
NorthEast Monitoring, Inc.
DR180+ Digital Recorder
Operator’s Manual
May 2006
Copyright 2006 - NorthEast Monitoring, Inc.
Part number: NEMM001 revision I


Table of contents
Physical Specifications 2
Electrical Specifications 2
Power Supply 2
Patient Leads 2
Operator Interface 2
Storage Capacity 2
Hooking up the Patient 3
Preparing the Recorder 5
How Patients use the Event Button 8
Erasing a Compact Flashcard 9
Other Recorder Settings 10
Recording Continuous 12-lead 13
Memory Requirements for 12-Lead Recordings 16
Processing Data Collected in Continuous 12-lead Modes 17
Error Messages 19
Appendix A: Maintenance and Care of the DR180+ Digital Recorder 20
Appendix B: Batteries for the DR180+ Digital Recorder 21
Appendix C: Pacemaker Detection with the DR180+ Digital Recorder 23
Appendix D: DR 180+ Accessories 24

H
OW
TO
U
SE THE
DR180+ D
H
OLTER
WARNING: Federal law restricts this device to sale by or on the
order of a physician.
The NorthEast Monitoring, Inc. DR180+ Digital Recorder is a Holter
monitor designed to facilitate the ambulatory cardiac monitoring, on
order of a physician, of those patients who may benefit from such monitoring, including but not limited to those with complaints of palpitations, syncope, chest pains, shortness of breath, or those who need to be
monitored to judge their current cardiac function, such as patients who
have recently received pacemakers. Only a trained Holter technician
should do patient hookups.
R
IGITAL
ECORDER
The data obtained by monitoring is not analyzed at the time of recording. After the recording is complete, the data must later be downloaded
to a compatible NorthEast Monitoring, Inc. Holter analysis system to be
analyzed.
Note: The DR180+ is not intended to replace real-time telemetry
monitoring for patients suspected of having life-threatening
arrhythmias.
NorthEast Monitoring, Inc. is an FDA Registered Facility (1224919)
that follows all FDA CGMP Manufacturing Practices. The DR180+
Digital Recorder has FDA 510K Approved Product Certification
(K001288 and K004007) and meets the AAMI EC-11/EC-38 standard
for frequency response.
Guide to NorthEast Monitoring, Inc.’s DR180+ Digital Holter Recorder

Physical Specifications
The DR180+ Digital Recorder meets the following physical specifications:
• 12.5 cm (length) x 7.0 cm (width) x 2.5 cm
(depth)
• 4-7/8 inches (length) x 2-3/4 inches (width)
x 1 inch (depth)
• Weight: 142 g (5.0 oz) without batteries;
200 g (6.9 oz) with batteries
Electrical Specifications
• Recording bandwidth: 0.05 to 70 hertz in 3channel mode
• Prefilter sampling rate: 360 samples/second
in 3-channel mode
• Data stored: 180 samples/second. In high
resolution mode, signal processing ensures
capture of peaks of narrow QRS complexes.
• Pacemaker sensitivity: 2 millivolts
• Pacemaker pulse duration: 150 to 2,000
microseconds
• The degree of protection against electric
shock is Type BF
• The recorder has not been tested for use in
the presence of a Flammable Anaesthetic
mixture and, therefore, is not suitable for
use in the presence of a Flammable Anaesthetic mixture with air or with oxygen or
nitrous oxide
Power Supply
The DR180+ is powered by two 1.5 volt AA
alkaline batteries (MN1500 or the equivalent),
two AA rechargeable NiMH (nickel metal
hydride) batteries, or two AA Eveready Lithium L91 batteries.
Patient Leads
DR180+ uses patient cables with either seven
leads or five leads for a 3-channel Holter
recording, or ten leads for a 3-channel Holter
plus 12-lead data. The cable connects to the
recorder via a 15-pin female connector on the
recorder.
An oximetry lead set with an oximetry sensor
replacing the channel 3 leads is also available.
The oximetry lead set consists of five leads for
a 2-channel Holter recording and a detachable
Nonin Medical lead with a pulse oximetry sensor. The cable connects to the recorder via a
15-pin female connector on the recorder.
Note: Please be sure to not pull on or stretch
the patient cables when you clean them or
attach them to the recorder or the patient.
