Ohio Medical 6700-1277-901, 6700-1275-901, 6700-1276-901, 6700-1278-901 User Manual
Thoracic Vacuum
Regulators
Service Manual
O(O)
|(On)
35
30
40
25
20
_
15
10
5
N
I
cmH O
E
R
C
45
2
50
55
60
A
S
E
40
50
5
60
4
High Flow
Low Vacuum
6
30
3
20
2
1
10
a
P
k
-
O
2
H
m
c
-
Thoracic
Thoracic
6700-0011-000 (Rev. 8.1) 08/19

Table of Contents
Safety Instructions ......................................................... 2
Receiving / Inspection.................................................... 2
User Responsibility ....................................................... 3
1/Precautions .................................................................. 4
1.1 Denitions ................................................................... 4
1.2 Warnings ....................................................................4
1.3 Cautions ..................................................................... 5
1.4 Intended Use .............................................................. 5
2/Scope ............................................................................ 6
2.1 North American Thoracic Vacuum Regulator ............. 6
2.2 English Thoracic Vacuum Regulator ..........................6
2.3 French Thoracic Vacuum Regulator ........................... 6
2.4 Spanish Thoracic Vacuum Regulator ......................... 6
3/Description and Specications .................................. 7
3.1 Description................................................................. 7
3.2 Specications ............................................................ 8
3.2.1 Technical Specications ................................... 8
3.2.2 Environmental Specications ........................... 8
3.2.3 Standards ......................................................... 8
4/Operation...................................................................... 9
4.1 Equipment Set-Up ...................................................... 9
4.1.1 Attaching the Overow Safety Trap (OST) ......10
4.2 Operation .................................................................. 10
4.2.1 Mode Selection .............................................. 10
4.2.2 Setting the Suction Level ............................... 10
4.2.3 Pre-use Checkout Procedure ......................... 10
4.2.4 Patient Set-Up ................................................ 11
8/Service Checkout Procedure.................................... 21
8.1 Set-Up ...................................................................... 21
8.2 Flow Test .................................................................. 21
8.3 Gauge Test ............................................................... 21
8.4 Regulation Test ......................................................... 21
8.5 Vacuum Limit Test .................................................... 21
8.6 Positive Pressure Relief Test .................................... 22
8.7 Leak Test .................................................................. 22
9/Maintenance ............................................................... 23
9.1 General Maintenance of Suction Equipment ............ 23
9.2 Recommended Maintenance.................................... 23
9.3 Repair Policy ............................................................ 24
9.4 Technical Assistance ................................................ 24
9.5 Return Instructions ................................................... 24
9.6 Installation Procedure for Adapters/Probes .............. 24
9.7 Disposal Instructions ................................................ 24
Safety Instructions
This manual provides you with important information about the
Thoracic Vacuum Regulators. To ensure the safe and proper
use of this device, READ and UNDERSTAND all of the safety
and operating instructions. IF YOU DO NOT UNDERSTAND
THESE INSTRUCTIONS, OR HAVE ANY QUESTIONS,
REFER TO THIS SERVICE MANUAL, CONTACT YOUR
SUPERVISOR, DEALER OR THE MANUFACTURER
BEFORE ATTEMPTING TO USE THE DEVICE.
Receiving / Inspection:
Remove product from package and inspect for damage. If
product is damaged, DO NOT USE and contact your dealer
or equipment provider.
5/Cleaning, Disinfection and Sterilization .................. 13
5.1 Cleaning ................................................................... 13
5.1.1 Routine Exterior Cleaning & Disinfection ........ 13
5.1.1.1 Approved Cleaning Solutions .............13
5.1.2 Internal Component Cleaning & Disinfection .. 13
5.1.2.1 Approved Flush Solutions ................... 13
5.1.3 Cold Flush Procedure .................................... 13
5.2 Sterilization ............................................................... 13
6/Troubleshooting ........................................................14
7/Service – Disassembly and Assembly ....................15
7.1 Service Tools and Equipment ................................... 15
7.2 Disassembly ............................................................. 15
7.2.1. Part Numbers ................................................ 16
7.3 Assembly ..................................................................20
2 6700-0011-000 (Rev. 8.1) 08/19
WARNINGS
⚠ This device is to be used ONLY by persons who
have been properly trained on the operation of the
device. Incorrect use of this device may cause
serious injury to a patient.
