Page 1

POOLPRO
Operation
Manual
™
MODEL PS6FC
E
17 June 2013
Page 2

i
PLEASE NOTE:
Because of our commitment to product improvement, the substance
and style of this manual may change. When changes are made,
the updated manual is posted for download in PDF format from the
Myron L Website: www.myronl.com
Page 3
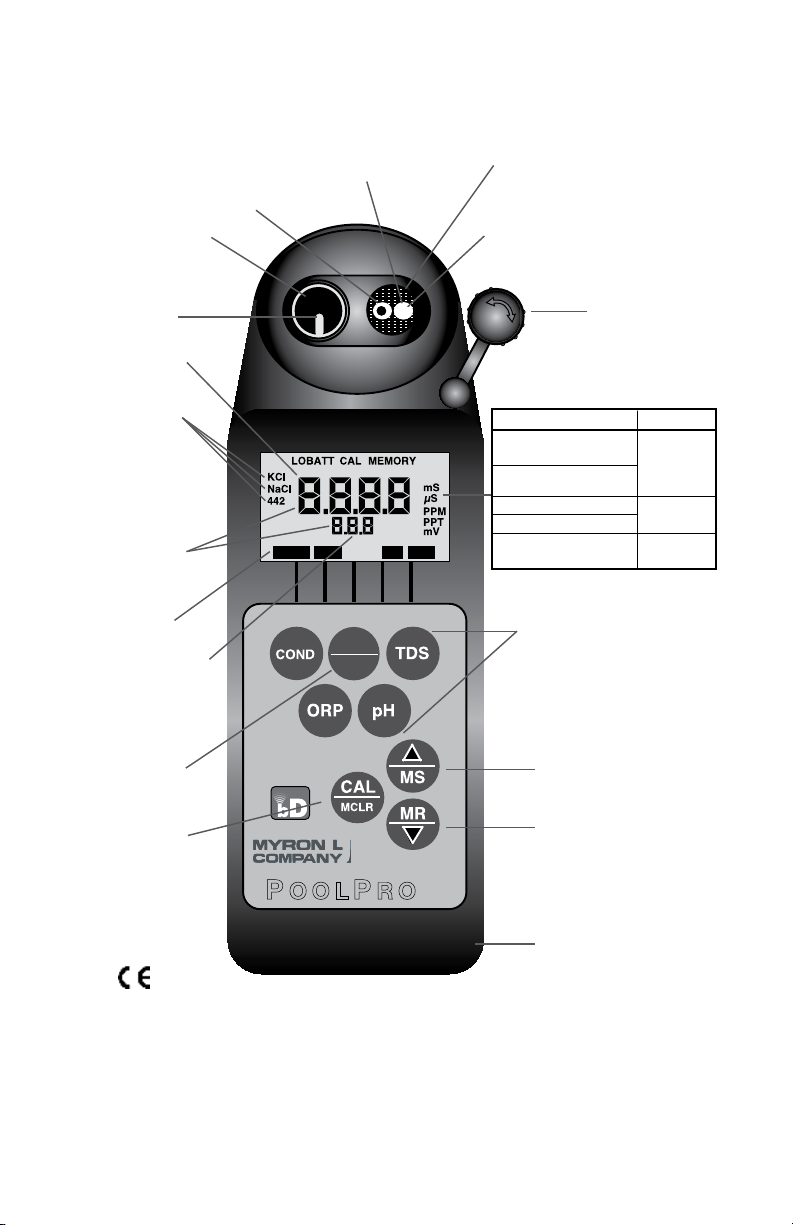
BUFFER
°C°F
ORP TDSpHCOND
PS6FC
E
MIN
SALT
bluDock Enabled
Reference
Junction under
Glass pH Bulb
These Measurement keys will:
• Turn instrument on
• Measure parameter
• Exit any function
(Built-in
Electrodes)
Preprogrammed
variable
conductivity/
TDS ratios
Parameters
Wrist/neck strap slot
(strap user supplied)
pH/ORP Sensor
Protective Cap
This key for:
• Calibration
• Memory Clear
• Solution Selection
• Confirmation
Up key/Memory Store
Down key/Memory Recall
Conductivity Cell
Displayed here:
• Temperature
readout
• Memory Storage/
Recall
• pH Calibration
ORP
Electrode
pH Glass
Electrode
pH/ORP Sensor
(Replaceable)
Instrument
Illustration
Temperature
Sensor
Date & Time
displayed here
Measurement
P OOLP RO
™
Units Of Measurement
Parameter
mS - millisiemens/cm
(millimhos/cm)
µS - microsiemens/cm
(micromhos/cm)
PPM - parts per million
PPM - parts per million
PPT - parts per thousand
mV - millivolts
Conductivity
MIN/SALT
TDS
ORP
Mineral/Salt
TDS of NaCl
24 January 2012
Free Chlorine
For detailed explanations see Table of Contents
MODEL PS6FCE
Shown with bluDock™ option installed
i
Page 4

ii
1
Page 5

I. INTRODUCTION
Thank you for selecting the feature-packed Po o l Pr o ™, one of the
Myron L Company’s latest in an increasing line of instruments utilizing
advanced microprocessor-based circuitry and SMT manufacturing
processes. This circuitry makes the instrument extremely accurate,
reliable and very easy to use.
The Po o l Pr o now includes Myron L Company’s exclusive Free
Chlorine Equivalent (FCE) feature for making ORP-based free chlorine
measurements, as well as
bluDock™ option. You can also measure conductivity, Mineral/SALT
(Sodium Chloride/NaCl), Total Dissolved Solids (TDS), pH, ORP/Redox
and Temperature, all with one simple-to-use instrument. Additional
features include a clock with time and date, a memory of up to 100
locations with time and date stamp, the ability of the user to adjust the
timeout “Auto OFF”, and enhanced performance. See Features and
Specications on pages 2 & 3.
The most exciting new feature is data logging with the ability to download
the memory or stored test data wirelessly with its corresponding time, date
and instrument name. This feature allows the user to create spreadsheets
and graphs with ease, and quickly and accurately manipulate data more
effectively. The optional bluDock™ and accompanying U2CI software is
compatible with most computers using either Microsoft Windows XP™,
Vista™ or 7™, or Macintosh OSX™. The data may be imported into a
variety of spreadsheet formats like Microsoft Excel CSV™.
Please Note: Although the Myron L Company has performed extensive
testing, we cannot guarantee compatibility of all applications and formats.
We suggest testing your application and format for compatibility before
relying on it.
Bluetooth®
wireless data transfer with the
For your convenience, a brief set of instructions is provided on the
bottom side of your Po o l Pr o .
Special note.....Conductivity, Mineral/Salt, and TDS require mathematical
correction to 25°C values (ref. Temperature Compensation, pg. 37). On
the left of the Po o l Pr o ’s liquid crystal display is shown an indicator of the
salt solution characteristic used to model temperature compensation of
conductivity and its TDS conversion. The indicator may be KCl, NaCl, or
442™. Selection affects the temperature correction of conductivity, and
the calculation of TDS from compensated conductivity (ref. Conductivity
Conversion to Total Dissolved Solids (TDS), pg. 40). The selection can
affect the reported conductivity of hot or cold solutions, and will change
the reported TDS of a solution. Generally, using KCl for conductivity,
NaCl for Mineral/Salt, and 442 for TDS will reect present industry
practice for standardization. This is how your instrument, as shipped
from the factory, is set to operate.
1
Page 6

