Nuvectra 4300, 4100 User Manual
Material Specification
Title:
IFU, Algovita SCS, Trial Stimulator Manual for Clinicians
Document Number and Revision
0300-000026-06
Page 1 of 2
Prepared By:
Approved By:
Amy Kosbau
Do not print the material specification notes pages when printing the literature piece.
Do not count the material specification notes pages in the literature piece page count.
1.0 PURPOSE
To define all parameters necessary to assure conformance to appropriate specifications.
2.0 SCOPE
This specification describes the c onfiguration and content for the IFU, Algovita SCS, Trial Stimulator Manual for Clinicians
3.0 RESPONSIBILITY
It is the responsibility of the product development engineer, manufacturing engineer or labeling engineer to maintain this
document in accordance with Algostim requirements.
4.0 MATERIAL CHARACTERISTIC S
4.1 Content: The content of the instructions for use document shall be de fined in the files and PDF provided by
Algostim.
4.2 Material: The paper shall be:
4.2.1 Front/Back Cover: 80# uncoated cover
4.2.2 Inner Pages: 40# white smooth opaque text stock.
4.3 Color:
4.3.1 4-color
4.4 Physical Size:
4.4.1 All dimensions are in inches unles s otherwise noted.
4.4.2 7.25 ± .20 length x 6.50 ± .20 width
4.5 Type of Binding: Perfect Bound
4.6 Other:
4.6.1 Literature Piece Page Count (including covers, excluding specification notes pages) 34
4.7 Language Translation R equirements & Configuration:
4.7.1 English (en)
5.0 STORAGE CONDITION
Store in dry location.
The information contained in this document is the sole property of QIG Group. Any reproduction in part or whole without the written permission of
QIG Group
QIG Group is prohibited.
Title:
IFU, Algovita SCS, Tria l St imulator Manual for Clinicians
Document Number and Revision
0300-000026-06
Page 2 of 2
6.0 QUALITY CHARACTERISTICS
Material Specification
6.1 Clarity of Text
: The text shall be easily readable and free of smears and smudges. Graphics content, layout and text
shall be consistent with that in the document (pdf) provided by the customer.
6.2 Workmanship: The booklet shall be uniformly cut along its edges and free of significant rough edges or paper
slivers.
6.3 Color: Color shall be uniform throughout each lot.
6.4 Lot Quality: Any lot of material, which does not meet the requirements o f this sp e c ification, is subject to re turn.
Greatbatch Medical reserves the right to return entire lots of material which fail to meet the requirements of this
specification.
7.0 PACKAGING/LABELING
7.1 Each package provided by the supplier shall be labeled with: Greatbatch part number, revision level of this
specification document, quantity, supplier name, and date of manufacturing.
7.2 A Certificate of Conformance is required with each shipment to include quantity, material characteristics,
Greatbatch part number, revision, PO, lot and date of manufacturing.
NOTE: Graphics Content and Layout to be shown and per Algostim file.
The information contained in this document is the sole property of QIG Group. Any reproduction in part or whole without the written permission of
QIG Group
QIG Group is prohibited.

Algovita™
2014
Y
Spinal Cord
Stimulation
System
Trial Stimulator
Model 4300
External Pulse
Generator (EPG)
Trial Stimulator Manual for Clinicians
ONL

Algovita™ is a trademark of QIG Group, LLC
FCC Information (US Only)
e following is communications regulation information about the Algovita Trial Stimulator and
Pocket Programmer.
Trial Stimulator FCC ID: 2ABU84300
Pocket Programmer FCC ID: 2ABU84100
ese devices comply with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) ese devices may not cause harmful interference, and (2) ese devices must accept
any interference received including interference that may cause undesired operation.
Important: Changes and modications to the products not authorized by Algostim, LLC could void
the FCC certication and negate your authority to operate these products.
Refer to the Information for Prescribers Manual for indications, contraindications, warnings, precautions, adverse events, clinical study results, and related information.