⚠ DO NOT operate this device in the presence of
ammable anesthetics. Static charges may not
dissipate and a possible explosion hazard exists
in the presence of these agents.
⚠ Thoracic Vacuum Regulator vacuum pressures
used should not exceed the recommendations of
the Chest Drainage System Manufacturer. Use of
excessive vacuum pressure may render the Chest
Drainage System ineective and may result in
patient harm.

User Responsibility
This Product will perform as described in this operating
manual and accompanying labels and/or inserts, when
assembled, operated, maintained and repaired in accordance
with the instructions provided. This Product must be checked
periodically. A defective product should not be used. Parts that
are broken, missing, worn, distorted or contaminated should
be replaced immediately. Should such repair or replacement
become necessary, see the Ohio Medical service manual for
service or repairs to this product. For service advice, Ohio
Medical recommends that a telephone request be made
Technical Competence
The procedures described in this service manual should
be performed by trained and authorized personnel only.
Maintenance should only be undertaken by competent
individuals who have a general knowledge of and
experience with devices of this nature. No repairs should
ever be undertaken or attempted by anyone not having such
qualications.
to the nearest Ohio Medical Regional Service Center. This
product and any of its parts should only be repaired using
written instructions provided by Ohio Medical or by Ohio
Medical trained personnel. The Product must not be altered
without the prior written approval of Ohio Medical’s Quality
Assurance Department. The user of this Product shall have
the sole responsibility for any malfunction which results from
improper use, faulty maintenance, improper repair, damage,
or alteration by anyone other than Ohio Medical.
Genuine replacement parts manufactured or sold by Ohio
Medical must be used for all repairs.
Read completely through each step in every procedure
before starting the procedure; any exceptions may result
in a failure to properly and safely complete the attempted
procedure.
Abbreviations used in this manual
O OFF
| ON (Regulate)
CCW Counter-clockwise (decrease)
CW Clockwise (increase)
in Inches
kPa Kilopascals (kPa x 7.50 = mmHg) (kPa x 10.197 = cmH2O)
LPM Liters per minute
mm Millimeters
mL Milliliters
mmHg Millimeters of mercury (mmHg x 0.133 = kPa ) (mmHg x 1.3595 = cmH2O)
cmH2O Centimeters of water (cmH2O x 0.098 = kPa) (cmH2O x 0.7355= mmHg)
°C Degrees Celsius
°F Degrees Fahrenheit
N-m Newton-Meter (N-m x .737 = ft-lb)
ft-lb Foot-Pound Force (ft-lb x 1.356 = N-m)
oz Ounces
NPTF National Pipe Thread Female (USA)
MPTS Multi-Purpose Therapy Stand
g grams
m meters
6700-0011-000 (Rev. 8.1) 08/19 3

1/Precautions
1.1 Denitions
Note: A Note provides additional information to clarify a
point in the text.
Important: An Important statement is similar to a note but of
greater emphasis.
⚠ CAUTION: A CAUTION statement is used when the
possibility of damage to the equipment exists.
⚠ WARNING: A WARNING statement is used when the
possibility of injury to the patient or the operator exists.
1.2 Warnings
This device is to be used only by persons who have been
adequately instructed in its use.
After patient use, regulators may be contaminated. Handle
in accordance with your hospital’s infection control policy.
Clean and disinfect all suction equipment before shipment
for service to ensure transportation personnel and/or service
personnel are not exposed to any hazardous contamination.
Clamping the tubing between the patient and the collection
bottle may result in pressure buildup in the catheter and
tubing.
Connect the vacuum regulator to the vacuum source only.
Connection to positive pressure sources, even momentarily,
could injure the patient or operator and damage the
equipment.
Always connect the regulator to the vacuum source and
check its operation before attaching the patient connection.
Do not use this device in the presence of ammable
anesthetics. Static charges may not dissipate and a possible
explosion hazard exists in the presence of these agents.
Following sterilization with ethylene oxide, quarantine parts
in a well ventilated area to allow dissipation of residual
ethylene oxide gas absorbed by the material. Aerate for 8
hours at 130°F (54°C).