This can cause premature failure of the
cable.
Operator Interface
The DR180+ has a 13-key keypad on the face
of the recorder around a liquid crystal display
(LCD). Use the keypad to interact with and
program the recorder. The function of each key
on the keypad changes depending on the display.
Storage Capacity
The patient’s Holter data is stored on a removable compact flashcard. To store 24 hours at
either normal or high resolution, the minimum
capacity of the compact flashcard should be 32
megabytes. Although 48 hours of 3-channel
Holter signal might fit in 32 megabytes, we
recommend that you use 64-megabyte flashcards instead. Compact flashcards of up to 512
megabytes can be used.
For details about flashcard capacity for recording 12-lead data, see the tables on page 16.
The DR180+ is compatible with standard silver/silver-chloride ECG electrodes.The
2 Guide to NorthEast Monitoring’s DR180+ Digital Holter Recorder
Hooking up the Patient
Hooking up the
Patient
The most important element in Holter monitoring is recording a clean long-term ECG signal.
Because a clean signal is directly dependent on
the hookup procedure, great care should be
taken when hooking up the patient. Poor
hookup causes poor signal quality and artifact.
To ensure proper hookup, follow these steps:
1. Using either the 5-electrode (3-channel) or
the 7-electrode (3-channel) diagram shown
below or the 10-electrode (3-channel
Holter, 12-lead) diagram on the following
page, identify sites for the electrodes. For
oximetry patients, use only channels 1 and
2 on the 7-electrode hookup; two channels
of Holter data will be recorded, and the
oximetry lead will use channel 3.
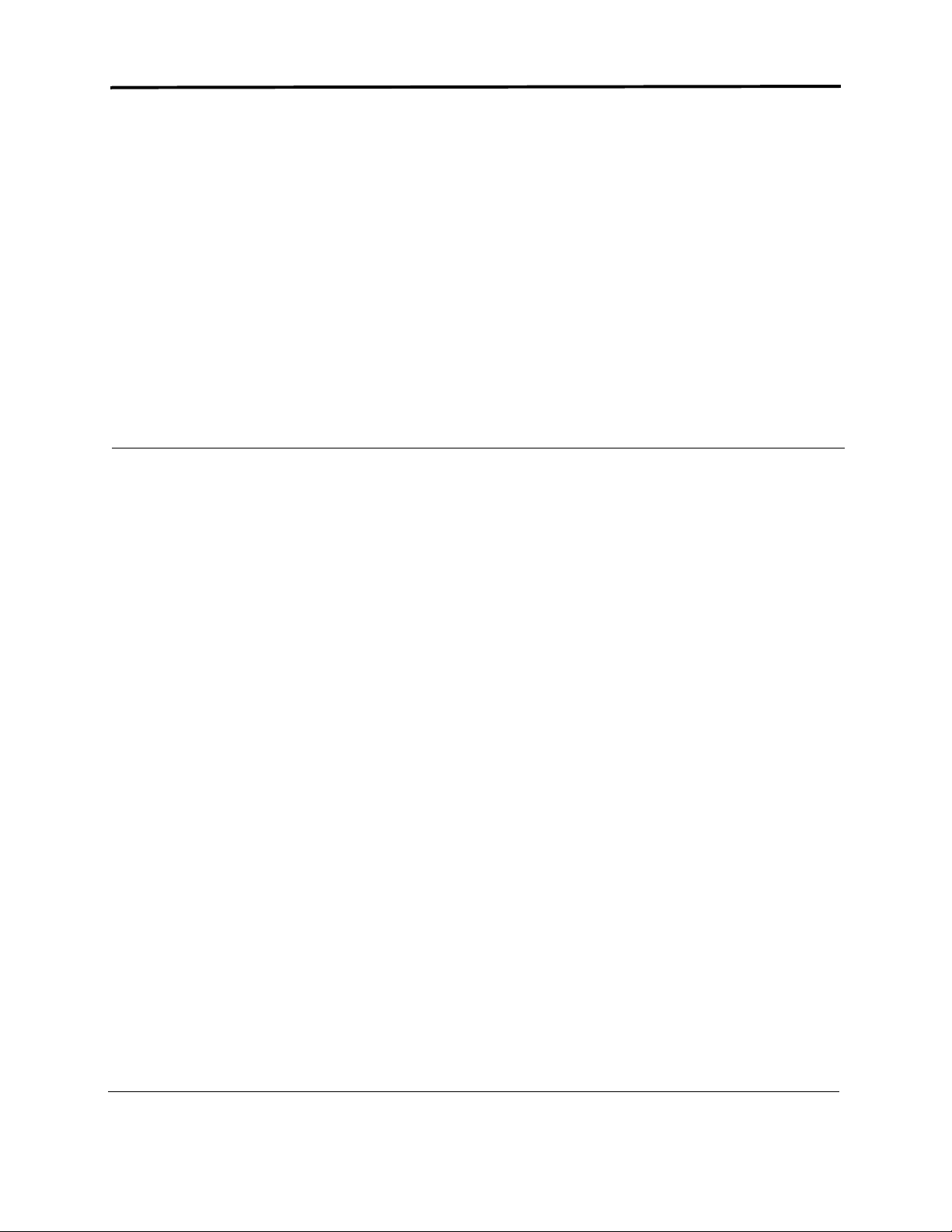
Channel 1:
+ Brown 5th rib, left anterior axillary line
-
Red
Channel 2:
+ Black
-
Red
Channel 3:
+ Black
-
Ground:
centered on manubrium
5th rib, left of mid-clavicular line
White right manubrium
Green
centered over rib
5-electrode placement
Channel 1:
+ Red 5th rib, left anterior axillary line
- White right manubrium
Channel 2:
+ Brown 2 cm. right of xiphoid process
- Black left manubrium
Channel 3:
+ Orange 5th rib, left of mid-clavicular line
- Blue centered on manubrium
Ground:
Green centered over rib
Guide to NorthEast Monitoring, Inc.’s DR180+ Digital Holter Recorder 3
