Olympus Winter and Ibe ESG200 User Manual
INSTRUCTIONS FOR USE
DRAFT – 2017-01-17
ELECTROSURGICAL GENERATOR
CELON ELITE
ESG-200
WA90001A
WA90002A
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.

DRAFT – 2017-01-17

Contents
DRAFT – 2017-01-17
1 General information ........................................................................................................... 9
1.1 User instructions ...............................................................................................................9
1.2 Signal words ......................................................................................................................9
1.3 Conventions throughout this document .............................................................................9
1.4 List of abbreviations ........................................................................................................10
1.5 Manufacturer ...................................................................................................................10
2 Safety information ............................................................................................................11
2.1 Intended use ...................................................................................................................11
2.2 Contraindications ............................................................................................................11
2.3 High frequency surgery ................................................................................................... 11
2.4 User qualication ............................................................................................................11
2.5 Environment of use .........................................................................................................12
2.6 General dangers, warnings and cautions........................................................................12
2.6.1 User-related error prevention .................................................................................12
2.6.2 Risks regarding environmental conditions .............................................................13
2.6.3 Risks regarding accessories ..................................................................................14
2.6.4 Risks regarding electric shock ...............................................................................14
2.6.5 Risks regarding burns ............................................................................................15
2.6.6 Potential hazards to the heart ................................................................................ 16
2.6.7 Risks regarding re and explosion .........................................................................17
2.6.8 Procedural hazards and complications .................................................................. 18
3 Product description and functional principles ..............................................................20
3.1 Scope of delivery ............................................................................................................20
3.2 Symbols ..........................................................................................................................20
3.3 Nomenclature and functional principles of the software features ....................................21
3.3.1 CQM – Contact Quality Monitoring for the neutral electrode .................................22
3.3.2 HPCS – High Power Cut Support ..........................................................................22
3.3.3 Autostart for the bipolar mode SoftCoag ................................................................22
3.3.4 FSM – Fast Spark Monitor .....................................................................................22
3.4 Features for selected CELON bipolar applicators ...........................................................22
3.4.1 RFITT – Radiofrequency induced thermotherapy .................................................. 23
3.4.2 RCAP – Resistance Controlled Automatic Power for RFITT .................................. 23
3.4.3 Automatic instrument recognition ...........................................................................23
3.4.4 Automatic wear-out detection .................................................................................23
3.5 Nomenclature and functional principles of the hardware ................................................25
3.5.1 The front panel ....................................................................................................... 25
3.5.2 The rear panel ........................................................................................................ 26
3.5.3 The double pedal foot switch .................................................................................27
3.5.4 The connecting cable for the neutral electrode ......................................................27
3.6 Nomenclature and functional principles of the touch screen ...........................................28
3.6.1 The All Screen .......................................................................................................28
3.6.2 The Set Screen ......................................................................................................30
3.6.3 The Mode Screen ..................................................................................................31
3.6.4 The screens for system settings ............................................................................31
3.6.5 Icons displayed on the touch screen ...................................................................... 33
3.7 Warranty ..........................................................................................................................34
4 Installation ........................................................................................................................35
4.1 General inspection ..........................................................................................................35
4.2 Placement of the electrosurgical generator .....................................................................35

4.3 Connecting the electrosurgical generator to the mains electricity ...................................35
DRAFT – 2017-01-17
4.3.1 Before connecting ..................................................................................................36
4.3.2 Connecting ............................................................................................................. 36
4.4 Connecting the double pedal foot switch ........................................................................37
4.4.1 Before connecting ..................................................................................................37
4.4.2 Connecting ............................................................................................................. 37
5 System settings ................................................................................................................39
5.1 Operation of the touch screen .........................................................................................39
5.2 Overview of system settings ...........................................................................................39
5.3 Select Procedure settings ...............................................................................................40
5.4 Foot switch assignment and Autostart ............................................................................41
5.5 Select Menu settings ....................................................................................................... 42
5.5.1 Save Procedure - Saving and overwriting procedure settings ...............................42
5.5.2 Delete Procedure ...................................................................................................44
5.5.3 Languages .............................................................................................................44
5.5.4 Touch Tone ............................................................................................................45
5.5.5 Autostart Setup ......................................................................................................45
5.5.6 Software Version ....................................................................................................45
5.5.7 Safety Test ............................................................................................................. 46
5.5.8 Bipolar Cut Setup ................................................................................................... 46
5.5.9 Service ................................................................................................................... 46
5.5.10 Volume control .....................................................................................................47
5.5.11 Brightness control ................................................................................................47
6 Connecting HF instruments ............................................................................................ 49
6.1 Safety information for connecting HF instruments ..........................................................49
6.2 Description of the output sockets ....................................................................................50
6.3 Connecting ...................................................................................................................... 51
7 Neutral electrode operation ............................................................................................53
7.1 Safety information for neutral electrode operation ..........................................................53
7.2 Split type and non-split type neutral electrodes and CQM .............................................. 54
7.3 Meaning of CQM indicator condition ............................................................................... 55
7.4 Correct usage of neutral electrodes ................................................................................55
7.5 Connecting to the electrosurgical generator ...................................................................56
7.5.1 Connecting a neutral electrode with a pre-attached cable .....................................56
7.5.2 Connecting a neutral electrode without a pre-attached cable ................................57
7.6 Verifying the contact quality monitor indicator ................................................................. 58
8 Output settings................................................................................................................59
8.1 Safety information on output settings ..............................................................................59
8.2 Overview of output modes ..............................................................................................59
8.2.1 Monopolar modes ..................................................................................................59
8.2.2 Bipolar modes ........................................................................................................ 60
8.3 Tissue effects depending on the output power level .......................................................61
8.3.1 Monopolar modes ..................................................................................................61
8.3.2 Bipolar modes ........................................................................................................ 61
8.4 Selecting the appropriate output settings ........................................................................62
8.5 The bipolar RFITT coagulation modes ............................................................................ 63
8.6 Output settings for selected CELON bipolar applicators ................................................. 65
9 Inspection before operation ............................................................................................ 67
9.1 Inspecting the supply of power ........................................................................................67
9.2 Inspecting the touch screen ............................................................................................67
9.3 Inspecting the foot switch assignment ............................................................................68

9.4 Inspecting the alarm system ...........................................................................................69
DRAFT – 2017-01-17
10 Operation ........................................................................................................................70
10.1 Safety information for operation ....................................................................................70
10.2 Activating .......................................................................................................................71
10.2.1 Prior to activating .................................................................................................71
10.2.2 Limited activation time .........................................................................................71
10.2.3 Functional principles of activation ........................................................................72
10.3 After use ........................................................................................................................ 74
10.3.1 Disconnecting ......................................................................................................75
11 Reprocessing .................................................................................................................76
11.1 General information for reprocessing ............................................................................76
11.2 Cleaning ........................................................................................................................ 76
11.3 Disinfection ....................................................................................................................77
11.4 Other HF equipment......................................................................................................77
12 Troubleshooting .............................................................................................................78
12.1 General problems ..........................................................................................................78
12.2 Error messages ............................................................................................................. 80
12.2.1 Overview of error messages ................................................................................ 82
13 Maintenance, repair and shipment ............................................................................... 86
13.1 Annual safety check ......................................................................................................86
13.2 Repair ............................................................................................................................86
13.3 Shipment ....................................................................................................................... 86
14 Storage and disposal ..................................................................................................... 87
14.1 Storage ..........................................................................................................................87
14.2 Disposal ........................................................................................................................87
15 Compatible equipment ..................................................................................................88
15.1 System chart .................................................................................................................88
15.2 Compatible neutral electrodes ......................................................................................89
16 Specications ................................................................................................................. 90
16.1 Specications for the CELON ELITE ESG-200 .............................................................90
16.2 Specications for the double pedal foot switch (WB50402W) .......................................91
16.3 Specications for the cord set .......................................................................................91
17 Electromagnetic compatibility ...................................................................................... 92
Appendix A ..........................................................................................................................97
Alarm system ........................................................................................................................97
Output tone information .........................................................................................................98
Touch tone and start melody ................................................................................................. 99
Appendix B ........................................................................................................................101
Mode characteristics according to IEC 60601-2-2 ..............................................................101
Characteristics of High Power Cut Support (HPCS) ...........................................................102
Output characteristics .........................................................................................................103