Algovita Spinal Cord Stimulation System
Contents
Explanation of Symbols Used on Packaging and Trial Stimulator 3
Introduction 5
Package Contents 5
Important Safety Information 6
Contraindications 6
Warnings 6
Precautions 7
Adverse Events 8
EPG Description 10
Turning the EPG On or Off 11
Quick Stimulation Off 12
Using the EPG During Intraoperative Test Stimulation 13
Connecting the Trial Cable to the EPG 13
Disconnecting the Trial Cable from the EPG 14
Stimulation Trial 15
Preparing the EPG for a Stimulation Trial 15
After the Stimulation Trial 15
Changing the EPG Batteries 16
Checking the Battery Charge Status 16
Changing the Batteries 17
EPG Care and Storage 18
General EPG Cleaning 18
Cleaning the EPG After Use 18
Cleaning the EPG Battery Contacts 18
Trial Stimulator Manual 1

EPG Service and Replacement 19
Disposal 19
Troubleshooting 20
Algostim Customer Service 21
Specications 22
Electromagnetic Compatibility Declaration 24
Wireless Information 28
Algovita Spinal Cord Stimulation System
2014
ONL
Y
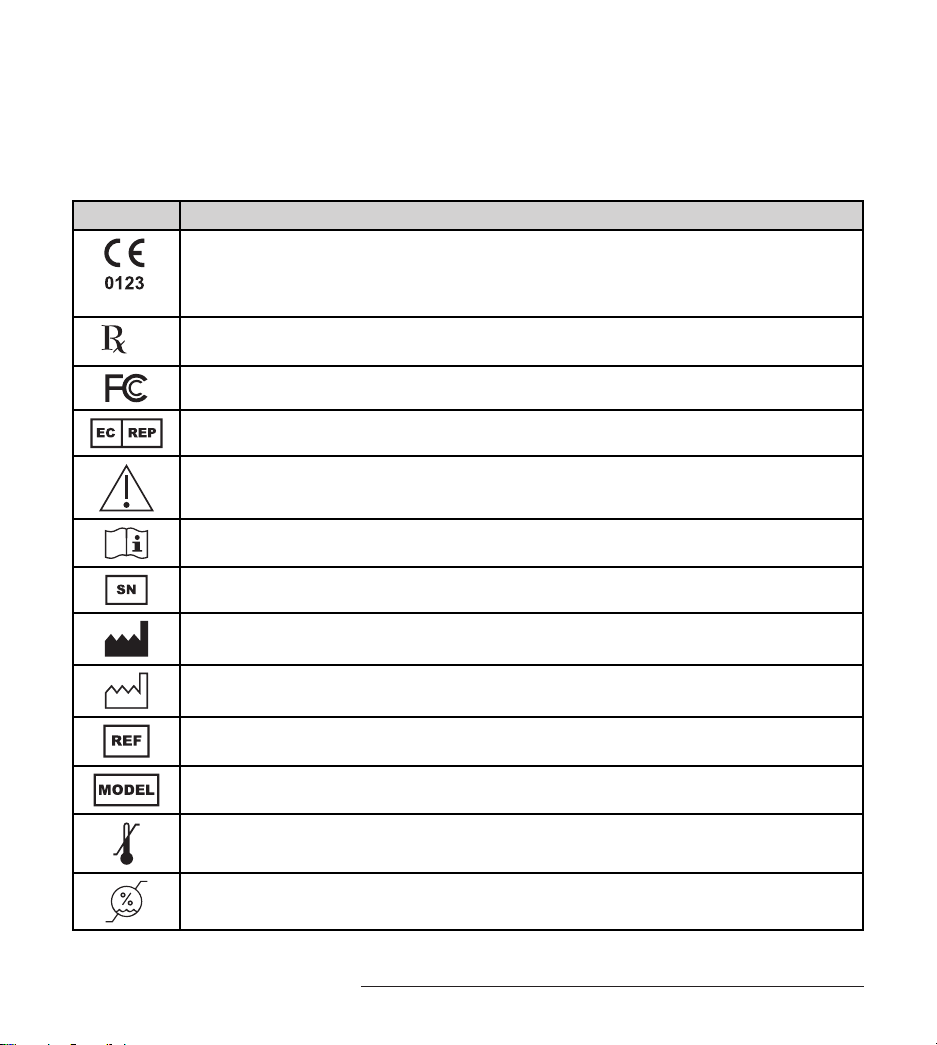
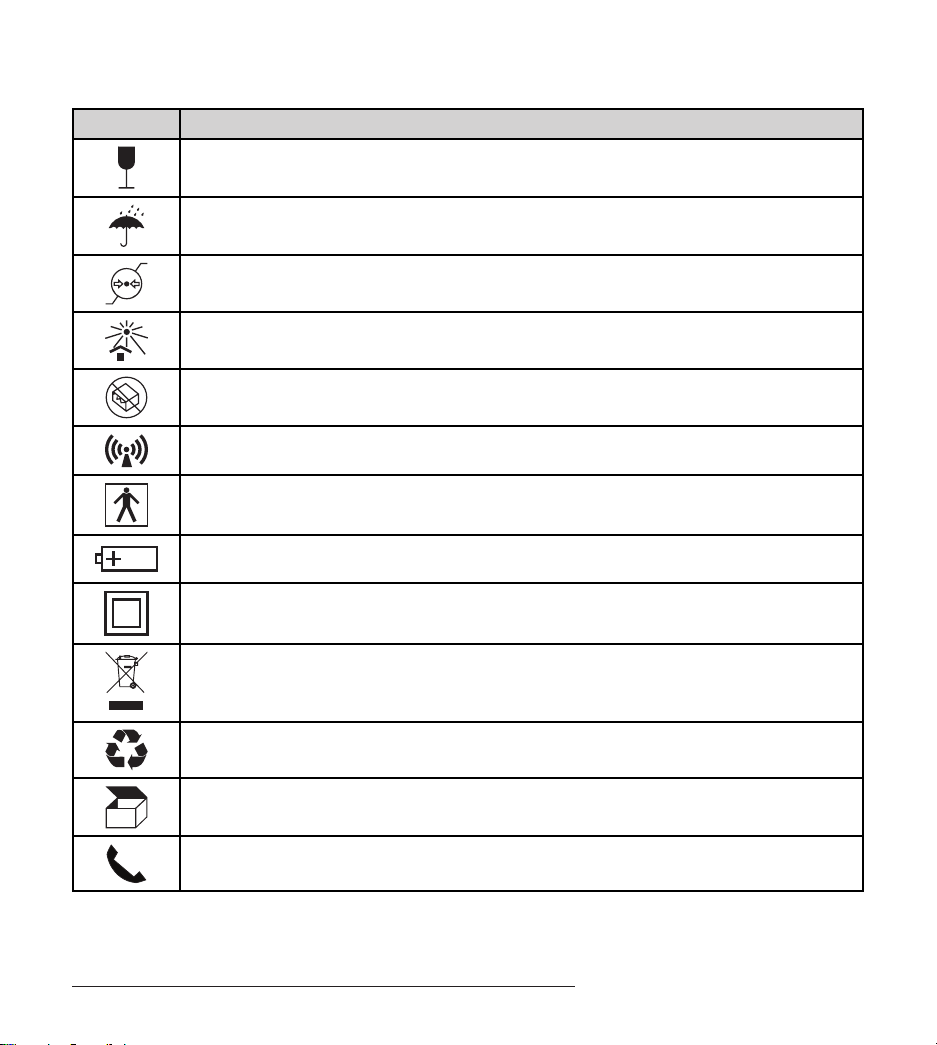
Explanation of Symbols Used on Packaging and Trial
Stimulator
Symbol Explanation
Conformité Européenne (European Conformity). is symbol means that the device fully
complies with European Directive AIMD 90/385/EEC and R&TTE Directive 1999/5/EC
Caution: Federal Law (USA) restricts this device to sale on or by the order of a physician
is device complies with Part 15 of the Federal Communication Commission rules
Authorized representative in Europe
Caution
Consult instructions for use
Serial number
Manufacturer
Date of manufacture
Catalogue number
Model
Temperature limit
Humidity limitation
Trial Stimulator Manual 3
Explanation of Symbols Used on Packaging and Trial Stimulator
Symbol Explanation
Fragile, handle with care
Keep dry
Atmospheric pressure limitation
Keep away from sunlight
Do not use if package is damaged
Non-ionizing radiation
Type BF equipment
Battery
Class II equipment
Not for general waste
Recycle
Contents
Telephone
4 Trial Stimulator Manual

Algovita Spinal Cord Stimulation System
Introduction
e Algovita™ Trial Stimulator Model 4300 (Figure 1) is part of the Algovita Spinal Cord
Stimulation (SCS) System, a rechargeable, 24-electrode, SCS system for the treatment of chronic
pain.