7-electrode placement
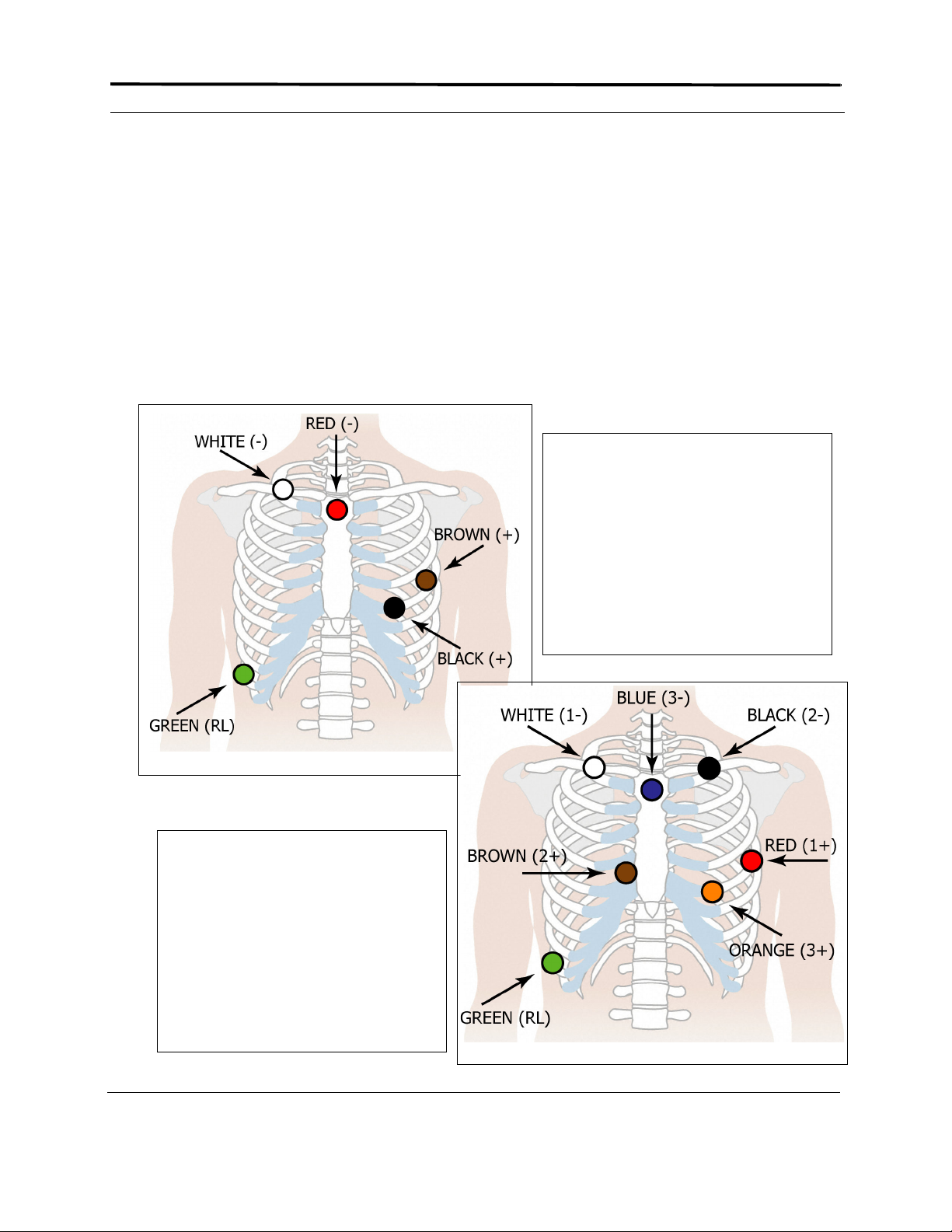
10-electrode, 12-lead hookup
2. Prepare the patient’s skin. If the patient has
hair in any of the electrode areas, shave it
with a safety razor. Use an alcohol pad and
rub the sites briskly until the skin reddens.
Let the skin air dry before proceeding.
For oximetry patients, determine the site for
the sensor. Recommended application sites
include the index fingers and toes, with a
tissue thickness of 5 to 21 mm.
3. Attach the patient cable to the recorder, then
snap a lead wire from the patient cable to
each of the electrodes.
4. Attach the electrodes to the patient by
securing an electrode at each of the prepared sites. Be sure to refer to the diagrams
for correct placement of each colored lead.
The electrodes should be placed over bone
at each of the sites. Press the center of each
electrode against the patient’s skin, then rub
the outer circle of each electrode to secure
it.
RA right mid-clavicular
LA left mid-clavicular
RL right iliac crest
LL left iliac crest
V1 4th intercostal space, right of sternum
V2 4th intercostal space, left of sternum
V3 between V2 and V4
