Nuvectra 4110 User Manual
Algovita® Spinal Cord Stimulation
Y
Clinician Programming Manual
Mobile Clinician Programmer Application Model 4510
Bridge Communicator Model 4110-xx
XXXX
ONL
0300-000176-001 Rev.2 SCS CP_Mobile.indb 1 7/12/19 9:20 AM

Algovita® is a registered trademark of Nuvectra Corporation
e Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by
Nuvectra is under license.
Refer to the Information for Prescribers Manual for indications, contraindications, warnings, precautions, adverse events,
clinical summary, and related information.
FCC Information (US Only)
Refer to the Apple® iPad User Guide at support.apple.com for its communications regulation information.
e following is communications regulation information about the Bridge Communicator.
Bridge Communicator FCC ID: 2ABU84110 contains FCC ID: QOQ11
is device complies with part 15 of the FCC rules. Operation is subject to the following two conditions: (1) is device
may not cause harmful interference, and (2) is device must accept any interference received including interference that
may cause undesired operation.
Important: Changes and modications to the products not authorized by Nuvectra could void the FCC certication and
negate your authority to operate these products.
0300-000176-001 Rev.2 SCS CP_Mobile.indb 2 7/12/19 9:20 AM

Contents
Packaging Symbols Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .v
Terminology. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .vi
1. Introduction ........................................................................7
About this Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
Spinal Cord Stimulation System ...................................................................7
Related Documents. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
2. Important Safety Information .........................................................11
Warnings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Precautions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
3. About the Clinician Programmer ......................................................13
Using the Ribbon. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
Using the Navigation Menu. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
4. Getting Started .....................................................................17
Logging In to the Clinician Programmer ............................................................17
Pairing a Bridge Communicator to the Clinician Programmer. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
5. Managing Patients ..................................................................19
Using the Patient Directory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19
Adding a Patient ..............................................................................20
6. ConguringStimulatorsandLeads ....................................................23
Adding a Stimulator. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23
Adding Leads ................................................................................24
Deleting Leads ...............................................................................25
Deleting a Stimulator ...........................................................................26
7. Programming ......................................................................27
Understanding the Programming Screen ...........................................................28
Using Programming Functions ...................................................................30
Conguring the Subprogram. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .30
Shifting the Amplitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
Performing Impedance Checks ...................................................................33
8. Managing Programs ................................................................. 35
Adding a Program. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .37
Adding a Subprogram. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38
Deleting a Program or Subprogram ...............................................................39
Managing a Disabled Program ...................................................................39
9. Patient Report. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .41
10. Stimulator Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .43
Accessing Stimulator Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .43
Stimulator Battery .............................................................................44
Auto Tune ...................................................................................45
Impedance Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .46
Patient Adjustments ...........................................................................47
Patient Devices ............................................................................... 48
11. Care, Cleaning, Replacement, and Servicing ............................................49
Care and Handling ............................................................................49
Cleaning ....................................................................................49
0300-000176-001 Rev.2 SCS CP_Mobile.indb 3 7/12/19 9:20 AM

Replacement and Servicing .....................................................................49
Disposal ....................................................................................49
12. Charging the Bridge Communicator. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .51
13. Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .53
Nuvectra Customer Service .....................................................................53
Clinician Programmer Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .53
Charging a Fully Discharged Implantable Stimulator ..................................................56
Setting the Implantable Stimulator Depth ...........................................................56
14. SystemSpecications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .57
iPad Specications ............................................................................57
Bridge Communicator Specications ..............................................................57
Wireless Information ...........................................................................60
Wireless Quality of Service. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .61
Medical Implant Communication Service (MICS) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .61
Bluetooth Wireless Technology ...............................................................61
Wireless Security .............................................................................61
Medical Implant Communication Service (MICS) .................................................61
Bluetooth Wireless Technology ...............................................................61
Electromagnetic Compatibility Declaration ..........................................................61
Compliances and Authorizations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .62
15. Glossary ..........................................................................63
0300-000176-001 Rev.2 SCS CP_Mobile.indb 4 7/12/19 9:20 AM
Packaging Symbols Glossary
Symbol Details
Title: Catalog Number
Standard: ISO 15223-1 Reference Number: 5.1.6
Description: Indicates the manufacturer’s catalog number so that the medical device can be identied.
