Page 1

OLYMPUS POLARIZING MICROSCOPE
[
INSTRUCTION
MANUAL
I
MODEL
BHSP
Page 2

This instruction manual has been written for the use of the Olympus Polarizing Microscope Model
BHSP.
It
is
recommended to read the manual carefully in order to familiar-
ize yourself fully with the use of the microscope, so that you can obtain optimum per-
formance from it.
VL.__n_NWW___---x--
IMPORTANT
Observe the following points carefully:
Operation
1. Always handle the microscope with the care it deserves, and avoid abrupt motions.
2.
Avoid exposure of the microscope to direct sunlight, high temperature* and humidity,
dust and vibration.
If the microscope is used in an ambient temperature higher than 40°c (104"F), it
may cause a trouble to the microscope.
3.
Only use the tension adjustment ring for altering the tension of the coarse adjustment.
Do not twist the two coarse adjustment knobs in the opposite directions simultane-
ously, which might cause damage.
4. Ascertain that the line voltage selector switch on the base plate is set to conform with
the local mains voltage.
Maintenance
1. Lenses must always be kept clean.
Fine dust on lens surfaces should be blown or
wiped off by means of an air blower or a clean brush. Carefully wipe'off oil or fingerprints deposited on the lens surfaces with gause moistened with a small amount of
xylene, alcohol or ether.
2.
Do not use organic solutions to wipe the surfaces of various components. Plastic
parts, especially, should be cleaned with a neutral detergent.
3.
Never disassemble the microscope for repair. Only authorized Olympus service per-
sonnel should make repairs.
4.
The microscope should
be
stored in its container immediately after use. If this is not
possible, it should be covered with'a vinyl dust cover.
Page 3

CONTENTS
I . STANDARD EQUIPMENT
.
II
NOMENCLATURE
.
Ill
ASSEMBLY 4
IV . IDENTIFICATION AND FUNCTION OF VARIOUS COMPONENTS 6
V
. OPERATION
Switching on the Light Source
Adjusting the Observation Tube
Use of the Orientation Plate
Centering the Condenser
Centering the Stage
Centering the Objectives
Use of Iris Diaphragms
Focus Adjustment 14
...............................................
..............................................
.....................................
..........................................
........
10
................................
...................................
......................................
11
12
13
.......................................
2
3
Use of Immersion Objectives
Orthoscopic Observation
Conoscopic Observation
Photomicrography
VI . OPTICAL DATA
VII . TROUBLESHOOTING ........................................ 18
............................................
................................
...................................
15
16
17
Page 4

1.
STANDARD EQUIPMENT
-
Component
Microscope stand (with Allen wrench,
KB-4 filter, immersion oil, and vinyl
dust cover, one each)
Line cord UYCP
Observation
tubes
Quadruple revolving nosepiece BH-PRE
Polarizing intermediate attachment
(with pin hole cap)
Centerable circular stage
(with stage plate, centering wrenches,
paired, and stage clips, paired)
Test plates
100-watt halogen lamp housing
(with collector lens and filter holder,
one each)
Pre-centered halogen bulbs 12VlOOWHAL-L
Abbe polarizing condenser BH2-POC
Objectives
Eyepieces
Photo eyepiece NF K3.3X
Binocular tube BH2-B130
Trinocular tube
Quarter wave plate
(R
=
-
147.3my)
Sensitive tint plate
(R
=
530my)
PO-D Ach. 4X
PO-D Ach. 10X
PO-D Ach. 20X (spring)
PO-D Ach. 40X (spring)
PO-D Ach. 100X (spring, oil)
PO-D Plan 4X
PO-D Plan 10X
PO-D Plan 20X (spring)
PO-D Plan 40X (spring)
PO-D Plan 100X (spring, oil)
WHK 10X
Cross-WH
Micro-WH
K
K
1 OX
1 OX
-------__-
BHSP-F
BH2-TR30
BH2-PA
BH2-SRP
AH-TP147
AH-TP530
BHS-LSH
Model
651P
1
1
1
-
1
1
1
1
1
1
2
1
1
1
1
1
1
-
-
-
-
-
1
1
1
-
653P
1
1
1
-
1
1
1
1
1
1
2
1
-
-
-
-
1
1
1
1
1
1
1
1
-
BHS-
751P
1
1
-
1
1
1
1
1
1
1
2
1
1
1
1
1
1
-
-
-
-
-
1
1
1
1
753P
1
1
-
1
1
1
1
1
1
1
2
1
-
-
-
-
-
1
1
1
1
1
1
1
1
1
Optional Accessories:
Berek compensator AH-CT P-3
Attachable mechanical stage AH-FMP
Page 5

