Page 1
EC
REP
Instructions for Use—English
• do not apply the pulse oximeter on the
same arm as a blood pressure cuff,
arterial catheter or infusion line(s) (IVs)
• excessive light, such as sunlight or
direct home lighting
• excessive motion
• moisture in the device
• improperly applied device
• finger is outside recommended size
range
• poor pulse quality
• venous pulsations
• anemia or low hemoglobin
concentrations
• cardiogreen and other intravascular
dyes
• carboxyhemoglobin
• methemoglobin
• dysfunctional hemoglobin
• artificial nails or fingernail polish
0123
Onyx® II Model 9560 Finger Pulse Oximeter
Indications for Use
The Nonin Onyx II Model 9560 Finger Pulse Oximeter is a small, lightweight, portable, wireless device indicated for
use in measuring and displaying functional oxygen saturation of arterial hemoglobin (%SpO
patients who are well or poorly perfused. It is intended for spot-checking of adult and pediatric patients on fingers
(other than the thumb) between 0.3 – 1.0 inch (0.8 – 2.5 cm) thick. The device’s intended use environments include
hospitals, clinics, long-term care facilities, skilled nursing facilities, emergency medical services and home
healthcare services.
Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed practitioner.
Contraindications
• Do not use the device in an MR environment, in an explosive atmosphere, or on infant or neonatal patients.
• This device is not defibrillation proof per IEC 60601-1.
Warnings
• Use the Model 9560 within its designated range (approximately 328 feet/100 meters, spherical radius, line of sight when
connected to a class I device, from patient module to the display). Moving outside this range may cause missing, lost, and/
or inaccurate data.
• This device is intended only as an adjunct in patient assessment. It must be used in conjunction with other methods of
assessing clinical signs and symptoms.
• The device must be able to measure the pulse properly to obtain an accurate SpO
hindering the pulse measurement before relying on the SpO2 measurement.
• Operation of this device below the minimum amplitude of 0.3% modulation may cause inaccurate results.
• General operation of the device may be affected by the use of an electrosurgical unit (ESU).
• The use of accessories other than those specified in these instructions may result in increased electromagnetic emission
and/or decreased immunity of this device.
• This device should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the
device should be observed carefully to verify normal operation.
• Keep the oximeter away from young children. Small items such as the battery door, battery, and lanyard are choking hazards.
• Certain activities may pose a risk of injury, including strangulation, if the lanyard should become wrapped around your neck.
!
Cautions
• This device has no audible alarms and is intended only for spot-checking.
• This device is designed to determine the percentage of arterial oxygen saturation of functional hemoglobin. Factors that may
degrade pulse oximeter performance or affect the accuracy of the measurement include the following:
measurement. Verify that nothing is
2
) and pulse rate of
2
• The device may not work when circulation is reduced. Warm or rub the finger, or re-position the device.
• This device’s display will go blank after 30 seconds of no readings or poor readings.
• In some circumstances, the device will interpret motion as good pulse quality. Minimize patient motion as much as possible.
• Clean the device before applying it to a patient.
• Do not sterilize, autoclave, or immerse this device in liquid. Do not pour or spray any liquids into the device.
• Do not use caustic or abrasive cleaning agents, or any cleaning products containing ammonium chloride or isopropyl alcohol.
• A flexible circuit connects the two halves. Do not twist or pull the flexible circuit or overextend the device’s spring. Do not
hang the lanyard from the device’s flexible circuit.
• A functional tester cannot be used to assess the accuracy of a pulse oximeter monitor.
• This equipment complies with IEC 60601-1-2 for electromagnetic compatibility for medical electrical equipment and/or systems.
This standard is designed to provide reasonable protection against harmful interference in a typical medical installation.
However, because of the proliferation of radio-frequency transmitting equipment and other sources of electrical noise in health
care and other environments, it is possible that high levels of such interference due to close proximity or strength of a source
might disrupt the performance of this device. Medical electrical equipment needs special precautions regarding EMC, and all
equipment must be installed and put into service according to the EMC information specified in this manual.
