Nuvectra 24082412 User Manual

Algovita
™
Spinal Cord
Stimulation
System
Stimulator Models
2408 and 2412
For IPG
replacement
Implant Manual
for Stimulator
IPG implant.indb 1 3/3/14 8:46 AM
Algovita™ is a trademark of QIG Group, LLC
CoreGuard™ is a trademark of Greatbatch, Inc.
Refer to the Information for Prescribers Manual for indications, contraindications, warnings,
precautions, adverse events, and related information.
FCC Information (US Only)
e following is communications regulation information about the Algovita Implantable Pulse
Generator (IPG).
2408 and 2412 IPG FCC ID: 2ABU824082412
ese devices comply with part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) ese devices may not cause harmful interference, and (2) ese devices must
accept any interference received including interference that may cause undesired operation.
Important: Changes and modications to these products not authorized by Algostim, LLC could
void the FCC certication and negate your authority to operate these products.
IPG implant.indb 2 3/3/14 8:46 AM

Contents
Explanation of Symbols Used on Packaging 5
Component Description 7
Package Contents 8
Component Sterilization 8
About this IPG Replacement Manual 8
Implant Procedure 8
Preparing for Surgery 8
Preoperative IPG Preparation 9
Explanting the IPG 13
Connecting the Leads or Extensions to the New IPG 13
Checking System Integrity 16
Implanting the New IPG 16
Completing the Implant Procedure 17
Patient Counseling Information 17
SCS System Implant: IPG Replacement 17
Registration Form and Temporary Patient ID Card 18
Returning Explanted Components 18
Algostim Customer Service 18
Specications 19
Wireless Information 20
Algovita SCS System Component Compatibility 21
Algovita Spinal Cord Stimulation System
Stimulator Model 2408 and 2412 Implant Manual
IPG implant.indb 3 3/3/14 8:46 AM
3

4 Stimulator Model 2408 and 2412 Implant Manual
IPG implant.indb 4 3/3/14 8:46 AM
Algovita Spinal Cord Stimulation System
Explanation of Symbols Used on Packaging
Symbol Explanation