11.
NOMENCLATURE
The Model
Revolving
nosepiece
BHSP
consists of various components as shown in the photo below:
Observation tube
,
Objective
Stage
Condenser
Base
Page 6

Ill.
ASSEMBLY
This picture illustrates the sequential procedure of assembly. The numbers indicate the
order of assembly
Take care to keep all glass surfaces clean, and avoid scratching the glass surfaces.
of
various components. Remove dust caps before mounting components.
NOTE:
Quarter wave plate
Sensitive wave plate
**Berek compensator
**Mechanical
For numbers
page.
0,
0,
0
and 0 please refer to explanations in detail on the next
Observation tube
Condenser
*
Screw the
**
Optionally available.
10X
Collector lens
Microscope stand
objective into theJfixed aperture of the nosepiece.
Page 7

Explanations
@
Mounting the halogen bulb
1)
Releasing the bulb clamping levers @ of
the collector lens
the arrow, insert two contact pins of the
halogen bulb into the socket
(Recommended to use a glove or gauze
to handle the halogen bulb.)
2)
Secure the bulb in position with the two
levers.
*
Before use, wipe off any fingerprints or
stains on the bulb.
0
Mounting the stage
1) Prior to mounting the stage, rack down
the stage mounting dovetail
way. (Fig.
2)
Loosen the stage clamping screw @ by
rotating counterclockwise with Allen
wrench provided. (Fig.
Insert the stage into the mounting dove-
tail
a
3)
Lower the stage until it comes in contact
with the stop pin
screw
in
detail
a
in the direction of
2)
2)
from above slowly. (Fig.
0;
then clamp with
@
.
(Fig.
2)
@.
a
2)
(Fig.
all the
1)
Fig. 1
Fig.
2
0
Mounting the revolving nosepiece
1)
Loosen the nosepiece clamping screw
(Fig.
3)
2)
Aligning the nosepiece dovetall slide to
the mounting block
piece
slowly all the way
*
Do
inserting into the mounting block.
Mounting the intermediate polarizing attachment
1)
Loosen the clamping screw
spring-loaded clamping screw
will cause the locating pin
draw. (Fig.
the screw further until the pin withdraws.
2)
With clamping.screw 0 pulled out, insert
the circular dovetail of the intermediate
attachment into the ring dovetail.
3)
Tighten the clamping screw.
not
tilt
or lock the nosepiece while
4)
@,
push in the nose-
a
fully. Pull
@.
@
to with-
If the pin does not, loosen
a
This
Fig.
3
!
Fig.
4
Page 8

IDENTIFICATION AND FUNCTION OF VARIOUS COMPONENTS
IV.
1,
!
Light path selector
The knob can be operated in
3
positions to deflect the light
as desired.
knob
Field
iris
diaphragm
Arrow mark
cates increase in diaphragm intensity. vided.
diameter.
@
ring
-t
0
indi- For continuously variable light Accepts the filter holder pro-
Page 9

Diopter adjustment ring
Photo tube
45'
click
stop lever
Stage centerinq knob
Pre-focusing lever
adjustment knob
P
ertrand lens turret ring
Engraved letters "IN" for insertion of Bertrand lens into
the liqht path.
removal of. the Bertrand lens
from the light path.
Bertrand lens focusing ring
'.
Clamping screw for stage
rotation
Polarizer clamping screw
After optimum extinction is
attained, clamp the polarizer.
"OUT"
for
Swing-out knob for
lens
Condenser
(when top lens swings
out, N.A. is
N.A.
0.25).
is
0.9
top
/
Y
Main switch
Page 10

holder*
Line cord
*Do not replace filters for about a few minutes after use, in order to give time to cool.
**The circuit breaker protrudes its central part to cut off the electrical power in case of the
dimmer circuit trouble (due to short circuit, etc.) or overcurrent. To restore the breaker, press
the central part. If the breaker is actuated again, disconnect the line cord from the
AC
outlet
and contact the Olympus service center.
Page 11