Figure 1. Algovita Trial Stimulator Model 4300
e trial stimulator is the external pulse generator (EPG) for the Algovita SCS System. e EPG is
used by the physician during intraoperative test stimulation and is used by the patient as part of the
Algovita Trial Stimulation System.
e EPG allows the physician to program system congurations identical to either the Algovita
Stimulator Model 2408 (3x8 channel) or the Stimulator Model 2412 (2x12 channel).
e EPG is programmed using the Clinician Programmer. During a stimulation trial, the patient
controls the EPG using the Pocket Programmer.
Package Contents
• Trial Stimulator Model 4300
• Trial Stimulator Pouch
• AAA Batteries (2)
• Product Literature
Trial Stimulator Manual 5
Important Safety Information
Important Safety Information
Contraindications
Diathermy. Shortwave, microwave and/or therapeutic ultrasound diathermy must not be used
on SCS patients. e energy generated by diathermy can be transferred through the SCS system,
causing tissue damage at the lead site which may result in severe injury or death.
Warnings
Electrocautery. Electrocautery devices should not be used in close proximity to an SCS trial
system. Contact between an active electrode and an implanted SCS system component can cause
direct stimulation of the spinal cord, which may result in severe injury to the patient
If use of electrocautery is necessary:
1. Turn the EPG o.
2. Use bipolar cautery.
3. Verify system and therapy function aer electrocautery use.
Electromagnetic Interference. Strong electromagnetic elds can potentially turn stimulation o
or change the strength of stimulation, which may cause an uncomfortable or jolting sensation. If
uncomfortable stimulation occurs, advise patients to move away from the area or turn stimulation
o.
Patients should also exercise care around:
• e detectors or security screeners such as those used at entrances or exits of department
stores, libraries, and other public establishments, and airport security screening devices.
Patients should exercise caution when approaching such a device and should request
assistance to bypass the device. If the patient must proceed through the device, the patient
should turn the EPG o and proceed with caution, moving through the center of the screener
as quickly as possible.
• Power lines or power generators
• Electric steel furnaces and arc welders
• Large, magnetized stereo speakers
• erapeutic magnets
6 Trial Stimulator Manual
Algovita Spinal Cord Stimulation System
Interaction with Implanted Sensing Stimulators and Other Implanted Devices. SCS systems
may interfere with the operation of implanted sensing stimulators such as pacemakers or
cardioverter debrillators (ICDs). If other implanted devices are indicated for the patient, careful
screening is required to determine if safe results can be achieved before permanently implementing
concurrent electrical therapies. e eects of implanted SCS systems on other implanted devices
are unknown.
Magnetic resonance imaging (MRI). Patients with the Algovita SCS system must not be exposed
to MRI. e electromagnetic eld generated by an MRI may forcefully dislodge the IPG or leads,
damage the IPG electronics, and induce voltage through the lead that may cause an uncomfortable
or jolting sensation or serious injury. e Algovita SCS System components have not been tested
for heating or migration in the MR environment. Introducing an Algovita SCS patient into an MRI
scanner may result in severe patient injury, death, or device malfunction.
Modication. Do not modify the EPG. Modication of any SCS system component may result in
damage to the system, compromised system integrity, and harm or injury to the patient.
Radio-frequency or microwave ablation. Safety has not been established for radiofrequency (RF)
or microwave ablation in patients who have an SCS system. Induced electrical currents may cause
heating, especially at the lead electrode site, resulting in tissue damage.
Precautions
System Interaction with Other Medical Treatments and Procedures. An IPG may interact with
the following therapies or procedures:
• Diagnostic x-rays. e eects of diagnostic x-rays on a stimulator are typically transient
because interference occurs only during the time of x-ray exposure. In some cases, the EPG
may need to be reprogrammed.
e following therapies or procedures may turn your stimulation o or may cause permanent
damage to your stimulator, particularly if used in close proximity to the EPG.
• Radiotherapy
• Lithotripsy
• External debrillation
• Radiation therapy
• Ultrasonic scanning
• High-output ultrasound
Trial Stimulator Manual 7
 Loading...
Loading...