Nevro WAND1001 Users Manual
Physician Implant
Manual
Senza®
Senza II®
ONLY

10186-ENG Rev P 2
NEVRO CORP.
All questions or concerns about Nevro Corp. products should be forwarded to:
Nevro Corp.
1800 Bridge Parkway
Redwood City, CA 94065, USA
Tel: +1.650.251.0005
Fax: +1.650.251.9415
info@nevro.com
MDSS GMBH
Schiffgraben 41
D-30175 Hannover,
Germany
Australian Sponsor
Emergo Australia
Level 20, Tower II, Darling Park
201 Sussex Street,
Sydney, NSW 2000
Australia
© Copyright 2018, Nevro Corp. All rights reserved.
No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system or translated into
any language or computer language, in any form or by any means, including, but not limited to, electronic, magnetic,
optical, chemical, manual, or otherwise without written permission of Nevro Corp.
Registered Trademarks: Nevro, Senza, Senza II, HF10, and the Nevro Logo are trademarks of the Nevro Corp.
CE Mark effective on 4 May 2010
Nevro hereby declares that the Senza® system is in compliance with the essential requirements and other relevant
provisions of the Radio Equipment Directive (2014/53/EU).
IMPORTANT: Do not change or modify any component of the Senza or Senza II Spinal Cord Stimulation system, unless
expressly approved by Nevro Corp.
CAUTION: Federal law restricts this device to sale, distribution and use by or on the order of a physician.
10186-ENG Rev P 3
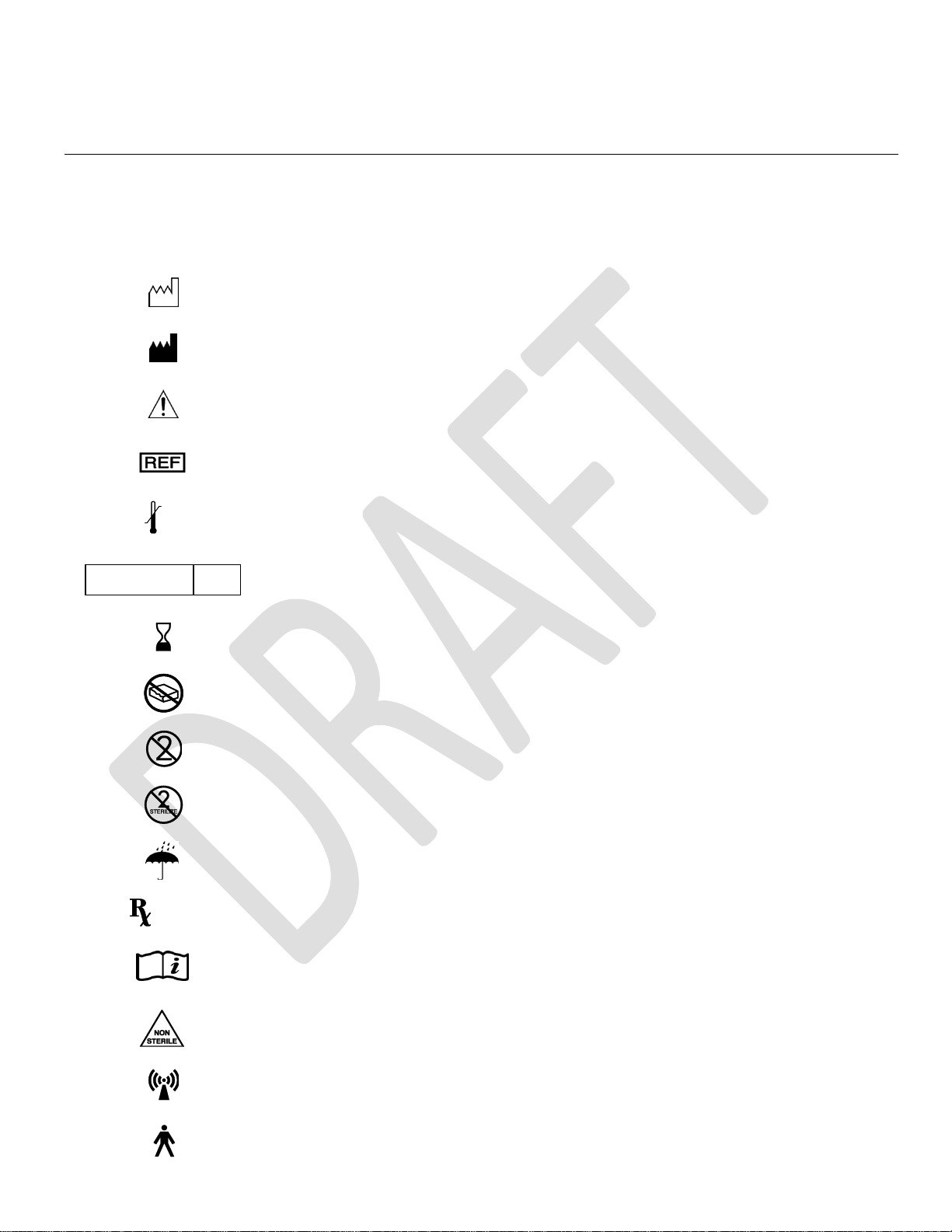
Explanation of symbols. Refer to the product for symbols that apply.