Summary of Putting the Microscope in Operation
Model BHSP
2
@
+
0
Match the line voltage selector switch to local mains voltage (see page
Switch on the light source.
Place a specimen slide on the stage.
Remove the Bertrand lens and analyzer from the light path.
10X
Coarse focus with the
Make interpupillary and diopter adjustments (page
Set the analyzer to optimum extinction position (page
Center the condenser (page
Center the stage (page
Center objectives other than
J
.
Swing in the desired objective.
K.
L.
Set the condenser, analyzer and Bertrand lens correctly according to your microscopic
purpose (pages
M. Fine focus.
Adjust aperture iris diaphragm and field iris diaphragm (page
N.
15
and
Microscopic
application
1
orthosco~ic
observation
objective.
10).
11).
12).
13).
10X
(page
13).
16)
/
Adjustment of Illumination System
Bertrand lens
Objective
/
4X
to
loox
in intermediate
polarizing at-
tachment
I
OUT
13).
Condenser
top lens
I
I
OUT
10).
i
1
Conoscopic
observation
Generally for biological use, however, remove the analyzer, Bertrand lens and test plates
from the light path.
*
Cut off this page at dotted line and put
reminder of microscopic procedure.
20X
to
100X
IN
I
it
on the wall near the microscope for use as a
IN
OLYMPUS
Page 12

V.
OPERATION
Switching on the Light Source
A.
1) Ascertain that the voltage selector switch
is set to conform with the local mains volt-
age. (Fig.
set, adjust it by means of the Allen wrench
provided or a screwdriver.
2)
Place the sliding voltage control lever @ on
the right side of the microscope base to a
position closest to you (low voltage position)
(Fig.
3) Actuate the main switch
(
Voltage Adjustment and Light Intensity
As you push the control lever
rection of the arrow in order to obtain in-
creasing intensity (Fig.
@
will display the lamp voltage.
Two LEDs on the left side indicate the volt-
age from
right side from
ments. The indication with the letters
"PHOTO"
color photomicrography.
6)
5)
If the switch is not correctly
@.
(Fig.
a
6),
the LED readout
OV
to
6V,
and twelve LEDs on the
6.5V
to 12V in
can
be used as a guide line for
0.5V
@
5)
/
in the di-
incre-
Fig.
Fig.
5
-
6
B.
Adjusting the Observation Tube
1) At the time of inserting the eyepieces into
the observation tube, take care to insert the
eyepiece with cross hairs or micrometer of
your choice into the right eyepiece tube,
aligning the positioning slot
ing pin
2) Looking through the both eyepieces with
both eyes, adjust the interpupillary distance,
sliding the knurled dovetail slides of the
right and left eyepiece tubes, until perfect
binocular vision is obtained. (Fig.
3) Looking through the right eyepiece (with
cross hairs or micrometer) with your right
eye, rotate the upper helicoid ring
eyepiece until the cross hairs (or micrometer)
are sharply focused. (Fig.
4)
Focus on the specimen with the coarse and
fine adjustment knobs so that the sharp
images of the specimen and cross hairs (or
micrometer) can be obtained simultaneously.
5)
Now look at the image through the left eyepiece with your left eye and rotate the
diopter adjustment ring
specimen without using the coarse and fine
adjustment knobs. (Fig.
@.
(Fig.
7)
a
and position-
8)
Q)
8)
@
to focus on the
8)
of the
Fig.
Fig.
7
8
Page 13