• Portable and mobile RF communications equipment can affect medical electrical equipment.
• Batteries may leak or explode if used or disposed of improperly. Remove batteries if the device will be stored for more than
30 days. Do not use different types of batteries at the same time. Do not mix fully charged and partially charged batteries at
the same time. These actions may cause the batteries to leak.
• Follow local, state, and national governing ordinances and recycling instructions regarding disposal or recycling of the device
and device components, including batteries.
• In compliance with the European Directive on Waste Electrical and Electronic Equipment (WEEE) 2002/96/EC, do not
dispose of this product as unsorted municipal waste. This device contains WEEE materials; please contact your distributor
regarding take-back or recycling of the device. If you are unsure how to reach your distributor, please call Nonin for your
distributor’s contact information.
Nonin Medical, Inc.
13700 1st Avenue North
Plymouth, Minnesota 55441-5443 USA
+1 (763) 553-9968 (outside US and Canada)
+46 650 401500 (Europe)
(800) 356-8874 (US and Canada)
Fax: +1 (763) 553-7807
+46 650 401514 (Europe)
E-mail: info@nonin.com
infointl@nonin.se (Europe)
www.nonin.com
MPS, Medical Product Service GmbH
Borngasse 20
Nonin and Onyx are registered trademarks of Nonin Medical, Inc.
U.S. Patents 5,490,523; 5,792,052
D-35619 Braunfels, Germany
© 2012 Nonin Medical, Inc.
6742-001-04
Page 2

Declaration of Conformity with FCC and Canadian Ministry of Health Rules for
!
0123
SN
Electromagnetic Compatibility
• Nonin Medical, Inc., of 13700 1st Avenue North, Plymouth, Minnesota, 55441, declares under its sole
responsibility that Model 9560, to which this declaration relates, comply with part 15 of the FCC Rules. Operation
is subject to the following two conditions: (1) this device may not cause harmful interference, and (2) this device
must accept any interference received, including interference that may cause undesired operation.
• Ministry of Health (Canada), Safety Code 6: standards include a substantial safety margin designed to ensure
the safety of all persons, regardless of age and health. The exposure standard for wireless mobile phones
employs a unit of measurement known as the Specific Absorption Rate, or SAR. The SAR limit set by the FCC
is 1.6W/kg.
Federal Communications Commission (FCC) Notice
This equipment has been tested and found to comply with the limits for a class B digital device, pursuant to part 15
of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a
residential installation. This equipment generates, uses, and can radiate radio frequency energy. If not installed
and used in accordance with the instructions, it may cause harmful interference to radio or television reception,
which can be determined by turning the equipment off and on. The user is encouraged to try to correct the
interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the distance between the equipment and the receiver.
• Connect the equipment to an outlet on a circuit different from the outlet where the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for assistance.
• RF Exposure: For body worn operation, to maintain compliance with FCC RF exposure guidelines, use only
accessories that contain no metallic components and provide a separation distance of 15 mm (0.6 inches) to the
body. Use of other accessories may violate FCC RF exposure guidelines and should be avoided.
• The Model 9560 is designed and manufactured not to exceed the emission limits for exposure to radio frequency
(RF) energy set by the United States FCC. These limits are part of comprehensive guidelines and establish
permitted levels of RF energy for the general population. The guidelines are based on the safety standards
previously set by both U.S. and international standards bodies. This EUT has been shown to be capable of
compliance for localized specific absorption rate (SAR) for uncontrolled environment/general population
exposure limits specified in ANSI/IEEE Std. C95.1-2005 and has been tested in accordance with the
measurement procedures specified in FCC/OET Bulletin 65 Supplement C (2001) and IEEE Std. 1528-2003.
• The FCC requires the user to be notified that any changes or modifications to this device that are not expressly
approved by Nonin Medical, Inc. may void the user’s authority to operate the equipment.
A Guide to Symbols
Symbol Definition of Symbol
Follow Instructions for Use.
Consult Instructions for Use
Caution!