Symbols
Description
SN
Serial number
LOT
Batch code
Date of Manufacture
Manufacturer
Caution
Catalog number
Temperature limitation (storage)
Sterilized using ethylene oxide
Use by
Do not use if package is damaged
Do not reuse
Do not re-sterilize
Keep dry
Prescription only
www.nevro.com
Consult Electronic Instructions for Use
Non-sterile
Non-ionizing radiation
Type B Applied Part
XX C
XXX F
XX C
XX F
STERILE
EO
ONLY

10186-ENG Rev P 4
Type BF Applied Part
Do not dispose of this product in the unsorted municipal waste stream. Dispose of this product according to
local regulations.
Conditional
MR Conditional
MR unsafe
CE Marking of Conformity
Authorized representative in the European Community

10186-ENG Rev P 5
Table of Contents
1. Device Description ......................................................................................................................... 6
2. Indications for Use ......................................................................................................................... 8
3. Contraindications ........................................................................................................................... 8
4. Warnings ....................................................................................................................................... 8
5. Precautions .................................................................................................................................. 12
6. Adverse Effects ............................................................................................................................ 15
7. Technical Specifications ................................................................................................................ 17
a. System Specifications .......................................................................................................................... 17
b. Charger Specifications ......................................................................................................................... 17
c. Stimulation Parameter Ranges ............................................................................................................ 17
d. Quality of Wireless Service ................................................................................................................. 17
e. Wireless Security ................................................................................................................................. 17
f. Telemetry Information ......................................................................................................................... 18
g. Wireless Charging Information ........................................................................................................... 18
h. Electromagnetic Interference ............................................................................................................. 18
8. Patient Identification .................................................................................................................... 21
9. Instructions for Use ...................................................................................................................... 22
10. Guidelines for Trial-phase Implantation ...................................................................................... 22
a. Temporary vs. Permanent Trials ..................................................................................................... 22
b. Pre-op Instructions .......................................................................................................................... 22
c. Percutaneous Lead Placement ........................................................................................................ 23
d. Surgical Lead Placement ................................................................................................................. 24
e. Performing Intra-operative Testing ................................................................................................ 24
f. Preparing for Temporary Trial......................................................................................................... 25
g. Anchoring the Lead ......................................................................................................................... 25
h. Connecting an Extension to the Lead ............................................................................................. 26
i. Percutaneous Tunneling of the Extension ...................................................................................... 26
j. Closing the Incision Sites ................................................................................................................. 27
k. Connecting the Lead or Extension to the Trial Stimulator .............................................................. 27
11. Guidelines for Permanent Implantation ...................................................................................... 27
a. Pre-op instructions .......................................................................................................................... 27
b. If the patient was given a temporary trial ...................................................................................... 27
c. If the patient was given a Permanent trial...................................................................................... 28
d. IPG Implantation ............................................................................................................................. 29
e. IPG Explant or Replacement ........................................................................................................... 31
12. Device Information for the Clinical Staff ...................................................................................... 31
a. Trial Stimulator .................................................................................................................................... 31
b. Rechargeable IPG ................................................................................................................................ 31

10186-ENG Rev P 6
1. Device Description
The Senza® and Senza II® Spinal Cord Stimulation (SCS) Systems are neuromodulation devices designed to
deliver electrical stimulation for the treatment of chronic intractable pain of the trunk and/or limbs.
