Page 1

Pain Reliever
E3 Intense (HV-F021-EW)
E3 Intense (HV-F021-ESL)
Instruction Manual
• Pain Reliever. Instruction Manual.
• Appareil anti-douleur. Mode d’emploi.
• Schmerztherapiegerät. Gebrauchsanweisung.
• Dispositivo per il trattamento del dolore. Manuale di istruzioni.
• Dispositivo para alivio del dolor. Manual de instrucciones.
• Apparaat ter verlichting van pijn. Gebruiksaanwijzing.
• ɗɥɟɤɬɪɨɧɟɣɪɨɦɢɨɫɬɢɦɭɥɹɬɨɪ ɞɥɹ ɨɛɟɡɛɨɥɢɜɚɧɢɹ.
Ɋɭɤɨɜɨɞɫɬɜɨ ɩɨ ɷɤɫɩɥɭɚɬɚɰɢɢ.
• A÷rÕ Giderici. KullanÕm KÕlavuzu.
IM-HV-F021-E-05-03/2018
EN
FR
DE
IT
ES
NL
RU
TR
AR
9200776-0E
Page 2

Table of contents
Before using the unit
Introduc tion ........................................................................................................................................3
Intended use ......................................................................................................................................3
Important safety precautions and warnings ......................................................................................4
Know your unit ...................................................................................................................................8
Package contents ..........................................................................................................................8
Optional medical accessories .......................................................................................................8
Buttons and their functions ............................................................................................................9
Operating instructions
Assembly steps ................................................................................................................................10
STEP 1 – Insert batteries ............................................................................................................10
STEP 2 – Attach the electrode cord to the main unit ..................................................................10
STEP 3 – Snap either electrode cord to each of the pads ..........................................................11
STEP 4 – Remove and discard plastic ¿ lm from pads (in case of new pads) ............................11
STEP 5 (Optional) – Attach the unit to the belt clip .....................................................................11
Get started with your therapy .........................................................................................................12
STEP 1 – Pad placement ............................................................................................................12
STEP 2 – Select 1 of 9 modes ....................................................................................................15
STEP 3 – Select the correct intensity level (1 low – 15 high) ......................................................16
How to manage and reduce your pain .............................................................................................17
When should I start therapy? ......................................................................................................17
How long should you use it? .......................................................................................................17
When to stop using the unit? .......................................................................................................17
Care and maintenance
Cleaning and storage .......................................................................................................................17
Cleaning the pads ........................................................................................................................17
When should you replace your pads? ........................................................................................18
Cleaning the unit ..........................................................................................................................18
Storing the pads ...........................................................................................................................18
Storing the unit and pads .............................................................................................................18
Troubleshooting ...............................................................................................................................19
Technical data ..................................................................................................................................21