[
Light Path Selection
The trinocular tube is provided with a light
a
path selector knob
the observation tube and/or to the photo
tube in
3
positions. (Fig.
to direct the light to
9)
Knob position
I
Amount of light
I
I
Application "Crossed filter" (1 ) Normal
An indicator plate is provided at the knob port to summarize the usage of the above table;
it
can be consulted before operating the knob.
V: Viewer (white letter)
C.V: Camera and viewer (yellowish green letters)
C: Camera (red letter)
The colors of the letters correspond with the color bands on the knob shaft.
Use
C.
2)
3)
4)
5)
6)
of the Orientation Plate
The analyzer built in the intermediate attachment should be adjusted for optimum extinction by means of the orientation plate provided in the following steps:
1) Bring the 10X objective into the light path, and make sure that the red dots
intermediate attachment and microscope stand are aligned. (Fig. 10)
Set both polarizer and analyzer at position "0" to attain the "crossed filter" position
Place the orientation plate on the center of the stage.
Looking at the orientation plate through the eyepieces, rotate the stage (as you rotate the
stage, the orientation plate darkens and brightens alternately) until it most darkens or attains
the extinction position; then, touch up the position ot the orientation plate manually so
that the lower edge (fiducial line) of the orientation plate nears the cross line
Disengage the analyzer @ from the light path, which makes the field of view bright.
Loosening the observation tube clamping screw @ ,
until the fiducial line of the orientation plate is in parallel with the cross line; then, reclamp
the observation tube. (Fig. 10)
Pushed in all the way
1
100% into binocular tube 1 20% into binocular tube / 100% into photo tube
(V) (C.V)
1 1
observation
Pulled out halfway
80% into photo tube
/
observation
(2)
Photomicrography
(focusing through
the binocular tube)
rotate the observation tube slightly
Pulled out all the way
(C)
I I
1
Photomicrography
a
(X
-
Fig.
Orientation
Plate Cross line
'10
I
1)))
on both
axis).
0
Fiducial line
parallel
Page 14
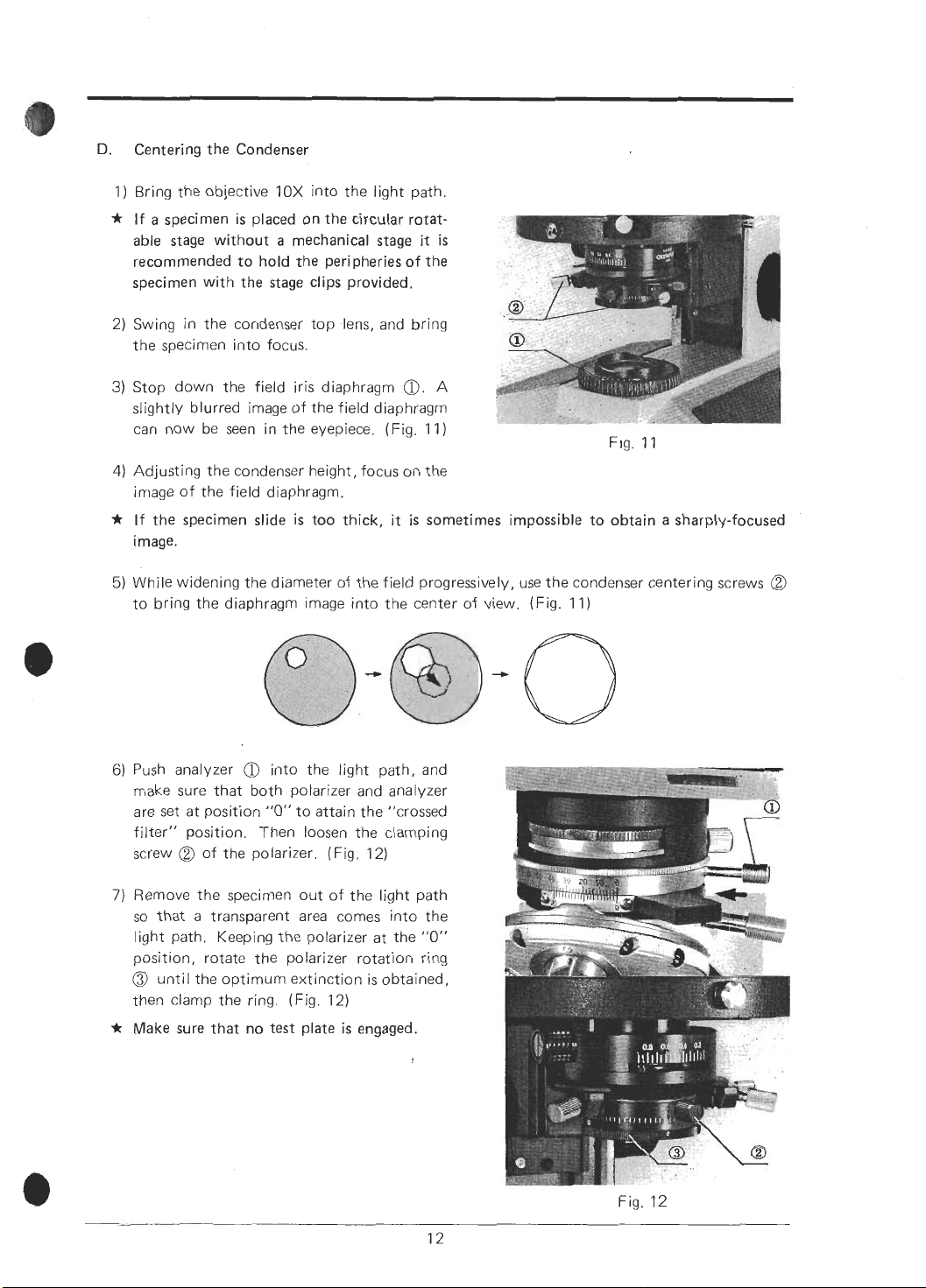
D.
Centering the Condenser
1) Bring the objective 10X into the light path.
*
If a specimen is placed on the circular rotatable stage without a mechanical stage
recommended to hold the peripheries of the
specimen with the stage clips provided.
2) Swing in the condenser top lens, and bring
the specimen into focus.
3)
Stop down the field iris diaphragm
slightly blurred image of the field diaphragm
can now be seen in the eyepiece. (Fig. 1 1
4)
Adjusting the condenser height, focus on the
image of the field diaphragm.
*
If the specimen slide is too thick, it is sometimes impossible to obtain a sharply-focused
Image.
5)
While widening the diameter of the field progressively, use the condenser centering screws
to bring the diaphragm image into the center of view. (Fig. 11)
it
O.
A
is
)
Fig.
11
@
6)
Push analyzer
make sure that both polarizer and analyzer
are set at position
filter" position. Then loosen the clamping
screw
@
7)
Remove the specimen out of the light path
SO
that a transparent area comes into the
light path. Keeping the polarizer at the
position, rotate the polarizer rotation ring
@
until the optimum extinction is obtained,
then clamp the ring. (Fig. 12)
*
Make sure that no test plate
a
into the light path, and
"0"
to attain the "crossed
of the polarizer. (Fig. 12)
is
engaged.
"0"
5
..
--.
7773?w-,*
Fig.
12
Page 15