Type BF Applied Part (patient isolation from electrical shock)
UL Mark for Canada and the United States with respect to electric shock, fire, and mechanical
hazards only in accordance with IEC 60601-1, UL 60601-1 and CAN/CSA-C22.2 No. 601.1.
CE Marking indicating conformance to EC Directive No. 93/42/EEC concerning medical devices
Radio Equipment Class Identifier
Serial Number
Battery Orientation
Non-ionizing electromagnetic radiation. Equipment includes RF transmitters. Interference may
occur in the vicinity of equipment marked with this symbol.
Indicates separate collection for electrical and electronic equipment (WEEE).
2
Page 3
Symbol Definition of Symbol
R
A
B
C
Remote Alarms; Not for Continuous Monitoring.
Protected against vertically falling water drops when enclosure is tilted up to 15 degrees and
IP32
ingress of solid foreign objects greater than or equal to 2.5 mm (0.1 in.) in diameter per
IEC 60529.
Bluetooth
Indoor use (France only)
Continua Certified™ signifies that this product has been tested and proven to be interoperable
with other products that carry the “Continua Certified” symbol.
RoHS Compliant (China)
®
Installing Batteries
pin
BDA
Ver
NOTE: Where applicable, an additional label bearing your country radio communications license information will
appear on the side of your device. This is not a serial number or device identifier.
Two 1.5 volt AAA-size batteries power the Model 9560 for approximately 600 spot checks. Nonin recommends using
alkaline batteries (included with each new Model 9560). When batteries are low, the numeric displays flash once per
second. Remove batteries if the device will be stored for more than 30 days. Replace low batteries as soon as
