Nevro WAND1000, EXTS1000, IPG1000, PTRD1000, IPG1500 Users Manual

Patient Manual
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 1
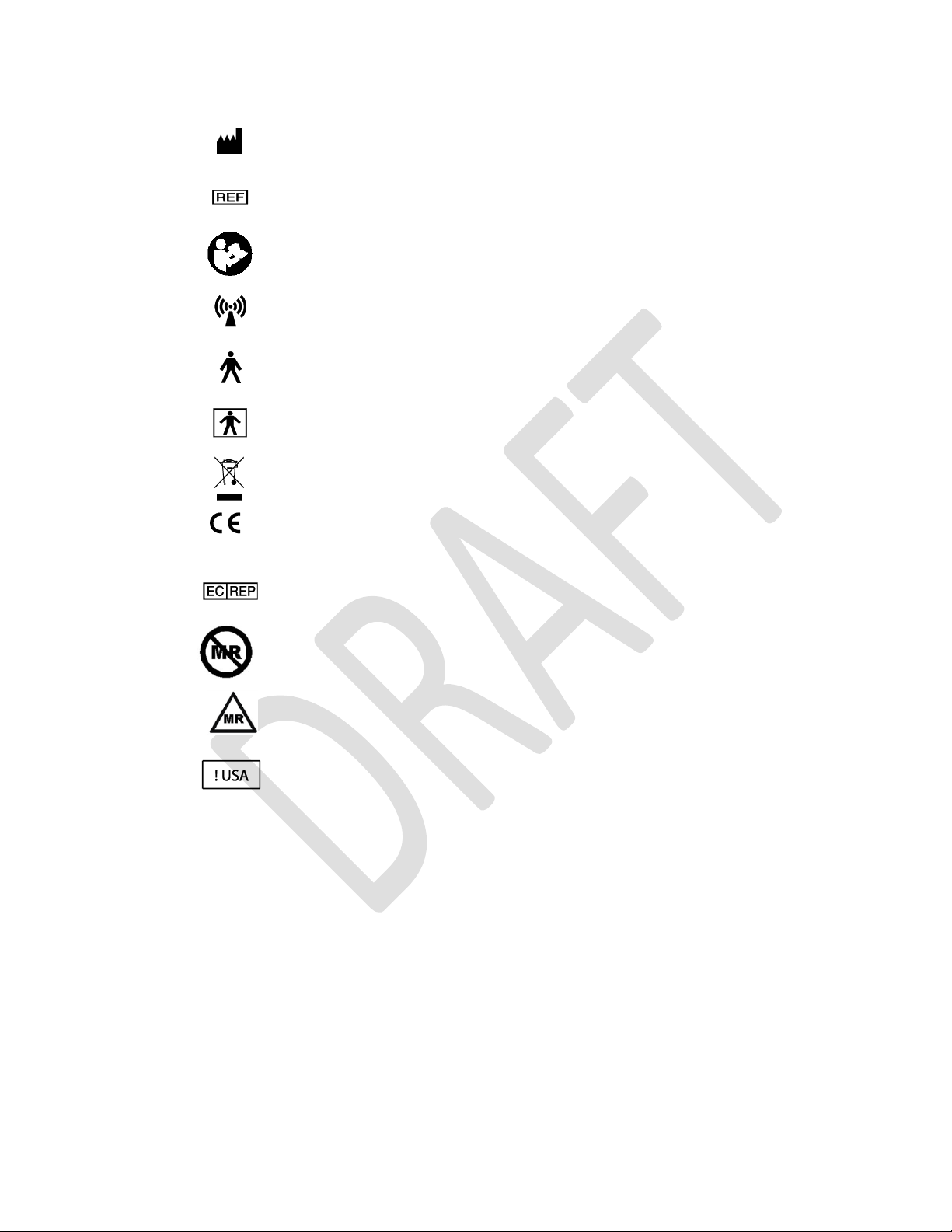
Symbols
Description
Manufacturer
Catalog number
Consult Instructions for Use
Non-ionizing radiation
Type B Applied Part
Type BF Applied Part
Do not dispose of this product in the unsorted municipal
waste stream. Dispose of this product according to local
regulations.