Title: Serial Number
Standard: ISO 15223-1 Reference Number: 5.1.7
Description: Indicates the manufacturer’s serial number so that the medical device can be identied.
Title: Batch Code
Standard: ISO 15223-1 Reference Number: 5.1.7
Description: Indicates the manufacturer’s batch codes so that the medical device’s batch or lot can be identied.
Title: Manufacturer
Standard: ISO 15223-1 Reference Number: 5.1.1
Description: Indicates the medical device manufacturer, as dened in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC.
Title: Date of Manufacture
Standard: ISO 15223-1 Reference Number: 5.1.3
Description: Indicates the date when the medical device was manufactured.
Title: Authorized Representative in the European Community
Standard: ISO 15223-1 Reference Number: 5.1.2
Description: Indicates the Authorized Representative in the European Community.
Title: Consult Instructions for Use
Standard: ISO 15223-1 Reference Number: 5.4.3
Description: Indicates the need for the user to consult the instructions for use.
Title: Caution
Standard: ISO 15223-1 Reference Number: 5.4.4
Description: Indicates the need for the user to consult the instructions for use for important cautionary information such as
warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself.
Title: Packaging Unit
Standard: ISO 7000:2014 Reference Number: 2794
Description: Indicates the number of pieces in the package.
Title: Do not use if package is damaged.
Standard: ISO 15223-1 Reference Number: 5.2.81
Description: Indicates a medical device that should not be used if the package has been damaged or opened.
Title: Keep Dry
Standard: ISO 15223-1 Reference Number: 5.3.4
Description: Indicates a medical device that needs to be protected from moisture.
Title: Temperature Limit
Standard: ISO 15223-1 Reference Number: 5.3.7
Description: Indicates the temperature limits to which the medical device can be safely exposed.
Title: Humidity Limitation
Standard: ISO 15223-1 Reference Number: 5.3.8
Description: Indicates the range of humidity to which the medical device can be safely exposed.
Title: Atmospheric Pressure Limitation
Standard: ISO 15223-1 Reference Number: 5.3.9
Description: Indicates the range of atmospheric pressure to which the medical device can be safely exposed.
Algovita Spinal Cord Stimulation System
1
1
1
1
1
1
1
1
2
1
1
1
1
Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 5 7/12/19 9:20 AM
v

Terminology
ONLY
Symbol Details
Title: Fragile, handle with care.
Standard: ISO 15223-1 Reference Number: 5.3.1
Description: Indicates a medical device that can be broken or damaged if not handled carefully.
Title: MR Unsafe
Standard: ASTM F 2503-13 Reference Number: 7.3.3
Description: An item which poses unacceptable risks to the patient, medical sta, or other persons within the MR
environment.
Title: Non-ionizing Electromagnetic Radiation
Standard: IEC/TR 60878 Reference Number: 5140
Description: To indicate generally elevated, potentially hazardous, levels of non-ionizing radiation, or to indicate equipment
or systems, e.g., in the medical electrical area that include RF transmitters or that intentionally apply RF electromagnetic
energy for diagnosis or treatment.
Title: Class II Equipment
Standard: IEC/TR 60878 Reference Number: 5172
Description: To identify equipment meeting the safety requirements specied for class II equipment according to IEC 61140.
Title: WEEE Waste of Electrical and Electronic Equipment Symbol for the Marking on EEE
Standard: BS EN 50419:2006
5
Description: Indicates that when end user wishes to discard this product it must be sent to separate collection facilities for
recovery and recycling in the EU.
1
3
4
4
Title: Type BF Applied Part
Standard: ISO 7000/IEC 60417 Reference Number: 5333
2
Description: To identify a type BF applied part complying with IEC 60601.
Title: Rx Only
Standard: 21 CFR Part 801.109 paragraph (b)(1)
Description: Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.
Title: European Conformity
Standard: 93/42/EEC Annex 12
Description: Indicates manufacturer declaration that the product complies with the essential requirements of the relevant
European health, safety, and environmental protection legislation.
