer. The
central computer should be placed outside the treatment room. An
observation room has to be arranged from where the personnel can
control the patient's treatment.
25. The personnel, responsible for the treatment/equipment, should check
the cables before each treatment. At any doubt about the intact
isolation, stop the treatment and call for an immediate service checkup.
26. Clean the electrodes before each treatment. Follow the procedure
written in the “disinfecting the accessories” part of this user’s manual.
Alcohol (or its substitute) has to be used for disinfecting.

plus
UM - EHY2000plus-R24
19
27. Before the treatment any sharp objects (knives, scissors, needles, pens
pencils, glasses etc.) have to be left far away from the waterbed.
28. Using waterbed mattress without checking its sufficient amount of water
can be dangerous, the patient could be burnt.
29. The water in the mattress must be heated up before the treatment. You
can find its detailed description in the ‘Preparation before treatment’
section of this user’s manual.
30. Accessory equipment connected to the analogue and digital
interfaces must be certified according to the respective IEC standards
(e.g. IEC 60950 for data processing equipment and IEC 60601-1 for
medical equipment). Furthermore, all configurations shall comply with
the system standard IEC 60601-1-1. Everybody who connects additional
equipment to the signal input part or signal output part configures a
medical system, and is therefore responsible that the system complies
with the requirements of the system standard IEC 60601-1-1. If in doubt,
consult the technical service department or your local representative.
31. Oncothermia does not substitute the conventional therapies only
supports those.
Resident risks:
The user has to follow the instructions mentioned above otherwise burning or
overheating of the tissue can occur.
The temperature calculation (displayed temperature) is only a calculation. Note,
Bear in mind that the temperature of some points of the heated local area can be
considerably higher than the average. This effect is called “hot spots” in the
literature. This inaccuracy can cause overheating of the tissue.
The calculation however is based on the so-called “equivalent temperature”
idea. This means that Electro-Hyperthermia heats up the tissue by a dynamic,
gradient method. The calculated temperature - has the same distortion ability of
the cells as the static overheating. This temperature (due to the dynamical
effects) in reality could be lower than the statically measurable one. Tolerated
accuracy of forwarded and reflected power (±30%) is checked at the start but is
not checked continuously during the treatment. This can cause overheating of
the tissue.

plus
UM - EHY2000plus-R24
20
Written consent for the treatment
According to medical regulation in some countries, a written consent has to be
signed by the patient before the start of the first treatment. This consent has to
contain the following points:
a. Clear capacity (or ability) to make the decision.
b. The medical provider must disclose information on the treatment,
test, or procedure in questions, including the expected benefits
and risks, and the likelihood (or probability) that the benefits and
risks will occur.
c. The patient must comprehend the relevant information.
d. The patient must voluntarily grant consent, without coercion or
duress.
Doctors must give information to the patients about a particular treatment or test
so that the patient can decide whether or not they wish to undergo the
treatment or the test. This process of understanding the risks and benefits of
treatment is known as informed consent. It is based on the moral and legal
premise of patient autonomy: Patients have the right to make decisions about
their own health and medical conditions.
• The patient must give their voluntary, informed consent for the
treatment and for most medical tests and procedures. The legal term for
failing to obtain informed consent before performing a test or
procedure on a patient is called battery (a form of assault).
• For many types of interactions (for example, a physical exam with your
doctor), the implied consent is assumed.
• For more invasive tests or for those tests or treatments with significant
risks or alternatives, you will be asked to give explicit (written) consent.
• Under certain circumstances, there are exceptions to the informed
consent rule. The most common exceptions are these:
I. An emergency in which medical care is needed
immediately to prevent serious or irreversible harm.
II. Legal incompetence in which someone is unable to
give permission (or to refuse permission) for testing or treatment.
For clear orientation the FDA (USA) and MHRA (UK) guidelines for written consent
are attached in Appendix 7.
plus
UM - EHY2000plus-R24
21
Device control
The Oncotherm EHY-2000plus is devoted to the high level requirements of modern
medical practice. The equipment is isolated from the common power-network for
safety purposes and supported by a specially developed software.