Important information regarding Electro Magnetic Compatibility (EMC) ....................................... 23
Warranty ...........................................................................................................................................24
2
Page 3
Introduction
Thank you for purchasing OMRON E3 Intense.
In order to use the unit safely, read the complete manual carefully before using the unit for the ¿ rst
time.
How does it work?
E3 Intense uses Triple Action TENS (Transcutaneous Electrical Nerve Stimulation) technology that
helps to:
- block the pain message
- trigger the release of endorphins (natural pain killers)
- improve the blood circulation (as result of repeated muscle contracting and relaxing)
Intended use
Medical purpose
OMRON E3 Intense is a Pain Reliever intended for reducing and relieving muscles and joints pain,
stiffness and numbness in the back, arms, legs, shoulders and feet by applying electrical nerve
stimulation to the surface of the skin near the site of the pain. It should be applied to normal, healthy,
dry, and clean skin of adult patients.
Any of the modes can safely be used on body parts or pains described in this manual. Just ¿ nd one
that feels good and is comfortable on your pain. It can be successfully used in conjunction with any
other pain treatment or medication.
Since the 1970s, pain relief based on TENS has been widely used by many healthcare
professionals, such as physiotherapists and pain specialists.
Intended User
This unit is intended to be operated by adults who can understand this instruction manual. It is not
for professional use in hospitals or other medical facilities, it is intended for home use only.
EN
3
Page 4
Important safety precautions and warnings
It is important that you read all the warnings and precautions included in this instruction
manual because they are intended to keep you safe, prevent injury and avoid a situation that
could result in damage to the unit.
SAFETY SYMBOLS USED IN THIS INSTRUCTION MANUAL
DANGER
WARNING
CAUTION
Do not use this unit with these other devices:
(1) If you have a pacemaker, implanted de¿ brillator, or other implanted metallic or electronic device.
Such use could cause electric shock, burns, electrical interference or death.
(2) Do not use this device while using another TENS device.
(3) Together with a life-supporting medical electronic device such as an arti¿ cial heart or lung or respirator.
(4) For Hospitals and Clinics, in the presence of or when attached to the body, electronic monitoring equipment
(e.g. cardiac monitors, ECG alarms), which may not operate properly when the electrical stimulation device is in
use.
(5) For Hospitals and Clinics, simultaneous connection of a patient to a high frequency surgical Medical Electronic
equipment may result in burns at the site of the stimulator electrodes and possible damage to the stimulator.
(6) For Hospitals and Clinics, operation in close proximity (e.g. 1 m) to a shortwave or microwave therapy Medical
Electronic equipment may produce instability to the stimulator output.
Do not use this unit under these conditions
Consult with your healthcare professional before using this unit. The unit may cause lethal rhythm
disturbances in certain susceptible individuals. If you have had a recent surgical procedure, the
stimulation may disrupt the healing process.
Do not use on these individuals
Pregnant women.
Do not use on children or infants because this device has not been evaluated for pediatric use.
Keep out of reach of young children because the electrode cord could cause strangulation.
Persons incapable of expressing their thoughts or intentions.
Persons incapable of operating the unit by themselves.
Use caution if you have a tendency to bleed internally, such as following an injury or fracture.
If you have suspected or diagnosed heart disease, you should follow precautions recommended
by your healthcare professional.
If you have suspected or diagnosed epilepsy, you should follow precautions recommended by
your healthcare professional.
Improper use may cause danger resulting in death or serious injury. These are situations
in which the device should not be used.
Indicates a potentially hazardous situation which, if not avoided, could result in death or
serious injury.
Indicates a potentially hazardous situation which, if not avoided, may result in minor or
moderate injury to the user or patient or damage to the equipment or other property.
DANGER
4
Page 5