The Senza and Senza II Systems are implantable systems and deliver stimulation using implantable leads
and a rechargeable, implantable pulse generator (IPG). The IPG is implanted in a subcutaneous pocket
and is capable of stimulating the spinal cord nerves when used with one or more leads. The IPG is
controlled by a Patient Remote and/or the Clinician Programmer. Other components of the Senza and
Senza II Systems include an external Trial Stimulator capable of delivering the same stimulation as the
IPG, Lead Extensions, Adaptors, Charger and charging system, operating room (OR) cables and surgical
accessories. Details regarding the Senza and Senza II Systems are as follows:
Senza System Details – Major Components
• Implantable Pulse Generator (Models Senza and Senza II): The Implantable Pulse Generator
(IPG) is a rechargeable implantable device with 16 output channels capable of stimulating the
spinal cord nerves through electrode leads. The IPG is designed to produce current-regulated,
charge-balanced, biphasic, capacitively-coupled, rectangular output pulses. The IPG header
contains the charging coil and two ports to allow the insertion of leads. The rechargeable
battery is contained in a hermetically sealed housing, which is inside the hermetic IPG Titanium
enclosure.
• Trial Stimulator: The Trial Stimulator is a battery-powered, handheld device capable of
providing the same stimulation as the IPG. During the Trial Phase of SCS, the subject wears this
external Trial Stimulator for a period of time to evaluate the effectiveness of the stimulation
prior to receiving a permanent implant. The Trial Stimulator is connected to the subject’s
implanted leads by the use of OR cables.
• IPG or Trial Stimulator interface with other Senza components: The Charger transmits energy
transcutaneously to recharge the IPG battery. The IPG and Trial Stimulator communicate with
the Patient Remote or Clinician Programmer via the Programmer Wand. Patients are also able
to send commands to the IPG or Trial Stimulator directly using the Patient Remote. The IPG also
includes a magnetic switch for turning the therapy off by using an external magnet.
• Patient Remote: The Patient Remote is a handheld battery operated unit able to communicate
with the IPG or Trial Stimulator. The Patient Remote includes multiple controls and indicators
for the purpose communicating with these components.
• Charger: This Charger is used by the subject to transcutaneously charge the IPG battery. It is a
portable device powered by a rechargeable battery and can be held in one hand.
• Programmer: The Clinician Programmer programs the IPG or Trial Stimulator via the
Programmer Wand via a graphical user interface (GUI).
• Programmer Wand: The Programmer Wand is the Clinician Programmer interface that allows
the communication with the IPG or Trial Stimulator.
• Leads, Lead Extensions and Lead Adaptors: There are two types of leads: Percutaneous and
Surgical. The Nevro Leads are intended to be used with an IPG or Trial Stimulator for use in
delivering stimulation. The Leads are for single use and interface with the IPG, Lead Extensions,
OR Cable, and lead accessories.

10186-ENG Rev P 7
The Percutaneous Lead has an isodiametric body made out of Pellethane 55D, which carries
eight low impedance cables. The proximal connector end has eight (8) individual contacts which
interface with the Nevro IPG and Lead Extensions.
The proximal end of the Surgical Lead has two legs each with 8 contacts. The proximal end of the
Surgical Lead is identical to the proximal end of the Percutaneous Lead. The distal end of the
lead is molded out of Silicone material and has 16 distal electrodes.
The proximal end of the Lead Extension is identical to the proximal ends of the Percutaneous
and Surgical Leads. The distal end of the Lead Extension is designed to accept the proximal end
of the Percutaneous or Surgical Lead. The construction of the Lead Extension is identical along
its length to the Percutaneous Lead.
The M8 and S8 Lead Adaptors allow a physician to connect a specific implanted Medtronic or St.
Jude Medical lead, respectively, with the Nevro Lead Extension or IPG. The construction of the
Lead Adaptors is identical to the Lead Extension.
Senza System Details - Surgical Accessories
• Torque Wrench: The Torque Wrench is used to tighten the setscrews that lock the Lead into the
IPG, to lock the Lead into a Lead Extension/Adaptors, or to activate the retention mechanism on
the Active Anchors.