possible, using the instructions below.
NOTE: Rechargeable batteries may be used; however, they require more frequent replacement.
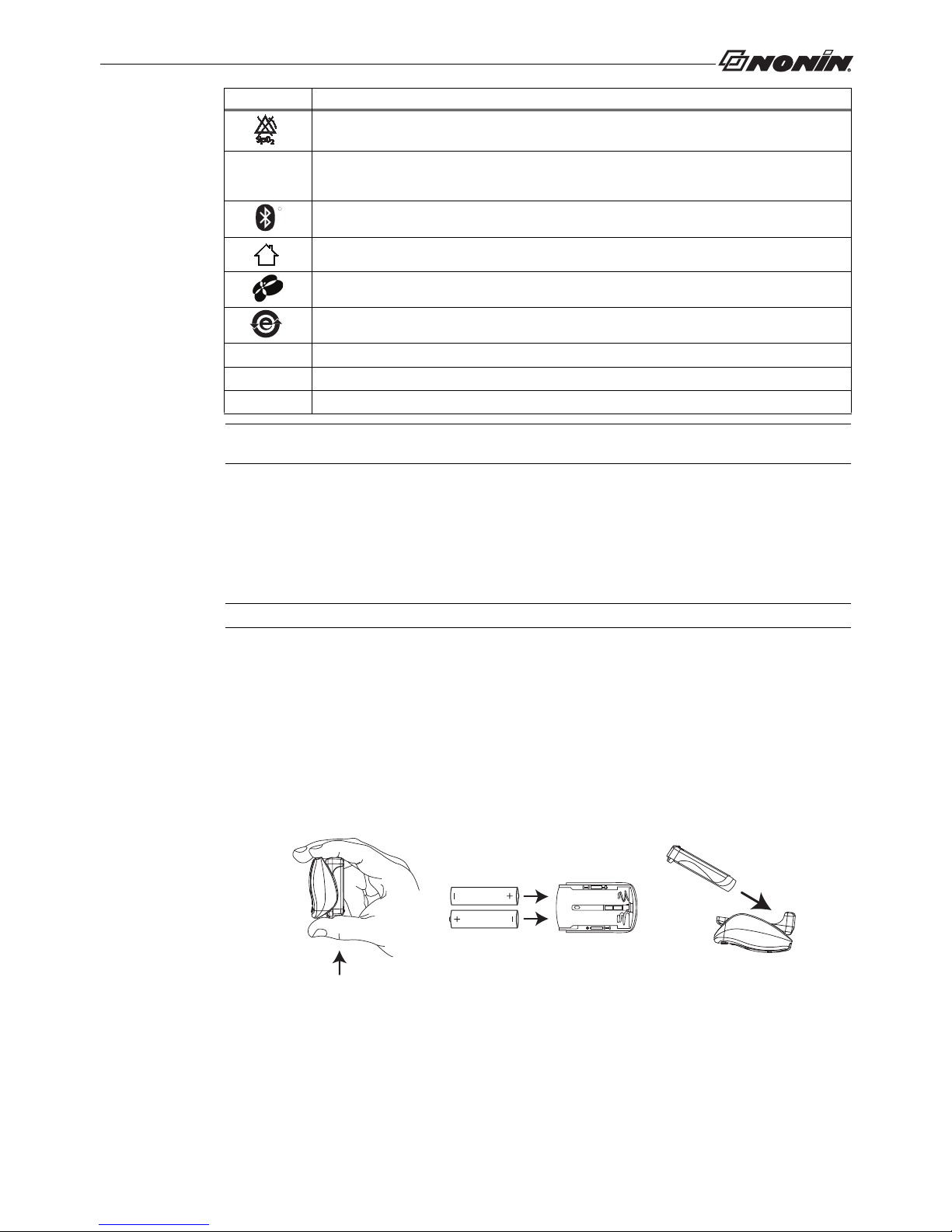
1. Hold the Model 9560 as shown in figure A. To release the device’s battery tray, press upward and then pull
outward slightly with the thumb.
2. Remove the old batteries from the battery tray. Dispose of the batteries properly.
3. Insert two new 1.5 volt AAA-size batteries. Follow the polarity markings (+ and -) as illustrated in figure B.
Proper positioning of the batteries is essential for operation.
4. Carefully guide the battery tray back onto the device. Press downward and then push inward slightly to resecure the battery tray. Do not force it into place; it fits only when properly positioned.
5. Visually inspect to ensure that the battery cover is properly placed.
6. Insert your finger in the device to verify operation. See the Activating the Onyx II 9560 and Verifying Operation
section for more information.
Personal Identification Number
Bluetooth Device Address
Versi on
3
Page 4

Activating the Onyx II 9560 and Verifying Operation
Pulse Quality
Indicator
!
The Model 9560 contains numeric Light-Emitting Diodes (LEDs) that display oxygen saturation and pulse rate. A
tricolor LED display (pulse quality indicator, shown at left) provides a visual indication of the pulse signal quality,
while blinking at the corresponding pulse rate. This display changes colors to alert you to changes in pulse quality
that may affect the readings:
• Green indicates a good pulse signal.
• Yellow indicates a marginal pulse signal.
• Red indicates an inadequate pulse signal.
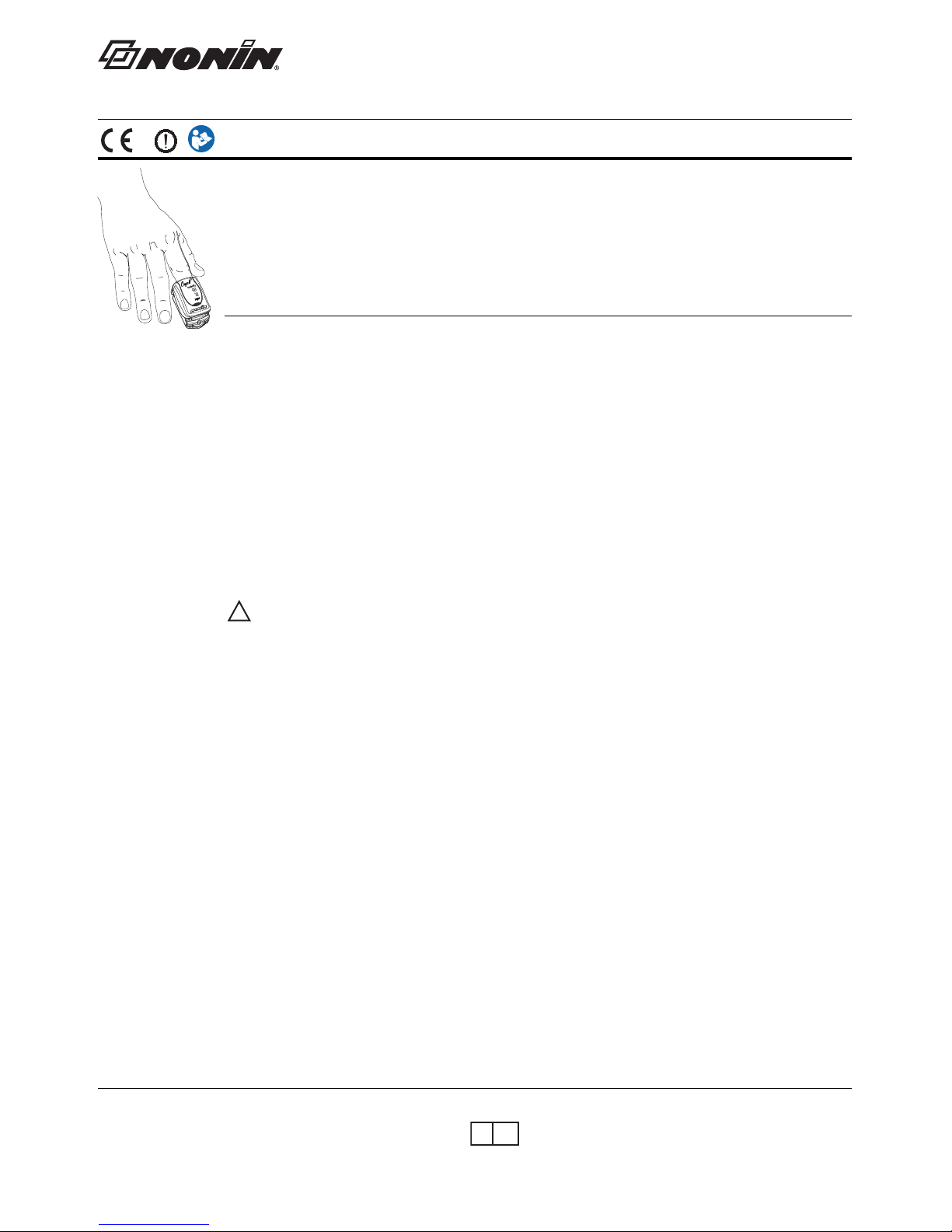