Important safety precautions and warnings
Use caution if stimulation is applied over the menstruating uterus.
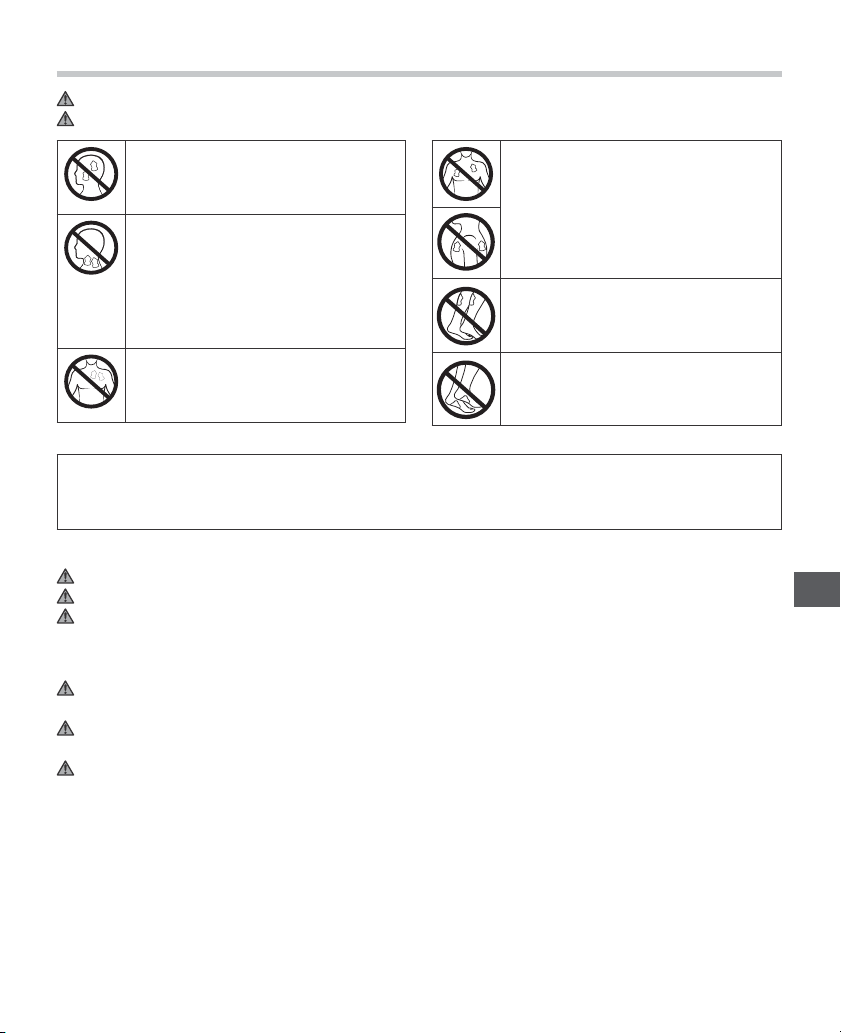
NEVER APPLY THE PADS TO THESE BODY AREAS:
The head, the mouth, or any area
of the face.
The neck or any area of the throat
because this could cause severe
muscle spasms resulting in closure
of the airway, dif¿ culty in breathing,
or adverse effects on heart rhythm
or blood pressure.
Do not use near the heart, or on
genital area.
Open wounds or rashes or over swollen, red, infected or inÀ amed areas or skin eruptions (such
as varicose veins, phlebitis, thrombophlebitis and thrombosis), or on top of or close to cancerous
lesions, or over areas of skin that lack normal sensation.
Both sides of the thorax
simultaneously (lateral or front
and back), or across your chest
because the introduction of
electrical current may cause rhythm
disturbances which could be lethal.
On the calves of both legs at the
same time because this may cause
cardiac disturbance.
On the bottom of both feet at the
same time because this may cause
cardiac disturbance.
Do not use this unit during these activities
When in the bath or shower;
While sleeping;
While driving, operating machinery, or during any activity in which electrical stimulation can put
you at risk of injury.
Pain management warnings
If you have had medical or physical treatment for your pain, consult with your healthcare
professional before using this unit.
If your pain does not improve, becomes seriously chronic or severe, or continues for more than 5
days, stop using the unit and consult with your healthcare professional.
The mere existence of pain functions as a very important warning telling us that something is
wrong. Therefore, if you suffer from any serious illness, consult your healthcare professional in
order to con¿ rm that it is advisable for you to use this OMRON E3 Intense.
EN
5
Page 6
Important safety precautions and warnings
Do not alter the unit
Do not plug this cord into any other device that is not an OMRON E3 Intense.
No modi¿ cation of this unit is allowed.
Use this unit only with the leads, electrodes, and accessories recommended by the manufacturer
to avoid damage to the unit.
Warnings regarding the pads
Apply pads to normal, healthy, dry, and clean skin (of adult patients) because it may otherwise
disrupt the healing process.
If you experience any skin irritation or redness after a session, do not continue stimulation in that
area of the skin.
Precautions regarding the pads
Do not move the pads to another location while the unit is on.
Therapy won’t work with just 1 pad. You must use 2 pads at the same time.
Make sure the components are connected well and the pads are ¿ xed on the part of the body you
wish to treat or the therapy may not be effective.
Pads should not touch any metal object, such as a belt buckle, necklace, or other metal worn
under clothing.
Do not overlap pads or put pads on top of each other. It may weaken or stop therapy, or the unit
may stop working.
Gel pads may also stick together and cause gel to be removed when separating.
Do not share pads with another person. This may cause a skin irritation or infection. Pads are
intended for use by one person.
Do not leave pads attached to the skin after treatment.
Do not bend or fold because the gel may get damaged and it won’t stick or function properly.
To avoid damage to the adhesive surface of the pads, put the pads only on the skin or on the
plastic pad holder provided to avoid damage to the adhesive surface of the pads.
Always place clean pads in accordance with illustrations provided (Refer to pages 12-14, Pad
Placement).
Do not apply ointment or any solvent to the pads or to your skin because it will disrupt the pads
from functioning properly. The self-adhesive pads will adhere to your skin.
Place pads at least 3 cm apart for optimal results.
Caution while using the unit
Main unit
If the unit is not functioning properly or you feel discomfort, immediately stop using the unit.
Do not use for any other purpose except for what it is intended for.
Do not place in a room with high humidity, such as a bathroom. This will damage the unit. Ideal
temperature for using +10 - +40 °C, 30 - 80 % relative humidity.
Do not use the unit without proper lighting. You may not be able to operate the unit successfully.
While using this device, make sure that no mobile phone or any other electrical devices that
emit electromagnetic ¿ elds is within 30cm. This may result in degradation of performance of the
device.
6
Page 7