20XX
Conformité Européenne (European Conformity). is symbol means that the device
fully complies with European Directive AIMD 90/385/EEC.
Caution: Federal Law (USA) restricts this device to sale on or by the order of a physician.
Consult instructions for use
Caution
Serial number
Catalog number
Model number
Authorized representative in Europe
Use by date
Distributed by
Manufacturer
Temperature limit
Keep dry
Sterilized using ethylene oxide
Do not resterilize
Do not reuse
Implant Manual for Stimulator
IPG implant.indb 5 3/3/14 8:46 AM
5
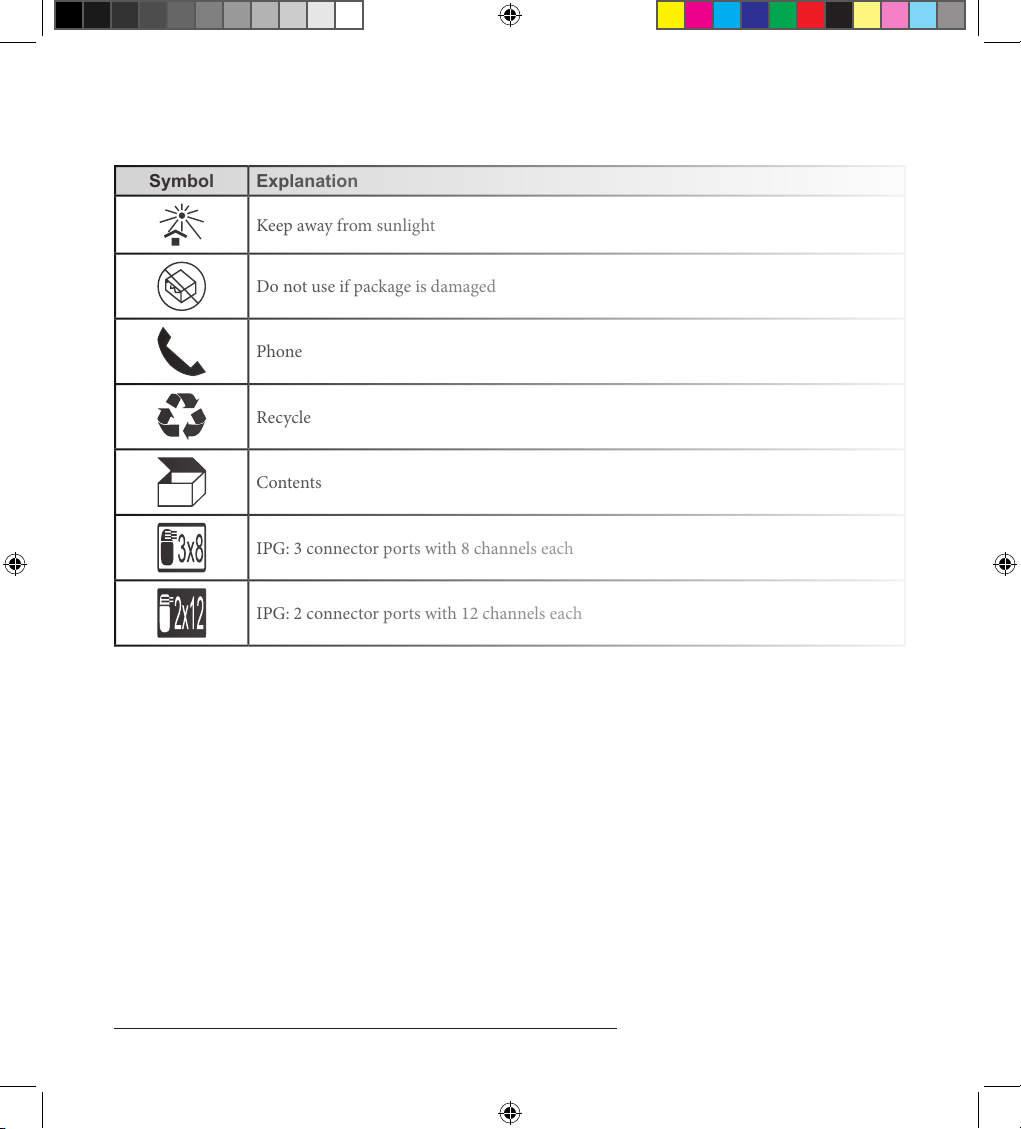
Explanation of Symbols Used on Packaging
Symbol Explanation
Keep away from sunlight
Do not use if package is damaged
Phone
Recycle
Contents
IPG: 3 connector ports with 8 channels each
IPG: 2 connector ports with 12 channels each
6 Implant Manual for Stimulator
IPG implant.indb 6 3/3/14 8:46 AM
Algovita Spinal Cord Stimulation System
1
2
1
2
3
,LLC
,LLC
Component Description
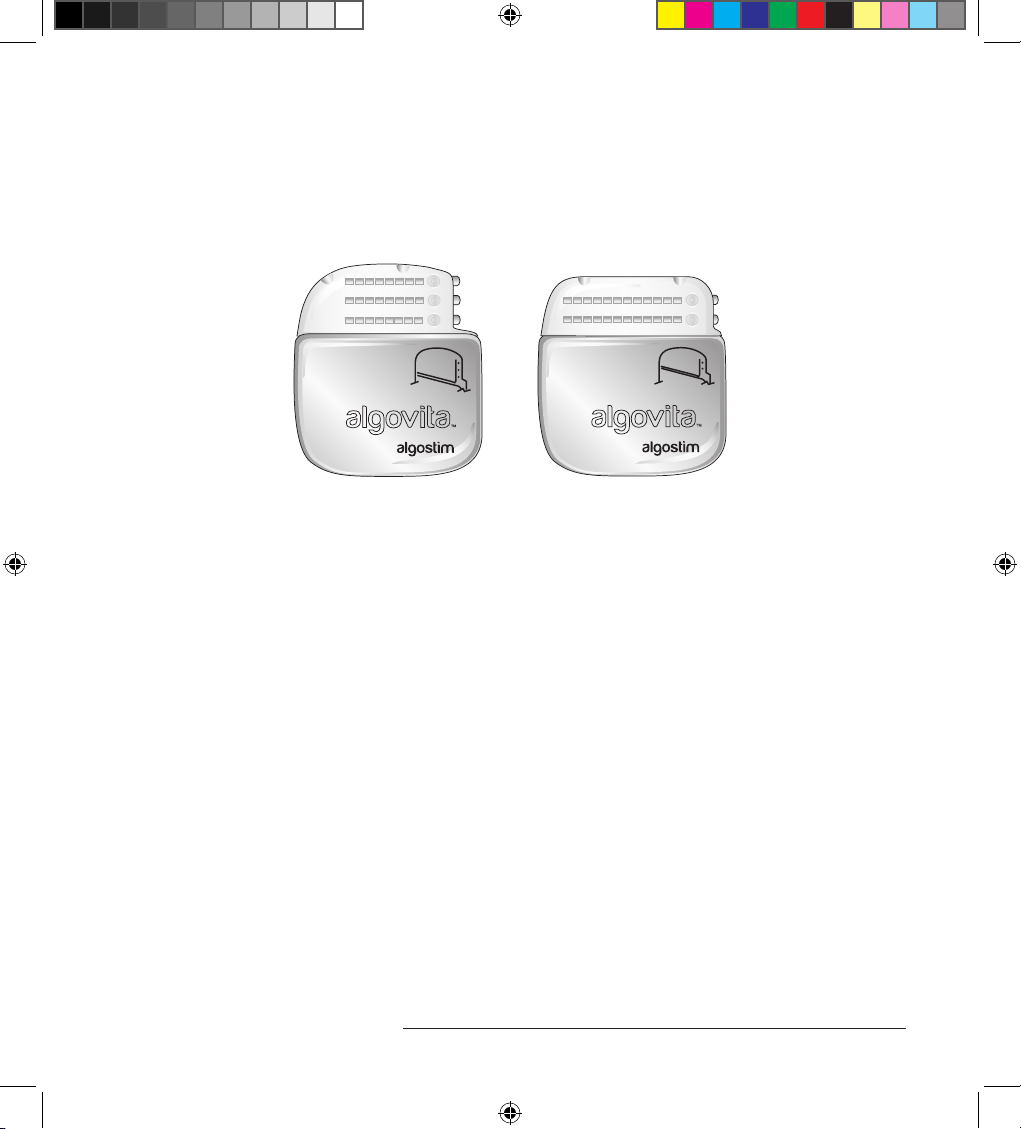
e Algovita™ Stimulator Model 2408 or 2412 (Figure 1) is part of the Algovita Spinal Cord
Stimulation (SCS) System, a rechargeable, 24-electrode, SCS system for the treatment of chronic
pain.
Figure 1. Algovita Stimulator Model 2408 and Model 2412
e Algovita Stimulator is the implantable pulse generator (IPG) for the Algovita SCS System. In
addition to the IPG, the implanted components of the SCS system consist of percutaneous leads or
paddle leads with optional extensions.
During the intraoperative test, an external pulse generator (EPG) is used in place of the IPG. e
clinician programs the IPG and the EPG using a Clinician Programmer. e patient adjusts the
system using either of 2 patient programmers.
Algovita IPGs are 24-channel rechargeable IPGs. Each channel allows the system 1 active electrode.
e channels are distributed over 2 or 3 connector ports, depending on the IPG model.
• Algovita Stimulator Model 2408 (3x8 channel)—ree connector ports accommodate 1 to 3
percutaneous leads, with each lead allowing up to 8 active electrodes.