If the vacuum regulator is repaired or disassembled in any
manner, the service checkout procedure (Section 8 Service
Checkout Procedure) must be performed before using the
equipment on a patient.
The pre-use checkout procedure (Section 4.2.3 Pre-use
Checkout Procedure) must be performed before using this
equipment on each patient. If the regulator fails any part of
the pre-use checkout procedure, it must be removed from
service and repaired by qualied service personnel.
When using a disposable chest drainage system, the
atmospheric vent at the top of the suction control chamber
must be occluded for proper suction regulation with the
Thoracic regulator.
The patient port of the regulator must be occluded when
setting the prescribed suction level so that the patient does
not receive higher than required suction levels.
A water seal system must be used with the Thoracic
Regulator to prevent atmospheric air from entering the
pleural cavity, and to show the presence of air movement
into the collection system.
The vacuum relief valve must be tested to ensure compliance
with the manufacturer’s specications before the unit is
placed in service. Remove the unit from service if it fails
the test, otherwise, excessive suction can cause injury to
a patient. Excess Loctite® may seal the steel ball to the
seat. This will disable the vacuum relief valve and may allow
suction to exceed the pre-set limit.
With the patient tubing occluded, all the bubbling in the water
seal system should stop. If bubbling does not stop, check all
connections to troubleshoot and eliminate leaks.
When a leak-free collection system connected to a patient
is turned on, after initial air in the system is eliminated, only
patient air will produce bubbles in the water seal.
4 6700-0011-000 (Rev. 8.1) 08/19

1/Precautions
1.3 Cautions
Cleaning the gauge may cause damage which will result in
inaccurate readings.
Do not steam autoclave or liquid sterilize the regulator.
Severe impairment of the operation of the regulator will
result. The only acceptable method of sterilization is with gas
(ethylene oxide).
To help prevent aspirate from entering the regulator, the
Thoracic Vacuum Regulator should always be used as part
of a water seal drainage system. If as a result of misuse,
water or aspirate gets into the regulator, it may impair the
regulator’s operation.
Do not use any Loctite® products, or any products which
contain methacrylate ester as an active ingredient to seal
the threads on the adapter/probe and ttings.
Only competent individuals trained in the repair of this
equipment should attempt to service it.
Sterilization with ethylene oxide mixtures may cause crazing
(minute supercial cracking) of some plastic parts. Crazing
will be more pronounced when mixtures containing Freon
are used.
1.4 Intended Use
The Ohio Medical® Thoracic Vacuum Regulator is a vacuumpowered suction apparatus that is intended for use with Chest
Drainage Systems in Thoracic, Cardiovascular, Trauma and
Critical Care applications.
®
Prior to placing the unit back into service, after disassembly
or cleaning, perform the service checkout procedure (Section
8 Service Checkout Procedure).
The gauge assembly must be handled with utmost care to
retain its precision. If the lens is removed, do not rest the
gauge on its face.
Use care when unhooking the tension spring from the
regulator. Excessive tension on the spring can crack the
plastic at the base of the mounting post.
To prevent stripping of the plastic threads, place the screw
in the hole and turn counter-clockwise until it drops into the
original threads, then tighten the screw.
Use of lubricants other than those recommended may
degrade plastic or rubber components (Section 7.1 Service
Tools and Equipment).
Not for eld or transport use*.
* The categories of Field and Transport Use are specically dened in ISO
10079-3 (Section 5.1.2), “Field” means use at accidents or emergencies
outside a hospital. “Transport” means use in ambulances, cars or airplanes.
These situations may expose the equipment to uneven support, dirt,
water, mechanical shock and temperature extremes. Ohio Medical suction
equipment has not been tested to comply with the specic requirements of
these categories.
6700-0011-000 (Rev. 8.1) 08/19 5
2/Scope
This service manual contains service, maintenance and
parts information for four models of the Thoracic Vacuum
Regulator.
Note: Parts numbers are for vacuum regulators without
adapters/probes.