3
II. FEATURES and SPECIFICATIONS
A. Features
• ORP-based FCE free chlorine measurement, displayed as ppm
concentration
• Ranges:
Conductivity, Min/Salt, TDS — 0-200,000 µS/ppm
pH − 0-14
ORP − ±999 mV; 0.00-9.99 ppm free chlorine
• Superior resolution 4 digit LCD displays full 9999 µS/ppm
• Accuracy of BETTER than ±1% of reading in a handheld instrument
±0.1% at calibration point
• All sensors are internal for maximum protection
• Improved 4 electrode sensor technology
• Waterproof to 1 meter/3 feet
• Autoranging conductivity/TDS
• Factory calibrations stored in microprocessor
• Prompts for easy pH calibration
• 3 conductivity/TDS solution conversions preprogrammed into
microprocessor
• Real Time Clock with Time and Date
• Data Logging with TIME and DATE in memory
• Memory stores 100 readings
• User adjustable timeout “Auto OFF”
•
Bluetooth®
wireless download capability with optional bluDock™
B. General Specications
Display 4 Digit LCD
Dimensions (LxWxH) 196 x 68 x 64 mm/
7.7 x 2.7 x 2.5 in.
Weight 352 g/12.4 oz.
Case Material VALOX*
Cond/MIN/SALT/TDS Cell Material VALOX*
Cond/TDS Electrodes (4) 316 Stainless Steel
Cond/MIN/SALT/TDS Cell Capacity 5 ml/0.2 oz.
pH/ORP Sensor Well Capacity 1,2 ml/0.04 oz.
Power 9V Alkaline Battery
Battery Life >100 Hours/5000 Readings
Operating/Storage Temperature 0-55°C/32-132°F
Protection Ratings IP67/NEMA 6 (waterproof to
1 meter/3 feet)
EMI/EMC Ratings EN61326-1: 2006 + Annex A: 2008
(hand-held devices)
(Conformité Européenne)
CISPR 11: 2003
IEC 61000-4-2: 2001 and,
IEC 61000-4-3: 2002
* ™ SABIC Innovative Plastics IP BV
Additional information is available on our website:
www.myronl.com
MADE IN USA
2
Page 7
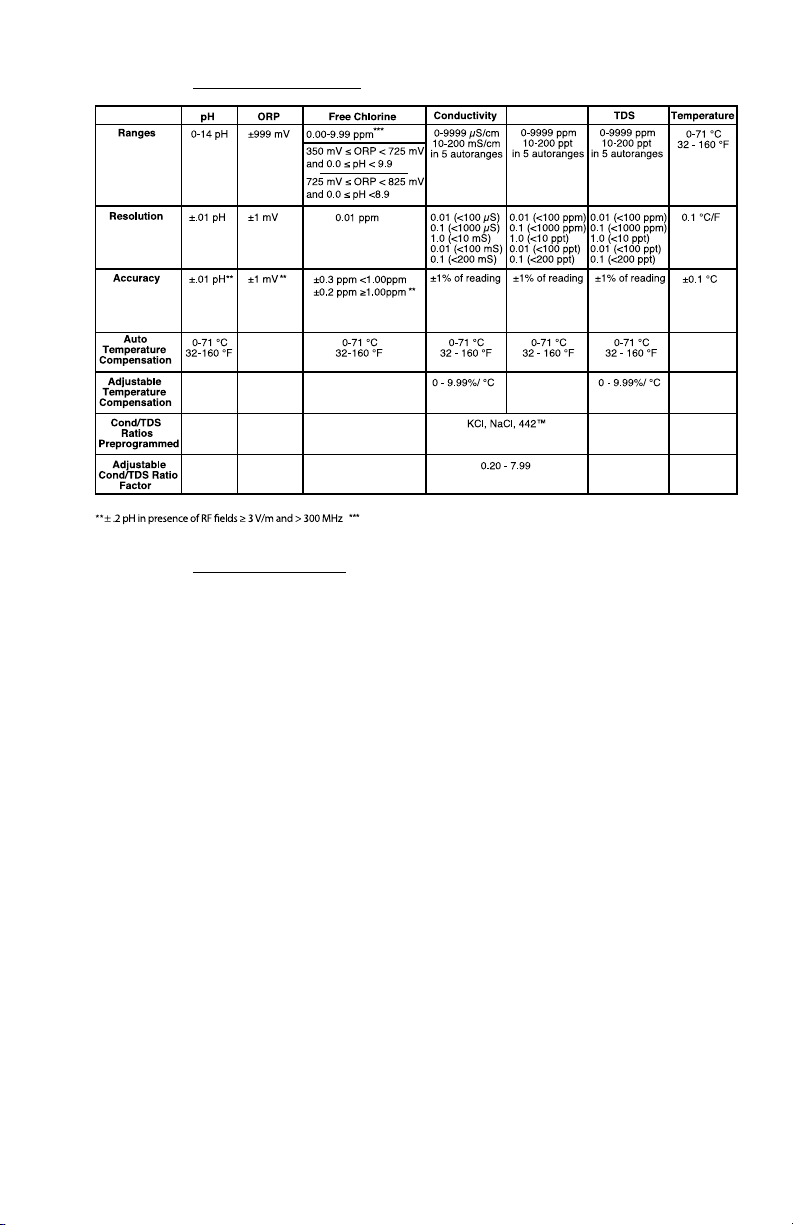
C. Specication Chart
If either ORP or pH is outside the specied limits, the instrument will display “-Or-”.
Mineral/Salt*
*
NaCl - Sodium Chloride
D. Warranty/Service
The Myron L Po o l Pr o ™, excluding the pH/ORP sensor, has a Two
(2) year limited warranty. The pH/ORP sensor has a six (6) month
limited warranty for materials and workmanship. If an instrument fails
to operate properly, see Troubleshooting Chart, pg. 34. The battery
and pH/ORP sensor are user-replaceable. For other service, return the
instrument prepaid to the Myron L Company.
MYRON L COMPANY
2450 Impala Drive
Carlsbad, CA 92010-7226 USA
+1-760-438-2021
E-Mail: info@myronl.com
techquestions@myronl.com
www.myronl.com
If, in the opinion of the factory, failure was due to materials or
workmanship, repair or replacement will be made without charge. A
reasonable service charge will be made for diagnosis or repairs due to
normal wear, abuse or tampering. This warranty is limited to the repair
or replacement of the Po o l Pr o only. The Myron L Company assumes
no other responsibility or liability.
3
Page 8

5
TABLE OF CONTENTS
Instrument Illustration .......................................i
I. INTRODUCTION ................................... 1
II. FEATURES and SPECIFICATIONS ....................2
A. Features ................................2
B. General Specications .....................2
C. Specication Chart ........................3
D. Warranty/Service..........................3
III. RULES of OPERATION.............................. 7
A. Operation ...............................7
B. Characteristics of the Keys ..................7
C. Operation of the Keys ......................7
1. Measurement Keys in General......... 7
2. COND, MIN/SALT & TDS Keys ........7
3. pH and ORP/Fr Chl Keys .............8
4. CAL/MCLR Key .................... 8
5. UP or DOWN Keys..................9
IV. AFTER USING the Po o l Pr o .......................... 9
A. Maintenance of the Conductivity Cell ..........9
B. Maintenance of the pH/ORP Sensor........... 9
V. SPECIFIC RECOMMENDED MEASURING
PROCEDURES .............................9
A. Measuring Conductivity, MIN/SALT & TDS ......9
B. Measuring pH ...........................10
C. Measuring ORP..........................10
1. ORP/FCE Mode Selection............. 10
2. Measuring ORP .................... 11
D. Measuring Free Chlorine Using FCE..........12
1. Prepare for FCE Measurement ......... 12
2. FC
E
Flow Method ................... 12
3. FCE Immersion Method............... 13
4. FCE Best Practices .................. 14
VI. SOLUTION SELECTION............................14
A. Why Solution Selection is Available ..........14
B. The 3 Solution Types .....................14
C. Calibration of Each Solution Type ............ 14
D. Procedure to Select a Solution .............. 15
VII. CALIBRATION.................................... 16
A. Calibration Intervals ......................16
B. Rules for Calibration of the Po o l Pr o .......... 16
1. Calibration Steps .................. 16
2. Calibration Limits ..................17
C. Calibration Procedures .................... 17
4
Page 9

1. Conductivity, MIN/SALT &TDS
Calibration....................17
2. Reloading Factory Calibration ........ 18
3. pH Calibration ....................18
4. ORP/Fr Chl Calibration .............21
5. Temperature Calibration............. 21
VIII. CALIBRATION INTERVALS .........................21
A. Suggested Intervals ......................21
B. Calibration Tracking Records ............... 21
C. Conductivity, MIN/SALT, TDS Practices .......22
D. pH and ORP Practices ....................22
IX. MEMORY........................................22
A. Memory Storage .........................23
B. Memory Recall ..........................23
C. Clearing a Record/Memory Clear ............ 23
X. TIME and DATE................................... 24
A. Setting TIME ............................24
B. Setting DATE............................25
C. Date Format (US & International) ............ 26
XI. TEMPERATURE FORMAT “Centigrade & Fahrenheit” ..... 26
XII. TOTAL RETURN to FACTORY SETTINGS..............27
XIII. CELL CHECK ....................................27
XIV. AUTO OFF ......................................28
XV. bluDock™ WIRELESS DATA TRANSFER INSTRUCTIONS ...29
A. Software Installation ......................29
B. Hardware Setup .........................30
C. Memory Stack Download ..................30
XVI. CARE and MAINTENANCE .........................31
A. Temperature Extremes ....................31
B. Battery Replacement...................... 31
C. pH/ORP Sensor Replacement ..............31
D. Cleaning Sensors ........................32
XVII. TROUBLESHOOTING .............................34
XVIII. ACCESSORIES...................................36
A. Conductivity/TDS Standard Solutions .........36
B. pH Buffer Solutions .......................36
C. pH Sensor Storage Solution ................ 36
D. ORP Sensor Conditioner Solution............36
E. Soft Protective Carry Cases ................37
F. Hard Protective Carry Cases ...............37
G. Replacement pH/ORP Sensor ..............37
H. bluDock™ Wireless Data Transfer
Accessory Package ................... 37
XIX. TEMPERATURE COMPENSATION (Tempco)
of Aqueous Solutions ........................37
5
Page 10