V4 5th intercostal space, mid-clavicular line
V5 5th intercostal space, anterior axillary line
V6 5th intercostal space, mid-axillary line
Positioning oximetry sensor on index finger
For oximetry patients, attach the oximetry
sensor to the patient. If you use the Nonin
Medical sensor wraps, follow the directions
on the sensor wrap insert. If you do not use
the Nonin Medical sensor wraps, follow the
directions on the oximetry sensor insert.
4 Guide to NorthEast Monitoring’s DR180+ Digital Holter Recorder
Preparing the Recorder
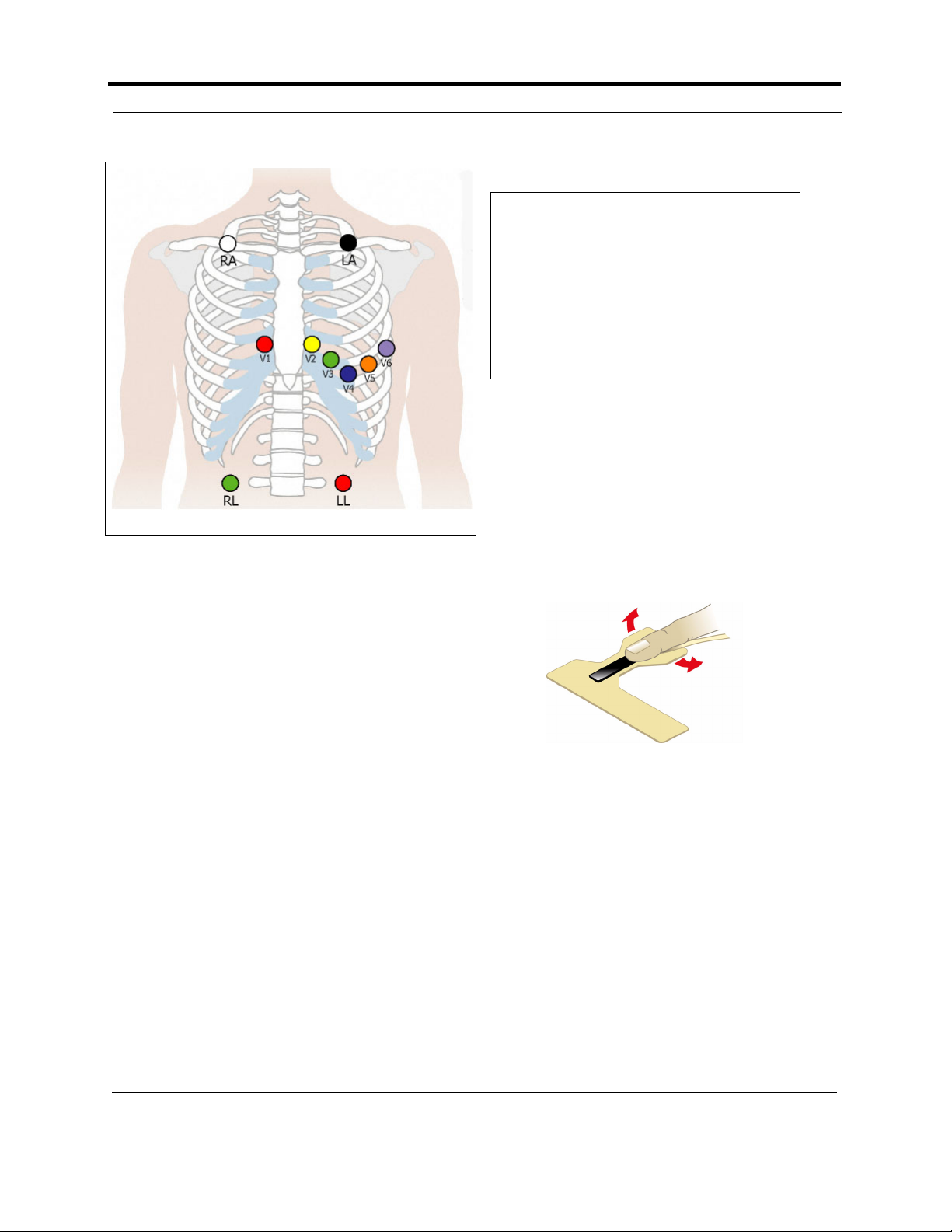
5. If you use
lead lock
or clip
lock electrodes, be
sure to use
the lock or
clip to
relieve
stress on
each lead
wire; refer
to the diagram at right for proper use. Otherwise,
tape each lead wire into a stress loop (see
the diagram below) to help prevent movement of the electrode.
Tape
Using a clip lock electrode
Electrode
up for reader” on the side that should be up
as you slide it into the recorder.
Note: The flashcard should slide in easily.
Make sure you do not force the flashcard in;
if you force the flashcard in upside-down, it
can damage the connector inside the
recorder.