Important safety precautions and warnings
Cord
Do not insert the electrode plug into any place other than the jack on the unit.
Do not pull on the electrode cord during treatment. Do not bend or pull the end of the cord.
When pulling out the cord from the unit, hold the plug and pull.
Replace the cord when broken or damaged.
Battery
Do not throw the batteries into a ¿ re. The batteries may explode.
Dispose of the unit, batteries, and components according to applicable legal regulations.
Unlawful disposal may cause environmental pollution.
Do not mix alkaline and manganese batteries as this will shorten the battery life.
During therapy, do not remove the battery cover and do not touch the battery terminals.
Use the battery within recommended period mentioned to it.
Pads
Detach the pads before replacing the batteries.
General precautions
The long-term effects of electrical stimulation are unknown.
Apply stimulation to only normal, intact, clean, dry, and healthy skin.
TENS is not effective in treating the original source or cause of the pain, including headache.
TENS is not a substitute for pain medications and other pain management therapies.
TENS devices do not cure disease or injuries.
TENS is a symptomatic treatment and, as such, suppresses the sensation of pain that would
otherwise serve as a protective mechanism.
You may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical
conductive medium (gel).
Keep the unit away from young children. The unit contains small pieces that may be swallowed.
Immediately contact your healthcare professional.
Possible adverse reactions
You should stop using the unit and consult with your healthcare professional if you experience
adverse reactions from the unit.
You may experience skin irritation beneath the stimulation electrodes applied to your skin.
Do not use to treat one region for extended periods of time (more than 2 × 15 minutes a session,
up to 3 times/day) or muscles in that region may become exhausted and sore.
EN
7
Page 8
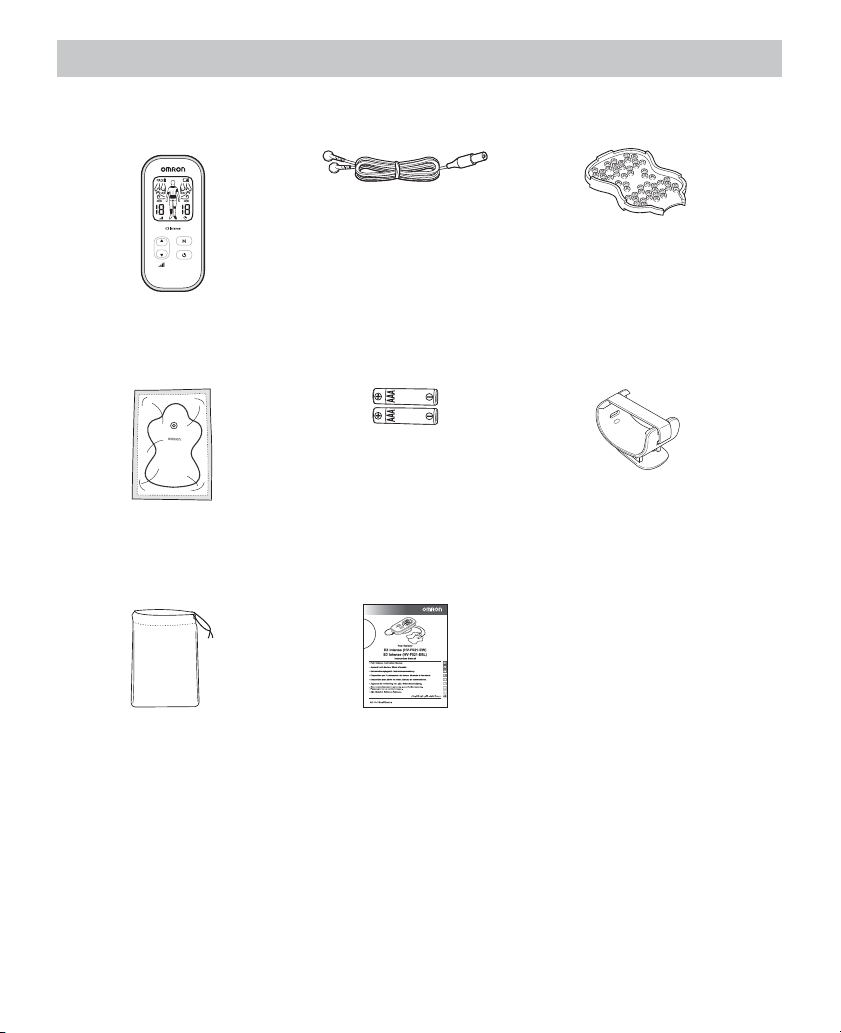
Know your unit
Package contents
Main unit Electrode cord Pad holder
Long Life Pads Batteries for trial use Belt clip
Soft pouch Instruction manual
IM-HV-F021-E-05-03/2018
9200776-0E
Optional medical accessories
(within the scope of EC Medical Device Directive 93/42/EEC)
• Long Life Pads
8
Page 9