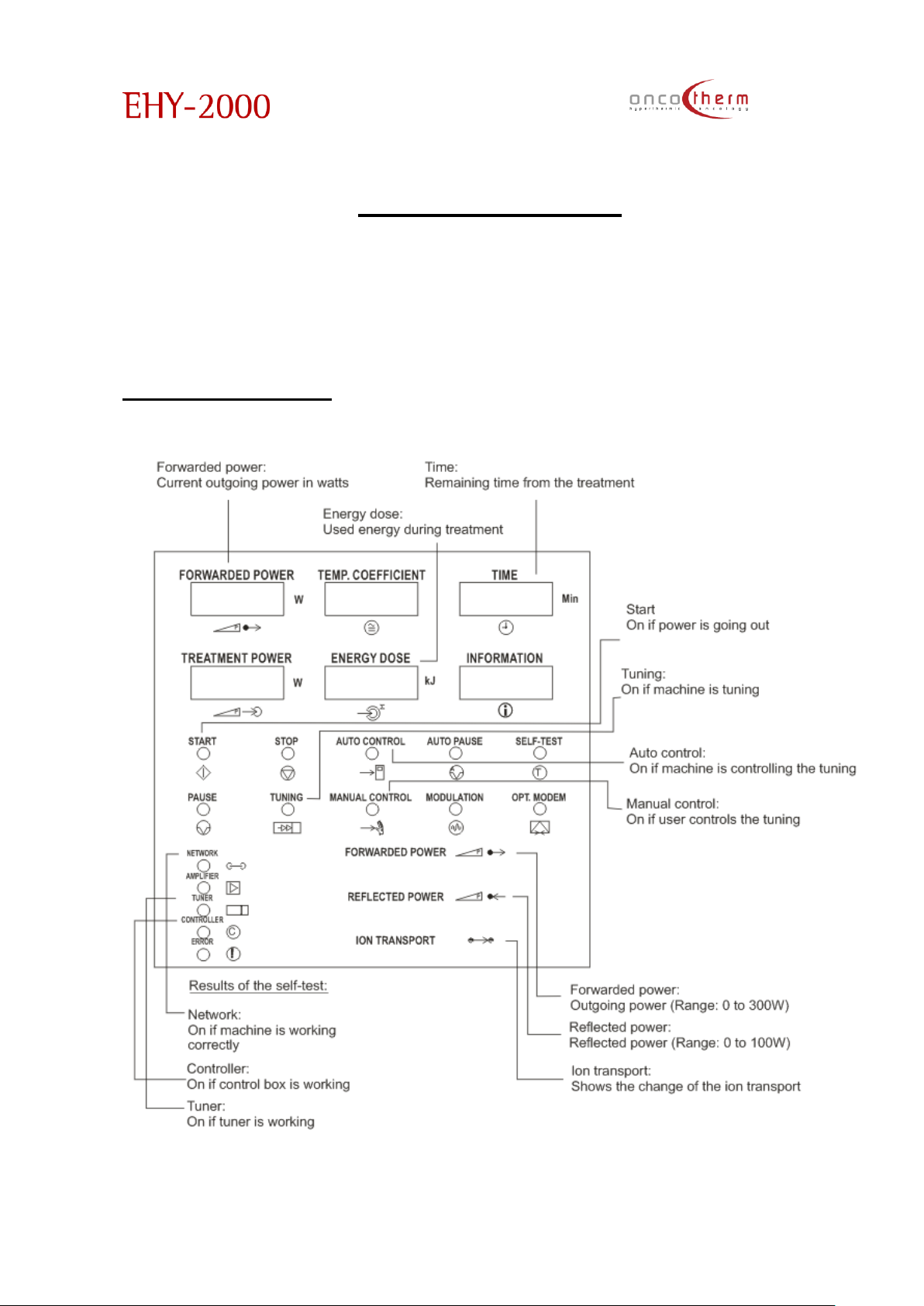
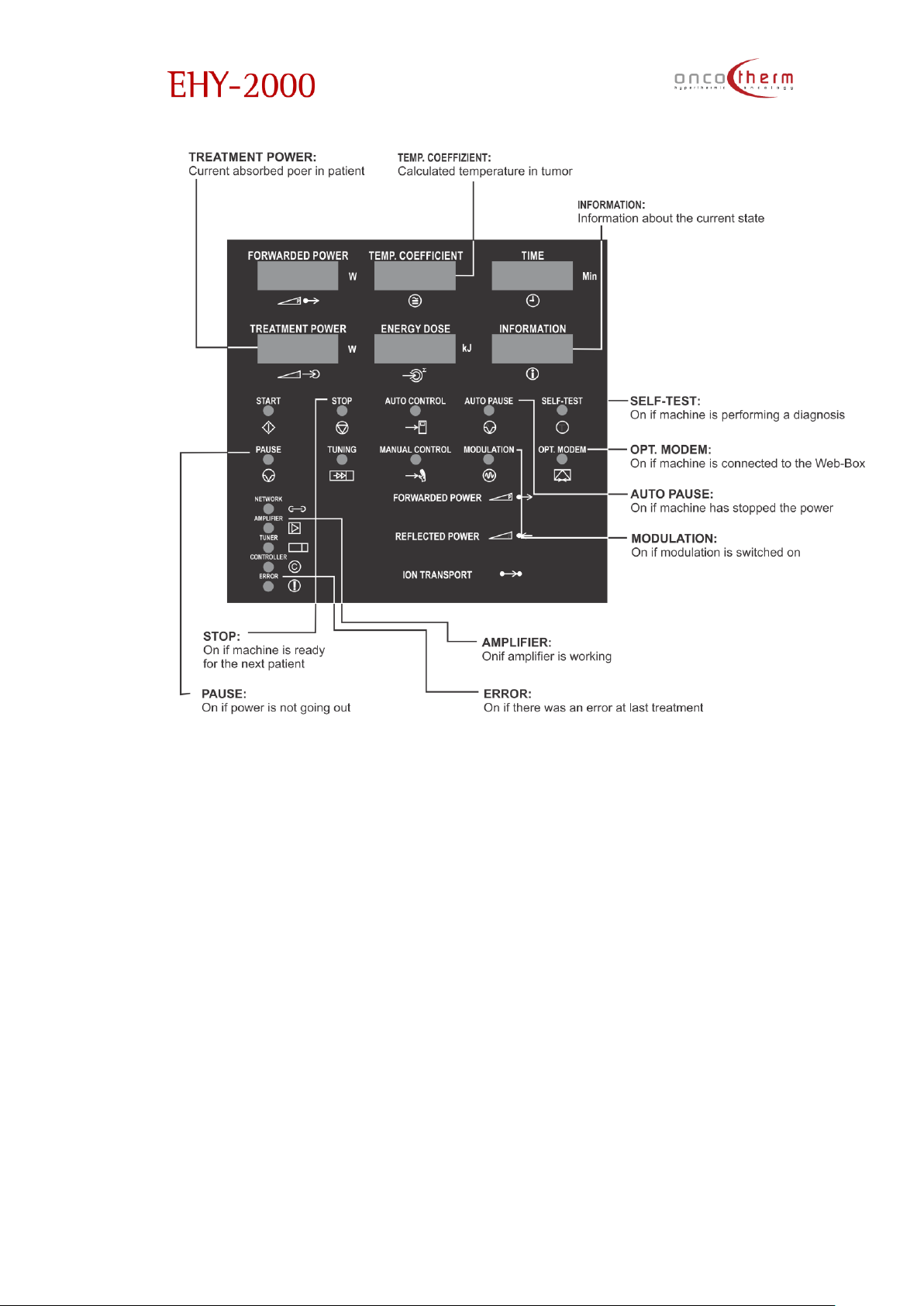
Display-EHY-2000plus
plus
UM - EHY2000plus-R24
22
The “temperature coefficient” s equivalent with the static temperature, which does the same distortion which
is the actual distortion rate in the tissue. In this meaning the temperature coefficient is the parameterization of the
actual distortion rate in the tumor.