Activate the Model 9560 by inserting the patient’s finger into the device. The device detects the inserted finger and
automatically illuminates the displays. Correct positioning of the finger is critical for accurate measurements.
NOTE: While on the finger, do not press the device against any surface and do not squeeze or hold it together. The
internal spring provides the correct pressure; additional pressure may cause inaccurate readings.
1. Insert the patient’s finger, nail side up, into the 9560 until the fingertip touches the built-in stop guide.
2. Make sure the finger is lying flat (not on its side) and is centered within the device. For best results, keep the
device at the patient’s heart or chest level.
3. If the device does not turn on, remove the finger and wait a few seconds before reinserting it.
When a finger is inserted, the device performs a brief startup sequence. Verify that all LEDs illuminate during the
startup sequence. If any LED is not lit, do not use the Model 9560; contact Nonin Technical Service for repair or
replacement. After the startup sequence, the device begins sensing the pulse (indicated by the blinking pulse quality
indicator). Allow the device to stabilize and observe about 4 seconds of continuous green-colored pulse quality
before relying on the displayed values. It is common for the displayed values to fluctuate slightly over a period of
several seconds. If the pulse quality indicator blinks yellow or red, try another finger.
A minus sign (-) appears in the left-most digit of the %SpO
removed. The last measured SpO
off. The device will automatically shut off (to conserve battery life) approximately 10 seconds after the finger is
removed, or after a 2-minute period of inadequate pulse signals.