E.
Centering the Stage
1) Place a specimen on the stage, and insert two centering wrenches
into the stage center-
ing screws (Fig. 13). Looking through the eyepiece and objective IOX, fix your eyes on
some particular point, for instance, at the point A or the center of the cross hairs.
2)
As you rotate the stage, the particular point in the specimen image moves f rom the point
in a circular path (A
circular path
E.
+ B + C +
(Diag. a)
D + A or A
+ D + C + B +
A) around the center of the
3) When the stage is turned by 180° from the
point A, the particular point coincides with
the point C.
4) Coincide the particular point to the point
E
by means of the two centering wrenches
(Fig. 13). (Diag. b)
5)
Next, move the particular point from the
point
E
to the center A of the cross hairs
(Diag.
c).
Ir
Repeat this procedure until the stage centra-
tion is complete. After completing the cen-
Fig. 13
tration, remove the wrenches.
Diag. a
Diag.
b
Diag.
c
A
F.
Centerins the Obiectives
This objective centration is necessary to all
the PO objectives except P010X. After
completing the stage centration, insert two
centering wrenches
a
into the objective
centering screws provided at each centerable
objective aperture
in
the nosepiece. (Fig.
14) The other procedure is just the same as
with stage centration.
G.
Use of Iris Diaphragms
Fig. 14
When the top lens of the polarizing condenser is swung out for orthoscopic observation, the
aperture iris diaphragm serves as a field iris diaphragm and the field iris diaphragm as an aperture iris diaphragm.
For conoscopic observation, generally the aperture iris diaphragm
is
fully opened and the
field iris diaphragm can be effectively used for reduction of glares and conoscopicobservation of very small objects.
Page 16