• Lead anchors: The Lead Anchors are used to anchor the Lead to the fascia or supraspinous
ligament.
• Insertion Needle: The Insertion Needle is used during implant surgery to introduce the
Percutaneous Lead between the vertebrae into the epidural space.
• Coiled Lead Blank: The Coiled Lead Blank is optionally used during surgery to clear a path for
the introduction of the Percutaneous Lead into the epidural space.
• Stylets: The Stylets are used to maneuver the Lead through the epidural space to the desired
implant location.
• IPG Port Plug: The IPG Port Plug is provided to seal the port of the IPG that is not in use when
only one Lead is implanted.
• OR Cables: The Operating Room (OR) Cables make electrical and mechanical connections
between the Trial Stimulator and the Leads or Lead Extensions.
• Tunneling Tool: The Tunneling Tool creates a subcutaneous tunnel for the leads from the IPG
site to the midline incision.
• IPG Template: The IPG Template acts as an optional aid for physicians in proper sizing of the IPG
implant pocket.
• Mx Trial Adaptor: The Mx Trial Adaptor is intended to connect a Medtronic OR cable to the
Nevro Trial Stimulator.

10186-ENG Rev P 8
2. Indications for Use
The Senza® and Senza II® neuromodulation systems are intended to aid in the management of chronic
intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following:
• Failed back surgery syndrome
• Intractable low back pain
• Upper back pain
• Leg pain
• Upper limb and neck pain
3. Contraindications
The Senza and Senza II systems should not be used for those patients who:
• Are poor surgical candidates.
• Fail to receive effective pain relief during trial stimulation.
• Are unable to operate the SCS system.
4. Warnings
Pediatric Use – The safety and effectiveness of spinal cord stimulation has not been established for pediatric
use.
Other Active Implanted Devices – The Senza and Senza II systems may interfere with other implanted
stimulators, such as cardiac pacemakers and defibrillators which have sensing features, and may result in
sensing problems or inappropriate responses. The effect of other implanted devices, including deep brain
stimulators, peripheral nerve stimulators, implanted drug delivery pumps, and cochlear implants on the Senza
and Senza II systems are unknown.
Sleep – Patients using therapy that generates paresthesia (tingling sensations caused by stimulation) may
choose to turn stimulation off to avoid uncomfortable sensations during sleep. Therapy at 10 kHz does not
generate paresthesia and therefore stimulation can remain on during sleep.
Operation of Vehicles (e.g., driving) or Machinery – Patients using therapy that generates paresthesia should
not operate motorized vehicles such as automobiles or potentially dangerous machinery and equipment with
the stimulation on. Stimulation must be turned off first in such cases. For these patients, any sudden
stimulation changes may distract patients from proper operation of the vehicle, machinery, or equipment.
Therapy at 10 kHz does not generate paresthesia and it is less likely that sudden stimulation changes resulting
in distraction could occur while having stimulation on when operating moving vehicles, machinery, and
equipment.
Heat From Charging – The charging coil may become warm while charging. Patients may experience
discomfort or burn if they charge while sleeping or do not use the provided charging belt. Additionally, the
charger should not be placed over insensate skin.

10186-ENG Rev P 9
Diathermy Therapy – Do not use shortwave diathermy, microwave diathermy or therapeutic ultrasound
diathermy on patients implanted with a neuromodulation system. Energy from diathermy can be transferred
through the implanted system and can cause tissue damage at the location of the implanted electrodes,
resulting in severe injury or death. The neuromodulation system, whether it is turned on or off, may be
damaged.
Computed Tomography (CT) – Before beginning a CT scan, the operator should use CT scout views to
determine if implanted or externally worn electronic medical devices are present and if so, their location
relative to the programmed scan range.
For CT procedures in which the medical device is in or immediately adjacent to the programmed scan range,
the operator should:
• Determine the device type;
• If practical, try to move external devices out of the scan range;
• Ask patients with neurostimulators to shut off the device temporarily while the scan is performed;
• Minimize x-ray exposure to the implanted or externally worn electronic medical device by:
o Using the lowest possible x-ray tube current consistent with obtaining the required image
quality; and
o Making sure that the x-ray beam does not dwell over the device for more than a few seconds;
Important note: For CT procedures that require scanning over the medical device continuously for more than a
few seconds, as with CT perfusion or interventional exams, attending staff should be ready to take emergency
measures to treat adverse reactions if they occur.