Figures
DRAFT – 2017-01-17
Figure 3.1 Front panel of the CELON ELITE ESG-200..................................................25
Figure 3.2 Rear panel ....................................................................................................26
Figure 3.3 Double pedal foot switch ..............................................................................27
Figure 3.4 Cable for neutral electrode, MAJ-814 ...........................................................27
Figure 3.5 BIPOLAR pane ............................................................................................. 28
Figure 3.6 MONOPOLAR pane .....................................................................................28
Figure 3.7 Buttons for system settings ........................................................................... 29
Figure 3.8 Buttons and symbols on the All Screen ........................................................29
Figure 3.9 Set Screen ....................................................................................................30
Figure 3.10 Mode Screen for monopolar cutting .............................................................31
Figure 3.11 Hierarchy list of the 3 right-hand sided buttons on the All Screen ................ 32
Figure 4.1 Connecting the cord set to the electrosurgical generator .............................36
Figure 4.2 Connecting the foot switch to the electrosurgical generator ......................... 38
Figure 5.1 Buttons for system settings ........................................................................... 39
Figure 5.2 Screen [Select Procedure] ............................................................................40
Figure 5.3 Screen [Assign Foot Switch] .........................................................................41
Figure 5.4 Screen [Select Menu] ...................................................................................42
Figure 5.5 Onscreen keyboard (letters and numbers) ...................................................43
Figure 5.6 Onscreen keyboard (upper/lower key and backspace key) ..........................43
Figure 5.7 Volume control ..............................................................................................47
Figure 5.8 Brightness control ......................................................................................... 48
Figure 6.1 CAUTION - Correct connection of a monopolar 1-pin plug with a 4 mm pin
diameter ........................................................................................................49
Figure 6.2 CAUTION - Do not connect a 2-pin plug with 4 mm pin diameters to the MO-
NOPOLAR socket ......................................................................................... 50
Figure 6.3 MONOPOLAR output socket specications for WA90001A ......................... 51
Figure 6.4 MONOPOLAR output socket specications for WA90002A ......................... 51
Figure 6.5 Bipolar output socket specications..............................................................51
Figure 7.1 Error message E0202 “Insufcient neutral electrode contact” ...................... 54
Figure 7.2 Message box “Neutral electrode” ..................................................................54
Figure 7.3 Connecting the plug of the neutral electrode ................................................57
Figure 7.4 Fixing the neural electrode to the connecting cable .....................................57
Figure 7.5 Split type indicator for CQM shining red on the Set Screen and on the All
Screen ..........................................................................................................58
Figure 8.6 All Screen .....................................................................................................62
Figure 8.7 Set Screen for monopolar modes .................................................................63
Figure 8.8 Mode Screen for monopolar cutting modes ..................................................63
Figure 8.1 Counters on the Set Screen and during activation .......................................64
Figure 8.2 Error message E0660 “No bipolar cutting” ...................................................65
Figure 8.3 Automatic instrument recognition .................................................................65
Figure 8.4 Incompatible settings .................................................................................... 66
Figure 9.1 Changing from the All Screen to the Set Screen ..........................................67
Figure 9.2 Changing from the All Screen to the screen [Select Menu] ..........................68
Figure 9.3 Changing from the All Screen to the screen [Assign Foot Switch] ...............68
Figure 9.4 Error message E0202 “Insufcient neutral electrode contact” ...................... 69
Figure 10.1 Error message E0115: "Activation time limit exceeded" ............................... 72
Figure 10.2 Background color for active coagulation on the All Screen ..........................73
Figure 10.3 Background color for active coagulation on the Set Screen .........................73
Figure 10.4 Error message E0141 "Power set to zero (--)" .............................................. 74
Figure 12.1 Error message E0002 “Short circuit” ............................................................81
Figure 15.1 System chart for CELON ELITE ESG-200 ...................................................88
Characteristics of HPCS ..................................................................................................102

DRAFT – 2017-01-17

DRAFT – 2017-01-17

1 General information
DRAFT – 2017-01-17
1.1 User instructions
• Before use, thoroughly read these instructions for use, and the instructions for use of all
other products that will be used during the procedure.
• If the required instructions for use are missing, immediately contact an Olympus
representative.
• Keep the instructions for use in a safe, accessible location.
1.2 Signal words
The following signal words are used throughout this document.
DANGER
Indicates a hazardous situation which, if not avoided, will result in death or serious injury.
WARNING
Indicates a potentially hazardous situation which, if not avoided, could result in death or
serious injury.
General information
CAUTION
Indicates a potentially hazardous situation which, if not avoided, may result in minor or
moderate injury. It may also be used to alert against unsafe practices or potential equipment
damage.
NOTICE
Indicates a property damage message.
1.3 Conventions throughout this document
This is the safety alert symbol. It is used to alert the user to potential physical injury hazards.
Observe all safety messages that follow this symbol to avoid possible injury.
This symbol indicates additional helpful information.
1. A numeration indicates a sequence of actions.
2. ...
• Bullet points indicate individual actions or different options for action.
- Dashes indicate the listing of data, options or objects.
1) Numbers with right parenthesis name elements in illustrations.
[example]
Bracketed terms refer to elements in the graphical user interface. Such elements can be:
- icons
- buttons
- menu items
- dialog elements
9

General information
DRAFT – 2017-01-17
1.4 List of abbreviations
AC Alternating current
CQM Contact Quality Monitor
DC Direct current
EMC Electromagnetic compatibility
ESD Electrostatic discharge
ESG Electrosurgical generator
FSM Fast Spark Monitor
GSM Global system for mobile communication
HF High frequency
HPCS High Power Cut Support
ICD Implanted cardioverter debrillator
IED Implanted electronic device
RFID radio-frequency identication
RFITT Radiofrequency induced thermotherapy
RCAP Resistance Controlled Automatic Power
UDI Unique device identier
1.5 Manufacturer
Olympus Winter & Ibe GmbH
Kuehnstr. 61
22045 Hamburg
Germany
10

2 Safety information
DRAFT – 2017-01-17
2.1 Intended use
Electrosurgical generator intended for tissue cutting and coagulation in conjunction with
electrosurgical accessories and ancillary equipment.
2.2 Contraindications
Electrosurgical interventions are contraindicated if, in the judgment of the physician,
tissue coagulation and cutting could have a negative effect on the state of the patient.
Electrosurgical interventions may be contraindicated for patients with implanted electronic
devices (e.g. pacemakers or cardioverter-debrillators), a weakened immune system or
blood coagulation disorders.
2.3 High frequency surgery
If there is an ofcial standard on the applicability of high frequency treatment as dened by
a national or local medical administration or other institutions, such as an academic society,
follow that standard when performing the procedure.
Safety information
Before performing any high frequency treatment, thoroughly study the properties, purposes,
and effects as well as the nature, extent, probability and imminence of possible risks
associated with the planned treatment. Furthermore, study any alternative therapeutic
method that can be performed.
Carry out high frequency treatment only when its benets outweigh its risks. Fully explain
to the patient the possible benets and risks of high frequency treatment as well as those
of any therapeutic method that can be performed instead of electrosurgery. Perform high
frequency treatment only after patient consent is granted.
During high frequency treatment, continue to evaluate the potential benets and risks. Stop
the treatment if the risks become greater than the possible benets to the patient.
2.4 User qualication
Medical use
If the required qualication for medical personnel who uses electrosurgical generators
is dened by a national or local medical administration or other institutions, such as an
academic society, then follow that standard.
If there is no such standard:
- The user must be a physician or medical personnel under supervision of a physician.
- The user must have experience with electrosurgical procedures that are used for the
therapy.
- The user must have received appropriate training in using this electrosurgical generator.
This training is provided during installation and commissioning of the CELON ELITE
ESG-200 by trained qualied personnel that has been authorized by Olympus.
These instructions for use do not explain or discuss clinical procedures.
11

Safety information
DRAFT – 2017-01-17
Commissioning
The electrosurgical generator must be properly installed and commissioned by trained
qualied personnel that has been authorized by Olympus.
Annual safety check
The annual safety check may only be carried out by a qualied electrician with sufcient
experience in maintaining medical electrical devices.
Repair
Repair of the product may only be performed by authorized service centers. Otherwise,
Olympus cannot be held responsible for the safety and performance of the product.
2.5 Environment of use
Medical use
This product is intended to be used in operating rooms or physicians’ ofces.
Annual safety check
Appropriate equipment for performing the safety check must be available. Refer to the
section “Annual safety check” on page 86.
2.6 General dangers, warnings and cautions
High frequency leakage current or spark discharge may cause burns to the user and the
patient. Always prepare for an emergency operation if unintentional burns, bleeding and
2.6.1 User-related error prevention
perforation occur.
The following dangers, warnings and cautions apply to the general handling of the product.
This information is to be supplemented by the dangers, warnings and cautions given in each
chapter in this document or in the instructions for use of any product being used with this
product.
WARNING
Improper use
Safety and effectiveness of electrosurgery do not only depend on the design of the used
equipment. To a major extent, the success of electrosurgical interventions depends on
factors that are under the control of the user. Therefore, it is particularly important to read,
understand and follow the instructions that are supplied with the electrosurgical generator
and with the accessories. Improper use can cause severe procedural complications and
equipment damage and could result in injuries to the patient, the user and the medical
personnel.
Only use the electrosurgical generator as outlined in these instructions for use.
• Before each use, inspect the electrosurgical generator and all accessories according to
the corresponding instructions for use.
12
CAUTION
Annual safety checks
To maintain device performance and electrical safety, annual safety checks must be carried
out on the electrosurgical generator and the foot switch. Otherwise, malfunction of the
devices could occur which may result in injury to the patient.