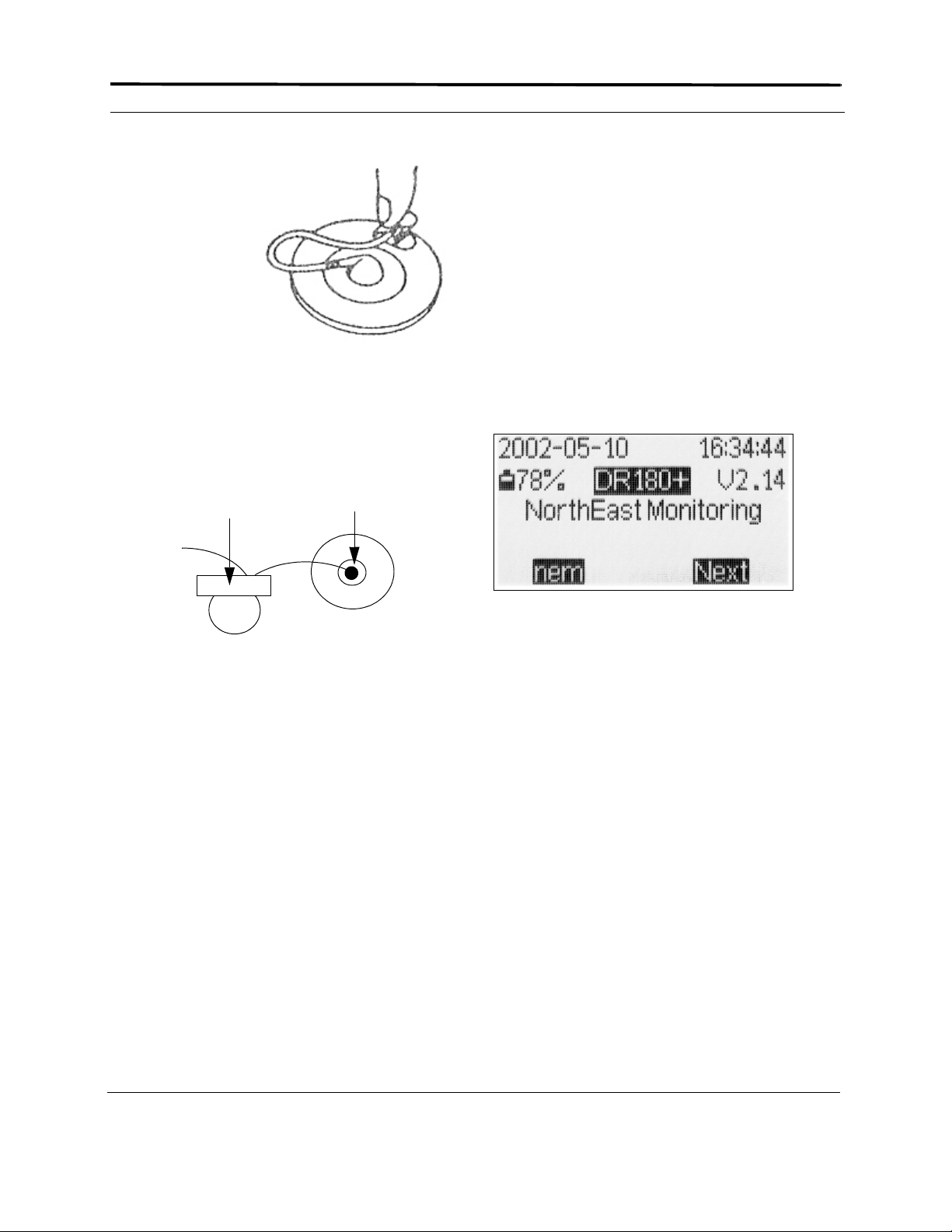
2. Insert two fresh AA batteries into the bat-
tery compartment, being sure to orient them
as indicated in the diagram inside the compartment. Replace the door to the battery
compartment. This information appears on
the LCD:
Start-up display
Stress loop
Preparing the
Recorder
After connecting the patient to the recorder,
follow these steps to start the recording:
1. Remove the door from the battery compart-
ment of the DR180+, then insert a compact
flashcard into the slot inside the compartment. Hold the flashcard by the edge with
the ridge and orient it so that the opposite
edge (with the connector) slides in first.
Looking at the bottom of the recorder, you
should see the bottom of the flashcard; if
the flashcard was supplied by NorthEast
Monitoring, Inc., its blue label reads “Caution: This side up for recorder. Other side
The display includes the current date and
time-of-day. Verify they are correct. It also
displays the percent of battery life remaining and the DR180+ software version.
Note: Some new, high-voltage batteries
(greater than 3.3 volts) can cause an incorrect
reading in the battery life remaining entry. If
you get a low (<10%) reading and you know
that the batteries are new, please ignore the
incorrect value and continue with the hookup
procedure.
Note: As you move through the process on the
LCD, use the Next key to move to the next step
in the procedure and use the Prev(ious) key to
display the screen one level up from the current display.
If, instead of the Start-up display, you see
the message, “Previous recording found,”
the compact flashcard holds a previous
Guide to NorthEast Monitoring, Inc.’s DR180+ Digital Holter Recorder 5
 Loading...
Loading...