7
A. Standardized to 25°C ..................... 37
B. Tempco Variation.........................37
C. An Example............................. 38
D. A Chart of Comparative Error ...............39
E. Other Solutions ..........................39
XX. CONDUCTIVITY CONVERSION to
TOTAL DISSOLVED SOLIDS (TDS) ............40
A. How it’s Done ...........................40
B. Solution Characteristics ...................40
C. When does it make a lot of difference?........ 40
XXI. TEMPERATURE COMPENSATION (Tempco)
and TDS DERIVATION .......................41
XXII. pH and ORP .....................................42
A. pH ....................................42
B. ORP/Oxidation-Reduction Potential/REDOX ...44
C. Free Chlorine ...........................45
1. FCE as an Indicator of Sanitizing
Strength ..................... 45
2. FCE Free Chlorine Units ............. 45
XXIII. SOFTWARE VERSION .............................46
XXIV. GLOSSARY......................................47
6
Page 11
III. RULES of OPERATION
A. Operation
Using the instrument is simple:
• Individual or multiple parameter readings may be obtained by
lling individual sensors or entire cell cup area.
• Rinse the conductivity cell or pH/ORP sensor well with test
solution 3 times and rell. Temperature and/or measurement
extremes will require additional rinses for maximum accuracy.
• Press the desired measurement key to start measurement.
Pressing the key again does no harm and restarts the 15
second auto “off” timer.
• Note the value displayed or press the MS key to store the
reading (ref. Memory Storage, pg. 23). It’s that simple!
B. Characteristics of the Keys
• Though your Po o l Pr o has a variety of sophisticated options,
it is designed to provide quick, easy, accurate measurements
by simply pressing one key.
• All functions are performed one key at a time.
• There is no “off” key. After 15 seconds of inactivity the
instrument turns itself off (60 seconds in CAL mode). User
adjustable up to 75 seconds.
• Rarely is it necessary to press and
to Select a Solution, pg. 15; or Cond. MIN/SALT or TDS
Calibration, pg.17).
hold
a key (as in Procedure
C. Operation of the Keys (See Instrument Illustration on pg. i)
1. Measurement Keys in General
Any of the measurement keys in the upper part of the keypad turns on
the instrument in the mode selected. The mode is shown at the bottom
of the display, and the measurement units appear at the right. Pressing
a measurement key does this even if you are in a calibration sequence
and also serves to cancel a change (ref. Leaving Calibration, pg. 17).
2. COND, MIN/SALT and TDS Keys
These 3 keys are used with solution in the Conductivity Cell.
Precautions:
• While lling cell cup ensure no air bubbles cling on the cell wall.
• If the proper solution is not selected (KCl, NaCl, 442),
refer to Why Solution Selection is Available, pg. 14 and
Procedure to Select a Solution, pg. 15.
a. COND Key
Solution to be tested is introduced into the conductivity cell and a press
of displays conductivity with units on the right. On the left is
shown the solution type selected for conductivity.
7
Page 12
9
b. MIN/SALT key
MIN
SALT
A press of
displays Total Dissolved Solids with units (PPM &
PPT).
on the right. On the left is shown solution type selected (NaCl) for
mineral/salt (ref. Solution Selection, pg. 14). An overrange condition
will show only [- - - -].
c. TDS key
A press of
displays Total Dissolved Solids with units on the right.
This is a display of the concentration of material calculated from
compensated conductivity using the characteristics of a known
material. On the left is shown solution type selected for TDS (ref.
Solution Selection, pg. 14).
3. pH and ORP/Fr Chl Keys
Measurements are made on solution held in the pH/ORP sensor well
(ref. pH and ORP, pg. 42). The protective cap is removed and the sensor
well is lled and rinsed with the sample enough times to completely
replace the pH Sensor Storage Solution.
After use, the pH/ORP sensor well must be relled with Myron L pH
Sensor Storage Solution, and the protective cap reinstalled securely
(ref. Maintenance of the pH/ORP Sensor, pg. 9 and Cleaning Sensors,
2. pH/ORP, pg. 32).
a. pH Key
A press of
right.
displays pH readings. No units are displayed on the
b. ORP/Fr Chl Key
A press of
displays Oxidation-Reduction Potential/REDOX
reading in millivolts, “mV” is displayed.
4. CAL/MCLR Key
A press of
allows you to enter the calibration mode while
measuring conductivity, TDS or pH. Once in CAL mode, a press of this
key accepts the new value. If no more calibration options follow, the
instrument returns to measuring (ref. Leaving Calibration, pg. 17).
If
is held down for about 3 seconds, CAL mode is not entered, but
“SEL” appears to allow Solution Selection (ref. pg. 14) with the Up or
Down keys. As in calibration, the CAL key is now an “accept” key.
While reviewing stored records, the MCLR side of the key is active to
allow clearing records (ref. Clearing a Record/Memory Clear, pg. 23).
8
Page 13
5. UP or DOWN Keys
MIN
SALT
While measuring in any parameter, the
or
keys activate
the Memory Store and Memory Recall functions.
While in CAL mode, the keys step or scroll the displayed value up or
down. A single press steps the display and holding either key scrolls the
value rapidly.
While in Memory Recall, the keys scroll the display up and down through
the stack of records (ref. Memory Recall, pg. 23).
IV. AFTER USING the Po o l Pr o
A. Maintenance of the Conductivity Cell
Rinse out the cell cup with clean water. Do not scrub the cell. For oily
lms, squirt in a foaming non-abrasive cleaner and rinse. Even if a
very active chemical discolors the electrodes, this does not affect the
accuracy; leave it alone. (ref. Cleaning Sensors, pg. 32)
B. Maintenance of the pH/ORP Sensor
The sensor well must be kept wet with a solution. Before replacing
the rubber cap, rinse and ll the sensor well with Myron L pH Sensor
Storage Solution. If unavailable, you can use an almost saturated KCl
solution, pH 4 buffer or at least a strong table salt solution. NEVER
USE DISTILLED WATER. (ref. pH and ORP Practices, pg. 22).
V. SPECIFIC RECOMMENDED MEASURING
PROCEDURES
If the proper solution is not selected (KCl, NaCl, 442), see Solution
Selection, pg. 14.
NOTE: After sampling high concentration solutions or temperature
extremes, more rinsing may be required. When sampling low conductivity
solutions, be sure the pH cap is well seated so that no solution washes
into the conductivity cell from around the pH cap.
A. Measuring Conductivity MIN/SALT & Total Dissolved Solids
(TDS)
1. Rinse cell cup 3 times with sample to be measured. (This
conditions the temperature compensation network and
prepares the cell.)
2. Rell cell cup with sample.
3. Press
,
or
.
4. Take reading. A display of [- - - -] indicates an overrange
condition.
9
Page 14
11
B. Measuring pH
1. Remove protective cap by squeezing its sides and pulling up.
2. Rinse sensor well 3 times with sample to be measured. Shake
out each sample to remove any residual liquid.
3. Rell both sensor wells with sample.
4. Press .
5. Note value displayed.
6. IMPORTANT: After use, ll pH/ORP sensor well with Myron L
pH Sensor Storage Solution and replace protective cap. If
Myron L pH Sensor Storage Solution is unavailable, you can
use a strong KCl solution, a pH 4 buffer, or a saturated solution
of table salt and tap water (ref. Cleaning Sensors, 2. pH/ORP,
pg. 32).
C. Measuring ORP
The PS6 features the ability to measure the activity of oxidizing or
reducing chemicals in solution as ORP mV. The instrument also includes
an innovative Free Chlorine Equivalent (FCE) feature (Measuring Free
Chlorine Using FCE, pg. 12) that uses ORP and pH to measure free
available chlorine (FAC) concentration in ppm. ORP mV and ppm of
free available chlorine (FAC) are the two most commonly used sanitizer
units of measure in water quality management.
Do not allow pH/ORP sensor to dry out.
1. ORP / FCE Mode Selection
The PS6 allows the user to choose between measuring oxidizing
sanitizers using either ORP mV or as parts per million (ppm) of equivalent
free chlorine. Use ORP to directly measure the oxidizing power of all
sanitizers like ozone, bromine, peracetic acid or chlorine. Use FCE to
measure the strength of oxidizing sanitizers as ppm of equivalent free
chlorine. To select between ORP and Free Chlorine modes:
1. Press
2. Press and hold
The current preference for ORP units of measure is displayed.
Factory setting for this preference is ORP mV. (See Figure 1, next
page.)
10
.
for approximately 3 seconds.
Page 15
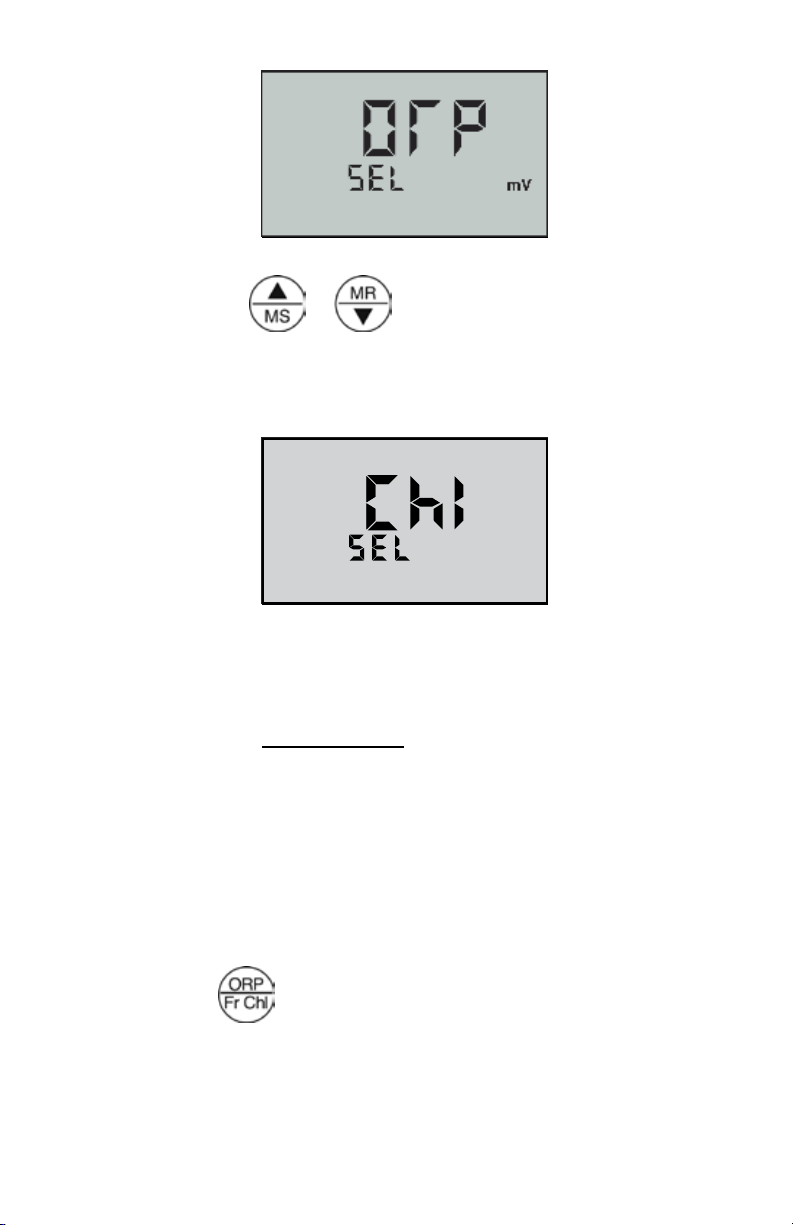
Figure 1
3. Press the or
Figure 2
PPM
keys to toggle between mV (standard
ORP mode) and FCE ppm. The setting chosen is displayed. (See
Figure 2.)
4. Press any parameter key to exit ORP unit preference selection
or let the unit time out. ORP unit preference will be saved.
2. Measuring ORP
1. Ensure the PS6FCE is in ORP mode (ref. ORP/FCE Mode
Selection, pg. 10).
2. Remove protective cap by rotating while grasping and pulling up.
3. Rinse sensor well and cell cup 3 times with sample to be
measured. Shake out each sample to remove any residual liquid.
4. Rell both sensor well and cell cup with sample.
5. Press .
6. Take reading.
7. Press MS to store reading in memory, if desired.
IMPORTANT: After use, ll pH/ORP sensor well with Myron L
11
Page 16
13
pH Sensor Storage Solution and replace protective cap. If
Myron L pH Sensor Storage Solution is unavailable, you can
use a strong KCl solution, a pH 4 buffer, or a saturated solution
of table salt and tap water (ref. Cleaning Sensors, 2. pH/ORP,
pg. 32). Do not allow pH/ORP sensor to dry out.
D. Measuring Free Chlorine Using FC
E
The FCE function can be used to measure discrete samples, owing
solution and bodies of water. Measurement technique is particular to the
type of sample. For accurate results, use the FCE Flow Method described
in section 2 below to measure discrete or owing samples. Use the FCE
Immersion Method described in section 3 below in situations where the
PS6FCE can be dipped to obtain a sample. Read through section 4. FCE
Best Practices before you begin.
1. Prepare for FCE Measurement
1. For ease of measurement, set the instrument’s Auto oFF feature
to 75 sec (ref. Auto oFF, pg. 28).
2. Ensure the FCE mode has been activated (ref. ORP/FCE Mode
Selection, pg. 10).
3. Remove protective cap from the pH/ORP sensor by rotating
while grasping and pulling up.
2. FCE Flow Method
1. Empty the pH/ORP sensor well of all storage solution.
2. Hold the PS6FCE at a 30º angle (cup sloping downward).
3. Thoroughly ush the sensor well and cell cup with a steady
stream of the solution you intend to measure by allowing the
solution to ow into and out of the sensor well and cell cup for at
least 10 seconds.
4. Let sample ow continuously into conductivity cell with no
aeration.
5. Allow both the sensor well and cell cup to remain lled with
sample.
6. Press
. The instrument will begin alternating between a
predicted nal ORP value and a free chlorine equivalent
concentration in ppm. Both readings will change rapidly at
rst.
12
Page 17