1. ISO 15223-1:2016 Medical Devices – Symbols to be used with medical device labels, labelling and information to be supplied Part 1: General
Requirements
2. ISO 7000:2014 Graphical symbols for use on equipment-Registered symbols
3. ASTM F 2503-13 Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment
4. IEC/TR 60878:2015 Graphical symbols for electrical equipment in medical practice
5. BS EN 50419:2006 Marking of Electrical Equipment in accordance with Article 11(2) of Directive 2012/19/EU (WEEE)
Terminology
is manual and the Clinician Programmer use the following terms interchangeably:
• trial stimulator and External Pulse Generator (EPG)
• implantable stimulator and Implantable Pulse Generator (IPG)
• stimulator to represent both a trial stimulator and an implantable stimulator
• Clinician Programmer to represent the Clinician Programmer Application
vi Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 6 7/12/19 9:20 AM

Algovita Spinal Cord Stimulation System
1. Introduction
About this Manual
is Clinician Programming Manual provides instructions on using the Algovita® Clinician Programmer Application and
on using, charging, and caring for the Bridge Communicator. e Algovita® Spinal Cord Stimulation (SCS) System delivers
mild electrical pulses to a specic area in the spinal cord. Patients may feel stimulation as a tingling sensation (paresthesia)
that masks or covers their pain.
is manual applies to Model 4510 Algovita Clinician Programmer Application and Model 4110-xx Bridge Communicator
for use with Algovita Stimulator Models 2408 and 2412, and Algovita Trial Stimulator Model 4300.
Clinicians use the Clinician Programmer intraoperatively to check system integrity and determine lead placement. ey
use it postoperatively to evaluate and program stimulation therapy, and to associate the Bridge Communicator, Patient
Programmer Charger, and Pocket Programmer with a stimulator.
Spinal Cord Stimulation System
e Algovita SCS System is a rechargeable, 24-electrode, SCS system for the treatment of chronic pain. e implanted
components of the SCS system consist of an implantable stimulator and leads with optional extensions. During a
stimulation trial, trial leads are placed percutaneously, with the end of the trial lead externalized and connected to a trial
stimulator. e trial stimulator replaces the implantable stimulator during intraoperative test stimulation and during the
stimulation trial.
Note: During stimulation trials, you can use a commercially available ground pad with the Algovita SCS System.
Clinician
Programmer
Model 4500
Clinician Programmer
Model 4510 with Bridge
Communicator Model 4110-xx