2.1 North American
Thoracic Vacuum Regulator
O(O)
35
30
40
25
45
20
_
cmH O
2
15
10
5
50
55
60
E
A
R
S
C
E
N
I
2.3 French
Thoracic Vacuum Regulator
|(On)
30
40
3
4
High Flow
50
Low Vacuum
5
6
60
Thoracique
6700-1277-901
20
2
1
10
a
P
k
-
O
2
H
m
c
-
Thoracic
6700-1275-901
2.2 International
Thoracic Vacuum Regulator
|(On)
30
40
3
4
High Flow
50
Low Vacuum
5
6
60
20
2
1
10
a
P
k
-
O
2
H
m
c
-
2.4 Spanish
Thoracic Vacuum Regulator
|(On)
30
40
3
4
High Flow
50
Low Vacuum
5
6
60
Thorácico
6700-1278-901
20
2
1
10
a
P
k
-
O
2
H
m
c
-
Thoracic
6700-1276-901
6 6700-0011-000 (Rev. 8.1) 08/19
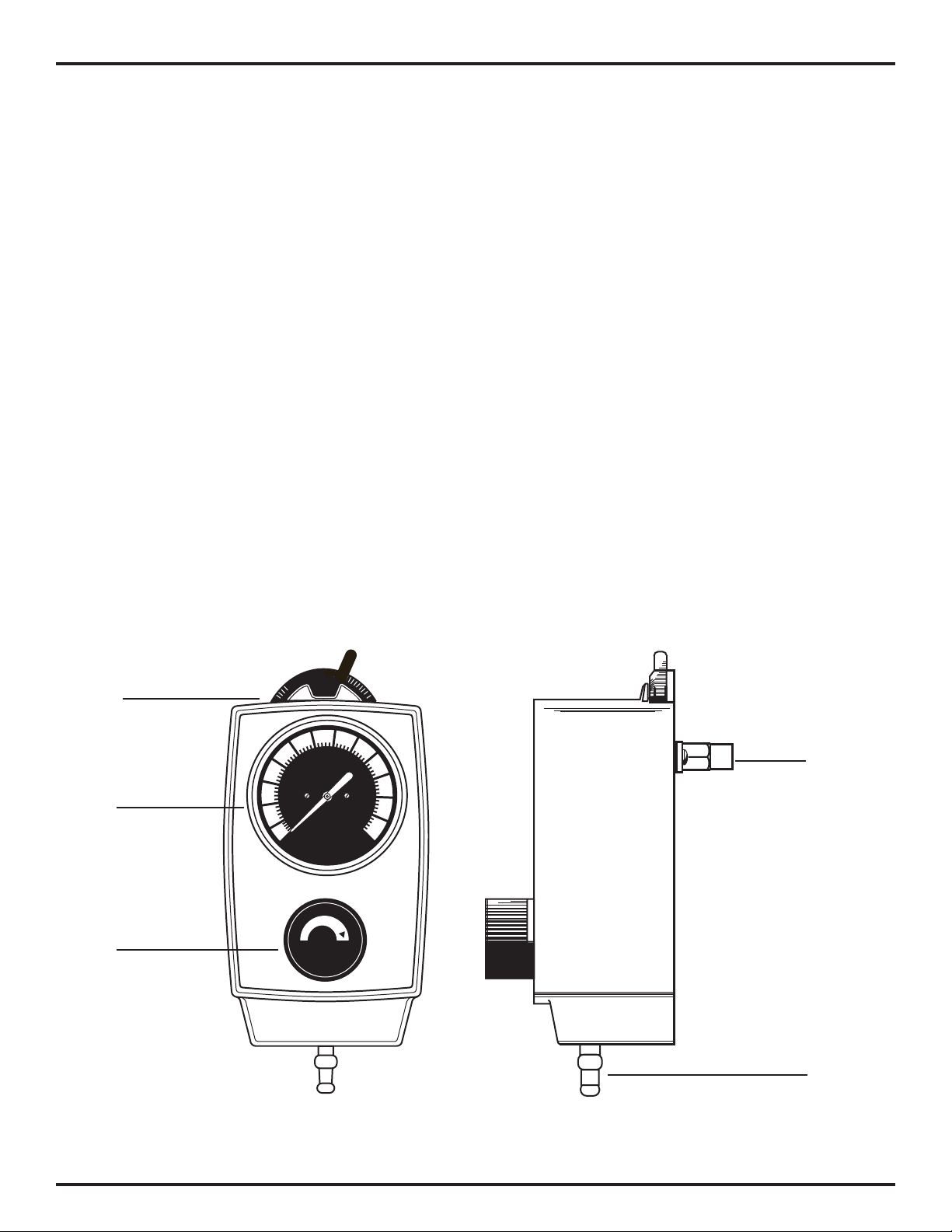
3/Description and Specications
3.1 Description
⚠ WARNING: Do not use this device in the presence
of ammable anesthetics. Static charges may not
dissipate and a possible explosion hazard exists in
the presence of these agents.