CE Marking of Conformity
Authorized representative in the European Community
MR unsafe
MR Unsafe
Conditional
MR Conditional
For USA audiences only
0086
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 2

NEVRO CORP.
All questions or concerns about Nevro products should be forwarded to:
Nevro Corp.
4040 Campbell Avenue, Suite 210
Menlo Park, CA 94025
USA
Tel: +1.650.251.0005
Fax: +1.650.251.9415
info@nevro.com
© Copyright 2015, Nevro Corp. All rights reserved.
No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system or
translated into any language or computer language, in any form or by any means, including, but not
limited to, electronic, magnetic, optical, chemical, manual, or otherwise without written permission of
Nevro Corp.
Registered Trademarks: Senza, HF10, Nevro, and the Nevro logo are trademarks of Nevro Corp.
CE Mark effective on 4 May 2010
Nevro hereby declares that the Senza® system is in compliance with the essential requirements and other
relevant provisions of the R&TTE Directive (1999/5/EC).
IMPORTANT: Do not change or modify any component of the Nevro® Senza system, unless expressly
approved by Nevro Corp.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 3

Table of Contents
INTRODUCTION ............................................................................................................................................. 5
ABOUT CHRONIC PAIN................................................................................................................................... 5
YOUR SENZA SYSTEM .................................................................................................................................... 5
INDICATIONS .................................................................................................................................................. 7
WARNINGS .................................................................................................................................................... 7
WARNINGS ABOUT OTHER MEDICAL TREATMENTS ..................................................................................... 9
PRECAUTIONS .............................................................................................................................................. 12
ADVERSE EVENTS ......................................................................................................................................... 14
IMPLANTATION SURGERY ............................................................................................................................ 15
TRIAL PHASE ................................................................................................................................................ 15
How to Set Up Your Trial Stimulator, Cables, and Remote Control ........................................................................ 16
The Remote Control ................................................................................................................................................ 19
YOUR PATIENT ID CARD ............................................................................................................................... 23
IMPLANTED STIMULATOR PHASE ................................................................................................................ 23
Battery Status ......................................................................................................................................................... 23
How to Charge the IPG ........................................................................................................................................... 24
How to Charge the Charger .................................................................................................................................... 26
Optimizing Charging ............................................................................................................................................... 26
Charging Tips .......................................................................................................................................................... 27
ASK YOUR DOCTOR ...................................................................................................................................... 27
TROUBLESHOOTING .................................................................................................................................... 28
Troubleshooting Therapy........................................................................................................................................ 28
Troubleshooting the Remote Control ..................................................................................................................... 28
Troubleshooting the Trial Stimulator...................................................................................................................... 29
Troubleshooting the Recharging Process ............................................................................................................... 29
All of the Lights are Blinking! .................................................................................................................................. 30
DEVICE DISPOSAL ......................................................................................................................................... 30
Device Specifications .............................................................................................................................................. 31
System Specifications ............................................................................................................................................. 31
System Components ............................................................................................................................................... 35
RC2000 Remote Control Instructions ..................................................................................................................... 38
INDEX ........................................................................................................................................................... 40
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 4

INTRODUCTION
This booklet was written for people who are considering or have received a Nevro® Senza® Spinal Cord
Stimulator (SCS) system to help treat pain. Every person is unique and your medical needs differ from
those of others, even people with the same condition and the same SCS system. For this reason, always
talk to your doctor if you have questions about your condition. This booklet presents general information
and can help you better communicate with your doctor.
The first part of this booklet discusses chronic pain, spinal cord stimulation, and the Senza system. It is
based on common questions that patients have about their condition, this particular treatment option,
and the Senza system.
The second part of this booklet explains how to use the devices.
In the back of the booklet, we have added some information in the appendices. The first appendix
contains technical information about this product. This information may be useful to you, but it is not
necessary for you to understand it in order to use your device. The second appendix shows pictures of
the parts of the Senza system.