7. Wait for the readings to stabilize. When the mV and ppm values
are unchanging for 5 consecutive readings, the FCE reading
has reached a stable level. This may take 1 to 2 minutes.
NOTE: If the reading takes more than 1 minute to stabilize,
press the
from disturbing the measurement process. Annunciators will
alert you when either the pH or ORP of the nal FCE ppm value
are Out of Range (“-Or-”).
8. Press MS to store reading in memory if desired.
3. FCE Immersion Method
NOTE: Use this method for pools, spas and other large standing bodies
of water.
1. Hold instrument beneath the surface of the water to avoid
surface effects on the water’s chemistry.
2. Swirl the instrument around for at least 10 seconds to thoroughly
rinse the cell cup and sensor well.
3. Continue holding the instrument under the surface while taking
the reading.
4. Press .
5. The instrument will begin alternating between a predicted nal
ORP value and a free chlorine equivalent concentration in ppm.
Both readings will change rapidly at rst.
after 1 minute to prevent Auto oFF feature
6. Wait for the readings to stabilize. When the mV and ppm values
are unchanging for 5 consecutive readings, the FCE reading
has reached a stable level. This may take 1 to 2 minutes.
NOTE: If the reading takes longer than 1 minute to stabilize,
press after 1 minute to prevent Auto-OFF feature from
disturbing the measurement process. Annunciators will alert
you when either the pH or ORP of the nal FCE ppm value are
Out of Range (“-Or-”).
7. Press MS to store reading in memory if desired.
13
Page 18

15
4. FCE Best Practices
For best results it is recommended that you:
1. Take 3 consecutive FCE measurements and record the
readings.
2. Calculate the average of the 3 measurements. Use this value.
3. Ignore measurements that are signicantly different from the
others. Ex: 3.20 ppm, 1.15 ppm, 3.10 ppm
IMPORTANT: After use, ll pH/ORP sensor well with Myron L pH
Sensor Storage Solution and replace protective cap. If Myron L pH
Sensor Storage Solution is unavailable, you can use a strong KCl
solution, a pH 4 buffer, or a saturated solution of table salt and tap
water (ref. Cleaning Sensors, 2. pH/ORP, pg. 32). Do not allow pH/
ORP sensor to dry out.
VI. SOLUTION SELECTION
A. Why Solution Selection is Available
Conductivity, MIN/SALT, and TDS require temperature correction to
25°C values (ref. Standardized to 25°C, pg. 37). Selection determines
the temperature correction of conductivity and calculation of TDS from
compensated conductivity (ref. Cond. Conversion to TDS, pg. 40).
B. The 3 Solution Types
On the left side of the display is the salt solution characteristic used
to model temperature compensation of conductivity and its TDS
conversion. Generally, using KCl for Conductivity, NaCl for Mineral/
Salt, and 442 (Natural Water characteristic) for TDS will reect present
industry practice for standardization. This is the setup as shipped from
the factory (ref. Solution Characteristics, pg. 40).
C. Calibration of Each Solution Type
There is a separate calibration for each of the 3 solution types. Note
that calibration of a 442 solution does not affect the calibration of a
NaCl solution. For example: Calibration (ref. Conductivity, MIN/SALT
or TDS Calibration, pg. 17) is performed separately for each type
of solution one wishes to measure (ref. Conductivity/TDS Standard
Solutions, pg. 36).
14
Page 19

D. Procedure to Select a Solution
MIN
SALT
In these first six sections, you have learned
all you need to take accurate measurements.
The following sections contain calibration,
advanced operations and technical information.
F igure 3
K C l
442
Na C l
NOTE: Check display to see if solution displayed (KCl, NaCl, 442) is
already the type desired. If not:
1. Press
,
or
to select the parameter on
which you wish to change the
solution type.
2. Press and hold
key
for 3 seconds to make
“SEL” appear (see Figure 3).
(For demonstration purposes,
all 3 solution types are shown simultaneously.)
3. Use the
or
key to select type of solution desired
(ref. Solution Characteristics, pg. 40). The selected solution
type will be displayed: KCl, NaCl, or 442.
4. Press
to accept new solution type.
15
Page 20

17
VII. CALIBRATION
KCl, NaCl or 442
Cond Gain only
MIN/SALT
TDS Gain only
pH 7, acid and/or base
ORP Zero set with pH 7 automatically
Gain only
A. Calibration Intervals
Generally, calibration is recommended about once per month with
Conductivity or TDS solutions. Calibration with pH solutions should
be checked twice a month. Calibration of ORP is not necessary (ref.
CALIBRATION INTERVALS, pg. 21).
B. Rules for Calibration of the Po o l Pr o
1. Calibration Steps
a. Starting Calibration
Calibration is begun by pressing
while measuring Conductivity,
MIN/SALT, TDS or pH. Measuring continues, but the CAL icon is on,
indicating calibration is now changeable.
The reading is changed with the
and to match the
known value. The calibration for each of the 3 solution types may be
performed in either conductivity or TDS mode.
Depending on what is being calibrated, there may be 1, 2 or 3 steps to
the calibration procedures.
16
Page 21

The
MIN
SALT
Figure 4
°C
NaCl
COND
CAL
µS
7582
23.8
accepts the new calibration value and steps you to the next adjustment
(or out of CAL mode if there are no more steps).
To bypass a calibration step, just press to accept the present
value as is.
b. Leaving Calibration
Calibration is complete when the “CAL” icon goes out. Pressing any
measurement key cancels changes not yet accepted and exits calibration
mode.
Leaving pH after the 2nd buffer results in the same gain being entered
in place of the 3rd buffer.
2. Calibration Limits
There are calibration limits. A nominal “FAC” value is an ideal value
stored by the factory. Attempts to calibrate too far, up or down, from
there will cause the displayed value to be replaced with “FAC”. If you
accept it (press the “Cal” key), you will have the original default factory
calibration for this measurement. The need to calibrate so far out that
“FAC” appears indicates a procedural problem, wrong standard solution,
a very dirty cell cup or an aging pH/ORP sensor (ref. Troubleshooting
Chart, pg. 34).
C. Calibration Procedures
1. Conductivity, MIN/SALT or TDS Calibration
becomes an “ACCEPT” key. At each point, pressing
a. Rinse conductivity cell three times with proper standard (KCl,
NaCl, or 442) (ref. Cond/TDS Standard Solutions, pg. 36).
b. Rell conductivity cell with same standard. NACL-7500 shown.
c. Press
press
appear on the display
(see Figure 4).
d. Press or to
to step the displayed value toward the standard’s value (7582
,
, “CAL” icon will
or
or
, then
17
Page 22

19
>7501) or hold a key down to cause rapid scrolling of the
MIN
SALT
reading.
e. Press
once to conrm new value and end the
calibration sequence for this particular solution type. If another
solution type is also to be measured, change solution type now
and repeat this procedure.
2. Reloading Factory Calibration
(Cond, MIN/SALT or TDS)
If calibration is suspect or known to be incorrect, and no standard
solution is available, the calibration value can be replaced with the
original factory value for that solution. This “FAC” value is the same
for all Po o l Pr o s , and returns you to a known state without solution in
the cell. The “FAC” internal electronics calibration (which bypasses
the electrodes and cell) is not intended to replace calibration with
conductivity standard solutions. If another solution type requires resetting,
change solution type and repeat this procedure.
a. Press
b. Press
or
.
or
.
c. Press
key until “FAC” appears and release.
d. Press
to accept the factory calibration setting.
3. pH Calibration
Important: Always “zero” your Po o l Pr o with a pH 7 buffer solution
before adjusting the gain with acid or base buffers, i.e., 4 and/or 10.
a. pH Zero Calibration
1. Rinse sensor well 3 times with 7 buffer solution.
2. Rell both sensor wells with 7 buffer solution.
18
Page 23

3. Press
Figure 5
BUFFER
pH
CAL
to verify pH
calibration. If the display
shows 7.00, skip the pH
Zero Calibration and
proceed to section b.
pH Gain Calibration.
4. Press
to enter calibration mode. The “CAL”, “BUFFER”
and “7” annunciators will appear (see Figure 5). Displayed
value will be the uncalibrated sensor.
NOTES: If a wrong buffer is added (outside of 6-8 pH),“7” and
“BUFFER” will ash, and the P
o o lPr o
will not adjust.
The uncalibrated pH value displayed in step 4 will assist in determining
the accuracy of the pH sensor. If the pH reading is above 8 with pH 7
buffer solution, the sensor well needs additional rinsing or the pH sensor
is defective and needs to be replaced
5. Press
or
until the display reads 7.00.
.
NOTE: Attempted calibration of >1 pH point from factory calibration will
cause “FAC” to appear. This indicates the need for sensor replacement
(ref. Troubleshooting pg. 34) or fresh buffer solution. The “FAC” internal
electronic calibration is not intended to replace calibration with pH
buffers. It assumes an ideal pH sensor. Each “FAC” indicates a factory
setting for that calibration step (i.e., 7, acid, base).
You may press
reduce your variation from factory setting by pressing
to accept the preset factory value, or you may
or
.
6. Press
to accept the new value. The pH Zero Calibration
is now complete. You may continue with pH Gain Calibration or
exit by pressing any measurement key.
19
Page 24