Patient Programmer Charger
with Charging Paddle
Pocket
Programmer
1
2
1
2
Trial
Stimulator
3
Implantable
Stimulator with Leads
Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 7 7/12/19 9:20 AM
7
Related Documents
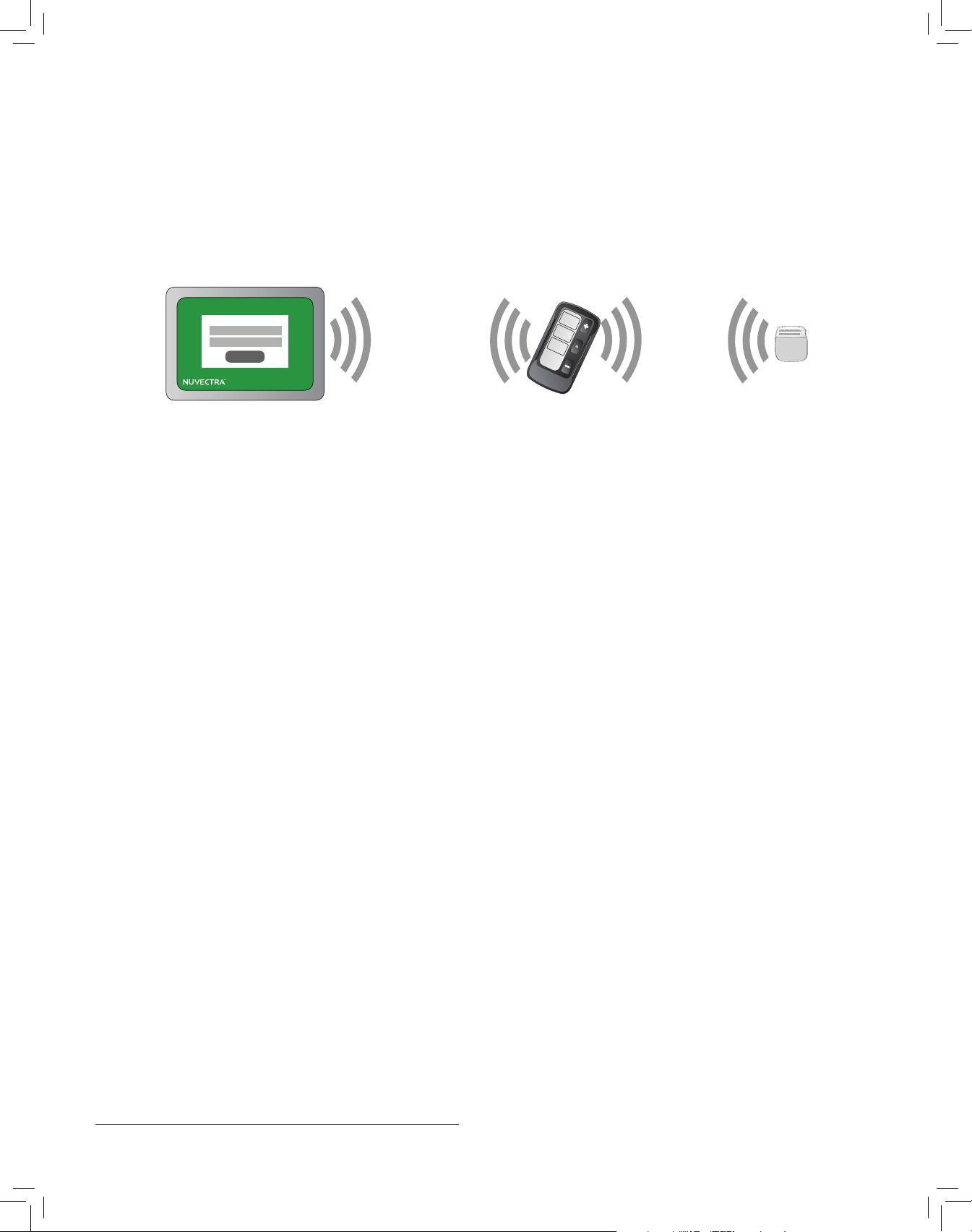
e Clinician Programmer Model 4510 runs on an Apple® iPad®. e Clinician Programmer Application communicates
with a stimulator via the Bridge Communicator. e Clinician Programmer Application communicates with the Bridge
Communicator Model 4110-xx via Bluetooth®, and the Bridge Communicator communicates with the stimulator via
Medical Implant Communication Service (MICS). e Bridge Communicator communicates with a stimulator within a
1 meter (3 feet) range, allowing the clinician to keep the Bridge Communicator outside the sterile eld during an implant
procedure. e Clinician Programmer Application communicates with a Bridge Communicator within a 5 meter (16 feet)
range.
LOG IN
Clinician Programmer
Application
Range:
5 meters
(16 feet)
Bluetooth
Bridge Communicator
Range:
1 meter
(3 feet)
MICS
Nuvectra
1
2
,LLC
Note:
• e Bridge Communicator Model 4110-xx only functions as a communication device for the Clinician Programmer
Application Model 4510. e Bridge Communicator cannot control stimulation.
• e patient’s Pocket Programmer Model 4100 cannot be used as a Bridge Communicator.
Related Documents
Refer to the appropriate Algovita SCS System manual for use instructions. Refer to the Information for Prescribers Manual
for indications, contraindications, warnings, precautions, adverse events, summary of clinical evaluation, and related
information.
e following documents are part of the Algovita SCS System documentation suite.
• System Implant Manual: Using Percutaneous Leads provides procedures for placing the leads for a stimulation trial,
implanting all components for a system implant with a percutaneous lead, and replacing percutaneous leads.
• System Implant Manual: Using Trial Leads provides procedures for placing the leads for a temporary lead trial.
• System Implant Manual: Using Paddle Leads provides the procedures for implanting all components for a system
implant with a paddle lead and a paddle lead replacement.
• Implant Manual for Extensions provides the procedures for replacing the extension.
• Implant Manual for Stimulator provides the instructions for the replacement of an Algovita Stimulator Model 2408
or 2412. For complete instructions on implanting an implantable stimulator as part of a system implant, refer to the
system manual packaged in the lead kit.
• Trial Stimulator Manual for Clinician provides instructions to the clinician on using the trial stimulator.