After CT scanning directly over the implanted or externally worn electronic medical device:
• Have the patient turn the device back on if it had been turned off prior to scanning.
• Have the patient check the device for proper functioning, even if the device was turned off.
• Advise patients to contact their healthcare provider as soon as possible if they suspect their device
is not functioning properly after a CT scan.
Magnetic Resonance Imaging (MRI) – The Senza and Senza II systems are MR Conditional which means that
safety has been demonstrated only within specifically defined conditions. Scanning under different conditions
may result in severe patient injury or device malfunction. Refer to the Senza System 1.5T and 3T MRI
Guidelines on Nevro’s website (www.nevro.com/physicianmanuals) for MRI-specific warnings and precautions
on conducting a MRI scan on a patient with the Senza or Senza II systems.
Devices in Hospital/Medical Environments – The use of following medical devices or procedures may damage
the SCS system or turn the stimulation off. After usage of these devices or procedures, the IPG may need to be
explanted as a result of permanent damage.
• Electrocautery: The IPG should not be exposed to electrocautery. If electrocautery is necessary with the
IPG implanted, use bipolar electrocautery. Do not use monopolar electrocautery.
• External defibrillation: The safety of discharge of an external defibrillator on patients implanted with an
SCS system has not been established.
• Lithotripsy or high-output ultrasonics: Do not use these devices in patients with an implanted IPG.
• Radiation therapy: If radiation therapy is needed near the IPG, shield the area over the IPG.
• Ultrasonic scanning: Do not use it over the IPG.

10186-ENG Rev P 10
If a patient is required to undergo lithotripsy, high-output ultrasound, electrocautery, external defibrillation,
radiation therapy, or ultrasonic scanning, follow these precautions.
• Turn off the IPG before the procedure.
• Use the equipment as far away from the IPG as possible.
• Keep fields, such as current, radiation, or high-output ultrasonic beams, away from the IPG.
• Equipment should be set to the lowest energy setting possible.
• After the therapy or procedure, check to see that the IPG is functioning properly by gradually increasing
the IPG’s stimulation to the desired level.
• If the patient suspects that the device is not functioning properly after the use of these therapies or
procedures, advise the patient to contact his or her healthcare provider.
Electromagnetic Interference (EMI) – Electromagnetic energy is generated by equipment found in the home,
work, medical or public environments. Electromagnetic interference may occur when the energy is strong
enough to interfere with neurostimulator function. Most electrical devices and magnets that patients will
encounter in a normal day are unlikely to affect the operation of the SCS system. However, some equipment
may generate strong electromagnetic fields that can turn the stimulator (IPG or TSM) off or cause shocks or
jolts (see below). Patients should keep away from areas of EMI and turn off the stimulator if they are in such
an area. The following are examples of sources that can potentially generate strong EMI.
• Theft detectors or security screeners such as airport security screening devices, retail store, and
libraries.
• Note: It is recommended that patients request assistance to bypass the theft detector or security
screener. If they must go through a screening device, the patient should turn off the stimulator and turn
it back on once they have passed through the security screener.
• Power lines and power generators
• Arc welders
• Large, magnetized stereo speakers
• Radio frequency identification devices (RFID)
Strong electromagnetic interference can result in the following:
• Serious patient injury, resulting from heating of the implanted components of the neurostimulation
system and damage to surrounding tissue.
• System damage, resulting in a loss of or change in symptom control and requiring surgical
replacement.
• Operational changes to the neurostimulator, causing it to turn on or off
• Unexpected changes in stimulation, causing a momentary increase in stimulation or intermittent
stimulation, which some patients have described as a jolting or shocking sensation. Although the
unexpected change in stimulation may feel uncomfortable, it does not damage the device or injure the
patient directly. In rare cases, as a result of the unexpected change in stimulation, patients have fallen
down and been injured.
Strong electromagnetic fields arising from closeness to electrical equipment such as mobile phones, satellite
phones and radio systems may interfere with the radio communication between the Remote Control and IPG.
Communication failure is indicated by three beeps. Communication can be restored by moving away from the
interfering electrical equipment and retrying the operation.
 Loading...
Loading...