• Make sure that the annual safety check is carried out regularly.
DRAFT – 2017-01-17
• Refer to the information in the section "Annual safety check" on page 86.
• Observe national statutory regulations.
2.6.2 Risks regarding environmental conditions
DANGER
Explosive atmosphere
The electrosurgical generator is not explosion proof. Using the electrosurgical generator in
explosive atmosphere will result in serious injury to the patient, the user and the medical
personnel.
• Do not use the electrosurgical generator within an explosive atmosphere.
CAUTION
The electrosurgical generator can disturb other equipment
The electrosurgical generator complies with the standards as described in the chapter
"Electromagnetic compatibility" on page 92. However, during activation, high frequency
signals or spark discharge noise generated by the electrosurgical generator can disturb
neighboring electrical equipment. Malfunction of the devices could occur, e.g. the monitor of
endoscopic imaging equipment might freeze or black out, which may result in injury to the
patient.
• Follow the instructions regarding electromagnetic ambient conditions given in the chapter
"Electromagnetic compatibility" on page 92.
• Make sure that electrosurgical generator is not used adjacent to or stacked with equipment
that is not part of this electrosurgical generator or system.
• Before use, thoroughly conrm the compatibility of all equipment.
• Do not use the electrosurgical generator in conjunction with:
- Electrical equipment for which the safety against leakage current is not conrmed.
- Electrosurgical equipment for which the safety in combined use is not conrmed.
• Do not loop cables.
• Do not bundle cables together with cables belonging to other medical equipment.
Safety information
CAUTION
Strong electromagnetic radiation can disturb the electrosurgical generator
Equipment that produces strong electromagnetic radiation can cause malfunction of the
electrosurgical generator which may result in injury to the patient. Equipment that produces
strong electromagnetic radiation is, e.g. microwave medical treatment equipment, shortwave medical treatment equipment, magnetic resonance imaging equipment, radio or
mobile phones.
• Do not use the electrosurgical generator in a location exposed to strong electromagnetic
radiation.
CAUTION
Unsuitable operating conditions
Using the electrosurgical generator under conditions other than specied can compromise
the performance and can damage the equipment which may result in patient injury.
• Only use the electrosurgical generator according the operating conditions as described in
the chapter "Specications" on page 90.
13

Safety information
DRAFT – 2017-01-17
2.6.3 Risks regarding accessories
WARNING
Mechanical stress
Excessive mechanical stress applied to the cords can damage the cords and its insulations.
This can result in electric shock and thermal injury to the patient, the user and the medical
personnel. Furthermore, mechanical stress to the cords can cause malfunction of the
electrosurgical equipment which can result in patient injury.
• Do not excessively bend, strain or squeeze the cords.
WARNING
Damaged equipment and accessories
The use of damaged equipment or of equipment with improper functioning may cause
electric shock, mechanical injury, infection and thermal injury to the patient and the user.
• Before each use, observe the instructions in the section "Inspection before operation" on
page 67.
• Do not use damaged equipment or equipment with improper functioning.
• Replace damaged equipment or equipment with improper functioning.
WARNING
Risk of sparkover
There is a risk of sparkover leading to electrical injury, thermal injury and/or unintended
nerve stimulation.
• Check the maximum rated voltage of the other HF equipment.
• Make sure that the maximum output voltage of the electrosurgical generator does not
exceed the lowest maximum rated voltage of any of the HF equipment used during the
procedure.
CAUTION
Incompatible equipment
Using incompatible equipment may lead to injury of the patient and/or the user as well as
damage to the product.
• For information on compatible equipment, refer to the chapter "Compatible equipment" on
page 88.
2.6.4 Risks regarding electric shock
WARNING
Grounding failure
There is a risk of electric shock if the housing of the electrosurgical generator is not
grounded.
• Only connect the cord set to a properly grounded xed socket-outlet.
• Do not use an adapter for an incompatible grounded xed socket-outlet as it can impair
safe operation of the electrosurgical generator.
WARNING
Liquids
If liquids get on or into the electrosurgical generator, then this could cause electric shock to
the user as well as damage to the electrosurgical generator which could result in injury to
the user.
• Keep liquids away from all electrical equipment.
• If liquids get on or into the electrosurgical generator, then immediately stop the operation.
14

CAUTION
DRAFT – 2017-01-17
Opening the housing
There is a risk of electric shock when opening the housing of the electrosurgical generator.
• Do not open the housing of the electrosurgical generator.
• Repair and service of the electrosurgical generator may only be performed by trained
qualied servicing personnel that has been authorized by Olympus.
2.6.5 Risks regarding burns
WARNING
Power settings
The selected output power should be as low as possible for the intended purpose. If the
output power initially is set to a level that is too high, then the electrode's insulation can be
damaged. This could result in burns to the patient and the user.
However, the PulseCut mode and all RFITT modes present an unacceptable risk when
used with power settings that are too low. The risk of excessive thermal effects rises if the
output power setting is too low when using these modes.
• For appropriate power settings refer to the tables with the modes characteristics in the
“Appendix A” on page 97.
• Prior to the procedure, perform basic testing with the electrosurgical generator.
Safety information
WARNING
Unintended tissue contact
When the output of the electrosurgical generator is active, then unintended contact between
tissue and the active part of the HF instrument can cause burns to the patient, the user and
the medical personnel.
• Store temporarily unused HF instruments in an electrically insulated container.
• Do not place unused HF instruments on the patient.
WARNING
Unintended current ow
Unintended current ow may cause injury to the patient and the user. The patient must be
insulated against all electrically conductive parts.
• Make sure that the patient does not come in contact with any metal parts.
• Ground the operating table.
• Place the patient on a dry, electrically insulating surface.
• Make sure that the patient’s clothes are dry.
• Prevent any contact between different skin surfaces (arms, legs) of the patient. Place dry
gauze between the body and arms and between the legs to prevent such contact.
• Prevent any skin contact between the patient and the user.
• Remove any metallic items from the patient, e.g. wristwatches, jewelry.
WARNING
Using two electrosurgical generators
Using the electrosurgical generator in conjunction with another electrosurgical generator
could result in patient and user injury due to the concentration of electric current.
• Keep HF instruments that are connected to the unused electrosurgical generator away
from the target area while the other generator is in operation.
• Do not activate output of both generators simultaneously.
15

Safety information
DRAFT – 2017-01-17
WARNING
Using physiological monitoring equipment
Current may ow to the monitoring electrodes and can cause thermal injury where the
monitoring electrodes are attached. Especially, the use of needle electrodes can result in
burns to the patient.
• Place the physiological monitoring electrodes as far away as possible from the electrodes
of the HF instrument.
• Do not use needle monitoring electrodes.
• Use physiological monitoring equipment with HF current limiting measures.
WARNING
Surgical gloves
During operation, HF current, especially leakage current, could ow via the user and the
assistant. This could result in burns to the user and the assistant.
• The user and the assistant must wear surgical gloves.
CAUTION
Unintended current ow and HF leakage current
Unintended current ow and HF leakage current may cause thermal injury to the patient.
The patient must be insulated against all electrically conductive parts.
• Ground the operating table.
• Make sure that the patient does not come in contact with metal parts, e.g. the operating
table.
• Place the patient on a dry, electrically insulating surface.
• Make sure that the patient’s clothes are dry.
• Prevent any contact between different skin surfaces (arms, legs) of the patient. Place dry
gauze between the body and arms and between the legs to prevent such contact.
• Prevent any skin contact between the patient and the user.
• Remove any metallic items from the patient, e.g. wristwatches, jewelry.
• Route all connecting cables so that they are not in direct contact with the patient.
• Route all connecting cables so that they are not in contact with other cables.
2.6.6 Potential hazards to the heart
DANGER
Malfunction of pacemakers and debrillators
Using HF equipment on patients with implanted electronic devices, e.g. cardiac pacemakers
or cardioverter debrillators, can cause failure of the implanted electronic device. Failure of
the implanted electronic device will affect the heart and could result in cardiac arrest.
• Prior to the HF procedure, conrm its safety with a cardiologist or the manufacturer of the
implanted electronic device.
• For monopolar procedures, position the neutral electrode so that the current pathway does
not pass through or near the implanted electronic device and its lead system.
• Do not apply the HF instrument in close proximity to the implanted electronic device.
WARNING
Electric shock hazard
When using the electrosurgical generator for procedures in the vicinity of the heart, note that
spark discharge can affect the heart and could result in cardiac arrest.
• Make sure to use the lowest necessary output power for procedures in the vicinity of the
heart.
16

WARNING
DRAFT – 2017-01-17
Cardiac emergency
• Keep ready a debrillator.
• Before operating the debrillator, remove all electrosurgical equipment from the patient.
2.6.7 Risks regarding re and explosion
The surgical application of electrical energy can ignite ammable gases, substances and
materials.
DANGER
Ignitable anaesthetics and gases
The igniting of ammable gases, especially anaesthetics, will cause serious injuries to the
patient, the user and the medical personnel.
• Take precautionary measures to keep away ammable gases from the site of intervention.
• Do not use ammable anaesthetics, e.g. nitrous oxygen or oxygen.
WARNING
Ignitable agents for cleaning and disinfection and other substances
The igniting of ammable substances, e.g. agents for cleaning and disinfection, can cause
serious injuries to the patient, the user and the medical personnel.
• If possible, only use non-ammable agents for cleaning and disinfection.
• Before the electrosurgical generator is used, allow ammable substances to evaporate.
• Make sure that there are no ammable solutions on the patient’s skin, e.g. under the
neutral electrode.
• Make sure that there are no ammable solutions in the body cavity of the patient.
WARNING
Ignitable materials
Sparks that occur during normal operation of the HF equipment can ignite ammable
materials, e.g. absorbent cotton or gauze and also body hair. This could result in serious
injuries to the patient, the user and the medical personnel.
• Use soluble surgical lubricating jelly to cover hair close to the site of intervention.
• Do not apply materials like cotton or gauze simultaneously with the HF instrument to the
site of intervention.
Safety information
WARNING
Ignitable gas in the gastrointestinal tract
Flammable gases within the intestines of the patient could cause re or explosion when
applying HF current. This could result in serious injury to the patient.
• Prior to the HF procedure, replace intestine gases by air or by other non-ammable gases.
WARNING
Fuses
Using inadequate fuses can cause electrical re within the electrosurgical generator. This
could result in injury to the patient, the user and the medical personnel.
• Fuses must only be replaced by a qualied electrician with sufcient experience in
maintaining medical electrical devices.
• Only use fuses as specied in the section “Specications for the CELON ELITE ESG-200”
on page 90.
• Before changing the fuses, disconnect the electrosurgical generator from the mains
electricity.
17