1)
Aperture iris diaphragm
Adjust the opening of the aperture iris diaphragm according to the various conditions such as the numerical
aperture of the objective, image contrast, depth of focus,
and flatness of field. Generally it is often preferable to
stop down the aperture iris diaphragm to about
80%
of the N.A. of the objective.
After the eyepiece is removed from the observation
tube, if necessary, look through the observation tube
and check the opening of the aperture diaphragm at the
objective pupil.
2) Field iris diaphragm
The field iris diaphragm controls the diameter of the ray bundle impinging on the specimen
surface and thus increases image definition.
Generally, it is preferable to slightly increase the diameter of the field iris diaphragm until
it is just outside the field of view.
H.
Focus Adjustment
1.
Tension of Coarse Adjustment Knobs
and Fine Adjustment
Although the tension of the coarse adjustment knobs has been already adjusted for
optimum performance by the manufacturer,
it is possible to personally adjust the tension
of the coarse adjustment for either heavy or
light movement depending on the operator's
preference by rotating the tension adjust-
ment ring
The ring can be rotated by inserting a screwdriver into one of the holes on the periphery of
the ring. The clockwise rotation (in the direction of the arrow) tightens the coarse adjust-
ment knobs. Do not loosen the ring too much, because the stage may drop or the fine
adjustment knobs may slip.
NOTE: Do not rotate the right and left coarse adjustment knobs in the opposite directions
a.
(Fig.
15)
simultaneously. If the stage drops and the specimen goes out of focus, the tension
adjustment ring is too loose. Tighten the ring.
70%
or
-.
Fig.
15
70-80%
30-
20%
2. Pre-focusing Lever
This lever
contact between specimen and objective as
well as to simplify coarse focusing. (Fig.
The lever is locked after coarse focus has
been accomplished. This prevents further
upward travel of the stage by means of the
coarse adjustment knobs, and automatically
provides a limiting stop if the stage is low-
ered and then raised again. The pre-focusing
lever does not restrict fine focusing.
@
is provided to prevent possible
16)
Fig.
16
Page 17

3.
Adjustment of Stage Block Height
In addition to the vertical movement of the
stage by means of coarse and fine adjustments, the stage block height can be changed
for observation of specimens which are
thicker than standard slides. To lower the
stage block;
1)
Loosen the stage block locking screw
with Allen wrench provided, and raise
the stage block until the stopping screw
a
can be seen, then reclamp.
2)
Replace the stopping screw into the lower
threaded hole@). (Fig. 17)
3)
Unclamping the stage block again, lower
until it stops, and clamp.
I.
Use of Immersion Objectives
1) Focus the specimen with a low power objective.
2)
Put a drop of immersion oil on the specimen slide and the front lens of the immersion
objective.
3)
Turn the revolving nosepiece to bring the immersion objective into the light path, and
focus with the fine adjustment knobs.
NOTE:
a
Care should be taken to prevent oil bubbles from forming in the oil film If
any, re-apply immersion oil, for these bubbles greatly deteriorate the lens
performance.
a
After use carefully wipe off the immersion oil deposited on the lens surfaces
with gauze moistened with xylene. Never leave oil on the lens surfaces after
use as oil remnants will seriously impair the performance of the lens system.
a
Fig. 17
'
4
1
J.
Orthoscopic Observation
1)
Swing out the top lens of the condenser.
In principle, polarized light enters the light path, parallel to the optical axis, to enable
observation of the optical characteristics of the specimen. However, this method will
darken the field of view and lower the resolving power of the objective extremely. Therefore, swing out the top lens of the condenser, using only the lower aperture of the lower
condenser lens (N.A.
2)
Insert the analyzer into the light path,
and attain the crossed filter position with
analyzer and polarizer at
this position, the polarizer vibration
the
X
direction, and the analyier vibra-
tion in the
position, pull out the analyzer rotation
screw.
3)
Rotate the stage until the extinction of
the image is attained, and move the
click stop lever
(Fig. 18)
0.25).
0
setting. At
Y
direction. To open the filter
a
toward the operator.
is
45"
in
Fig. 18
Page 18