• Patient System Manual describes for a patient the Algovita SCS System and provides important safety information
about living with the SCS system. is manual also provides instructions on how to use, charge, and care for the
programmers that patients use to adjust their SCS system.
• Patient Stimulation Trial Manual describes for a patient the trial Algovita SCS System and provides important safety
information about living with the SCS system. is manual also provides instructions on how to use, charge, and care
for the programmers that patients use to adjust their SCS system.
• Patient Magnet Manual describes for a patient how to use the patient magnet, optional with the Algovita SCS System.
• Programmer Charger Quick Reference is a quick reference guide for patients for the Programmer Charger, and includes
information on selecting programs, adjusting stimulation strength, and turning stimulation on and o.
8 Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 8 7/12/19 9:20 AM

Algovita Spinal Cord Stimulation System
• Pocket Programmer Quick Reference is a quick reference guide for patients for the Pocket Programmer, and includes
information on selecting programs, adjusting stimulation strength, and turning stimulation on and o.
• Algovita® Spinal Cord Stimulation System MRI Procedure Guidelines Manual provides complete instructions and
information on contraindications, warnings, precautions, and instructions for MR conditions of use.
Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 9 7/12/19 9:20 AM
9

Related Documents
10 Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 10 7/12/19 9:20 AM

Algovita Spinal Cord Stimulation System
2. Important Safety Information
Warnings
Programmer Interaction with Other Implanted Devices. Do not use the Clinician Programmer to change program
settings when near a person who has a pacemaker or other implanted devices. e eects of the Algovita System Clinician
Programmer on other implanted devices are unknown.
Modication. Do not modify the Clinician Programmer, Bridge Communicator, or charging accessories. Modication of
any Algovita System component may result in damage to the system, compromised system integrity, and harm or injury to
the patient.
Precautions
Adjusting Program Settings. Avoid adjusting stimulator program settings to levels far above the pain relief response
threshold. High settings may cause discomfort and increase the need for more frequent charging.
Component Compatibility. Use only the Clinician Programmer, Bridge Communicator and accompanying charger cord
to charge the Bridge Communicator or adjust stimulation. e eects of non-Algovita components on an Algovita SCS
System are unknown.
Electromagnetic Interference. Do not attempt to program near equipment that may generate electromagnetic interference
(EMI) as it may interfere with the programmer’s ability to communicate with other Algovita System components. If EMI
disrupts programming, move the iPad or Bridge Communicator away from the source of EMI. Examples of sources of
EMI are Magnetic Resonance Imaging (MRI), lithotripsy, computer monitors, cellular and cordless telephones, motorized
wheelchairs, x-ray equipment, and other monitoring equipment. Interrupting programming may result in incorrect or
incomplete programming.
Flammable Atmospheres. Avoid using the iPad or Bridge Communicator in ammable or explosive environments (e.g.,
an anesthetic mixture with air, oxygen, or nitrous oxide). Using a battery-powered device near ammable or explosive
atmospheres can produce a spark which may cause injury.
iPad Charge Depleted. Depleting the battery charge on the iPad during use will shut down the Clinician Programmer
Application. Any unsaved information is lost, but saved information is retained.
Magnetic Resonance Imaging (MRI). Do not take the iPad, Bridge Communicator, or power cord into an MR
environment, such as an MR scanner room. e iPad, Bridge Communicator, and power cord are MR Unsafe. External
devices are MR Unsafe, do not allow the external devices into the MRI scanner (magnet) room. Please refer to the Algovita
SCS System MRI Procedure Guidelines (0300-000175-001(USA) or 0300-000148-001 (OUS)) for full information.
Damaged Packaging. Do not use an Algovita System component if the package is damaged or open. If a component is
damaged, the Algovita System may not function properly.
Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 11 7/12/19 9:20 AM
11

Precautions
12 Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 12 7/12/19 9:20 AM
Algovita Spinal Cord Stimulation System
3. About the Clinician Programmer
A program is a combination of settings for delivering stimulation to one or more pain sites. Using the Clinician
Programmer, you congure programs before transferring them to the stimulator for a patient’s use. You assign (pair) a
Pocket Programmer and Patient Programmer Charger with the patient’s Algovita System using the Clinician Programmer.
You can also display and congure the current stimulator status, perform diagnostics on the stimulator, and display usage
logs.