21
b. pH Gain Calibration
Figure 6
BUFFER
pH
CAL
Figure 7
pH
BUFFER
CAL
Important: Always calibrate or verify your Po o l Pr o with a pH 7 buffer
solution before adjusting the gain with acid or base buffers, i.e., 4 and/or
10, etc. Either acid or base solution can be used for the 2nd point “Gain”
calibration and then the opposite for the 3rd point. The display will verify
that a buffer is in the sensor well by displaying either “Acd” or “bAS”.
1. The pH calibration mode is initiated by either completion of the
pH Zero Calibration, or verifying 7 buffer and pressing the
twice while in pH measurement mode.
2. At this point the “CAL”, “BUFFER” and “Acd” or “bAS”
annunciators will be displayed (see Figures 6 and 7).
NOTE: If the “Acd” and “bAS” indicators are blinking, the unit is indicating
an error and needs either an acid or base solution present in the sensor
well
.
3. Rinse sensor well 3 times with acid or base buffer solution.
4. Rell sensor well again with same buffer solution.
5. Press
6. Press
or
until display agrees with buffer value.
to accept 2nd point of calibration. Now the
display indicates the next type of buffer to be used.
Single point Gain Calibration is complete. You may continue for the 3rd
point of Calibration (2nd Gain) or exit by pressing any measurement key.
20
Page 25

Exiting causes the value accepted for the buffer to be used for both acid
and base measurements.
To continue with 3rd point calibration, use basic buffer if acidic buffer
was used in the 2nd point, or vice-versa. Again, match the display to the
known buffer value as in step 2 and continue with the following steps:
7. Repeat steps 3 through 6 using opposite buffer solution.
8. Press
completes the Calibration procedure. Fill sensor well with
Myron L Storage Solution and replace protective cap.
4. ORP/Fr Chl Calibration
ORP electrodes rarely give false readings without problems in the
reference electrode. For this reason, and because calibration solutions
for ORP are highly reactive and potentially hazardous, your Po o l Pr o
has an electronic ORP calibration. This causes the zero point on the
reference electrode to be set whenever pH 7 calibration is done.
5. Temperature Calibration
Temperature calibration is not necessary in the Po o l Pr o .
VIII. CALIBRATION INTERVALS
There is no simple answer as to how often one should calibrate an
instrument. The Po o l Pr o is designed to not require frequent recalibration.
The most common sources of error were eliminated in the design,
and there are no mechanical adjustments. Still, to ensure specied
accuracy, any instrument must be checked against chemical standards
occasionally.
A. Suggested Intervals
On the average, we expect calibration need only be checked monthly for
the Conductivity, MIN/SALT or TDS functions. The pH function should be
checked every 2 weeks to ensure accuracy. Measuring some solutions
will require more frequent intervals.
to accept 3rd point of calibration, which
B. Calibration Tracking Records
To minimize your calibration effort, keep records. If adjustments you
are making are minimal for your application, you can check less often.
Changes in conductivity calibration should be recorded in percent.
Changes in pH calibration are best recorded in pH units.
21
Page 26

23
Calibration is purposely limited in the Po o l Pr o to ±10% for the
conductivity cell because more than that indicates damage, not drift.
Likewise, calibration changes are limited to ±1 pH unit because more than
that indicates the end of the sensor’s lifetime, and it should be replaced.
C. Conductivity, MIN/SALT, TDS Practices to Maintain Calibration
1. Clean oily lms or organic material from the cell electrodes
with foaming cleaner or mild acid. Do not scrub inside the cell.
2. Calibrate with solutions close to the measurements you make.
Readings are compensated for temperature based on the type
of solution. If you choose to measure tap water with a KCl
compensation, which is often done (ref. An Example, pg. 38), and
you calibrate with 442 solution because it is handy,
the further away from 25°C you are, the more error you
have. Your records of calibration changes will reect
temperature changes more than the instrument’s accuracy.
3. Rinse out the cell with pure water after taking measurements.
Allowing slow dissolving crystals to form in the cell
contaminates future samples.
4. For maximum accuracy, keep the pH sensor cap on tight so
that no uid washes into the conductivity cell.
D. pH and ORP Practices to Maintain Calibration
1. Keep the sensor wet with Myron L Storage Solution.
2. Rinse away caustic solutions immediately after use.
ORP calibration solutions are caustic, and ±5% is considered very
accurate. By using the pH zero setting (0 mV = 7 pH) for ORP and
precision electronics for detection, the Po o l Pr o delivers better accuracy
without calibration than a simpler instrument could using calibration
solutions.
IX. MEMORY
This feature allows up to 100 readings with their temperatures to be
stored simultaneously for later recall. At the same time, the TIME and
DATE are also recorded. To download the memory to a computer, (ref.
bluDock™ Wireless Data Transfer Instructions, pg. 29).
22
Page 27

A. Memory Storage
Figure 8
°C
KCl
COND
MEMORY
1. While displaying a
measurement, press
to record the
displayed value.
2. “MEMORY” will appear
and the temperature display
will be momentarily replaced
by a number (1-100) showing the position of the record. Figure
8 shows a reading of 1806 µS stored in memory record #4.
B. Memory Recall
1. Press any measurement key.
2. Press , “MEMORY” will appear, and the display will
show the last record stored.
3. Press
or
to scroll to the record location desired
(the temperature display alternates between temperature
recorded and location number).
4. Press to display time and date stamp.
5. Press any measurement key to leave memory recall or allow to
automatically turn off.
C. Clearing a Record/Memory Clear
After recalling a certain record location, press and HOLD
to
clear that memory. This space will be the place for the next memory
record, unless you scroll to another empty position before ending the
recall sequence. The next memory stored will go into the next highest
available memory location.
Example:
You have locations 1-7 lled and wish to clear the
conductivity reading stored in record location #3 and replace it with a
pH reading.
1. Press and scroll to location #3.
2. Press and HOLD
to clear old record #3.
3. Fill pH/ORP sensor well with sample.
23
Page 28

25
4. Press
Figure 9
MEMORY
Figure 10
CAL
to measure sample and press
reading in location #3.
5. The next memory stored will go into location #8.
6. To clear all records: After
to store
pressing
,
scroll down.
“CLr ALL” will be displayed
(see Figure 9).
7. Press . All records will be cleared.
X. TIME and DATE
The Time and Date may easily be changed as you travel.
A. Setting TIME
Time is always displayed in 24 hour time.
Example shown in Figure 11, 16:05 equals 4:05 PM.
1. Press
.
2. Press
until the time is displayed (stored readings, PC
OFF, CLr ALL, time, i.e.,
“16:05”).
3. Press
to initiate. CAL
will be displayed along with
the time, (see Figure 10).
4. Press
or
to change the time.
5. Press to accept the change (new time).
24
Page 29

B. Setting DATE
Figure 11
Figure 12
CAL
Figure 13
CAL
Figure 14
CAL
Example shown in Figure 11,
is in US format, i.e., mo/dy/yr.
NOTE: The default format is US.
Date format may be changed
(ref. Date Format “US and
International (Int)”, pg. 26).
1. Press
.
2. Press repeatedly until the date is displayed (stored
readings, PC OFF, CLr ALL, time, date, i.e., 01/24/12
(January 24, 2012)).
3. Press
to initiate. CAL will be displayed along with the
YEAR, (see Figure 12).
4. Press
or
to
change the YEAR.
5. Press to accept the
change (new year).
6. Press
or
to
change the month.
7. Press
to accept the
change (new month),
(see Figure 13).
8. Press
or
to change the day.
9. Press
to accept
the change (new day)
(see Figure 14).
25
Page 30

27
C. DATE FORMAT “US & International (Int)”
Figure 15
Figure 16
Figure 17
Figure 18
1. Press
.
2. Press
repeatedly until the format is displayed (stored
readings, PC OFF, CLr ALL, time, date, date format).
3. Press
to change. Display will now indicate other format
(see Figures 15 & 16).
4. Press any measurement key or allow to automatically turn off.
XI. TEMPERATURE FORMAT “Centigrade & Fahrenheit”
1. Press
.
2. Press
to display the stored memory records.
3. Press
repeatedly until you pass the “US” or “Int” date
format location. The display will show a “C” or “F”
(see Figures 17 and 18).
26
Page 31

4. Press ; the display will change to the other unit.
Figure 19
5. Press
; all temperature reading are now in degrees last
shown.
NOTE: Tempco will still be shown in %/°C
.
XII. TOTAL RETURN to FACTORY SETTINGS “FAC SEL”
There may come a time when it would be desirable to quickly reset
all the recorded calibration values in the instrument back to the factory
settings. This might be to ensure all calibrations are set to a known
value, or to give the instrument to someone else free of adjustments or
recorded data for a particular application.
NOTE: All stored data will be lost.
1. Press
.
2. Press to display the stored memory records.
3. Press repeatedly until
you pass the CLr ALL
and the C-F locations. The
display will show a
“FAC SEL” (see Figure 19).
4. Press
to accept the resetting. Display will return to
Conductivity mode.
XIII. CELL CHECK
The cell check veries the cleanliness of the conductivity/TDS/MIN/SALT
sensor. In normal use the cell may become dirty or coated and require
cleaning. If the display is showing “.00” when the cell cup is dry, the sensor
is probably clean. No matter what a manufacturer claims, a sensor can
and will become contaminated or coated; therefore require cleaning. A
true 4-wire sensor, as in the Po o l Pr o , helps to mitigate contamination,
however, NO SENSOR IS 100% IMMUNE.
27
Page 32

29
1. Press
Figure 20
Figure 21
Figure 22
Figure 23
.
2. Press
to display the
stored memory records.
3. Press
repeatedly until
you pass the FAC SEL
location. The display will
show a “CELL ch”
(see Figure 20).
4. Press
to test.
If cell is clean, Good will
momentarily be displayed
(see Figure 21). If cell is
dirty, “CELL cLn” will be
displayed (see Figure 22),
(ref. Cleaning Sensors,
pg. 32).
XIV. AUTO OFF
Auto off allows the user to adjust the
time the instrument is ON (up to 75
seconds) after each press of a key.
Default time is 15 seconds with 60
seconds in CAL (calibration) mode.
1. Press
.
2. Press
to display the stored memory records.
3. Press
repeatedly until you pass the CELL ch location.
The display will show “Auto oFF” (see Figure 23).
28
Page 33