Throughout the booklet, we have provided definition of medical or electronic terms in a shaded box with
a definition.
Finally, the back of the booklet contains an index to help you look up specific information if you ever
need it.
STIMULATION. Small electrical pulses produced by the SCS system delivered to your spinal cord to
provide therapy for your pain. Spinal cord stimulation is sometimes called “therapy delivery.”
ABOUT CHRONIC PAIN
Everybody feels pain when there is a painful external stimulus such as a pinprick or touching something
hot. This is referred to as acute pain and is an important normal sensation that helps protect against
injury. Chronic pain is very different. People with chronic pain may also feel pain when there is no
obvious reason or may have pain that does not go away long after an injury.
CHRONIC. Something that persists or lasts for more than 3 months.. Chronic pain is pain that does not go
away with the passage of time or as the body heals from an injury.
Chronic pain can be intractable, which is the medical term meaning that it is hard to treat. You have
probably tried many treatments to control your pain and found that they did not work well or perhaps
they did not work at all.
INTRACTABLE. Any condition, such as chronic pain, which is very difficult to control or treat effectively.
YOUR SENZA SYSTEM
The Senza system works by delivering electrical energy from a device to an area around your spine. The
system is capable of delivering the HF10™ therapy, a therapy that does not produce tingling sensations
called paresthesia. It is also capable of providing stimulation that produces paresthesia at some therapy
settings. You will first go through a trial phase where you and your doctor evaluate the therapy to see if it
is right for you. The Senza system trial phase consists of several components:
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 5

Trial Stimulator is a temporary device that you use outside the body to test to see if the therapy
is helpful to you.
Lead is a thin insulated wire that connects to the Trial Stimulator at one end and with small
electrodes on the other end placed near your spine. A small amount of electrical energy from the
device travels through the lead and near the spine.
OR cable is an insulated wire used outside the body to temporarily connect the leads to the Trial
Stimulator.
Remote Control is a unit that can turn the stimulator ON or OFF and allows for some adjustments
of therapy settings.
PARESTHESIA. A sensation of tingling, “pins and needles,” prickling, or even burning. Paresthesia may be
brief or it may last a long time.
TRIAL PHASE. A time during which a person with chronic pain tests SCS therapy to see if and how well it
works. During the trial phase, the person will temporarily use a Trial Stimulator, which is not implanted in
the body.
If the Senza system is right for you, your doctor will discuss with you the implantation of a batterypowered device after the trial phase. The Senza system components will include:
Implantable pulse generator (IPG) is a small, battery-powered electronic device that is implanted
inside the body (see IPG in the diagram above).
Lead, instead of connecting to an external stimulator as occurred during the trial phase, will
connect to the implanted IPG. After implantation there are no external wires or connections as
occurred during the trial phase (see lead wires in diagram above).
Charger recharges the IPG after it is implanted.
Power Adaptor recharges the Charger.
Charger Belt and Charger Holster holds the Charger during recharging.
Remote Control can turn the IPG ON or OFF and allows for some adjustments of therapy settings.
For pictures of the Charger, Power Adaptor, Charger Belt and Remote Control, please see the “System Components” section at the
end of this manual.
IMPLANTABLE PULSE GENERATOR or IPG. A self-contained battery-powered device that is small enough
to fit in a person’s hand and delivers small amounts of electrical energy or pulses.
You will first use the Trial Stimulator and Remote Control. If the Senza system is right for you, your doctor
will then implant the IPG. You control the implanted device with the same Remote Control.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 6

INDICATIONS
The Senza system is not right for everyone. Indications for the use of this device are as follows:
The Senza™ neuromodulation system is indicated as an aid in the management of chronic intractable
pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following: failed
back surgery syndrome, intractable low back pain, and leg pain.
INDICATIONS. Reasons that you should get a device, drug, or treatment. Indications are determined by
medical experts, clinical studies, and the Food & Drug Administration (FDA).