Buttons and their functions
Know your unit
Pad Icon
If the pad dislodges, the pad icon will
appear.
Pad icon displays if the pad falls off.
15 Intensity Levels
(1 low to 15 high)
Intensity Button
Set according to your needs.
Press Ÿ for higher intensity.
Press ź for lower intensity.
Battery Icon
If the battery is low, the battery
icon will display.
: Battery is close to low.
: Battery is low.
Minutes remaining of therapy
Automatic 15 minute shut off.
Mode Button
Select 1 of the 9 pre-set modes that
feels good on your pain. Mode type
will show on screen.
Power Button
Push once for “ON” and again for
“OFF”.
EN
9
Page 10

Assembly steps
Before using, check these points to make sure everything is working properly.
1. The cord is not broken.
2. The pad adhesive sticks and is not damaged.
3. The electrode cord connection is not broken.
4. The unit is intact and in working order.
5. There is no battery leakage.
STEP 1 – Insert batteries
1. Remove the battery cover on the back using a coin.
2. Insert batteries. Make sure the
correspond when inserting batteries.
3. Reinstall the battery cover. Tighten with a coin.
signs
STEP 2 – Attach the electrode cord to the main unit
Attach the electrode cord plug to the bottom of the main unit.
Battery cover
10
Page 11

Assembly steps
STEP 3 – Snap either electrode cord to each of the pads
For the ¿ rst time, take the pads out of the sealed package.
Do not turn the unit on, until pads are on your skin.
You must use both pads or stimulation will not work.
STEP 4 – Remove and discard plastic ¿ lm from pads (in case of new pads)
Remove the clear plastic ¿ lm from the back of the pad.
Discard the plastic ¿ lm backing as well as the clear packaging.
STORING PADS ON PAD HOLDER
After using, put sticky side of pads on either side of the pad
holder.
STEP 5 (Optional) – Attach the unit to the belt clip
The clip may fall off if attached to soft or thin clothing.
11
Pad facing down
Pad Holder
Pad sticky side up
EN
Page 12

Get started with your therapy
(Use for a maximum of 2 x 15 minutes per session)
STEP 1 – Pad placement
For optimal therapy:
Place pads on either side of the pain, not directly on the pain.
Place 2 pads at least 3 cm apart.
Do not overlap pads or put on top of each other.
Do not add spray, lotions or creams to skin or pads.
Do not share pads.
Before starting your therapy, rate your pain from 1 low to 15 high. This mental check gives you a
basis you can compare to once the session is complete.
Examples of pads application
LOWER BACK
A B
A. Attach both pads on the lower back according to your pain.
Place pads on muscle of back, not on spine, for optimal therapy.
B. Attach 1 pad below and above the region in pain, both on same side.
ARM
Attach both pads on either side of the area with pain.
12
Page 13

JOINT (ELBOW)
Attach both pads on either side of the joint with pain.
JOINT (KNEE)
Attach both pads above the knee or above and below the
joint with pain.
LEG (HIP & THIGH)
Attach both pads on either side of the area with pain.
Get started with your therapy
EN
LEG (CALF)
Attach both pads on the calf with pain.
Pads should not be placed simultaneously on the
calves of both legs.
13
(continued)
Page 14

Get started with your therapy
STEP 1 – Pad placement (continued)
FOOT (ANKLE)
Attach the pads on the left for pain on the outside of your
ankle/foot. Attach the pads on the right for pain on the inside
of your ankle/foot.
Do not put the pads on the bottom of both feet at the
same time.
SHOULDER
AB
A. Attach both pads on the shoulder according to your pain.
B. Attach 1 pad on the front and on the back of your shoulder.
Do not use near the heart, on both sides of the thorax or across your chest because the
introduction of electrical current may cause rhythm disturbances which could be lethal.
Outside
Inside
14
Page 15

Get started with your therapy
STEP 2 – Select 1 of 9 modes
• Push “ ”.
• Choose 1 of the 9 modes. Modes cannot be combined.
Choose a massage-like mode
1. Tapping
2. Kneading
3. Rubbing
How to switch modes
The unit automatically defaults to the last mode selected. Each time you push the mode button
(“ ”), it switches to the next mode at the lowest intensity. You can only use ONE MODE at a time.
How to select the right mode
Any of the modes can be used on body parts or pains described in this manual.
Select the mode that feels right for your unique pain.
Arm Lower back Leg Foot
Or choose a pain mode:
4. Arm
5. Lower Back
6. Leg
7. Fo o t
8. Joint (Knee/elbow/wrist)
9. Shoulder
Push “ ”
Push “ ”
EN
Therapies
designed for
Potential
conditions
Sensation Series of low to
Arm Lower back Leg Foot
Swelling, stiffness,
sore or achy, muscle
or nerve pain.
medium rate tapping,
tingling and pulsing
sensations.
Stiffness, soreness,
muscle or nerve pain.
Series of high
rate to low tingling
sensations, followed
by tapping. With
higher intensity, you
may feel kneading
or massage-like
sensations.
15
Swelling, fatigue,
stiffness, muscle or
nerve pain.
Series of low to
medium tapping and
rubbing sensations.
Swelling, fatigue,
chilly feeling, sore or
achy.
Series of low rate
tapping, pulsing
sensations.
(continued)
Page 16

Get started with your therapy
STEP 2 – Select 1 of 9 modes (continued)
Joint Shoulder Tap Knead Rub
Therapies
designed for
Potential
conditions
Sensation Series of
Joint Shoulder Tap Knead Rub
Swelling,
stiffness, sore or
achy.
medium to
high rate
tapping, pulsing
sensations.
Stiffness, sore
or achy, tight
feeling.
Series of low
to high rate
tapping, pulsing,
kneading and
massage-like
sensations.
Stiffness,
soreness, tight
feeling.
Series of low
rate tapping
sensations.
Stiffness, sore
or achy, knotty
muscles, tight
feeling.
Series of
medium
rate pulsing
sensations to
mimic massage.
Stiffness, sore
or achy, knotty
muscles, tight
feeling.
Series of high
rate pulsing
sensations to
mimic hands
rubbing.
STEP 3 – Select the correct intensity level (1 low – 15 high)
Start at the lowest intensity level and slowly increase it by pushing the “Ÿ(Up)” arrow button. You
should feel a gentle pulsing sensation.
How do I pick the right intensity level for my pain?
Each time you push “Ÿ(Up)” or “ź(Down)” arrow, it moves to another level. If the stimulation
sensation becomes weaker or disappears, increase the intensity until it is restored. But, if the
sensation is at all uncomfortable, press the down arrow to decrease the intensity.
• Press Ÿ for higher intensity.
• Press ź for lower intensity.
How long is the therapy?
The unit will continue automatically for 15 minutes
before it shuts off. We recommend a total of 2 ×
15 minutes therapy in one sitting, up to 3 times/day.
The screen shows you how many minutes are remaining.
Intensity
Level
Minutes left out
of 15 minutes
16
Page 17