Using the Ribbon
A ribbon displays across the top of the application for most of the application screens.
Navigation
Menu
Patient Name
Bridge Communicator
Status
Stimulator
Status
Stimulation. Tap ON or OFF to turn stimulation on or o. e blinking green light indicates
stimulation is on. e light blinks quickly to indicate stimulation is ramping up, then
the blinking slows when ramping nishes. e blinking green light turns o to indicate
stimulation is o.
Bridge Communicator Status and Battery Level. Indicates when the Clinician Programmer is
connected to and communicating with the Bridge Communicator. Shows the battery level for
the Bridge Communicator.
Note: Before using the Clinician Programmer with a patient, make sure that it is connected
to and communicating with the Bridge Communicator. If the Clinician Programmer cannot
connect to the Bridge Communicator, verify that the Bridge Communicator is paired with the
Clinician Programmer and is within 5 meters (16 feet) of the Clinician Programmer.
Stimulator Status and Battery Level. Indicates when the Clinician Programmer is
communicating with the stimulator. Shows the battery charge level for the stimulator.
e Clinician Programmer automatically attempts to connect to the stimulator when you
navigate from the Patient Directory to another patient screen.
Note: If the Clinician Programmer cannot connect to a stimulator, verify that:
• the stimulator is on and not connected to another device, such as a Programmer Charger
or another Clinician Programmer;
• the Bridge Communicator is paired with the Clinician Programmer, is in Bluetooth mode,
and is within 1 meter (3 feet) of the stimulator;
• the Clinician Programmer is within 5 meters (16 feet) of the Bridge Communicator;
• the iPad Bluetooth is on.
Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 13 7/12/19 9:20 AM
13

Using the Navigation Menu
Using the Navigation Menu
Use the items in the Navigation menu to congure and view patient programs and stimulator settings. Access the
Navigation menu by tapping
e top list in the Navigation menu includes workow tasks. e clinician advances through the workow to congure
components, and create and manage programs. e task in progress is blue.
on the ribbon.
Completed
In progress
Workow tasks
Incomplete
1 - Stimulator Congure and view stimulator information. Refer to Adding a Stimulator on page 23.
2 - Leads Congure and view lead information and port connections. Refer to
Adding Leads on page 24.
3 - Programming Create and congure programs, including electrode conguration and settings (amplitude,
pulse width, and frequency). Optionally, conduct impedance checks. Refer to Programming on
page 27.
Note: When you nish programming, go to the Summary screen to save the changes and
upload the programs to the stimulator.
4 - Summary Save programs, review current program settings, and create or delete programs. Refer to
Managing Programs on page 35.
Report Generate a PDF report that includes information about a patient’s stimulator, leads, and
programs. Refer to Patient Report on page 41.
Note: Report is only available when you are viewing the Summary screen.
14 Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 14 7/12/19 9:20 AM

Algovita Spinal Cord Stimulation System
Stimulator Settings Congure and view stimulator settings. Refer to Stimulator Settings on page 43.
Patient Directory View patients added to the Clinician Programmer. Refer to
Using the Patient Directory on page 19.
Add Bridge Comm Pair a Bridge Communicator with the Clinician Programmer. Refer to Pairing a Bridge
Communicator to the Clinician Programmer on page 17.
About View information about the application such as model numbers, soware version numbers,
and trademarks and licenses information.
Log Out Log out of the Clinician Programmer.
Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 15 7/12/19 9:20 AM
15

16 Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 16 7/12/19 9:20 AM
Algovita Spinal Cord Stimulation System
4. Getting Started
Before using the Clinician Programmer, make sure that Bluetooth is on. Refer to the Apple iPad User Guide at
support.apple.com for instructions.
Aer you log in to the Clinician Programmer, pair the Bridge Communicator with the Clinician Programmer to enable
communication between the Clinician Programmer and a stimulator.
Logging In to the Clinician Programmer
e rst time you launch the Clinician Programmer, verify that you have an internet connection in order to use the login
credentials provided by Nuvectra. You can optionally set up a Touch ID® within iPad Settings and use your nger for future
log-ins. It is also recommended to turn o all Apple iPad system-level notications and alerts in the iPad settings feature.
Responding to any system-level alert or notication while using the Clinician Programmer Application results in the user
being automatically logged out of the Clinician Programmer Application.