CONTRAINDICATIONS
CONTRAINDICATIONS. Situations in which the device should not be used because the risk of use clearly
outweighs and possible benefit. Contraindications are determined by medical experts, clinical studies,
and the Food & Drug Administration (FDA).
The Senza system is contraindicated (not appropriate) for certain patients. Contraindications for the
Senza system include:
Not being able to operate the Nevro SCS system
Not being able to have the SCS surgery
Failing to receive effective pain relief during trial stimulation.
Your doctor can tell you if the Senza system might be appropriate for you. If you have questions about
whether the Senza system may be right for you, ask your doctor.
WARNINGS
Warnings are statements about safety of your device that you should take very seriously. If you do not
follow these warnings, it is possible that you could be hurt and/or the device could be damaged. The
following are some warnings for the Senza system:
Stimulation Frequencies - Stimulation frequencies in the range of 2 Hz to 1,200 Hz are indicated for
paresthesia-based therapy and the system must be configured to produce paresthesia. Stimulation at
10,000 Hz is indicated as paresthesia-free therapy and the system must be configured to deliver
paresthesia-free stimulation. Stimulation between 1,200 Hz and 10,000 Hz has not been evaluated for
safety, effectiveness and perception of paresthesia. It is unknown whether stimulation amplitude settings
at frequencies not studied (i.e., between 1200 Hz and 10,000 Hz) can produce injury at stimulation
output levels that produce paresthesia.
Stimulation at vertebral levels above T8 – Safety of Nevro SCS system at >2kHz to10 kHz program
settings above the T8 vertebral level has not been studied.
Pediatric Use - The safety and effectiveness of spinal cord stimulation has not been established for use in
children.
Other Active Implanted Devices – Please let your doctor know if you have any other active implanted
devices in your body. The Senza system may interfere with other implanted stimulators, such as cardiac
pacemakers and defibrillators which have sensing features, and may result in sensing problems or
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 7

inappropriate responses. The effect of other implanted devices, including deep brain stimulators,
peripheral nerve stimulators, implanted drug delivery pumps, and cochlear implants on the Senza system
are unknown.
Sleep – If you are using therapy that generates paresthesia (tingling sensations caused by stimulation)
you may choose to turn stimulation off to avoid uncomfortable sensations during sleep (see Warning
regarding Stimulation Frequency). If you are using therapy at 10 kHz which does not generate
paresthesia, stimulation can remain on during sleep.
Operation of Vehicles (e.g., driving) or Machinery - If you are using therapy that generates paresthesia
you should not operate motorized vehicles such as automobiles or potentially dangerous machinery and
equipment with the stimulation on when using paresthesia-causing programs. Stimulation must be
turned off first in such cases. Any sudden stimulation changes may distract you from proper operation of
the vehicle, machinery, or equipment. If you are using therapy at 10 kHz which does not generate
paresthesia, it is less likely that sudden stimulation changes resulting in distraction could occur.
Heat from Charging - You will have to recharge the battery in your device. Always use the special Charger
Belt when recharging. During recharging, the Charging Coil may become warm or even burn you. If you
feel warmth or discomfort when recharging the device, stop recharging and contact your doctor. Do not
place the charger over an area of skin where you do not feel any sensation.
Electromagnetic Interference (EMI) - Ordinary household appliances, magnets, and devices encountered
in everyday life will not affect your implanted Senza device. However, some equipment generates
electromagnetic interference (EMI) which may affect your Senza system.
ELECTROMAGNETIC INTERFERENCE (EMI). Invisible signals generated by some equipment, appliances,
and devices, also known as noise or static. Even if you cannot hear this noise, it may be picked up by your
implanted SCS system and can affect it.