Safety information
DRAFT – 2017-01-17
2.6.8 Procedural hazards and complications
DANGER
Procedural hazards and complications
Insufcient knowledge of the user about the medical literature and about the specic
procedural hazards will result in severe complications that can result in death of the patient.
• The electrosurgical generator must only be used by physicians who have experience with
the electrosurgical procedure that is used for the specic therapy.
• To respond to possible complications during the procedure consider the following:
• Prepare at least one hemostatic procedure: coagulation, clipping or local injection.
• Make sure that emergency equipment for life-saving, intubation and appropriate
pharmaceuticals are located in or near the procedure room.
• Prepare a spare electrosurgical generator or an alternative procedure to avoid
interruption of treatment due to an unexpected electrosurgical generator failure during
the treatment.
• If any abnormal output is suspected during the operation, immediately terminate the
use of the equipment.
• If the foot switch does not react, switch off the electrosurgical generator. Malfunction
of the equipment can cause an unintended increase of output energy.
• Use physiological monitoring equipment throughout the entire procedure.
• Consider the use of a bipolar mode for procedures where the HF current could ow
through parts of the body with a relatively small cross sectional area.
• It is not recommended to use electrosurgery for circumcisions because of the risk of
thermal injuries. The risk can be reduced if metal parts of any kind (e.g. clamps) or
monopolar HF instruments are avoided.
WARNING
Electrosurgical smoke
Electrosurgical interventions produce smoke. When unltered smoke is inhaled, then this
can affect the health of the user and the medical personnel.
• Wear surgical masks during the procedure.
• Make sure that adequate ventilation, surgical smoke evacuators or other means is
provided in the operating room.
WARNING
Output performance
Tissue coagulum that builds up at the HF instrument can impede the output performance
of the electrosurgical generator. Increasing the output level can damage the electrosurgical
generator and can result in injury to the patient.
• Carefully clean eschar affected areas of the HF instrument. Refer to the instructions for
use of the HF instrument.
• If necessary, replace the HF instrument.
If non-insulated grasping forceps are used during endoscopic interventions, then the HF
current is dispersed via the endoscope which impedes the output performance and which
can result in injury to the patient.
• Only use isolated grasping forceps during endoscopic interventions.
18

Safety information
DRAFT – 2017-01-17
WARNING
Electrical stimulation of nerves and muscles
Low frequency electrical currents or intense high frequency electrical currents can stimulate
nerves and muscles which may result in violent spasms or muscle contractions of the
patient. Low frequency electrical currents may be generated by a partial rectication of
intense high frequency electrical current, in particular when there is a spark discharge to the
tissue or to another metallic object. Intense high frequency electrical currents can occur at
the beginning of an electrosurgical cut or when using high output power settings.
• Use the lowest appropriate output power level. Be aware that certain modes present an
unacceptable risk with power settings that are too low. With the PulseCut mode and all
RFITT modes the risk of excessive thermal effects rises if the output power setting is too
low.
• For appropriate power settings refer to the tables with the modes characteristics in the
“Appendix B” on page 101.
CAUTION
Generator defect
Short circuits can cause malfunction or damage of the electrosurgical generator which may
cause an undesirably high output of power. This could result in injury to the patient.
• Make sure that the electrodes of an HF instrument do not come in contact with each other.
• Do not touch metallic parts with the HF instrument.
19

Product description and functional principles
DRAFT – 2017-01-17
3 Product description and functional principles
3.1 Scope of delivery
• Before use, check that all items listed below are available.
• Contact an Olympus representative or an authorized service center if any items are
missing or damaged.
WA90001A or WA90002A
- Electrosurgical generator CELON ELITE ESG-200
- Foot switch including connecting cable (WB50402W)
- Instructions for use
A cord set for connecting the electrosurgical generator to the mains electricity is not included
as the plug types depend on national standards. Refer to the section “Specications for the
cord set” on page 91.
3.2 Symbols
This section gives an explanation for each symbol used on the product and on the
packaging of the product.
Caution, consult accompanying documents
Follow instructions for use
HF isolated patient circuit – symbol for patient circuit where there are no components
installed to provide a low-impedance path to earth for HF currents
Debrillation proof type CF applied part (cardiac application)
Non-ionizing electromagnetic radiation
CE certication mark – symbol for the compliance with the Medical Device Directive 93/42/
EEC
In accordance with European Directive 2002/96/EC on Waste Electrical and Electronic
Equipment, this symbol indicates that the product must not be disposed of as unsorted
municipal waste, but should be collected separately.
20
cTUVus certication mark
Fuse rating
Potential equalization terminal

Product description and functional principles
DRAFT – 2017-01-17
Catalog number
Serial number
Batch code
Quantity of content
Manufacturer
Date of manufacture
Do not use if package is damaged
Fragile, handle with care
Indicates the range of humidity to which the medical device can be safely exposed
Indicates the temperature limits to which the medical device can be safely exposed
Keep away from rain. Keep dry
Type CF applied part
Indicates the stacking limit of a package by mass
Storage conditions
Transport conditions
Indicates a recovery/recyclable package or package material
Federal (USA) law restricts this device to sale by or on the order of a physician.
3.3 Nomenclature and functional principles of the software features
The electrosurgical generator is equipped with the following features:
- Contact Quality Monitor (CQM)
- High Power Cut Support (HPCS)
- Autostart
- Fast Spark Monitor (FSM)
21

Product description and functional principles
DRAFT – 2017-01-17
For selected CELON bipolar applicators the electrosurgical generator also provides:
- Radiofrequency induced thermotherapy (RFITT)
- Resistance Controlled Automatic Power (RCAP) for RFITT
- Automatic instrument recognition
- Automatic wear-out detection
The functional principles of theses features are described in the sections below.
3.3.1 CQM – Contact Quality Monitoring for the neutral electrode
When using split type neutral electrodes for monopolar electrosurgery, the CELON ELITE
ESG-200 is able to detect unintended detachment of the neutral electrode from the patient.
The indicator for the contact quality monitor (CQM) shines green while the contact between
the split type neutral electrode and the skin of the patient is within an acceptable resistance
range. If the contact between the split type neutral electrode and the patient’s skin is
insufcient, then an alarm tone sounds, a warning message is displayed and the contact
quality monitor indicator shines red.
Using non-split type neutral electrodes is not as safe as using split-type ones as CQM is not
able to detect any detachment of the neutral electrode from the patient.
For detailed information on the safe and correct use of neutral electrodes refer to the chapter
“Neutral electrode operation” on page 53.
3.3.2 HPCS – High Power Cut Support
All cutting modes are supported by HPCS. This feature optimizes the start of the cutting
procedure by applying high power to the tissue to support immediate spark ignition and to
reduce the risk of mechanical cutting.
3.3.3 Autostart for the bipolar mode SoftCoag
If the impedance is within a dened range, then Autostart feature permits automatic
activation of the output power as soon as both electrodes of the electrosurgical instrument
touch the tissue. It is possible to set a time delay so that the automatic activation does not
start immediately when the electrodes are in contact with the tissue but only after a certain
time. Consequently, activating by foot switch is not necessary.
Autostart is available only for the bipolar mode SoftCoag.
3.3.4 FSM – Fast Spark Monitor
This feature ensures smooth and reproducible cutting although the tissue characteristic are
varying, e.g. in muscle and fat.
3.4 Features for selected CELON bipolar applicators
The following features are compatible only with selected CELON bipolar applicators, e.g.
CelonProSleep plus or CelonProBreath.
22