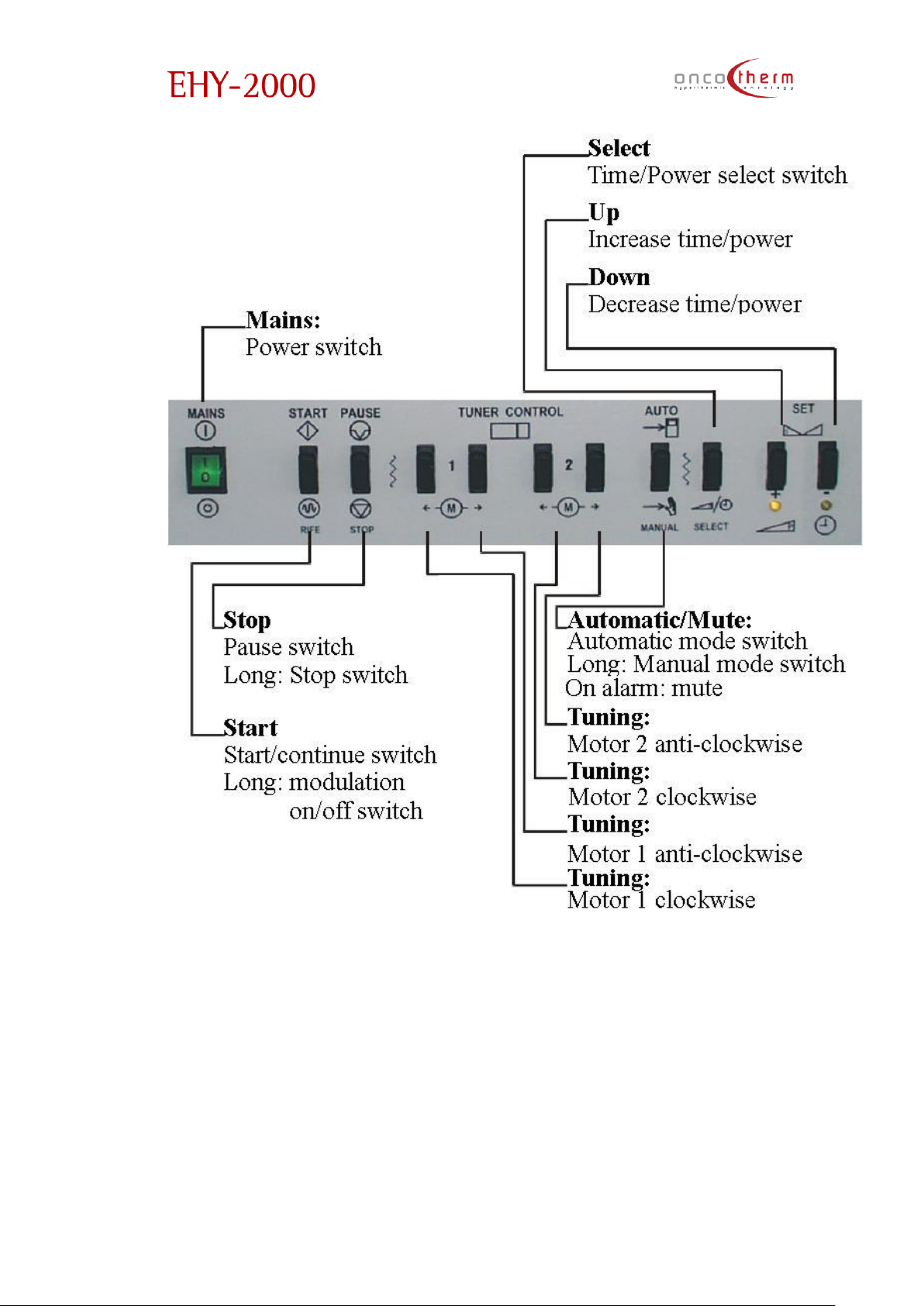
plus
UM - EHY2000plus-R24
23
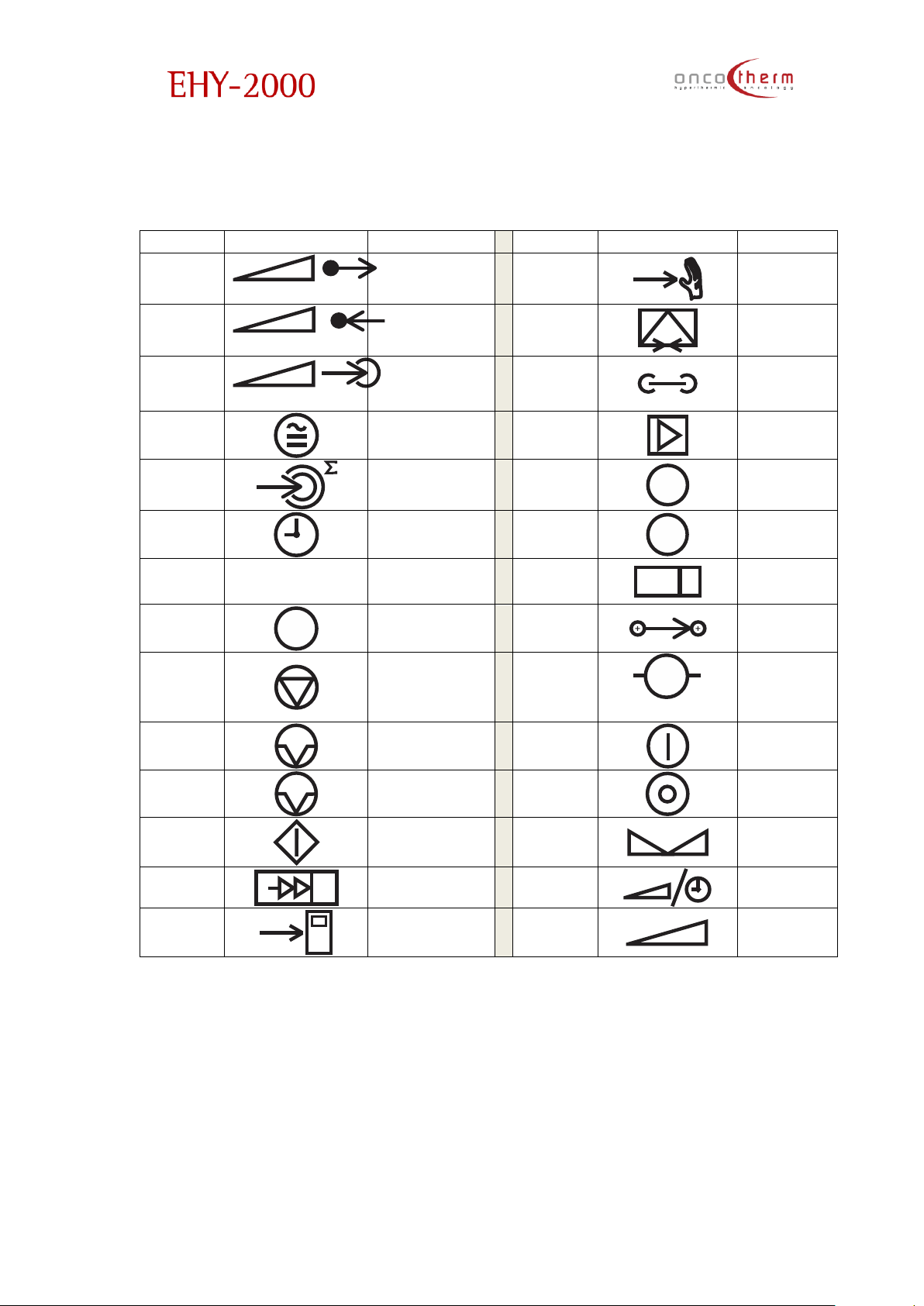
plus
UM - EHY2000plus-R24
24
The actual denotations are:
German:
English German
English
German
Forwarded
Power
P
Leistungsabgabe
Manual
Control
Manual mode
Reflected
Power
P
Leistungsreflexion
Opt.
Modem
Opt. Modem
Treatment
Power
P
Leistungaufnahme
Network
Netz
Temp.
Coefficient
Temp. Koeffizient
Amplifier
Verstärker
Energy
dose
Energiemenge
Controller
C
Controller
Time Zeit
Error
!
Fehler
Information
Information
Tuner
Tuner
Self-test
T
Test
Iontransport
Ionen
transport
Stop Stop
Tuner
➔
M
Tuner
Pause Pause
Mains Netz
Auto Pause
A
Pause
Mains Off
Netz aus
Start Start
Set
S
Einstellung
Tuning Tuning