• Algovita Stimulator Model 2412 (2x12 channel)—Two connector ports accommodate 1 or 2
percutaneous or paddle leads, with each lead allowing up to 12 active electrodes.
e IPG delivers stimulation using independent current distribution, a technology that allows
variable amounts of current to be delivered to each active electrode.
e IPG battery is a deep discharge recovery battery with CoreGuard™ technology. Even if the
patient allows the battery to completely discharge, the battery can be recharged with the Algovita
Programmer Charger.
IPG implant.indb 7 3/3/14 8:46 AM
Implant Manual for Stimulator
7

Package Contents
Package Contents
• Stimulator Model 2408 (3x8) or Model 2412 (2x12)
• Torque wrench
• Port plugs (3 for Model 2408, 2 for Model 2412)
• Product literature
• Temporary patient ID card
• Implant registration form and business reply envelope
Component Sterilization
e implantable and surgical accessory components were sterilized with ethylene oxide prior to
shipment. e SCS system sterile components are intended for single use only and must not be
resterilized.
Caution: Do not resterilize a system component or reimplant an explanted system component
because of risk of infection or malfunction.
About this IPG Replacement Manual
is manual provides the instructions for the replacement of an Algovita Stimulator Model 2408
or 2412. For complete instructions on implanting an IPG as part of a system implant, refer to the
system manual packaged in the lead kit.
Implant Procedure
Implanting clinicians should be thoroughly familiar with this manual and all other product
labeling.
Caution: Do not place the charging paddle on an unhealed wound. e charging paddle is not
sterile. Contact with an unhealed wound may result in an infection.
Preparing for Surgery
Before opening the sterile pack, verify the following on the sterile pack label:
• IPG—Model number and use-by date
8 Implant Manual for Stimulator
IPG implant.indb 8 3/3/14 8:46 AM
 Loading...
Loading...