Product description and functional principles
DRAFT – 2017-01-17
3.4.1 RFITT – Radiofrequency induced thermotherapy
The medical purpose of the radiofrequency induced thermotherapy, RFITT, is to achieve
controlled tissue coagulation. The CELON ELITE ESG-200 offers 4 different RFITT modes
that are used in conjunction with the CELON bipolar applicators. For further information refer
to the section “The bipolar RFITT coagulation modes” on page 63.
3.4.2 RCAP – Resistance Controlled Automatic Power for RFITT
RCAP is a feature that supports the mode Strong RFITT. Deep tissue coagulation without
signicant desiccation is achieved with RCAP.
RCAP determines the maximum power uptake of the tissue, adapted to the momentary
treatment status and automatically adjusts the electrosurgical generator. For this process,
the geometry of the bipolar applicator used as well as individual tissue characteristics,
e.g. blood perfusion, are taken into account. Therefore, premature tissue desiccation
is effectively avoided and subsequent manual adjustment of the supplied power is
unnecessary. Also refer to the section “The bipolar RFITT coagulation modes” on page
63.
3.4.3 Automatic instrument recognition
The electrosurgical generator is equipped with a radio transmitting and receiving technology.
Therefore, the selected CELON bipolar applicator that is connected to the electrosurgical
generator is recognized automatically. This means:
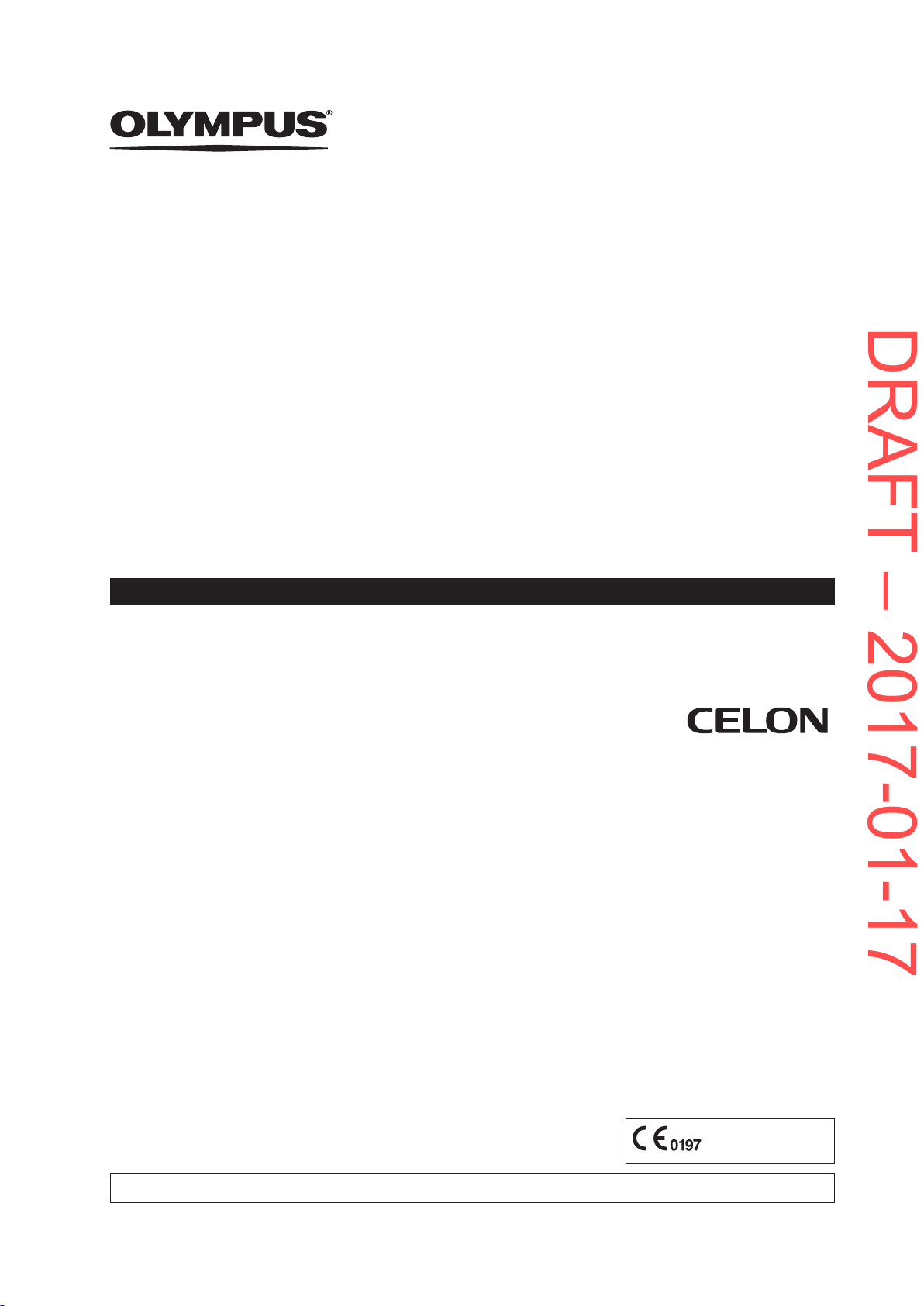
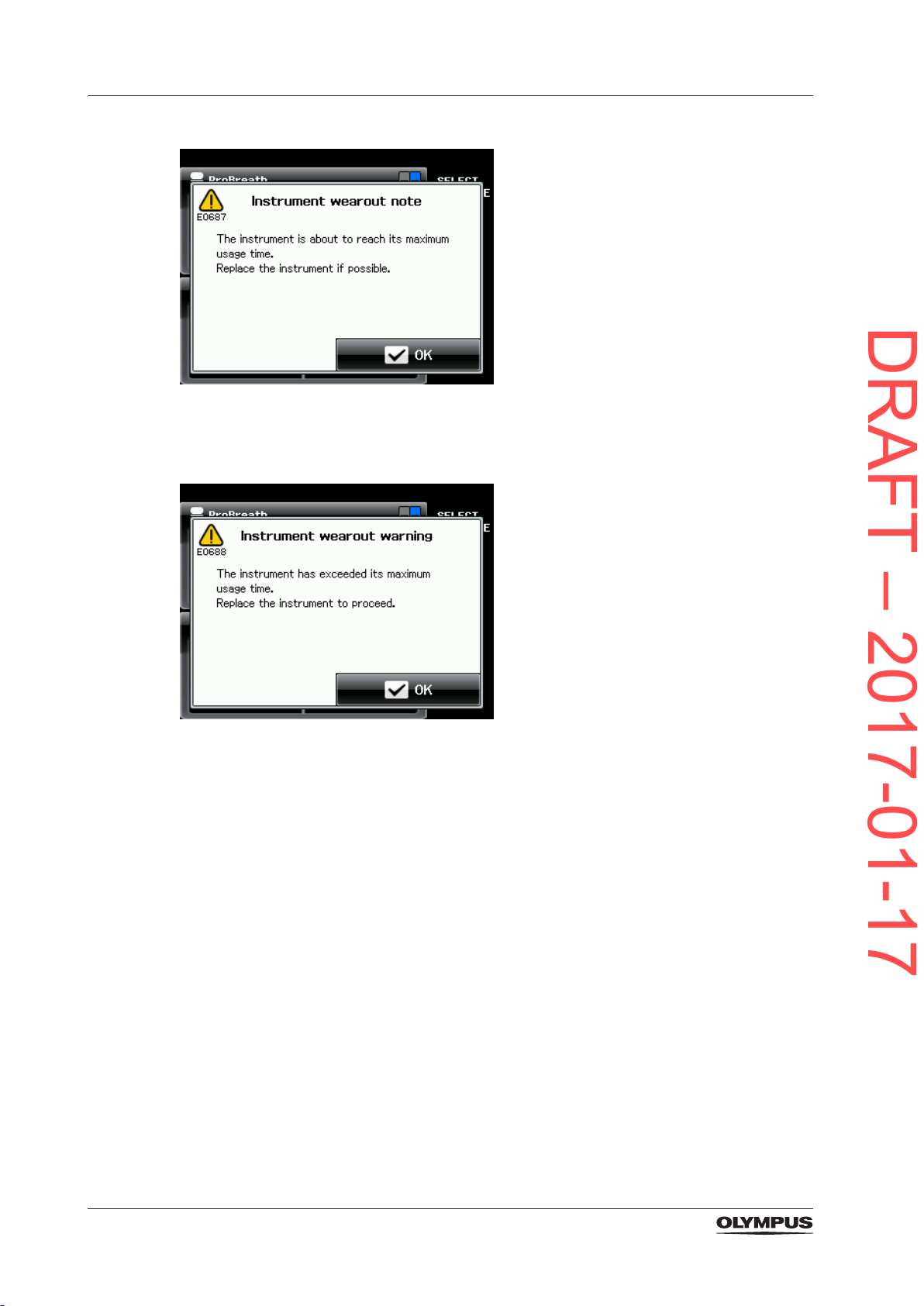
3.4.4 Automatic wear-out detection
- The name of the connected CELON bipolar applicator is displayed in the BIPOLAR pane
of the touch screen, e. g. [ProBreath] or [ProSleep plus].
- Only compatible modes and output power settings are available.
- The user can select one of these compatible modes and can change the output power
level within a restricted range.
Other radio transmitters and receivers, e. g. mobile phones, may interfere with the automatic
instrument recognition.