4. Press to initiate. CAL
Figure 24
CAL
Figure 25
CAL
will be displayed along with
the “15 SEC” (see Figure
24).
5. Press
or
to
change the time (see
Figure 25). Maximum
time is shown.
6. Press
to accept the
change (new time).
XV. bluDock™ WIRELESS DATA TRANSFER INSTRUCTIONS
NOTE:
bluDock
Bluetooth®
Bluetooth
is a registered trademark of Bluetooth SIG. The
module is a registered
Bluetooth
device.
Requires Myron L bluDock™ accessory package, Model # BLUDOCK.
Package includes Po o l Pr o hardware modication that allows the unit
to communicate wirelessly with a personal computer congured for
wireless device communication. Package also includes U2CI software
application that will operate on Windows XP, Vista and 71, and Macintosh
OSX2 based computer systems and
computers that do not have
Bluetooth
Bluetooth
capability.
USB adapter (dongle) for
A. Software Installation
1. Place Myron L Po o l Pr o U2CI Installation CD v2.0.0 & later
into your computer or download U2CI application from the
Myron L website:
http://myronl.com/main/U2CI_Application_DL.htm
2. Upon opening, select the folder for your operating system.
3. Install U2CI application. See detailed installation instructions
on CD or Myron L website:
http://myronl.com/main/U2CI_Application_DL.htm
4. Additional drivers may be required. See our website for the
latest information.
1 Windows, XP, Vista, 7 & Excel are registered trademarks of Microsoft Corporation.
2 Macintosh OSX is a registered trademark of Apple Computer, Inc.
29
Page 34

31
B. Hardware Setup
Figure 26
Figure 27
Figure 28
For a computer without
Bluetooth
capability: If you don’t have the
dongle that came with the
BLUDOCK, one can be ordered
separately from the Myron L
Company. Order Model # BDDO.
Plug in your dongle and install per
manufacturer’s instructions.
For computers with
Bluetooth
dongle installed:
Bluetooth
capability/
First time use of the bluDock:
1. Press any parameter button
to turn the Po o l Pr o on.
2. Put the Po o l Pr o in “PC
On” mode by pressing the
key until “PC OFF”
appears (see Figure 26).
3. Then press the
key.
“PC On” will be displayed
(see Figure 27).
NOTE: “PC Ini” may momentarily be
displayed while initializing (see Figure
28).
4. Add bluDock to your
Bluetooth
devices per your
operating system procedure.
THE BLUDOCK DEVICE
PASSKEY IS 1234.
5. After pairing, note the number
of the COM port assigned
by the computer.
In Windows XP, note the number of the
outgoing COM port assigned by the computer.
NOTE: The unit will automatically power down after 60 sec. If the unit
powers down during pairing, repeat steps 1-3 above and continue.
C. Memory Stack Download
1. With the Po o l Pr o in “PC On” mode, open the U2CI
software application.
2. Verify that the port selected matches the COM port number noted
(rst time only). This is the outgoing COM port on Windows XP.
3. In the U2CI application, click on the data download button. A
30
Page 35

data transfer bar will appear while the data is being
downloaded.
Once downloaded, the data may be manipulated, printed or stored within
the Myron L U2CI application, or the data may be exported to another
more powerful spreadsheet1 such as Excel2.
Additional features, such as assigning a name to the instrument, setting
time and date and erasing data are available. See U2CI software
installation CD or visit our website for the latest instructions:
http://myronl.com/main/U2CI_Application_DL.htm
4. Upon completion, click on the “disconnect” icon.
5. Turn off Po o l Pr o PC download mode by selecting any
measurement function. Failure to do so will reduce battery life.
XVI. CARE and MAINTENANCE
Po o l Pr o s should be rinsed with clean water after use. Solvents should
be avoided. Shock damage from a fall may cause instrument failure.
A. Temperature Extremes
Solutions in excess of 71°C/160°F should not be placed in the cell
cup area; this may cause damage. The pH sensor may fracture if the
Po o l Pr o temperature is allowed to go below 0°C/32°F. Care should be
exercised not to exceed rated operating temperature.
Leaving the Po o l Pr o in a vehicle or storage shed on a hot day can easily
subject the instrument to over 66°C/150°F. This will void the warranty.
B. Battery Replacement
Dry Instrument THOROUGHLY. Remove the four (4) bottom screws.
Open instrument carefully. Carefully detach battery from circuit board.
Replace with 9 volt alkaline battery. Replace bottom, ensuring the
sealing gasket is installed in the groove of the top half of case. Re-install
screws, tighten evenly and securely.
NOTE: Because of nonvolatile EEPROM circuitry, all data stored in
memory and all calibration settings are protected even during power
loss or battery replacement. However, loss of time and date may occur if
battery is removed for longer than 3 minutes (180 seconds).
C. pH/ORP Sensor Replacement
Order model RPR. When ordering, be sure to include the model and
1 Please Note: Although the Myron L Company has performed extensive testing, we
cannot guarantee compatibility of all applications and formats. We suggest testing your
application and format for compatibility before relying on it.
2
Windows, XP, Vista, 7 & Excel are registered trademarks of Microsoft Corporation.
31
Page 36

33
serial number of your instrument to ensure receipt of the proper type.
pH/ORP SENSOR
Top View
ORP
Electrode
pH Glass
Electrode
Sensor
Body
Reference
Junction
under Glass
pH Bulb
Complete installation instructions are provided with each replacement
sensor.
D. Cleaning Sensors
1. Conductivity/TDS/MIN/SALT
The conductivity cell cup should be kept as clean as possible. Flushing
with clean water following use will prevent buildup on electrodes.
However, if very dirty samples — particularly scaling types — are
allowed to dry in the cell cup, a lm will form. This lm reduces accuracy.
When there are visible lms of oil, dirt, or scale in the cell cup or on the
electrodes, use isopropyl alcohol or a foaming non-abrasive household
cleaner. Rinse out the cleaner and your Po o l Pr o is ready for accurate
measurements.
2. pH/ORP
The unique pH/ORP sensor in your Po o l Pr o is a nonrellable
combination type that features a porous liquid junction (see Figure 29).
It should not be allowed to dry out. If it does, the sensor may sometimes
be rejuvenated by rst cleaning the sensor well with Isopropyl alcohol or
a liquid spray cleaner such as Windex™ or Fantastic™ and rinsing well.
Do not scrub or wipe the pH/ORP sensor.
Then use one of the following methods:
1. Pour a HOT salt solution ~60°C/140°F, preferably potassium
chloride (KCI) solution (Myron L pH/ORP Sensor Storage
Solution) — HOT tap water with table salt (NaCl) will work ne
— in the sensor well and allow to cool. Retest.
2. Pour DI water in the sensor well and allow to stand for no more
32
Figure 29
or
Page 37

than 4 hours (longer can deplete the reference solution
and damage the glass bulb). Retest.
If neither method is successful, the sensor must be replaced.
“Drifting” can be caused by a lm on the pH sensor bulb and/or
reference. Use isopropyl alcohol (IPA) or spray a liquid cleaner such
as Windex™ or Fantastic™ into the sensor well to clean it. The sensor
bulb is very thin and delicate. Do not scrub or wipe the pH/ORP sensor.
Leaving high pH (alkaline) solutions in contact with the pH sensor for
long periods of time is harmful and will cause damage. Rinse such
liquids from the pH/ORP sensor well and rell it with Myron L Storage
Solution to extend the useful life of the sensor. If unavailable, you can
use a saturated KCl solution, pH 4 buffer, or a saturated solution of table
salt and tap water, but this should be replaced with storage solution as
soon as possible.
Samples containing chlorine, sulfur, or ammonia can “poison” any pH
electrode. If it is necessary to measure the pH of any such sample,
thoroughly rinse the sensor well with clean water immediately after
taking the measurement. Any sample element that reduces (adds an
electron to) silver, such as cyanide, will attack the reference electrode.
Replacement sensors are available only from the Myron L Company or
its authorized distributors.
33
Page 38

35
XVII. TROUBLESHOOTING CHART
Symptom Possible Cause Corrective Action
No display, even though
measurement key pressed
Inaccurate pH readings 1. pH calibration needed. Ref. pH Cal.,
No response to pH changes Sensor bulb is cracked or an
Will not adjust down to pH 7 pH/ORP sensor has lost KCl. Clean and rejuvenate sensor (ref. Cleaning Sensors, pg. 32) and recalibrate. If no
pH readings drift or respond slowly to
changes in buffers/samples or “FAC”
is displayed repeatedly
Unstable Conductivity/TDS/
MIN/SALT readings
Unable to calibrate Conductivity/
TDS/MIN/SALT
Low ORP Reading
Slow or no response to ORP changes
Battery weak or not connected. Check connections or replace battery. Ref. Battery Replacement, pg. 31.
pg. 18.
2. Cross-contamination from residual pH
buffers or samples in sensor well.
3. Calibration with expired pH buffers.
electromechanical short caused by an
internal crack.
1. Temporary condition due to “memory” of
solution in pH sensor well for long periods.
2. Bulb dirty or dried out.
3. Reference junction clogged or coated.
Dirty electrodes. Clean cell cup and electrodes. Ref. Cleaning Sensors, pg. 32.
Film or deposits on electrodes. Clean cell cup and electrodes. Ref. Cleaning Sensors, pg. 32.
ORP platinum electrode is dirty. Check the ORP sensor functioning. Take an ORP reading of Myron L pH/ORP
FCE responds very slowly or returns an
atypically high Predictive ORP value
34
1. Dirty platinum electrode (see above).
2. ORP sensor memory/battery effect.
Some ORP sensors exhibit a residual
charge when measuring LOW Free
Chlorine concentrations soon after
measuring a HIGH Free Chlorine
concentration.
Page 39

1. Recalibrate instrument.
2. Thoroughly rinse sensor well.
3. Recalibrate using fresh buffers. Ref. pH Buffer Solutions, pg. 36.
Replace pH/ORP sensor. Ref. Replacement pH/ORP Sensor, pg. 37.
improvement, replace pH/ORP sensor (ref. Replacement pH/ORP Sensor, pg. 37).
Clean and rejuvenate sensor (ref. Cleaning Sensors, pg. 32) and recalibrate. If no
improvement, replace pH/ORP sensor (ref. Replacement pH/ORP Sensor, pg. 37).
Sensor Storage Solution (ref. pH Sensor Storage Solution, pg. 36. If the reading is
outside the range of 350-400 mV, clean ONLY the platinum ORP
electrode with Myron L ORP Conditioner solution-soaked cotton swab (ref. ORP
Sensor Conditioner Solution, pg. 36), being careful not to touch the swab to the
glass bulb of the pH sensor.
1. Rinse the pH/ORP sensor well briey with a small amount of ORP Sensor
Conditioner Solution. DO NOT leave the conditioning solution in the sensor well
for more than 10 seconds.
2. Rinse the pH/ORP sensor 3 times with Sensor Storage Solution.
3. Fill the sensor well with Sensor Storage Solution and let rest for 5 minutes.
35
Page 40