From this position, it is easy to rotate the stage in
refer to the angular scale, and the stage clicks at the diagonal position, at which position,
the retardation angle is measured. (Take care if you rotate the stage too fast, it sometimes overrides the click stop position.) To release the
45'
click stop lever.
4)
Insert the quarter wave plate or sensitive tint plate into the slot, closest to you at your
right hand side in the intermediate polarizing tube.
To disengage the test plate, you can just pull
*
A
Berek compensator is optionally available to measure the birefringence of a specimen.
it
45'
increments without having to
45'
click stops, push back the
back to its click stop position.
Sensitive tint plate Quarter wave plate
K.
Conoscopic Observation
1)
Swing in the top lens of the condenser
need to immerse between the condenser and specimen slide.
2)
Bring the specimen into focus, rotate the Bertrand lens turret ring into the IN position.
3)
Focus on the interference figure formed at the back focal plane of the objective from
20X
to
100X.
objects.)
The pinhole cap provided may be used in place of the eyepiece to directly view the
interference figure mentioned above. In this case, the Bertrand lens is disengaged.
L.
Photomicrography
1)
Photomicrographic equipment
Photomicrography with the Model BHSP requires photomicrographic equipment such
as the photomicrographic system camera, exposure meter, photo eyepiece, etc. Read
the instruction manuals for each equipment.
2)
Photo eyepieces
graphy, and
Berek compensator
(N.A.
0.9).
and illuminate the specimen with no
(Recommended to stop down the field irisdiaphragm in case of very small
NFK3.3X
NFK2.5X
and
NFK5X
for conoscopic photomicrography.
are recommended for orthoscopic photomicro-
3)
Image magnification is obtained from the equation below:
Objective magnification
X N FK
photo eyepiece magnification.
Page 19

VI.
OPTICAL DATA
Objective
\
Eyepiece
\
WHKlOX
(Field number
WHKlOX
(Field number
Magnificat ion
W.
D.
(mm)
Focal length (mm)
Resolving power (p)
Total magnification
Focal depth
20)
Field of view dia-
f
meter (mm)
+
Magnification
Focal length (mm)
Resolving power (p)
Total magnification
Focal depth
20)
Field of view diameter (mm)
(p)
(p)
1
1
34.23
3.36
40X
172.5
5
1
1.34
1
OOX
27.6
2
PO
D
Ach.
PO D Plan
20X
0.4
0.83
8.99
0.84
200X
9.1
4
1
+
40X
0.65
0.23
4.67
0.52
400X
3.0
0.5 0.1
1 OOOX
1
OOX
1.25
0.17
1.75
0.27
1 OOOX
0.68
*
Immersion objective. Resolving power is obtained when the objective is used at the full
aperture diaphragm.
0
W.D. (Working distance):
The distance between the specimen or cover glass and the nearest point of the objective.
0
N.A.
(Numerical aperture):
The numerical aperture represents a performance number which could be compared to
the relative aperture (f-number) of a camera lens.
comparing the resolving powers of all types of objectives. The larger
resolving power.
0
Resolving power:
The ability of a lens to register small details. The resolving power of a lens is measured
by its ability to separate two points.
0
Focal depth:
The distance between the upper and lower limits of sharpness
an optical system.
0
Field number:
A
number that represents thq diameter in mm of the image
is formed by the lens in front of it.
0
Field of view diameter:
The actual size
of
the field
of
view in mm.
N.A.
values can be used for directly
N.A.,
the higher the
in
the image formed by
of
the field diaphragm that
Page 20

VII. TROUBLESHOOTING
If you are unable to obtain full performance from your microscope, please consult with the
table below as pointers for troubleshooting.
Troubles
1.
Optical System
(a) With illuminator
switched
field of view
cannot be seen.
(b) Field of view is
cut off or illumi-
nated
(c) Dust or dirt is
visible in field of
(d) Excessive image
contrast.
(e) Resolution prob-
lems:
0
Image is not sharp.
0
Insufficient con-
t rast.
o
lmage details lack
definition.
On,
irregularly.
Causes Remedies
Bertrand lens is engaged.
Analyzer and polarizer are in "cross
filter,n
position
Light path selector lever is stopped
midway.
Nosepiece is not click stopped.
Nosepiece
to stand.
Condenser is not correctly mounted
on ring mount.
Test plate is stopped midway.
In case of orthoscopic observation,
condenser top lens stays in light
path or stops midway.
Field iris diaphragm is stopped
down excessively.
Lamp is not correctly attached.
Dust or dirt on glass surface at light
exit on base.
Dust on condenser top lens.
Dirty specimens.
Dust on eyepiece.
Condenser is lower excessively.
Aperture iris diaphragm
down excessively.
Nosepiece is not correctly attached.
Objective is not correctly position- Slightly rotate nosepiece until it
ed in light path.
I
Dirt on objective front lens.
Immersion objective i$ used with-
out immersion oil.
Bubbles in immersion oil.
Olympus designated oil is not used.
Dirty specimen.
Dirt on condenser lens.
Specimen is not properly illumi- Adjust illumination.
nated.
is
OjJ,,
not correctly attached
is
stopped
Disengage.
Disengage analyzer.
V.
or
Push in lever up to C.
Slightly rotate nosepiece until it
clicks into position.
Insert sliding dovetail mount kto
stand all the way, until it stops,
then lock.
Re-insert condenser all the way.
Push plate all the way until it clicks.
Swing it out of light path.
Open diaphragm fully.
Re-insert lamp correctly.
Clean off dust or dirt.
Raise condenser.
Open diaphragm.
Insert sliding dovetail mount all
it
the way, until
clicks into position.
I
Clean objective.
Apply immersion oil.
Remove bubbles.
Use designated oil.
Clean.
stops, then lock.
V.
1
Page 21