• To avoid interference observe the instructions in the chapter “Electromagnetic
compatibility” on page 92.
To protect the patient from a worn-out instrument the bipolar applicators are equipped with
memory chips that record the usage. When the permitted usage span is nearly reached,
the electrosurgical generator alerts the user by emitting an alarm tone and displaying the
following error message:
23
Product description and functional principles
DRAFT – 2017-01-17
At this stage and as soon as the treatment situation allows, the user needs to replace the
bipolar applicator.
When the permitted usage span is exceeded, the following error message is displayed:
When this error message is displayed, it is impossible to activate the bipolar applicator
anymore.
24
Product description and functional principles
DRAFT – 2017-01-17
3.5 Nomenclature and functional principles of the hardware
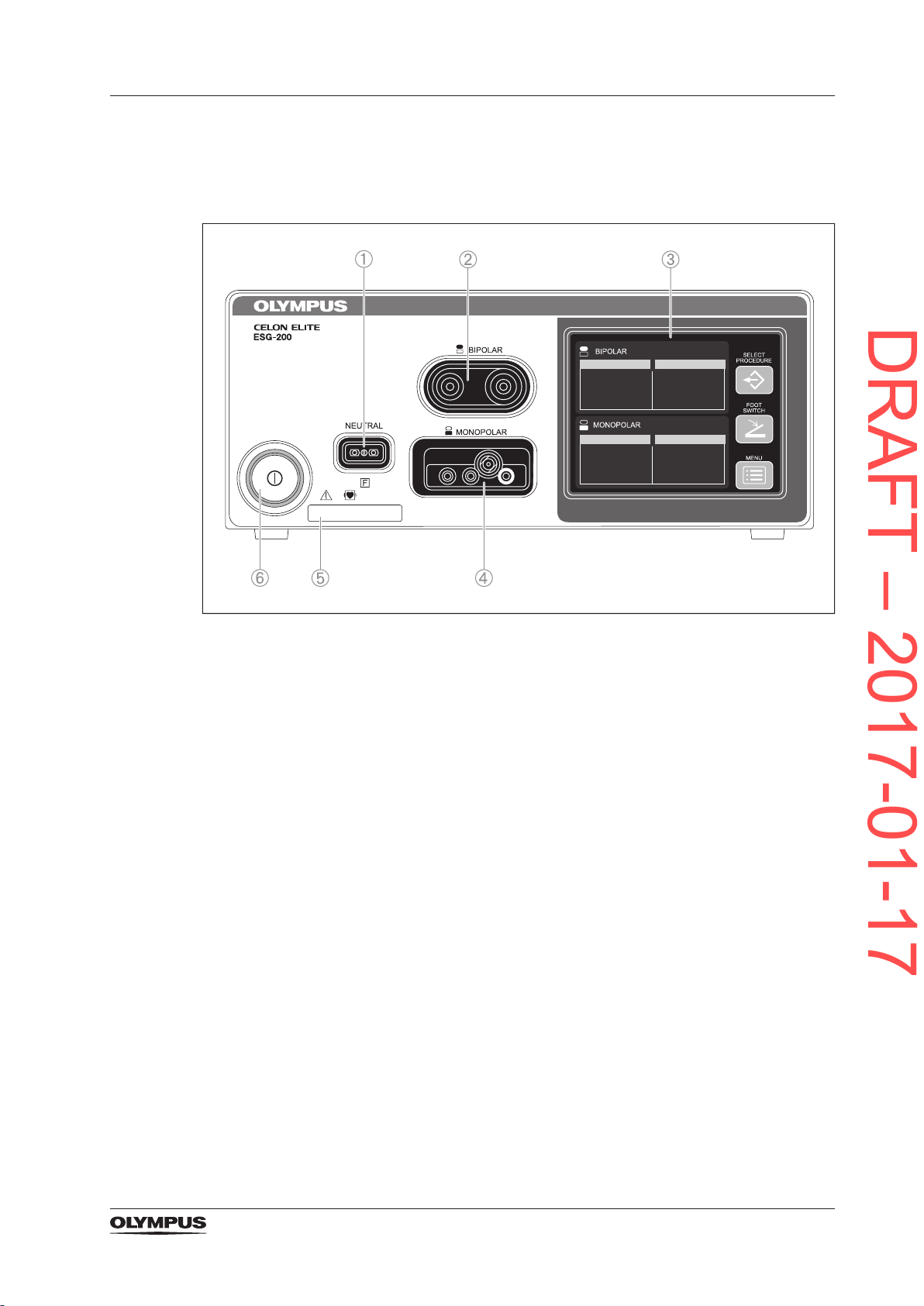
3.5.1 The front panel
Figure 3.1 Front panel of the CELON ELITE ESG-200
1) *Neutral electrode socket
To connect a neutral electrode.
2) *BIPOLAR output socket
To connect a bipolar HF instrument.
3) Touch screen
To display the status of connected accessories.
To show and modify settings.
4) *MONOPOLAR output socket
To connect a monopolar HF instrument.
5) UDI (unique device identier)
6) Power switch
To switch the electrosurgical generator on and off.
*Applied part according to standard IEC 60601-1.
25
Product description and functional principles
DRAFT – 2017-01-17
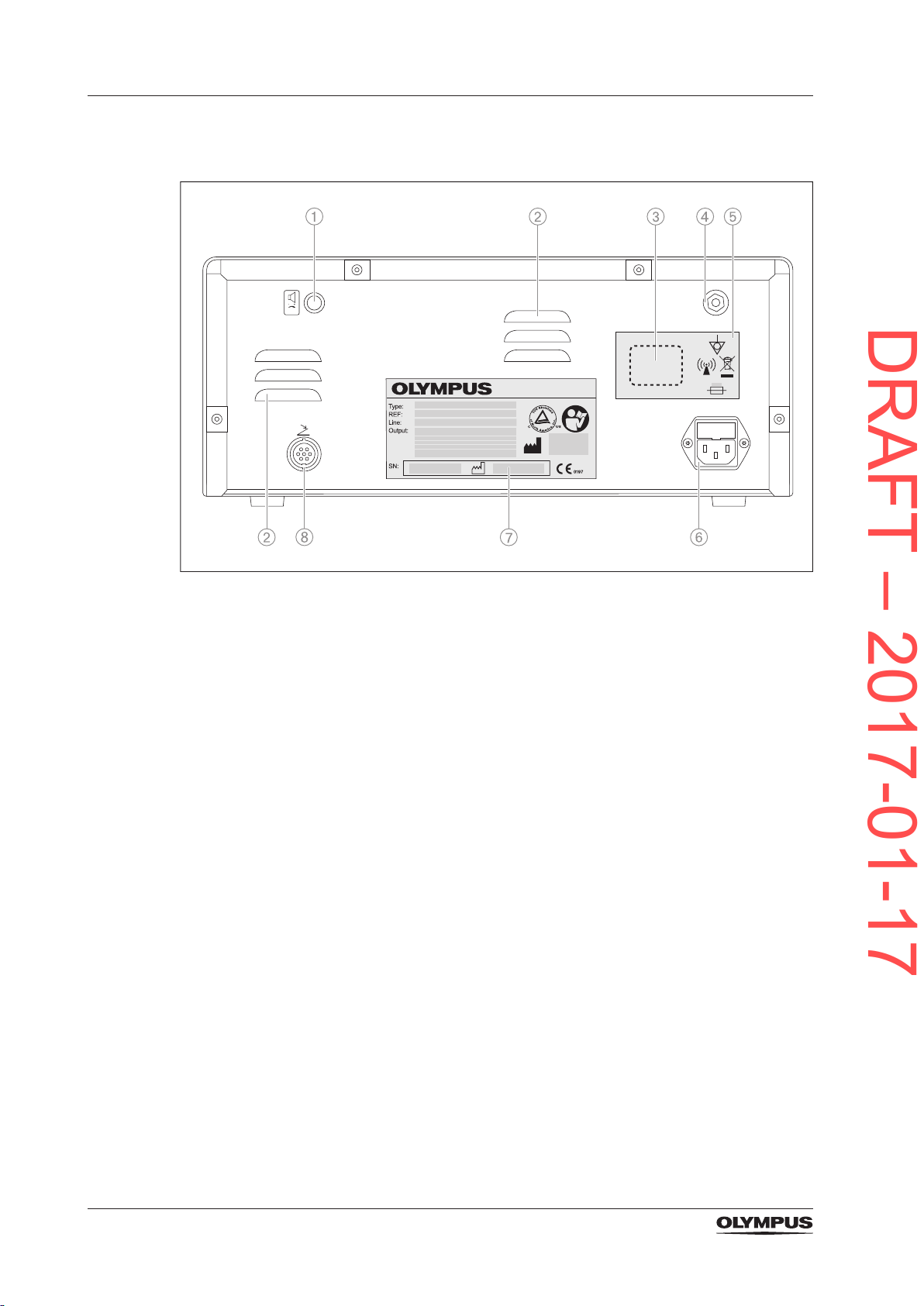
3.5.2 The rear panel
Figure 3.2 Rear panel
1) Volume control
To adjust the volume of the output tone.
2) Ventilation slots
3) Place holder for country specic RFID symbol and registration number
Only applicable for specic countries. Not applicable for the European Union.
4) Equipotential bonding point
To increase electrical safety by potential equalization
5) Symbol plate
6) Power socket with fuse holder
7) Rating plate