When the Model 9560 is placed on the finger and powered on, and not in a connection with an electronic medical
record (EMR) system, it will be available for a wireless connection (Bluetooth) for a minimum of 90 seconds. It will
remain in this mode until:
• a successful wireless connection occurs with your EMR system,
• two minutes have passed without a successful wireless connection with your EMR system, or
• the Model 9560 is powered off.
and pulse rate values display for 10 seconds while the device automatically turns
2
display when the Model 9560 senses the finger has been
2
Using the Lanyard and Carrying Case
WARNING: Certain activities may pose a risk of injury, including strangulation, if lanyard should become
wrapped around your neck.
CAUTION: A flexible circuit connects the two halves. Do not twist or pull the flexible circuit or
overextend the device’s spring. Do not hang the lanyard from the device’s flexible circuit.
A lanyard and carrying case are provided for convenience. The device will function with or without the lanyard.
If lanyard use is desired, thread the lanyard as shown below.
4
Page 5

Onyx II 9560 Care, Maintenance, and Cleaning
CAUTIONS:
!
The advanced digital circuitry within the Model 9560 requires no calibration or periodic maintenance other than
battery replacement. Field repair of the Model 9560 circuitry is not possible. Do not attempt to open the Model 9560
case or repair the electronics. Opening the case will damage the Model 9560 and void the warranty. Do not open
the Model 9560 more than 90°, and do not twist or pull on the device when cleaning.
Cleaning the Onyx II Model 9560
• Clean the device before applying it to a patient.
• Do not sterilize, autoclave, or immerse this device in liquid. Do not pour or spray any liquids into the device.
• Do not use caustic or abrasive cleaning agents, or any cleaning products containing ammonium chloride or
isopropyl alcohol.
1. Wipe the surfaces with a soft cloth dampened with a 10% bleach solution (household bleach [5.25% sodium
hypochlorite]). Do not use undiluted bleach or any cleaning solution other than those recommended here, as
permanent damage could result.
2. Dry with a soft cloth, or allow to air dry. Ensure that all surfaces are completely dry.
Testing Summary
SpO2 accuracy and low perfusion testing was conducted by Nonin Medical, Inc. as described below.
SpO2 Accuracy Testing
SpO2 accuracy testing is conducted during induced hypoxia studies on healthy, non-smoking, light-to-dark-skinned
subjects in an independent research laboratory. The measured arterial hemoglobin saturation value (SpO
sensors is compared to arterial hemoglobin oxygen (SaO
co-oximeter. The accuracy of the sensors is in comparison to the co-oximeter samples measured over the SpO
range of 70-100%. Accuracy data is calculated using the root-mean-squared (A
ISO 80601-2-61 and ISO 9919, Standard Specification for Pulse Oximeters for Accuracy.
) of the
) value, determined from blood samples with a laboratory
2
value) for all subjects, per
rms
2
2
Low Perfusion Testing
This test uses an SpO2 Simulator to provide a simulated pulse rate, with adjustable amplitude settings of various
levels. The module must maintain accuracy in accordance with ISO 80601-2-61 and ISO 9919 for pulse rate
SpO
2
and SpO
at the lowest obtainable pulse amplitude (0.3% modulation).
2
5
Page 6

Specifications
Oxygen Saturation Display Range: 0% to 100% SpO
2
Pulse Rate Display Range: 18 to 321 beats per minute (BPM)
Declared Accuracy:
The table below shows A
values measured using the Onyx II 9560 in a clinical study in non-motion conditions.
rms
Accuracy Summary by Decade
Decade
Oxygen Saturation
)
(A
rms
Low Perfusion Oxygen
Saturation (A
rms
)
70 – 80% ±2 ±2
80 – 90% ±2 ±2
90 – 100% ±2 ±2
70 – 100% ±2 ±2
This graph shows plots of the error (SpO2 – SaO2) by SaO2 using
the 9560 with a linear regression fit and upper 95% and lower
95% limits of agreement. Each sample data point is identified by
subject from a clinical study in non-motion conditions.