How to manage and reduce your pain
When should I start therapy?
Use as soon as your pain begins. Start with one session (the unit automatically turns off at 15
minutes). Turn off with pads still on and rate your pain again.
How long should you use it?
Start with one 15 minute session. Always turn the unit off with pads still on. Rate your pain to check
your progress. Stop therapy session if pain has reduced or stopped. Press “ ” button to continue
therapy for another 15 minute session.
1 session Max minutes/session Max times/day
15 minute 2 x 15 minutes 3 times per day
automatic shut-off
See warnings on page 7. Long-time treatment and strong stimulation may cause
muscular fatigue and may generate adverse effects.
When to stop using the unit?
1. If you experienced an adverse reaction (skin irritation/redness/burns, headache or other painful
sensation, or if you feel any unusual discomfort).
2. If your pain does not improve, becomes seriously chronic and severe, or continues for more than
5 days.
Cleaning and storage
The unit is designed for repeated use over time. The pads will last up to 150 uses, or 5 months
(based on use 1/day). Here are important cleaning and storage instructions:
Cleaning the pads
1. Turn the power off and remove the electrode cord from the pads.
2. Wash the pads when the adhesive surface becomes dirty and/or the pads are dif¿ cult to attach.
• Wash the pad softly with your ¿ ngertips under slow running
cold water for several seconds (do not use a sponge/cloth/
sharp object like a nail on the adhesive side, do not use
detergents, chemicals or soap).
EN
3. Pads can be washed after 15 uses, approximately 10 times for up to 150 uses. Do not wash the
pads too long or too frequently. If the adhesive surface becomes sticky or the pad peels off, leave
the pad in the refrigerator (do not freeze) overnight. The adhesion may be restored.
4. Dry the pads and let the adhesive surface air-dry completely. Do not wipe with a tissue paper or
cloth.
5. Pads are replaceable and can be purchased.
17
Page 18

Cleaning and storage
The life of the pads may vary by how often you wash the pads, the skin condition, and how you store
the pads.
When should you replace your pads?
If the pad no longer sticks to your skin or if more than 25 % of the pad’s surface is not in contact with
your skin.
Cleaning the unit
1. Turn the unit off and disconnect the electrode cord from the pads.
2. Clean with a cloth lightly moistened or soaked in a neutral (mild) cleaning solution and wipe
gently.
• Do not use chemicals (like thinner, benzene).
• Do not let water get into the internal area.
Storing the pads
1. Turn the unit off and remove the cord from the bottom of the unit.
2. Remove the pads from your body.
3. Leave the electrode cord connected to the pads.
Place the pads on the pad holder, 1 pad on each side
with the sticky side of each pad on the pad holder.
4. Wrap the electrode cord around the pad holder.
Storing the unit and pads
• Store the unit with the belt clip on.
Store the pads with the electrode cord on the pad holder and put
into the pouch.
• Do not keep in areas subject to direct sunlight, high or low
temperatures, humid area, near to ¿ re, vibration, or shock.
• Do not keep at places that can be easily reached by children.
• When not in use for a long period, remove the batteries before storage,
to avoid liquid discharge from batteries.
• Do not wrap the electrode cord around the unit because it may
damage the cord.
18
Page 19

Troubleshooting
In case of any of the below problems occurs during use, ¿ rst check that no other electrical device is
within 30cm. If the problem persists, refer to the table below.
If this happens... Possible causes... Try this solution...
The intensity is not
felt.
Very weak intensity
level.
The skin turns red
or feels irritated.
No power source. Are the polarities of battery (+ and -)
Power cut off
during use.
Battery icon is
empty or close to
empty.
Pad gel does not
stick to skin.
Are you using only 1 pad?
Have you removed the transparent ¿ lm
from the pads?
Are the pads stacked together or do pads
overlap?
Is the cord properly connected to the unit? Connect the cord plug correctly into the
Is the intensity setting too low? Press the Ÿ up button.
Is the gel damaged? Replace the pads.
Are the batteries weak? Replace both batteries.
Is the adhesive surface of pads dirty or
dry?
Is therapy time too long? Use less than 15 minutes.
Are the 2 pads attached properly to the
body?
Is the pad surface worn out? Replace both pads at the same time.
aligned in the wrong direction? Are the
batteries depleted?
Are the batteries weak?
Is the cord broken? Replace the cord.
Are the batteries weak? Replace both batteries at the same time.
Is the adhesive surface of pads dirty or
dry?
Have you removed the transparent ¿ lm
from the pads?
Is the pads wet? or Is your skin too wet? Air dry the pads. Or dry the skin.
The pad gel may be damaged. Replace the pads.
Are you using the pads during perspiring? Dry the pad placement area.
Have the pads been washed too long and/
or too frequently?
Were the pads stored under high temperature,
high humidity, or direct sunshine?
Put the other pad on your skin. You must
use both pads for therapy to work.
Peel off the ¿ lm on the adhesive sur face
of pads.
Check placement of pads. Refer to pages
12-14, Pad Placement.
jack at the bottom of this unit.
Wash the adhesive surface of pads softly
with your ¿ ngertips for about 3 seconds
under cold slow running water.
Refer to pages 12-14, Pad Placement
and attach correctly.
Check the batteries for correct alignment.
Replace the batteries.
Replace both batteries at the same time.
Wash the adhesive surface of pads softly
with your ¿ ngertips for about 3 seconds
under cold slow running water.
Peel off the ¿ lm on the adhesive sur face
of pads.
Leave the pads in refrigerator (do not
freeze) for overnight.
Replace the both pads.
EN
19
Page 20