8) Foot switch socket
To connect the double pedal foot switch.
26
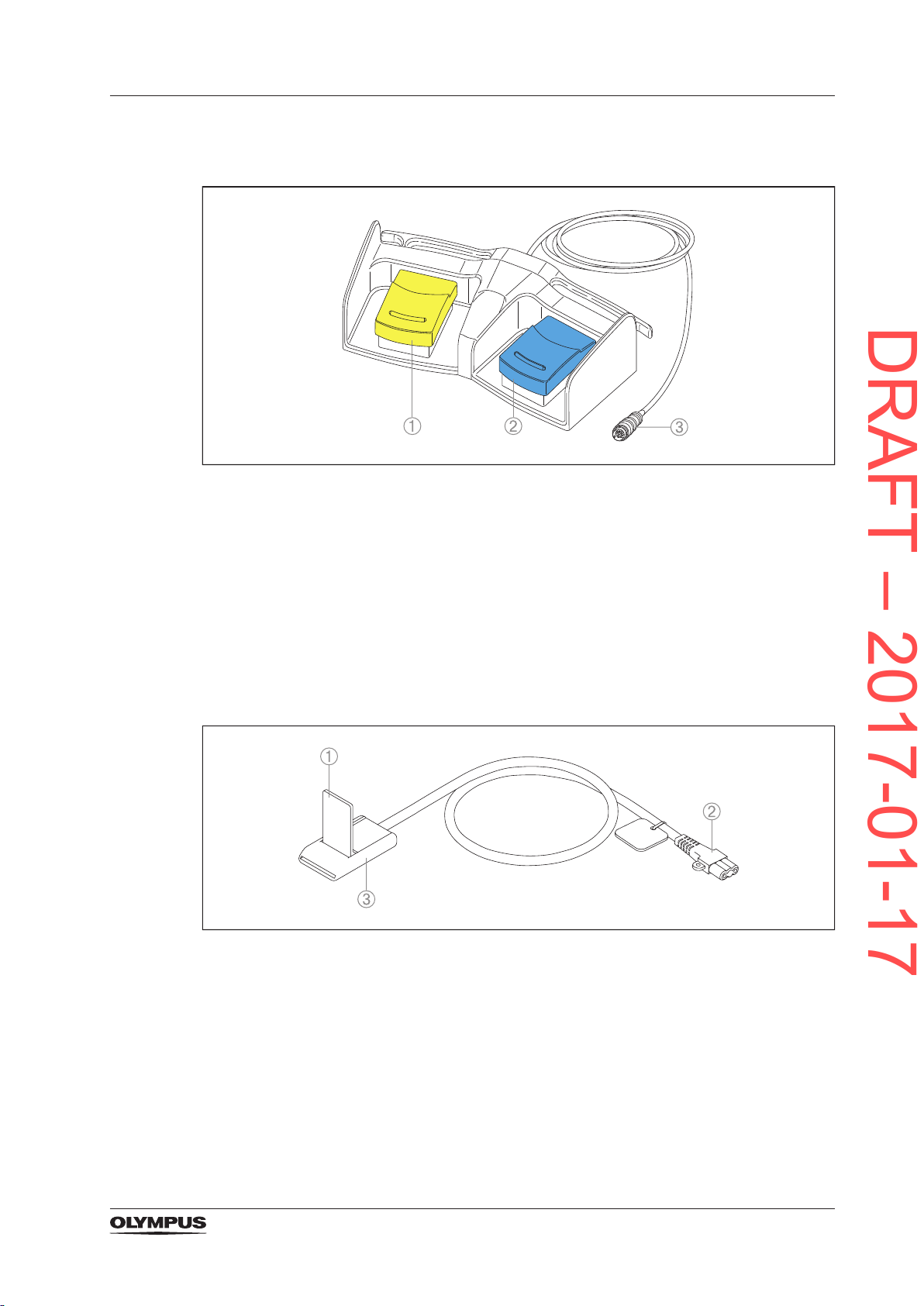
3.5.3 The double pedal foot switch
DRAFT – 2017-01-17
Figure 3.3 Double pedal foot switch
1) Cut pedal (yellow)
To activate the selected cutting mode.
2) Coagulation pedal (blue)
To activate the selected coagulation mode.
3) Foot switch plug
To connect the foot switch to the foot switch socket.
Product description and functional principles
3.5.4 The connecting cable for the neutral electrode
This cable is not included in the delivery scope and may be purchased separately.
Figure 3.4 Cable for neutral electrode, MAJ-814
1) Locking lever
To lock the neutral electrode in the clamp.
2) Plug to front panel of electrosurgical generator
To connect the neutral electrode to the neutral electrode socket.
3) Clamp
To connect the neutral electrode to the cable.
27
Product description and functional principles
DRAFT – 2017-01-17
3.6 Nomenclature and functional principles of the touch screen
The electrosurgical generator is equipped with a user friendly touch screen. The control
buttons are activated by tapping the corresponding part on the screen with the nger tip.
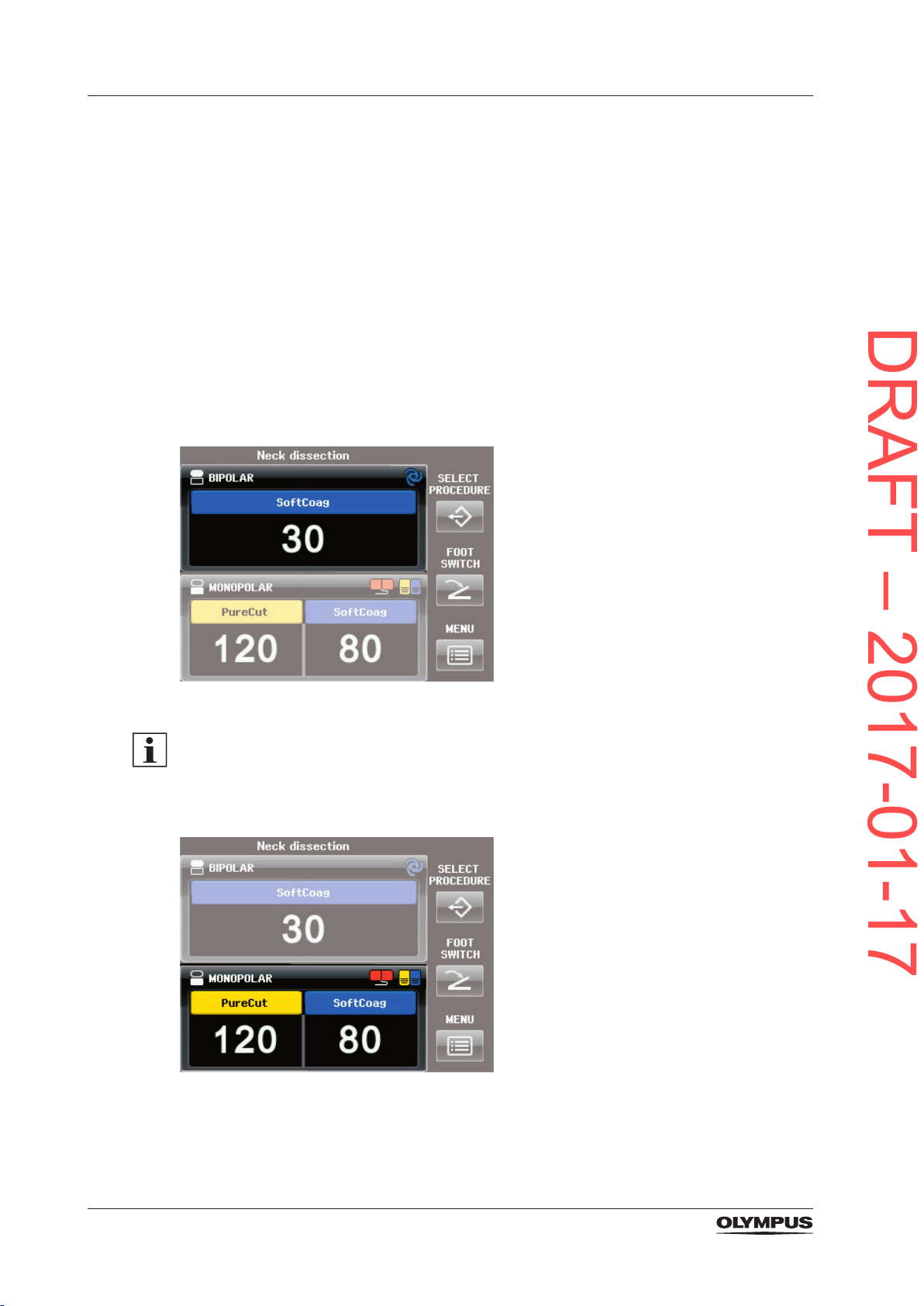
3.6.1 The All Screen
The All Screen is the initial screen of the electrosurgical generator. The All Screen displays
the current settings of the selected modes as well as the 3 buttons on the right-hand side for
system settings.
MONOPOLAR pane, BIPOLAR pane and buttons for system settings
Each pane covers all output socket related information as described below. Tap into the
pane to switch to the corresponding Set Screen. On the Set Screen the mode and the output
power levels for the corresponding output socket can be set.
Figure 3.5 BIPOLAR pane
As a default setting the cutting modes for the BIPOLAR socket are switched off, i.e. only the
blue coagulation button is displayed in the BIPOLAR pane. It is impossible to access and
activate any bipolar cutting mode.