Pulse Rate Declared Accuracy Range (A
rms
*):
20 to 250 BPM ±3 digits
Low Perfusion Pulse Rate Declared Accuracy Range
*):
(A
rms
40 to 240 BPM ±3 digits
Measurement Wavelengths and Output Power**:
Red:
Infrared:
660 nanometers @ 0.8 mW maximum average
910 nanometers @ 1.2 mW maximum average
Temperature:
Operating: 23 °F to 104 °F / -5 °C to 40 °C
Storage/Transportation: -40 °F to 158 °F / -40 °C to 70 °C***
Humidity:
Operating: 10% to 95% non-condensing
Storage/Transportation: 10% to 95% non-condensing
Altitude:
Operating: Up to 40,000 feet / 12,192 meters
Hyperbaric Pressure: Up to 4 atmospheres
Battery Life:
Operating: Two 1.5 volt AAA-size batteries power the Model 9560 for approximately
600 spot checks.
Storage: 12 months, minimum
Classifications per IEC 60601-1 / CAN/CSA-C22.2 No. 601.1/ UL 60601-1:
Degree of Protection: Type BF-Applied Part
Enclosure Degree of Ingress Protection: IP3
2
Mode of Operation: Continuous
This product complies with ISO 10993-1, Biological evaluation of medical devices – Part 1: Evaluation and testing.
This device is not made with natural rubber latex.
*± 1 A
represents approximately 68% of measurements.
rms
**This information is especially useful for clinicians performing photodynamic therapy.
*** When the Model 9560 is transferred from a non-operating temperature/humidity condition, allow one hour of stabilization to operating temperature/
humidity specifications prior to use.
6
Page 7

Antenna:
Type: L-shaped PWB whip-type antenna
Transmitter:
Bluetooth Compliance: Version 2.0
Operating Frequency: 2.4 to 2.4835 GHz
Output Power: <20 dBm
Operating Range: 328 feet/100-meter radius indoors (line of sight when connected to a
Network Topology: Star
Operation: Bluetooth Slave
Antenna Type: Internal
Modulation Type: Frequency Shift Keying
Frequency Hopping Spread Spectrum:
Band Width: 1 MHz
class I device)
Warranty
NONIN MEDICAL, INCORPORATED, (Nonin) warrants to the purchaser, for a period of 4 years from the date of purchase, each Model 9560
exclusive of the batteries, spring, carrying case, lanyard, and lanyard lock.
Nonin shall repair or replace any Model 9560 found to be defective in accordance with this warranty, free of charge, for which Nonin has
been notified by the purchaser by serial number that there is a defect, provided notification occurs within the applicable warranty period.
This warranty shall be the sole and exclusive remedy by the purchaser hereunder for any Model 9560 delivered to the purchaser which is
found to be defective in any manner whether such remedies be in contract, tort or by law.
This warranty excludes cost of delivery to and from Nonin. All repaired units shall be received by the purchaser at Nonin’s place of business.
Nonin reserves the right to charge a fee for a warranty repair request on any Model 9560 found to be within specifications.
Model 9560 is a precision electronic instrument and must be repaired by trained Nonin personnel only. Any sign or evidence of opening the
Model 9560, field service by non-Nonin personnel, tampering, or any kind of misuse of the Model 9560, shall void the warranty. All nonwarranty work shall be done at Nonin’s standard rates and charges in effect at the time of delivery to Nonin.
Nonin Medical, Inc.
13700 1st Avenue North
Plymouth, Minnesota 55441-5443 USA
+1 (763) 553-9968
(800) 356-8874 (USA and Canada)
Fax: +1 (763) 553-7807
E-mail: technicalservice@nonin.com
Nonin Medical AB
Fibervägen 2
82450 Hudiksvall, Sweden
+46 650 401500 (Europe)
Fax: +46 650 401514
E-mail: serviceintl@nonin.se
www.nonin.com
7
Page 8

Manufacturer’s Declaration
Refer to the following tables for specific information regarding this device’s compliance to IEC 60601-1-2.