If this happens... Possible causes... Try this solution...
Pad icon is
displayed.
Only 1 pad is attached, or both pads are
not attached.
Have you removed the transparent ¿ lm
from the pad?
Is the cord properly connected to the main
unit?
Is the adhesive surface of pads dirty or
dry?
Re-attach dislocated pad(s) onto the skin
¿ rmly. Power off-on after correction.
Peel off the ¿ lm on the adhesive sur face of
pads. Power off-on after correction.
Connect the cord plug correctly into the
jack at the bottom of the main unit. Power
off-on after correction.
Wash the adhesive surface of pads softly
with your ¿ ngertips for about 3 seconds
under cold slow running water. Power
off-on after correction.
2020
Page 21

Technical data
Product Category Electroanalgesic Transcutaneous Stimulator
Product Description Pain Reliever
Model (code) E3 Intense (HV-F021-EW)
Operation Mode Continuous operation
Power Source DC3 V
Battery Life New batteries (2 AAA alkaline batteries) will last for
Frequency Approx. 1 to 238 Hz
PULSE Duration 150 ȝsec
Maximum Output Voltage 70 V (during 500 ȍ load)
Power Control 15 intensity levels
Operating Temperature, Humidity and
Air Pressure
Storage and Transportation
Temperature, Humidity, Air Pressure
Weight Approx. 100 g (incl. batteries)
Outer Dimensions 52 (W) × 112 (H) × 25 (D) mm
Package Contents Main unit, Electrode cord, Pad holder, Long Life Pads,
Classi¿ cations Internally powered (Protection against electric shock)
This OMRON product is produced under the strict quality system of OMRON HEALTHCARE Co.,
Ltd., Japan.
Designed for a minimum of 5 years life expectancy.
E3 Intense (HV-F021-ESL)
(2 AAA alkaline batteries or 2 AAA manganese batteries)
approx. 4 months (when used for 15 minutes a day,
Lower Back Mode, max. intensity).
+10 to +40 °C / 30 to 80 % RH (non-condensing) /
700 to 1060 hPa
-20 to +60 °C / 10 to 95 % RH (non-condensing) /
700 to 1060 hPa
Batteries for trial use, Belt clip, Soft pouch, Instruction manual
Type BF (Applied part; Pads)
IP22 (Ingress Protection)
EN
21
Page 22

Description of symbols that, depending on a model, can be found on the product itself,
product sales package or IM
This product should not be used by persons with medical implants, e.g. heart
pacemakers, arti¿ cial heart, lung or other electronic life support systems.
Applied part - Type BF
Degree of protection against electric shock (leakage current)
IP XX
Ingress protection degree
provided by IEC 60529
Serial number Symbol of Eurasian Conformity
Indication of connector polarity
CE Marking Class II equipment
For indoor use only
Need for the user to consult the
instructions for use
GOST-R symbol
Humidity limitation
Direct current
Date of manufacture
Product production date is integrated in a Serial number, which is placed on the sales
package: the ¿ rst 4 digits mean year of production, the next 2 digits - month of production.
The device ful¿ ls the provisions of the EC directive 93/42/EEC
(Medical Device Directive).
NOTE: These speci¿ cations are subject to change without notice.
IP classi¿ cation is degrees of protection provided by enclosures in accordance with IEC 60529.
The device is protected against solid foreign objects of 12.5 mm diameter and greater such as a
¿ nger, and against oblique falling water drops which may cause issues during a normal operation.
Tem perature lim itat i on
Atmospheric pressure limitation
Alternating current
22
Page 23