• To switch on the bipolar cutting modes refer to the section “Bipolar Cut Setup” on page
46.
Figure 3.6 MONOPOLAR pane
28
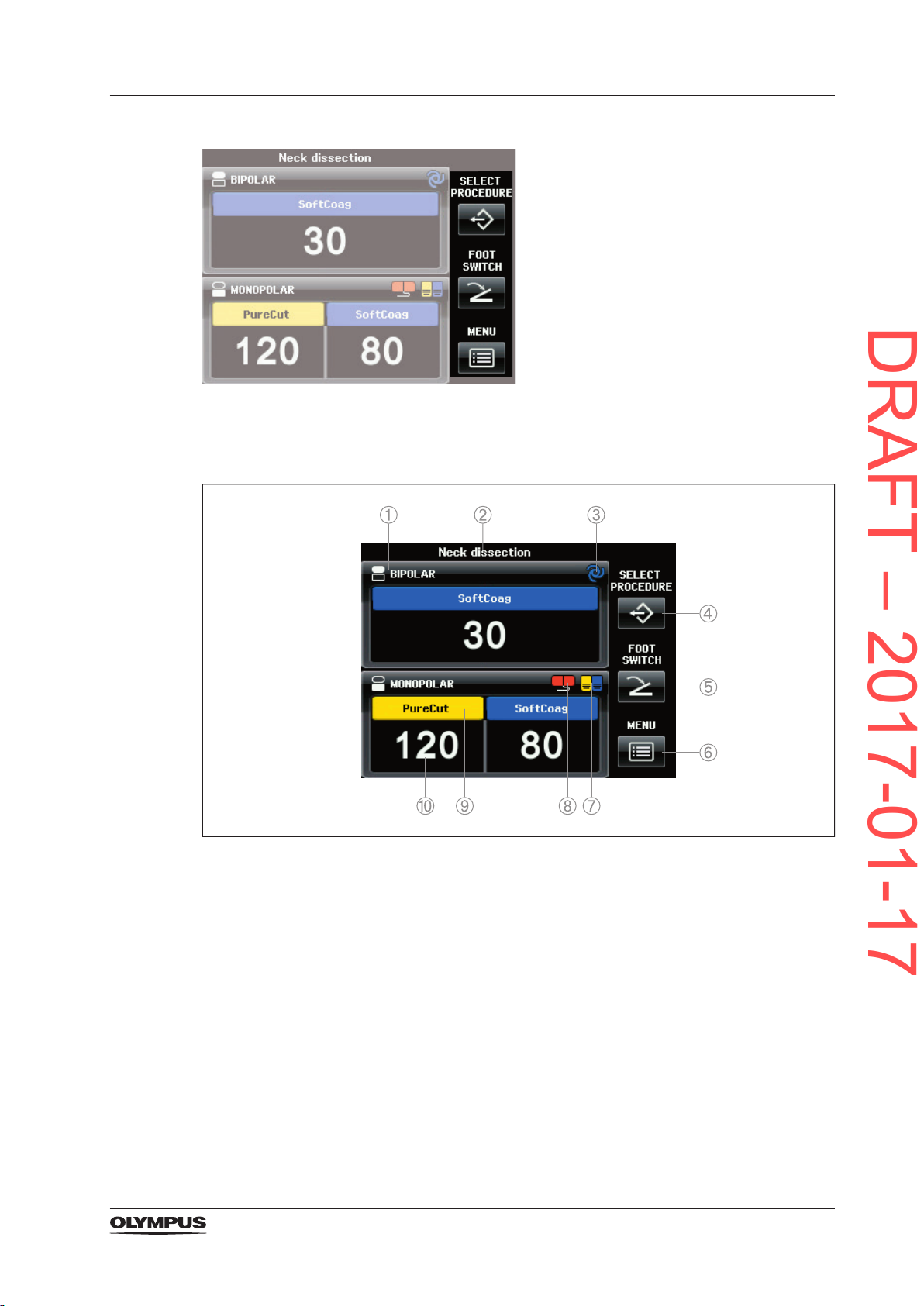
Figure 3.7 Buttons for system settings
DRAFT – 2017-01-17
Single elements of the All Screen
Product description and functional principles
Figure 3.8 Buttons and symbols on the All Screen
1) Output socket indicator and output socket name
Displays the symbol and the name of the corresponding output socket.
2) Procedure name
Displays the selected procedure. If no procedure is selected, then this line is blank.
3) Autostart indicator
Indicates that the Autostart feature is assigned to the corresponding output socket.
4) Button [SELECT PROCEDURE]
To open the screen [Select Procedure screen] for recalling saved settings.
5) Button [FOOT SWITCH]
To open the screen [Assign Foot Switch] for assigning the foot switch or the Autostart
feature to an output socket.
6) Button [MENU]
To open the screen [Select Menu] for controlling several functions.
7) Foot switch indicator
Indicates that a foot switch is assigned to the corresponding output socket.
29
Product description and functional principles
DRAFT – 2017-01-17
8) CQM indicator for neutral electrodes
Indicates the connection status of a neutral electrode.
9) Mode name
Displays the selected mode.
10) Output power level
Displays the selected output power level.
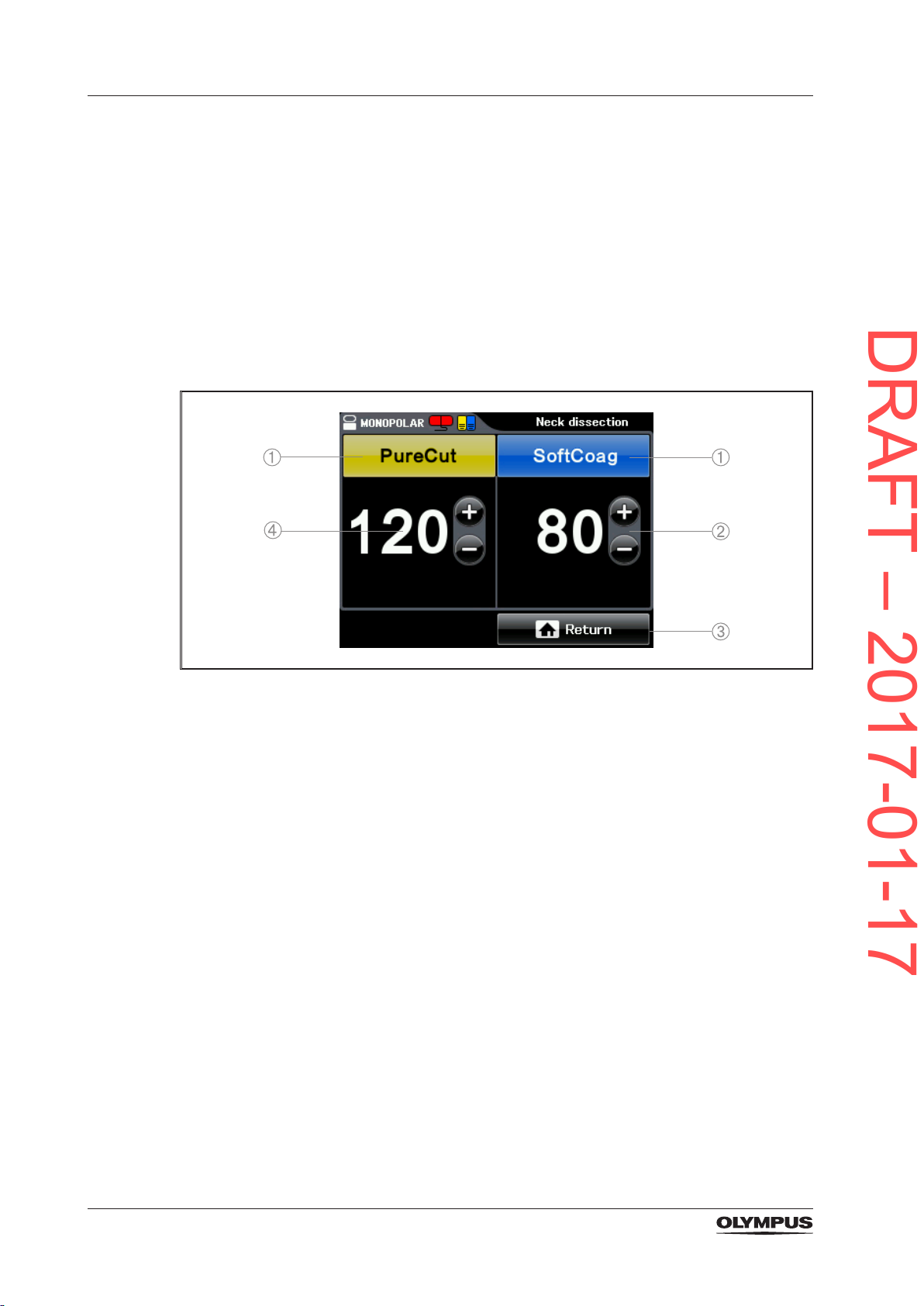
3.6.2 The Set Screen
On the Set Screen the mode and the output power levels for the corresponding output
socket are set. The Set Screen is displayed when tapping into the pane [BIPOLAR] or
[MONOPOLAR] on the All Screen.
Figure 3.9 Set Screen
1) Mode button
Displays the name of the output mode as selected in the Mode Screen.
2) Plus and minus button
To increase and decrease the level of output power.
3) Return button
To save the settings and to return to the All Screen.
4) Output power level
To show the selected level of output power.
30
 Loading...
Loading...