Electromagnetic interference (EMI) can affect the implanted Senza system in ways that are hard to
predict. For example, EMI might:
Turn your Senza system ON or OFF
Cause your Senza system to give you more stimulation (a “shock”)
Cause damage to the system that may result in loss of therapy and require reoperation to replace
the system
There are many sources of EMI today so it is not possible to give exact instructions as to how to avoid
them. Listed below are some well-known sources of EMI that you should avoid:
Power lines and power generators
Arc welders
Large, magnetized stereo speakers
Radiofrequency identification devices (RFID)
Exposure to strong EMI can result in serious patient injury or death, resulting from heating of the
implanted components of the SCS system and damage to the surrounding tissue.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 8

Theft Detectors and Security Screening Devices - Security checkpoints, metal detectors, screening
systems at airports, and theft detectors all produce EMI. If you must pass through such a system, tell the
personnel that you have an implanted medical device and show them your patient ID card. They may be
able to help you get through the checkpoint without going through the scanner. If that is not possible,
you may be able to pass through the scanner or detector by turning the device OFF and moving through
the scanner as quickly as possible.
Theft detection systems may also produce EMI. While some theft detection systems are obvious and are
located at store exits, others may be concealed within the store. If you are in a store or other
environment and suspect EMI is affecting your device, turn OFF the Senza system and move out of the
area. Once you are out of the area, check whether therapy is ON or OFF. You may have to recharge the
device. If you have specific questions about EMI sources, talk to your doctor.
Strong electromagnetic fields arising from closeness to electrical equipment such as mobile phones,
satellite phones and radio systems may interfere with the radio communication between the Remote
Control and IPG. As described in the “Troubleshooting” section of this manual, communication failure is
indicated by three beeps. Communication can be restored by moving away from the interfering electrical
equipment and retrying the operation.
Electrostatic Discharge (ESD) is a common source of electromagnetic interference that can occur when a
person or object accumulates a static charge. ESD is made worse by low humidity and synthetic
materials.
If the battery terminals of the Trial Simulator are exposed to ESD, the device may reset and stop
stimulation. Stimulation can be restarted by following the instructions in the “How to Turn ON
Stimulation” section of this manual. To avoid unintentionally stopping stimulation, do not open
the battery compartment while stimulation is ongoing.
ESD may cause the Charger to stop charging the IPG. If this happens, charging can be resumed by
repeating the steps in the “How to Charge the IPG” section of this manual. ESD events can be
minimized by keeping the charger in the Charger Holster while recharging the IPG.
WARNINGS ABOUT OTHER MEDICAL TREATMENTS
Always tell your doctors, nurses, and other clinicians (including dentists, physical therapists, occupational
therapists, and others) that you have the Senza Spinal Cord Stimulation system implanted in your body.
There are some procedures that are not recommended for people with the Senza system, and there are
other procedures which may be possible for you only with certain precautions. If you ever need any of
these treatments, be sure to discuss them with your pain doctor as well as with the clinical team doing
the procedure.
Procedures that are not recommended for you with an implanted SCS system include:
Diathermy
Computed tomography (CT scans)
Magnetic resonance imaging (MRI scans)
Lithotripsy
External defibrillation
Ultrasound procedures
Radiation
Radio-Frequency or Microwave Ablation
DIATHERMY. A medical treatment in which heat energy from shortwaves, microwaves, or ultrasounds
are used as treatment or in surgery.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 9

Diathermy Therapy - Energy from the diathermy device can be transferred to the SCS system and can
cause the lead to overheat, which may cause damage to the device, heating and damage to the body,
severe injury, and even death.
CT SCAN. A type of technology in which x-ray-like images are taken in sections (slices) and then reassembled by computer to provide detailed two- and three-dimensional pictures of inside the body.
Computed Tomography (CT) – Please inform your doctor and medical personnel conducting your CT scan
that you have an implanted SCS system. You must turn your device off temporarily while the scan is
being conducted. It is important that the person conducting your CT scan does the following:
• Determines the device type;
• If practical, tries to move external devices out of the scan range;
• Minimizes x-ray exposure to the implanted or externally worn electronic medical device by:
o Using the lowest possible x-ray tube current consistent with obtaining the required
image quality; and
o Making sure that the x-ray beam does not dwell over the device for more than a few
seconds;
Important note: For CT procedures that require scanning over the medical device continuously for more
than a few seconds, as with CT perfusion or interventional exams, attending staff should be ready to take
emergency measures to treat adverse reactions if they occur.