Table 1: Electromagnetic Emissions
Emissions Test Compliance Electromagnetic Environment—Guidance
This device is intended for use in the electromagnetic environment specified below. The customer and/or user of this device
RF Emissions
CISPR 11
RF Emissions
CISPR 11
Harmonic Emissions
IEC 61000-3-2
Voltage Fluctuations/Flicker
Emissions
IEC 61000-3-3
should ensure that it is used in such an environment.
Group 2
Class B
N/A
N/A
This device must emit electromagnetic energy in order to perform its
intended function. Nearby electronic equipment may be affected.
This device is suitable for use in all establishments, including
domestic and those directly connected to the public low-voltage
power supply network that supplies buildings used for domestic
purposes.
Table 2: Electromagnetic Immunity
Immunity Test IEC 60601 Test Level
This device is intended for use in the electromagnetic environment specified below. The customer and/or user of this device should ensure that it is
Electrostatic Discharge (ESD)
IEC 61000-4-2
Electrical Fast Transient/Burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short interruptions,
and voltage variations on power
supply input lines
IEC 61000-4-11
Power Frequency (50/60 Hz)
Magnetic Field
IEC 61000-4-8
NOTE: U
is the AC mains voltage before application of the test level.
T
±6 kV contact
±8 kV air
±2 kV for power supply lines
±1 kV for input/output lines
±1 kV differential mode
±2 kV common mode
±5% U
(>95% dip in UT) for 0.5 cycle
T
±40% U
(60% dip in UT) for 5 cycles
T
±70% U
(30% dip in UT) for 25 cycles
T
<5% U
(>95% dip in UT) for 5 sec.
T
3 A/m 3 A/m
used in such an environment.
Compliance
Level
±6 kV contact
±8 kV air
N/A
N/A
N/A
Electromagnetic Environment—Guidance
Floors should be wood, concrete, or ceramic tile. If
floors are covered with synthetic material, relative
humidity should be at least 30%.
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
Power frequency magnetic fields should be at levels
characteristic of a typical location in a typical
commercial or hospital environment.
8
Page 9

d1.17P=
d1.17P=
d2.33P=
d1.17P=
d1.17P=
d2.33P=
Table 3: Guidance and Manufacturer’s Declaration—Electromagnetic Immunity
Immunity Test
This device is intended for use in the electromagnetic environment specified below. The customer and/or user of this device should ensure that it is
Portable and mobile RF communications equipment should be used no closer to any part of the device, including cables, than the recommended
separation distance calculated from the equation applicable to the frequency of the transmitter.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
Radiated RF per ISO
9919 clause 36 and
ISO 80601-2-61 clause
202.6.2.3
a. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio,
AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment
due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the
device is used exceeds the applicable RF compliance level above, the device should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as reorienting or relocating the device.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [3] V/m.
NOTES:
IEC 60601 Test
Level
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
20 V/m
80 MHz to 2.5 GHz
Compliance
Level
used in such an environment.
N/A
[3] V/m
[20] V/m
80 MHz to 800 MHz
800 MHz to 2.5 GHz
where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic
site survey
Interference may occur in the vicinity of equipment marked with the following
symbol:
Electromagnetic Environment—Guidance
Recommended Separation Distance
a
, should be less than the compliance level in each frequency range.
• At 80 MHz and 800 MHz, the higher frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
b
The following table details the recommended separation distances between portable and mobile RF communications equipment and this
device.
Table 4: Recommended Separation Distances
This device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. Users
of this device can help prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communication equipment (transmitters) and the device as recommended below, according to maximum output
Rated Maximum Output
Power of Transmitter
W
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.2 1.2 2.3
10 3.7 3.7 7.4
100 12 12 23
power of the communications equipment.
Separation Distance According to Frequency of Transmitter
150 kHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2.5 GHz
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be
estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter
in watts (W) according to the transmitter manufacturer.
NOTES:
• At 80 MHz and 800 MHz, the higher frequency range applies.
• These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
9
 Loading...
Loading...