To launch the Clinician Programmer Application:
1. Tap on your iPad.
2. Log in with your Nuvectra username and password, or a Touch ID.
If you are unable to authenticate the Clinician Programmer Application for rst time use, the Clinician Programmer
should retry upon a predened timeout when a token is not received.
If you are unable to log in, call Nuvectra Customer Service toll-free at 1-844-727-7897 within the United States or
1-214-618-4980.
Pairing a Bridge Communicator to the Clinician Programmer
To pair the Bridge Communicator with the Clinician Programmer:
1. Place the Bridge Communicator in Bluetooth mode.
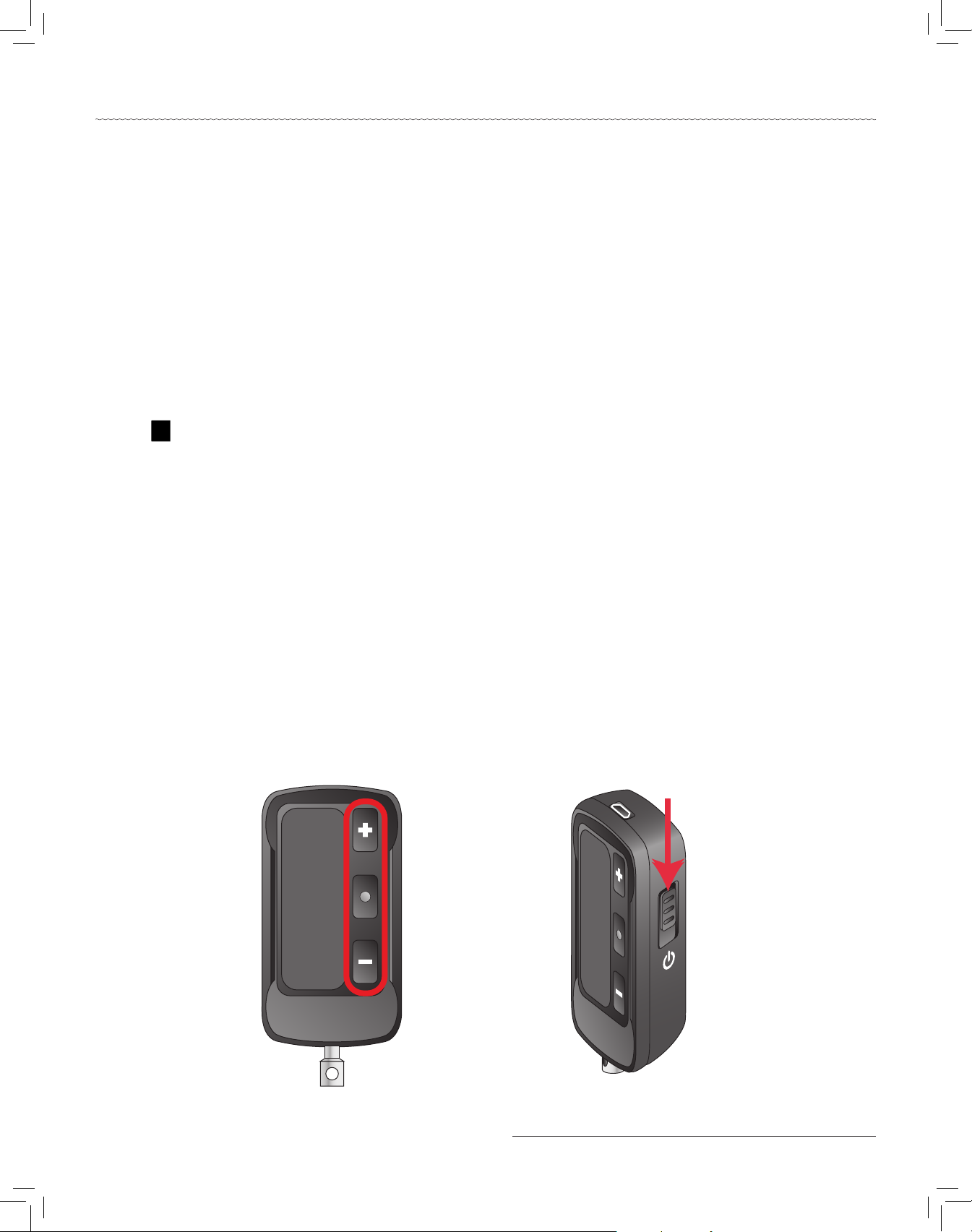
a. Tap and hold one of the buttons shown in image a. Do not use the red QSO button.