The Thoracic Vacuum Regulator is a lightweight, compact
unit used throughout the hospital for chest and mediastinal
drainage.
Each regulator contains a vacuum gauge which indicates
suction supplied by the regulator.
The Thoracic Vacuum Regulator operates only in a regulated
vacuum mode. The unit has a diaphragm-type regulator
which provides an adjustable vacuum of 0 to 50 cmH2O (0 to
4.9 kPa). The regulator has a vacuum gauge with increments
to 60 cmH2O (5.9 kPa) and is housed in impact-resistant
plastic for durability.
A vacuum limiting valve is incorporated which limits the
maximum vacuum between 50 and 60 cmH2O (4.9 and 5.9
kPa). If positive pressure is applied to the patient port (e.g.
patient coughing) a positive pressure relief valve operates to
relieve the pressure.
In the | (ON) mode, the vacuum source is connected through
the regulator module which functions as an automatic valve.
Turning the suction control knob adjusts the position of the
regulator module and allows selection of a predetermined
level of suction.
During use, as the ow requirement increases, the valve
automatically opens to maintain suction at the pre-set level.
Conversely, when the ow requirement decreases, the valve
automatically closes to maintain suction at the pre-set level.
The same mechanism compensates for changes in supply
vacuum and automatically maintains the pre-set suction
level.
1. Suction Control Knob - Allows adjustment of suction to
the patient.
2. Mode Selector Switch - Allows quick mode changes.
a. | (ON) - Suction can be adjusted with the suction
control knob.
b. O (OFF) - No suction is supplied to the patient.
3. Vacuum Gauge - Displays the suction level to the patient
during use.
Mode
Selector
Switch
Vacuum
Gauge
Suction
Control
Knob
O(O)
30
25
20
_
cmH O
15
10
5
R
C
N
I
Thoracic
35
40
45
2
50
55
60
E
A
S
E
Probe
Port
Patient
Port
Adapter/
6700-0011-000 (Rev. 8.1) 08/19 7

3/Description and Specications
3.2 Specications
3.2.1 Technical Specications
Gauge: Accuracy ± 3 cmH2O (± 0.3 kPa)
Flow Rate: 0 to 40 LPM without ttings at full increase setting
depending on the supply vacuum and open air ow
Positive Pressure Safety Relief Valve: Located in patient circuit to prevent pressurization of
patient chest cavity in excess of 10 cmH2O (1.0 kPa)
Regulated Suction Range: 0 to 50 cmH2O (0 to 4.9 kPa)
Vacuum Relief Valve: 55 cmH2O ± 5 cmH2O (5.4 kPa ± 0.5 kPa)
Weight: 24 oz (680 g)
(less ttings)
Dimensions Height: 7.2 in (185 mm)
(less ttings) Width: 3.5 in (90 mm)
Depth: 4.3 in (108 mm)
Latex tubing 0 to full vacuum
0.25 in (6.4 mm) ID Flow dependent on source and set-up
Disposable tubing 0 to full vacuum
(available separately in some markets; Flow dependent on source and set-up
6 mm ID x 450 mm, 750 mm and 2 m) to connect regulator and collection bottle
Disposable Suction Filter: 0 to full vacuum
0 to 100 LPM @ 650 mmHg (86.7 kPa)
3.2.2 Environmental Specications
Operating Temperature Range: 40°F to 120°F (4°C to 49°C)
Storage Temperature Range: 0°F to 160°F (–18°C to 71°C)
Operating and Storage Relative Humidity: 5 to 95%
3.2.3 Standards
ISO 10079-3 (Section 5.1.2)
8 6700-0011-000 (Rev. 8.1) 08/19
 Loading...
Loading...