Troubles
I
Causes
I
Remedies
i
(f) Field of view
is partially out
of focus.
of focuseccentrically.
(h) Light intensity
does not increase
although voltage
is raised.
(i) No conoscopic
image can be
seen.
(j)
Crossed filter
position is not
attained.
Nosepiece
Objective is not correctly positioned in light path.
Specimen is not correctly positioned on stage.
Nosepiece is not correctly attached.
Objective is not correctly positioned in light path.
Condenser is out of center.
Condenser is not correctly centered.
Condenser is lowered excessively.
Condenser top lens is not in light
path.
Analyzer is out of light path.
is
not correctly attached.
Insert sliding dovetail mount into
stand all the way, then lock.
Slightly rotate nosepiece until
clicks into position.
Place specimen on stage and secure
it
with specimen clips.
Insert sliding dovetail mount all the
it
way, until
Slightly rotate nosepiece until it
clicks into position.
Center condenser.
Center condenser.
Raise condenser.
I
Swing it in.
Push it in.
stops, then lock.
it
2.
Electric System
(a) Illuminator
too bright (or
too dark).
(b) Output voltage
for illuminator
cannot be regu-
lated.
(c) Lamp flickers
and intensity is
unstable.
(d) Reduced bulb
life.
is
Line voltage selector switch
matched to the mains voltage.
Mains voltage is too high (or too
low).
Voltage selector switch is not
matched to mains voltage.
Mains voltage is too low or too
high.
is
Mains voltage
-
Loose electrical connection.
Bulb is not a standard bulb. Use a standard bulb.
unstable.
is
Match selector switch with mains
not
voltage.
Adjust mains voltage with a variable
voltage transformer.
Adjust mains voltage selector switch
to mains voltage.
Adjust mains voltage with a variable
voltage transformer.
Use a variable voltage transformer.
Secure connection.
Page 22

1
Troubles
Causes
Remedies
(a) Coarse adjust-
ment is too tight.
(b) Stage drops or
specimen goes
out of focus.
(c) Stage cannot be
raised to upper
limit.
(d) Stage cannot be
lowered to lower
limit of working
range.
(e) Objective front
lens hits against
specimen.
4.
Observation Tube
(a) Incomplete
binocular vision.
Tension adjustment ring
ed too much.
User is trying to raise stage passing
over upper focusing limit imposed
by engaged pre-focusing lever.
Tension adjustment ring is too
loose.
Pre-focusing lever is engaged in
lower than focusing position.
Condenser mount is lowered too
much.
Specimen is mounted on stage upside down.
lnterpupillary distance is not cor-
rectly adjusted.
Diopter adjustment is incomplete.
is
tighten-
Loosen tension adjustment ring
proper1 y.
Unlock pre-focusing lever.
Tighten ring properly.
Unlock pre-focusing lever
Raise condenser mount.
!
Reverse specimen.
Correct interpupillary distance.
Complete diopter adjustment.
J
<
_i
/
5.
Stage
out of focus
when you touch
stacle.
(b)
Specimen stops
midway on the
X
or Y traverse.
(c) When stage is
rotated, image of
specimen goes
out of field of
1
view.
Right and left eyepieces are not
matched.
User is unaccustomed with a binocular vision.
Stage
is
not correctly clamped.
Specirnen is not corre'ctly posi-
tioned on stage.
Stage is not centered or objective
is not.
Use a pair of rriatched eyepieces.
Prior to looking at the image of
specimen, try to look entire field
of view, or look at a far away object before resuming microscopic
observation.
Clamp stage securely. (a) l mage easily goes
Adjust specimen position.
Center.
 Loading...
Loading...