b. While holding the button from step a, slide the Power On/O button down and hold for two seconds or until bl
displays (bl displays on the Bridge Communicator when it is communicating via Bluetooth).
c. Release all buttons aer bl displays.
b.a.
d. Verify that a P displays and is blinking (Bridge Communicator is ready to pair).
Note: Ready to pair status will time out aer two minutes.
0300-000176-001 Rev.2 SCS CP_Mobile.indb 17 7/12/19 9:20 AM
Clinician Programming Manual
17
Pairing a Bridge Communicator to the Clinician Programmer
2. On the Clinician Programmer, select Add Bridge Comm from the Navigation menu.
e application begins scanning for a Bridge Communicator that is within 5 meters (16 feet) of the Clinician
Programmer Application.
e Clinician Programmer displays Bridge Communicators that are in range.
3. On the Clinician Programmer, tap the le or right triangles to scroll through the carousel of communicators if more
than one is in range.
4. Tap Select for the serial number that matches your Bridge Communicator.
5. If prompted, tap a button (not the red QSO button) and slide the On/O button down and hold for two seconds until
P displays.
6. Enter the four-digit code displayed on the Bridge Communicator into the popup window on the Clinician
Programmer. Enter the code displayed from le to right and top to bottom.
7. Tap Pair in the popup window.
18 Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 18 7/12/19 9:20 AM
Algovita Spinal Cord Stimulation System
5. Managing Patients
To program stimulation for a patient, add the patient to the Patient Directory. For a new patient without an existing
stimulator, you manually create the patient. For a patient with an existing stimulator, you import the information from
their stimulator.
Using the Patient Directory
Add patients and select patients using the Patient Directory.
To access the Patient Directory:
1. Log in to the Clinician Programmer or tap the Navigation menu and select Patient Directory.
e Patient Directory screen displays.
2. Scroll up or down in the Patient Directory to view additional patients.
3. Tap to select a patient.
..
Item Description
Import from
Stimulator
Create Patient
Edit
Open
Delete
Add a patient using information stored on the stimulator.
Add a patient that does not have a programmed stimulator.
Modify name, ID, and date of birth for the selected patient.
Access lead, stimulator, and program information for the selected patient.
Delete the selected patient from the Clinician Programmer.
Note: is does not delete information from the stimulator assigned to the patient.
Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 19 7/12/19 9:20 AM
19

Adding a Patient
Adding a Patient
Note: When you add a patient with an existing stimulator, the Clinician Programmer imports patient information stored
on the stimulator.
For patients with an existing stimulator, add a patient by importing information from their stimulator. For new patients
with an empty stimulator, create the patient rst, then add the stimulator.
To add a patient from a stimulator:
1. Move the Bridge Communicator within 1 meter (3 feet) of the stimulator, and the Clinician Programmer Application
within 5 meters (16 feet) of the Bridge Communicator.
2. Tap Import from stimulator on the Patient Directory screen.
e Clinician Programmer scans for stimulators and Bridge Communicators within range. e pane on the right of
the screen shows stimulators that are in range and their communication status. It also shows Bridge Communicators
that are in range and whether the Clinician Programmer is actively communicating with them. Tap the patient’s
stimulator when found or optionally tap Stop Scan to stop the scanning process at any time.
3. Tap the triangles on either side of the displayed stimular to view stimulators in range.
4. Tap the stimulator that matches the patient’s stimulator.
e trial simulator serial number is on the label on the back of the stimulator. e implantable stimulator packaging
material and the front of the implantable stimulator includes the serial number.
Note: If a stimulator does not display in the list, make sure it is on, nearby, and not connected to another Clinician
Programmer, Programmer Charger, or Pocket Programmer by turning the programmers o or moving them out of
range.
To create a patient:
20 Clinician Programming Manual
0300-000176-001 Rev.2 SCS CP_Mobile.indb 20 7/12/19 9:20 AM
 Loading...
Loading...