After CT scanning directly over the implanted or externally worn electronic medical device:
• You should turn your Senza system device back on.
• Check that the Senza system is working properly.
• Contact your doctor as soon as possible if you suspect the Senza system is not functioning
properly after a CT scan.
MRI SCAN. A type of technology in which electromagnetic energy is used to take images of soft tissue in
the body.
Magnetic Resonance Imaging (MRI) - The Senza system is MR Conditional which means that safety has
been demonstrated only within specifically defined conditions. Scanning under different conditions may
result in severe injury, death or device malfunction. If your doctor determines it is safe for you to have an
MRI, your doctor will have you turn off the device before the scan and turn it back on when the scan is
complete. This should be done with extreme caution, since these scans may injure you or damage the
device. Never undergo an MRI scan without making sure the team doing the scan knows you have an SCS
device.
If your doctor recommends an MRI, inform the doctor that you have the Senza Spinal Cord Stimulation
system and show him/her your patient ID card (refer to Your Patient ID section in this manual). The
doctor will need to take specific precautions (as identified in the 1.5T and 3T MRI Guidelines) to prevent
patient injury and device damage. Do not take the trial stimulator, patient remote, or charger into the
MRI scan room. They are not considered safe for MRI and may be rapidly pulled into the MRI scanner. In
doing so, they may strike and injure a person.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 10

Before undergoing these or any other procedures, discuss them with your doctor to be sure they are safe
for you.
LITHOTRIPSY. The use of sound waves to help break up calcified stones in the body.
EXTERNAL DEFIBRILLATION. The emergency use of two large paddles placed on the chest to deliver a
large amount of electrical energy to “re-start” the heart.
ULTRASOUND PROCEDURES. Any number of procedures that use sound waves to get images of the soft
tissue in the body.
RADIATION. The use of radiation energy for therapy. There are many types of radiation treatments.
Radiation can be as simple as an x-ray of the body or it can be targeted therapy to kill cancer cells (radio
therapy).
Lithotripsy, External Defibrillation, Ultrasound Procedures and Radiation - If you are required to
undergo lithotripsy, external defibrillation, high-output ultrasound, radiation therapy, or ultrasonic
scanning, inform the medical personnel conducting the procedure that you have an implanted SCS
system and follow these precautions:
• Turn off the IPG before the procedure.
• Have medical personnel use the equipment as far away from the IPG as possible.
• Have medical personnel keep fields, such as current, radiation, or high-output ultrasonic
beams, away from the IPG.
• Equipment should be set to the lowest energy setting possible.
• After the therapy or procedure, check to see that the IPG is functioning properly by
gradually increasing the IPG’s stimulation to the desired level.
• If you suspect that the device is not functioning properly after the use of these therapies
or procedures, please contact your doctor.
Radio-Frequency or Microwave Ablation - An electrical current produced by a radio/micro wave is used
to heat up a small area of nerve tissue, thereby decreasing pain signals from that specific area.
Radio-Frequency or Microwave Ablation - Safety has not been established for Radio-Frequency or
Microwave Ablation in people who have an implanted neurostimulation system. Induced electrical
currents may cause heating, especially at the lead electrode site, resulting in tissue damage.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 11

PRECAUTIONS
Precautions are instructions about your device you should follow to avoid damage to the device, so that
it will function correctly and last longer.
Store the Trial Stimulator and Remote Control at normal temperatures in the range of -4 to 140°
F (-20 to 60°C).
Keep the Trial Stimulator and Remote Control dry.
Do not drop the Trial Stimulator and Remote Control. Although these devices are built for
constant use, they could break if dropped onto a hard surface.
Do not plug your Charger into a power source near water.
Use only Nevro or Nevro-approved accessories with your Senza system.
Caring for the Trial Stimulator, Remote Control, and Charging System - You can care for your Trial
Stimulator, Remote Control, and Charger by cleaning them with a soft, damp (not wet) cloth and mild
detergent. If you prefer, you can also clean these accessories with isopropyl alcohol, available at a drug or
department store. Do not use any harsh or abrasive cleansers and never let moisture get inside these
items.