Select
Time/Power
P
Zeit/Leistung
Wahl
Auto
Control
Auto mode
Power
P
Leistung
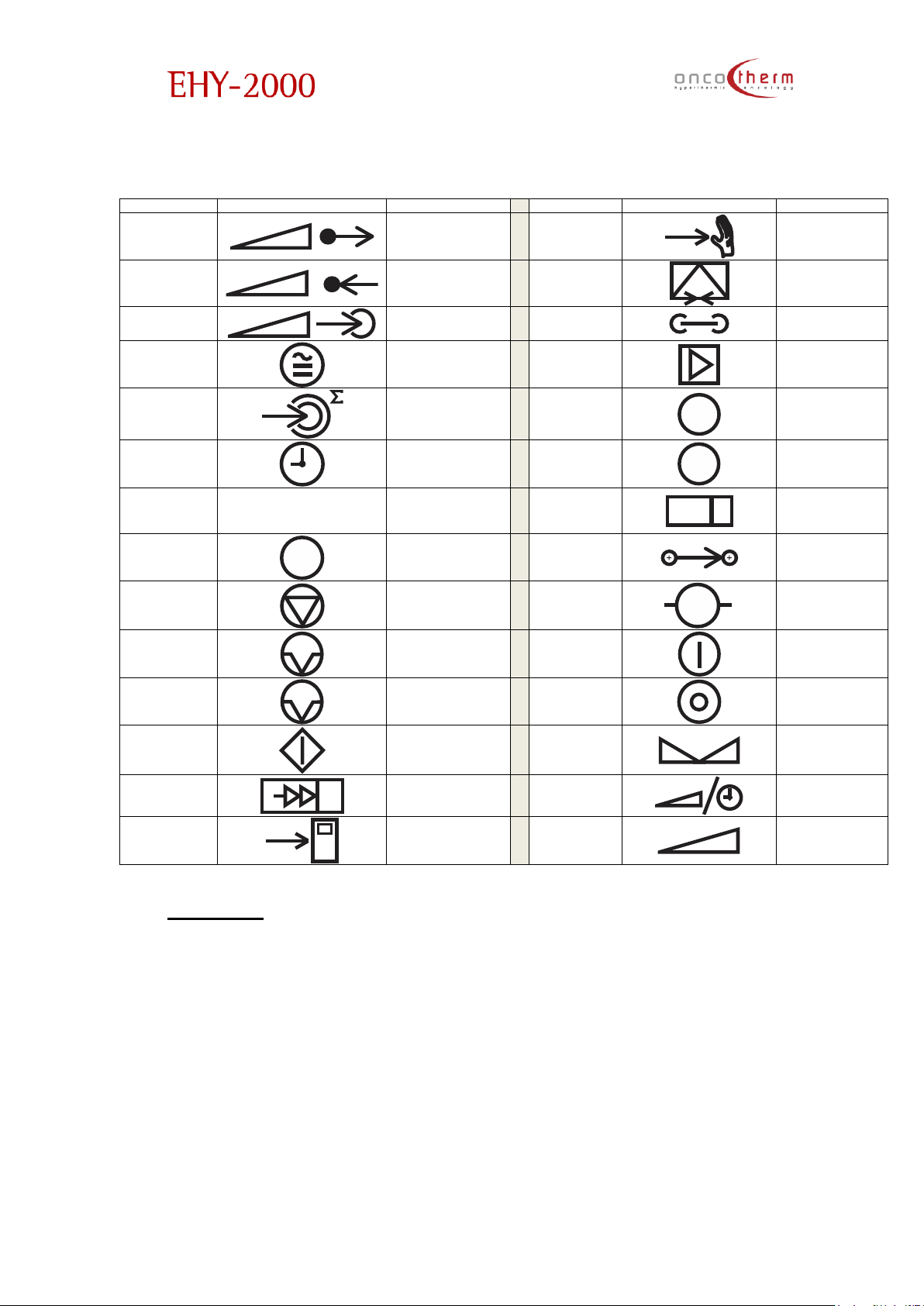
plus
UM - EHY2000plus-R24
25
Hungarian:
English
Hungarian
English
Hungarian
Forwarded
Power
P
Kiadott teljesítmény
Manual
Control
Kézi vezérlés
Reflected
Power
P
Visszavert
teljesítmény
Opt. Modem
Optikai Modem
Treatment
Power
P
Kezelési
teljesítmény
Network
Belső hálózat
Temp.
Coefficient
ECM hőmérséklet
Amplifier
Erősítő
Energy dose
Energia mennyiség
Controller
C
Vezérlő
Time Idő
Error
!
Hiba
Information
Információ
Tuner
Kézi hangolás
Self-test
T
Belső teszt
Iontransport
Ion transzportáció
Stop Leállítás
Tuner Motor
➔
M
Hangoló motor
Pause Szünet
Mains On
Hálózat be
Auto Pause
A
Automatikus szünet
Mains Off
Hálózat ki
Start Índítás
Set
S
Beállítás
Tuning
Automatikus
hangolás
Select
Time/Power
P
Idő/teljesítmény
választás
Auto Control
Automatikus
vezérlés
Power
P
Teljesítmény
Keyboard
Some keys may have many functions depending on the actual mode of the
device.
Power: Here you can switch on and off the device. If the device is switched
on the switch should lighten up. Note: if an emergency button is pressed –
that is indicated by a red light on the front panel – then the device cannot
be switched on.
Start/ReTune: The treatment can be started by pressing this button. By doing
it, the treatment starts and an automatic tuning also starts to set the correct
 Loading...
Loading...