37
XVIII. ACCESSORIES
NOTE: MSDSs are available on the Myron L website for all solutions:
http://www.myronl.com/main/Material_Safety_DS_DL.htm
A. Conductivity/TDS Standard Solutions
Your Po o l Pr o has been factory calibrated with the appropriate
Myron L Company NIST traceable KCl, NaCl, and our own 442™ standard
solutions. Most Myron L conductivity standard solution bottles show
three values referenced at 25°C: Conductivity in microsiemens/
micromhos, the ppm/TDS equivalents based on our 442 Natural
Water™ and NaCl standards. All standards are within ±1.0% of reference
solutions. Available in 2 oz., quarts/liters, and gallon/~3.8 liter bottles.
1. Potassium Chloride (KCl)
The concentrations of these reference solutions are calculated from
data in the International Critical Tables, Vol. 6. The 7000 µS is the
recommended standard. Order KCL-7000
2. 442 Natural Water™
442 Natural Water Standard Solutions are based on the following salt
proportions: 40% sodium sulfate, 40% sodium bicarbonate, and 20%
sodium chloride, which represent the three predominant components
(anions) in freshwater. This salt ratio has conductivity characteristics
approximating fresh natural waters and was developed by the Myron L
Company over four decades ago. It is used around the world for
measuring both conductivity and TDS in drinking water, ground water,
lakes, streams, etc. 3000 ppm is the recommended standard.
Order 442-3000
3. Sodium Chloride (NaCl)
This is especially useful in salt water pools and spas, as sodium chloride
is the major salt component. Most Myron L standard solution labels show
the ppm NaCl equivalent to the conductivity and to ppm 442 values. The
7500 ppm is the recommended standard. Order NACL-7500.
B. pH Buffer Solutions
pH buffers are available in pH values of 4, 7 and 10. Myron L Company
buffer solutions are traceable to NIST certied pH references and are
color-coded for instant identication. They are also mold inhibited and
accurate to within ±0.01 pH units @ 25°C. Order 4, 7 or 10 Buffer.
Available in 2 oz., quarts/liters, and gallon/~3.8 liter bottles.
C. pH Sensor Storage Solution
Myron L pH Sensor Storage Solution prolongs the life of the pH sensor.
Available in 2 oz., quarts/liters, and gallon/~3.8 liter bottles.
D. ORP Sensor Conditioner Solution
Myron L ORP Conditioner Solution removes contaminants and conditions
the ORP electrode.
36
Available in 1 oz. Order ORPCOND1OZ
.
Page 41

E. Soft Protective Carry Cases
Padded Nylon® carrying case features a belt clip for hands-free mobility.
Two colors to choose from:
Blue - Model #: UCC
Desert Tan - Model #: UCCDT ® Registered trade mark of DuPont
F. Hard Protective Carry Cases
Large case with 2 oz. bottles of calibration standard solutions (KCl-7000,
NaCl-7500, 442-3000, 4, 7, & 10 pH buffers and pH storage solution).
Model #: PKPS
Small case (no calibration standard solutions) - Model #: UPP
G. Replacement pH/ORP Sensor
pH/ORP sensor is gel lled and features a unique porous liquid junction.
It is user-replaceable and comes with easy to follow instructions.
Model #: RPR
H. bluDock™ Wireless Data Transfer Accessory Package
This accessory allows the operator to download the Po o l Pr o memory
stack to a spreadsheet on a computer. The package includes bluDock
modied circuit board in the unit, software CD, installation and operating
instructions, and dongle.
Model #: BLUDOCK
XIX. TEMPERATURE COMPENSATION (Tempco)
of Aqueous Solutions
Electrical conductivity indicates solution concentration and ionization
of the dissolved material. Since temperature greatly affects ionization,
conductivity measurements are temperature dependent and
are normally corrected to read what they would be at 25°C.
A. Standardized to 25°C
Conductivity is very accurately measured in the PoolPro by a method
that ignores ll level, electrolysis, electrode characteristics, etc., and
uses a microprocessor to perform temperature compensation. In simpler
instruments, conductivity values are usually assigned an average
correction similar to that of KCl solutions for correction to 25°C. The
correction to an equivalent KCl solution is a standard set by chemists
that standardizes the measurements and allows calibration with precise
KCl solutions. In the PoolPro, this correction can be set to other solutions
or tailored for special measurements or applications.
B. Tempco Variation
Most conductivity instruments use an approximation of the temperature
characteristics of solutions, perhaps even assuming a constant value.
The value for KCl is often quoted simply as 2%/°C. In fact, KCl tempco
37
Page 42

39
varies with concentration and temperature in a non-linear fashion. Other
Chart 1
0 5 10 15 20 25 30 35 40 45 50 55 60
1.500%
1.600%
1.700%
1.800%
1.900%
2.000%
2.100%
2.200%
2.300%
2.400%
2.500%
KCl % / °C
% / °C
Temperature
solutions have more variation still. The Po o l Pr o uses corrections that
change with concentration and temperature instead of single average
values. See Chart 1.
38
C. An Example of 2 different solution selections and the
resulting compensation
How much error results from treating natural water as if it were KCl at
15°C?
A tap water solution should be compensated as 442 with a tempco of
1.68 %/°C, where the KCl value used would be 1.90 %/°C.
Suppose a measurement at 15°C/59°F is 900 microsiemens of true
uncompensated conductivity.
Using a 442 correction of 10 (degrees below 25) x 1.68% indicates the
solution is reading 16.8% low. For correction, dividing by (.832) yields
1082 microsiemens as a compensated reading.
A KCl correction of 10 (degrees below 25) x 1.9% indicates the solution
is reading 19% low. Dividing by (.81) yields 1111 microsiemens for a
compensated reading. The difference is 29 out of 1082 = 2.7%.
Page 43

D. A Chart of Comparative Error
7%
Chart 2
55
(1)%
(2)%
0%
1%
2%
3%
4%
5%
6%
0 5 10 15 20
25
30 35 40 45 50
Temperature
NaCl error with KCl tempco
442 error with KCl tempco
In the range of 1000 µS, the error using KCl on a solution that should be
compensated as NaCl or as 442, is illustrated in the Chart 2 below.
Users wanting to measure natural water based solutions to 1% would
have to alter the internal compensation to the more suitable preloaded
“442” values or stay close to 25°C. Users who have standardized to
KCl-based compensation may want to stick with it, regardless of
increasing error as you get further from 25°C. The Po o l Pr o will provide
the repeatability and convertibility of data necessary for relative values
for process control.
E. Other Solutions
A salt solution like sea water acts like NaCl. An internal correction for NaCl
can be selected for greatest accuracy with such solutions. Many solutions
are not at all similar to KCl, NaCl or 442, however, are still referenced to
one of these for the purpose of commonality.
Clearly, the solution characteristics should be chosen to truly represent
the actual water under test for rated accuracy of ±1%. Many industrial
applications have always been relative measurements seeking a number
to indicate a certain setpoint or minimum concentration or trend. The
Po o l Pr o gives the user the capacity to take data in the “KCl conductivity
39
Page 44

41
units” to compare to older published data, in terms of NaCl or 442, or
may be appropriate. The Po o l Pr o can be used to reconcile data taken
with other compensation assumptions.
XX. CONDUCTIVITY CONVERSION to TOTAL
DISSOLVED SOLIDS (TDS)
Electrical conductivity indicates solution concentration and ionization
of the dissolved material. Since temperature greatly affects ionization,
conductivity measurements are temperature dependent and are
normally corrected to read what they would be at 25°C (ref. Temperature
Compensation, pg. 41).
A. How it’s Done
Once the effect of temperature is removed, the compensated conductivity
is a function of the concentration (TDS). Temperature compensation of
the conductivity of a solution is performed automatically by the internal
processor with data derived from chemical tables. Any dissolved salt at
a known temperature has a known ratio of conductivity to concentration.
Tables of conversion ratios referenced to 25°C have been published by
chemists for decades.
B. Solution Characteristics
Real world applications have to measure a wide range of materials and
mixtures of electrolyte solutions. To address this problem, industrial users
commonly use the characteristics of a standard material as a model for
their solution, such as KCl, which is favored by chemists for its stability.
Users dealing with sea water, etc., use NaCl as the model for their
concentration calculations. Users dealing with freshwater work with
mixtures including sulfates, carbonates and chlorides, the three
predominant components (anions) in freshwater that the Myron L
Company calls “natural water”. These are modeled in a mixture called
“442™” which the Myron L Company markets for use as a calibration
standard, as it does standard KCl and NaCl solutions.
The Po o l Pr o contains algorithms for these 3 most commonly
referenced compounds. In the LCD display, the solution type being used
is displayed on the left.
C. When does it make a lot of difference?
First, the accuracy of temperature compensation to 25°C determines the
accuracy of any TDS conversion. Assume we have industrial process
40
Page 45

water to be pretreated by RO. Assume it is 45°C and reads 1500 µS
uncompensated.
1. If NaCl compensation is used, an instrument would report 1035
µS compensated, which corresponds to 510 ppm NaCl.
2. If 442 compensation is used, an instrument would report 1024
µS compensated, which corresponds to 713 ppm 442.
The difference in values is 40%.
In spite of such large error, some users will continue to take data in
the NaCl mode because their previous data gathering and process
monitoring was done with an older NaCl referenced device.
Those who want true TDS readings that will correspond to evaporated
weight will select the correct Solution Type.
XXI. TEMPERATURE COMPENSATION (Tempco)
and TDS DERIVATION
The Po o l Pr o contains internal algorithms for characteristics of the 3
most commonly referenced compounds. In the display, the solution type
being used is shown to the left.
When taking conductivity measurements, the Solution Selection
determines the characteristic assumed as the instrument reports what a
measured conductivity would be if it were at 25°C. The characteristic is
represented by the tempco, expressed in %/°C. If a solution of 100 µS at
25°C increases to 122 µS at 35°C, then a 22% increase has happened
over this change of 10ºC. The solution is said to have a tempco of 2.2
%/ºC.
Tempco always varies among solutions because it is dependent on their
individual ionization activity, temperature and concentration. This is why
the Po o l Pr o features mathematically generated models for known salt
characteristics that also vary with concentration and temperature.
41
Page 46