Important information regarding Electro Magnetic Compatibility (EMC)
HV-F021-EW and HV-F021-ESL manufactured by OMRON HEALTHCARE Co., Ltd. conform
to EN60601-1-2:2015 Electro Magnetic Compatibility (EMC) standard. Further documentation
in accordance with this EMC standard is available at OMRON HEALTHCARE EUROPE at the
address mentioned in this instruction manual or at www.omron-healthcare.com.
Refer to the EMC information for HV-F021-EW and HV-F021-ESL on the website
www.omron-healthcare.com and Warranty card in the package if purchasing in Russia.
Correct Disposal of This Product
(Waste Electrical & Electronic Equipment)
This marking shown on the product or its literature, indicates that it should not be disposed
of, with other household wastes at the end of its working life. To prevent possible harm to the
environment or human health from uncontrolled waste disposal, please separate this product
from other types of wastes and recycle it responsibly to promote the sustainable reuse of material
resources.
Household users should contact either the retailer where they purchased this product, or their
local government of¿ ce, for details of where and how they can return this item for environmentally
safe recycling.
Business users should contact their supplier and check the terms and conditions of the purchase
contract. This product should not be mixed with other commercial waste for disposal.
EN
23
Page 24

Warranty
Thank you for buying an OMRON product. This product is constructed of high quality
materials and great care has been taken in its manufacturing. It is designed to give you
a high level of comfort, provided that it is properly operated and maintained as described
in the instruction manual.
This product is warranted by OMRON for a period of 3 years after the date of purchase.
The proper construction, workmanship and materials of this product is warranted by
OMRON. During this period of warranty OMRON will, without charge for labour or parts,
repair or replace the defect product or any defective parts.
The warranty covers only products purchased in Europe, Russia and other CIS
countries, Middle East and Africa.
The warranty does not cover any of the following:
a. Transport costs and risks of transport.
b. Costs for repairs and / or defects resulting from repairs done by unauthorised persons.
c. Periodic check-ups and maintenance.
d. Failure or wear of accessories or other attachments other than the main device itself,
unless explicitly warranted above.
e. Costs arising due to non-acceptance of a claim (those will be charged for).
f. Damages of any kind including personal caused accidentally or from misuse.
Should warranty service be required please apply to the dealer whom the product
was purchased from or an authorised OMRON distributor. For the address refer to the
product packaging / literature or to your specialised retailer. If you have dif¿ culties in
¿ nding OMRON customer services, visit our website (www.omron-healthcare.com) for
contact information.
Repair or replacement under the warranty does not give rise to any extension or renewal
of the warranty period.
The warranty will be granted only if the complete product is returned together with the
original invoice / cashticket issued to the consumer by the retailer. OMRON reserves the
right to refuse the warranty service if any unclear information has been given.
24
Page 25

25
EN
Page 26

Manufacturer
Fabricant
Hersteller
Fabricante
Produttore
Fabrikant
ɉɪɨɢɡɜɨɞɢɬɟɥɶ
Üretici
Δόϧλ˵ϣϟ Δϛέηϟ
OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, KYOTO,
617-0002 JAPAN
EU-representative
Mandataire dans l’UE
EU-Repräsentant
Representante en la UE
Rappresentante per l’UE
Vertegenwoordiging in de EU
ɉɪɟɞɫɬɚɜɢɬɟɥɶ ɜ ȿɋ
AB temsilcisi
ϲΑϭέϭϷ ΩΎΣΗϻΎΑ ϝϳΛϣΗϟ ΔϬΟ
Production facility
Site de production
Produktionsstätte
Planta de producción
Stabilimento di produzione
Productiefaciliteit
ɉɪɨɢɡɜɨɞɫɬɜɟɧɧɨɟ ɩɨɞɪɚɡɞɟɥɟɧɢɟ
Üretim Tesisi
ϊϳϧλΗϟ Γ΄ηϧϣ
Subsidiaries
Succursales
Niederlassungen
Empresas ¿liales
Consociate
Dochterondernemingen
Ɏɢɥɢɚɥɵ
Yan Kuruluúlar
ΔόΑΎΗϟ ΕΎϛέηϟ
Made in China Prodotto in Cina
Fabriqué en Chine Geproduceerd in China
Hergestellt in China ɋɞɟɥɚɧɨ ɜ Ʉɢɬɚɟ
Fabricado en China Çin'de Üretilmiútir
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR Hoofddorp,
THE NETHERLANDS
www.omron-healthcare.com
OMRON DALIAN Co., Ltd.
No. 3, Song Jiang Road,
Economic and Technical Development Zone,
Dalian 116600, China
OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG, UK
www.omron-healthcare.com
OMRON MEDIZINTECHNIK HANDELSGESELLSCHAFT mbH
Konrad-Zuse-Ring 28, 68163 Mannheim, GERMANY
www.omron-healthcare.com
OMRON SANTÉ FRANCE SAS
14, rue de Lisbonne, 93561 Rosny-sous-Bois Cedex, FRANCE
www.omron-healthcare.com
Uniquement pour le marché français:
OMRON Service Après Vente
Nº Vert 0 800 91 43 14
ϥϳλϟ ϲϓ ϊϧλ
 Loading...
Loading...