Pregnancy and Nursing - This device is not to be used in pregnant/nursing women, or women who may
become pregnant.
Patient Activities - Some therapy settings are known to cause tingling sensations (called “paresthesias”).
With such settings, you may feel a sudden increase in these sensations when you change your posture or
make large or sudden movements. You can lower the amplitude or turn off the stimulation before
making posture changes. If you are using stimulation at 10 kHz which does not generate paresthesia,
these postural changes should not affect you.
Patient Activities Related to Lead Movement - Do not make sudden and excessive bending, stretching,
or twisting movements, particularly within the first weeks after the surgery. An implanted lead can move
from its original location during such movements, which might affect delivery of therapy. In such cases,
your system may need to be reprogrammed or the lead may need to be repositioned through another
operation.
Scuba Diving and Hyperbaric Chambers – Your Senza system is sensitive to high pressure. To prevent
possible damage to the device, prior to beginning these activities turn OFF the Senza system and:
Do not scuba dive to depths greater than 115 feet (35 meters)
Do not enter a hyperbaric chamber with pressure above 4.5 atmospheres
HYPERBARIC CHAMBER. A special chamber or compartment in which 100% oxygen is delivered to a
person under very high pressures, far above the normal atmospheric pressure. Hyperbaric therapy is
used for some medical treatments, such as wound healing.
Transcranial Magnetic Stimulation (TMS) and Electroconvulsive Therapy (ECT) - Safety has not been
established for TMS or ECT in people who have an implanted neurostimulation system. Induced electrical
currents may cause heating, especially at the lead electrode site, resulting in tissue damage.
TMS. A non-invasive way that uses magnetic fields to stimulate nerve cells in the brain.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 12

ECT. A procedure in which electric currents are passed through the brain to intentionally cause a seizure.
Transcutaneous Electrical Nerve Stimulation - Do not place transcutaneous electrical nerve stimulation
(TENS) electrodes so that the TENS current passes over any part of the neurostimulation system. If you
feel that the TENS may be interfering with the implanted neurostimulator, discontinue using the TENS
and consult with your doctor.
TENS. A TENS unit is a device that sends small electrical currents to targeted body parts. These currents
are used to relieve pain.
Post-Operative Pain - In the days after the surgery, you may experience pain in the implant area, which is
typical in SCS surgeries.
IPG Location and Patient Manipulation - Do not to twist or rotate the IPG. If the IPG flips over in the
body, the charger may not be able to charge the IPG. Manipulation of the IPG in your body may cause the
skin over the IPG to become thinner over time.
Infection - If you experience persistent discomfort or excessive redness around the wound areas, please
advise your physician. You may need to be checked for infection. Infections related to the SCS may
require the implanted components to be explanted. Do not use the charger if the incision is not
sufficiently healed. The charger and the charging belt are not sterile and should not be in contact with the
incision.
Cell Phones - The impact of cell phones on the neuromodulation system is unknown at this time.
IPG Failure - If your IPG does not provide stimulation even after complete charging of the IPG or
replacement of the batteries in the Patient Remote Control, turn off the IPG and contact your physician.
When frequency of recharging becomes too inconvenient for you, the IPG may need to be replaced. You
should contact your physician if this occurs.
Device Disposal - Do not dispose the IPG, Patient Remote Control or Charger in fire. The battery in these
devices can explode in fire. The IPG should be explanted in the case of cremation. All explanted IPGs
should be returned to Nevro Corp. Do not dispose of electrical components, including batteries, in the
unsorted municipal waste stream. Dispose of electrical components, including batteries, according to
local regulations.
Long-Term Effectiveness of Spinal Cord Stimulation - The long-term effectiveness of spinal cord
stimulation has been documented. Not all patients realize long-term benefits from spinal cord
stimulation. Stimulation effectiveness at 10 kHz has been established for one year.
Patient Manual 11052 Rev A (2015-01-15) [DRAFT] 13
 Loading...
Loading...