43
XXII. pH and ORP
A. pH
1. pH as an Indicator
pH is the measurement of Acidity or Alkalinity of an aqueous solution. It
is also stated as the Hydrogen Ion activity of a solution. pH measures
the effective, not the total, acidity of a solution.
A 4% solution of acetic acid (pH 4, vinegar) can be quite palatable, but
a 4% solution of sulfuric acid (pH 0) is a violent poison. pH provides the
needed quantitative information by expressing the degree of activity of
an acid or base.
In a solution of one known component, pH will indicate concentration
indirectly. However, very dilute solutions may be very slow reading, just
because the very few ions take time to accumulate.
2. pH Units
The acidity or alkalinity of a solution is a measurement of the relative
availabilities of hydrogen (H+) and hydroxide (OH-) ions. An increase
in (H+) ions increases acidity, while an increase in (OH-) ions increases
alkalinity. The total concentration of ions is xed as a characteristic
of water, and balance would be 10
-
7
mol/liter (H+) and (OH-) ions in a
neutral solution (where pH sensors give 0 voltage).
pH is dened as the negative logarithm of hydrogen ion concentration.
Where (H+) concentration falls below 10-7, solutions are less acidic than
neutral, and therefore are alkaline. A concentration of 10-9 mol/liter of
(H+) would have 100 times less (H+) ions than (OH-) ions and be called
an alkaline solution of pH 9.
3. The pH Sensor
The active part of the pH sensor is a thin glass surface that is selectively
receptive to hydrogen ions. Available hydrogen ions in a solution will
accumulate on this surface and a charge will build up across the glass
interface. The voltage can be measured with a very high impedance
voltmeter circuit; the dilemma is to connect the voltmeter to solution on
each side.
The glass surface encloses a captured solution of potassium chloride
holding an electrode of silver wire coated with silver chloride. This is
the most inert connection possible from a metal to an electrolyte. It can
still produce an offset voltage, but using the same materials to connect
to the solution on the other side of the membrane causes the 2 equal
offsets to cancel.
42
Page 47

The problem is, on the other side of the membrane is an unknown test
Glass surface
Figure 30
KCl solution
Electrode wire
Electrode
wire
H
+
ions
Junction
Plug
KCl solution
Figure 31
Junction plug
Platinum button
H+ ions
Electrode wires
Glass
Glass
Surface
solution, not potassium chloride. The outside electrode, also called
the Reference Junction, is of the same construction with a porous
plug in place of a glass barrier to allow the junction uid to contact the
test solution without signicant
migration of liquids through the
plug material. Figure 30 shows
a typical 2 component pair.
Migration does occur, and this
limits the lifetime of a pH junction,
from depletion of solution inside
the reference junction or from
contamination. The junction may
be damaged if dried out because
insoluble crystals may form in a
layer, obstructing contact with test
solutions. See pH/ORP, pg. 42.
4. The Myron L Integral pH Sensor
The sensor in the Po o l Pr o (see
Figure 31) is a single construction
in an easily replaceable package.
The sensor body holds an oversize
solution supply for long life.
The reference junction “wick” is
porous to provide a very stable,
low permeable interface, and is
located under the glass pH sensing
electrode. This construction
combines all the best features of
any pH sensor known.
5. Sources of Error
The basics are presented in
pH/ORP, pg. 42.
a. Reference Junction
The most common sensor problem will be a clogged junction because
a sensor was allowed to dry out. The symptom is a drift in the “zero”
setting at 7 pH. This is why the Po o l Pr o does not allow more than 1 pH
unit of offset during calibration. At that point the junction is unreliable.
b. Sensitivity Problems
Sensitivity is the receptiveness of the glass surface, which can be
diminished by a lm on the surface. This problem also causes long
response time.
43
Page 48

45
c. Temperature Compensation
pH sensor glass changes its sensitivity slightly with temperature, so the
further from pH 7 one is, the more effect will be seen. A pH of 11 at
40°C would be off by 0.2 units. The Po o l Pr o senses the sensor well
temperature and compensates the reading.
B. ORP/Oxidation-Reduction Potential/REDOX
1. ORP as an Indicator
ORP is the measurement of the ratio of oxidizing activity to reducing
activity in a solution. It is the potential of a solution to give up electrons
(oxidize other things) or gain electrons (reduce).
Like acidity and alkalinity, the increase of one is at the expense of the
other, so a single voltage is called the Oxidation-Reduction Potential,
with a positive voltage showing, a solution wants to steal electrons
(oxidizing agent). For instance, chlorinated water will show a positive
ORP value.
2. ORP Units
ORP is measured in millivolts, with no correction for solution temperature.
Like pH, it is not a measurement of concentration directly, but of activity
level. In a solution of only one active component, ORP indicates
concentration. Also, as with pH, a very dilute solution will take time to
accumulate a readable charge.
3. The ORP Sensor
An ORP sensor uses a small platinum surface to accumulate charge
without reacting chemically. That charge is measured relative to the
solution, so the solution “ground” voltage comes from a reference
junction - same as the pH sensor uses.
4. The Myron L ORP Sensor
Figure 31, pg. 43, shows the platinum button in a glass sleeve. The
same reference is used for both the pH and the ORP sensors. Both
pH and ORP will indicate 0 for a neutral solution. Calibration at zero
compensates for error in the reference junction.
A zero calibration solution for ORP is not practical, so the Po o l Pr o
uses the offset value determined during calibration to 7 in pH calibration
(pH 7 = 0 mV). Sensitivity of the ORP surface is xed, so there is no
gain adjustment either.
5. Sources of Error
The basics are presented in pH/ORP, pg. 42, because sources of
error are much the same as for pH. The junction side is the same, and
though the platinum surface will not break like the glass pH surface,
its protective glass sleeve can be broken. A surface lm will slow
the response time and diminish sensitivity. It can be cleaned off with
detergent or acid, as with the pH glass.
44
Page 49

C. Free Chlorine
1. Free Chlorine as an Indicator of Sanitizing Strength
Chlorine, which kills bacteria by way of its power as an oxidizing agent,
is the most popular germicide used in water treatment. Chlorine is not
only used as a primary disinfectant, but also to establish a sufcient
residual level of Free Available Chlorine (FAC) for ongoing disinfection.
FAC is the chlorine that remains after a certain amount is consumed by
killing bacteria or reacting with other organic (ammonia, fecal matter)
or inorganic (metals, dissolved CO2, Carbonates, etc) chemicals in
solution. Measuring the amount of residual free chlorine in treated
water is therefore the best method for determining its effectiveness in
microbial control.
The Myron L Company FCE method for measuring residual disinfecting
power is based on ORP, the specic chemical attribute of chlorine (and
other oxidizing germicides) that kills bacteria and microbes.
2. FCE Free Chlorine Units
The PS6FCE is the rst handheld device to detect free chlorine directly,
by measuring ORP. The ORP value is converted to a concentration
reading (ppm) using a conversion table developed by Myron L Company
through a series of experiments that precisely controlled chlorine levels
and excluded interferants.
Other test methods typically rely on the user visually or digitally
interpreting a color change resulting from an added reagent-dye. The
reagent used radically alters the sample’s pH and converts the various
chlorine species present into a single, easily measured species. This
ignores the effect of changing pH on free chlorine effectiveness and
disregards the fact that some chlorine species are better or worse
sanitizers than others.
The Myron L Company PS6FCE avoids these pitfalls. The chemistry of
the test sample is left unchanged from the source water. It accounts
for the effect of pH on chlorine effectiveness by including pH in its
calculation. For these reasons, the PS6’s FCE feature provides the best
reading-to-reading picture of the rise and fall in sanitizing effectivity of
free available chlorine.
The PS6FCE also avoids a common undesirable characteristic of other
ORP-based methods by including a unique Predictive ORP value in
its FCE calculation. This feature, based on a proprietary model for
ORP sensor behavior, calculates a nal stabilized ORP value in 1 to
2 minutes rather than the 10 to 15 minutes or more that is typically
required for an ORP measurement.
45
Page 50

47
XXIII. SOFTWARE VERSION
Figure 32
Contact the Myron L Company to see if a software upgrade is available.
1. Press key.
2. Press
key until three numbers are displayed as shown
in Figure 32.
3. Press
key, instrument
will time out in ~15 seconds.
46
Page 51

XXIV. GLOSSARY
Anions Negatively charged ions.
See Solution Characteristics, pg. 40.
Algorithm A procedure for solving a mathematical problem.
See Temperature Compensation and TDS Derivation,
pg. 41.
FAC Free Available Chlorine. The amount of chlorine that
remains active in solution and is available for ongoing
disinfection. See Free Chlorine as an Indicator, pg. 45.
FCE FCE™ directly measures ORP, the germ killing
property of chlorine and other oxidizing germicides.
It displays both the ORP reading (in mVDC) as well
as an equivalent free chlorine concentration (in
E
familiar ppm). For more information see FC
™
:
Groundbreaking Measurement of Free Chlorine
Disinfecting Power in a Hand-Held Instrument on
the Myron L Company website.
Logarithm An arithmetic function. The inverse of an exponential
function. See pH Units, pg. 42.
Mineral A term used in the pool & spa industry for SALT
(Sodium Chloride - NaCl). Expressed in parts per
million (ppm).
ORP Oxidation-Reduction Potential or REDOX, See ORP/
Oxidation-Reduction Potential/REDOX, pg. 44.
REDOX An abbreviation for Reduction-Oxidation reactions.
Reaction This is the basic electrochemical process by which
chlorine destroys microbes by grabbing electrons from
the microbe’s proteins, denaturing the protein and
killing the organism. ORP directly measures the
strength of a solutions’ REDOX potential and,
therefore, sanitizing strength.
TDS Total Dissolved Solids or the Total Conductive Ions in a
solution. See Conductivity Conversion to TDS, pg. 40.
Tempco Temperature Compensation
See Temperature Compensation, pg. 41.
For details on specic areas of interest refer to the Table of Contents.
47
Page 52

MYRON L COMPANY
2450 Impala Drive
Carlsbad, CA 92010-7226
USA
Tel: +1-760-438-2021
Fax: +1-760-931-9189
www.myronl.com
Made In USA
PS6FCEOM 17JUN13
 Loading...
Loading...