Page 1

YOUR
LITHO-KIT
GUIDE
™
Page 2

Page 3
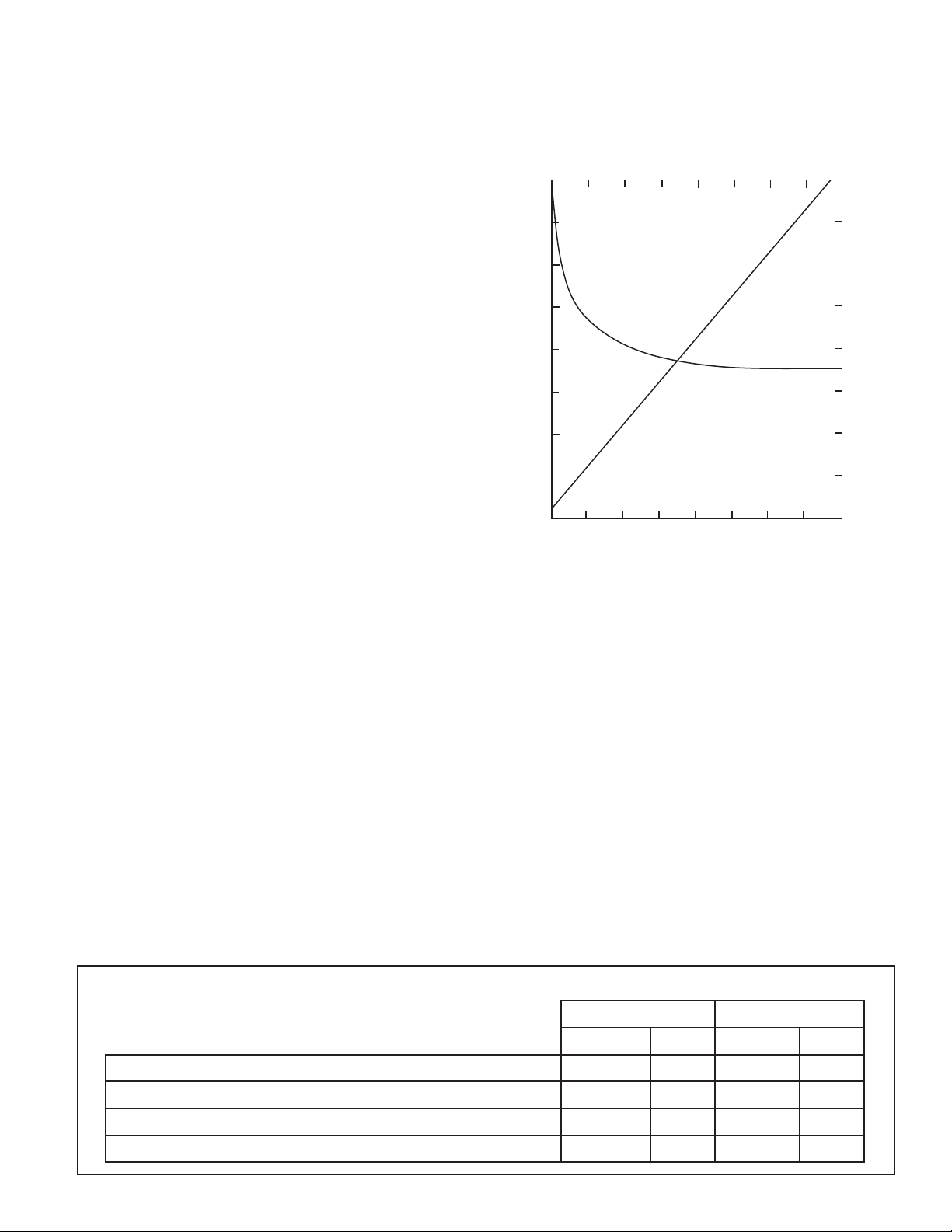
Conductivity (micromhos/cm)
pH
pH
Conductivity
Concentration (oz./gal.)
3500
3000
2500
2000
1500
1000
500
0 1 2 3 4 5 6 7 8
7
6
5
4
3
2
1
INTRODUCTION
Your Litho-Kit™ is a quality control "tool" to help you
print better. It and this Guide will improve your printing
through better control of fountain (dampening) solutions.
The rising popularity of alcohol-free solutions has
increased the need for very careful monitoring of their
conductivity, pH and temperature.
While this Guide offers information we hope will be very
useful, it makes no specic recommendations regarding
fountain solution temperature, concentration, pH or
conductivity values. A good source for such information
is your solution supplier, who is most familiar with
your local conditions. Another source is the Graphic
Arts Technical Foundation, a non-prot research and
educational organization which provided much of the
information in this Guide.
CONDUCTIVITY AND pH: HOW THEY CAN
HELP YOU
The Myron L instrument which is the "heart" of your kit
is either a conductivity instrument or a conductivity/pH
instrument. Both are industrial-quality instruments for
professionals. Reliable even in demanding conditions, they
feature electrodes mounted inside a cell cup for maximum
protection. Details of specications and operation can be
found in the instruction manual in each kit.
Conductivity is the ability of a solution to pass an
electrical current. The amount of current passed
depends on the concentration of ions, or electrically
charged particles in the solution. The higher the
concentration of ions, the higher the degree of
conductivity. The unit of conductivity measurement is the
microsiemen (also called the micromho).
Traditionally, pH, a measure of the degree of acidity
or alkalinity, was used to check fountain solution
concentration. Today, however, conductivity testing
is recognized as a much more accurate method.
Many modern dampening solutions are pH stabilized
(or buffered), so only small changes in pH are seen,
even when solution strength is dramatically changed.
The conductivity, however, increases as solution
concentration rises.
The advantage of checking fountain solution
concentration with conductivity, rather than pH, can be
seen in the following graph.
Concentration vs. pH and Conductivity for a hypothetical
combination of fountain solution concentrate and water
Notice how the pH levels off, but conductivity values rise
on a straight-line basis as the concentration increases.
This "linear" relationship allows you to easily match the
conductivity value to a specic concentration of your
own solution.
Even though pH usually is not the best method to check
the concentration of fountain solution, it is still very
important and must be checked regularly. The pH of acid
dampening solution affects sensitivity, plate-life, inkdrying, etc. Also, pH can change during a run if the paper
has a high acid or alkaline content. Conclusion: pH must
be maintained at the proper level for good printing.
The table below lists recommendations for checking
fountain solution conductivity and pH.
RECOMMENDED TESTING METHOD
TYPE OF FOUNTAIN SOLUTION
ACID X X X X
BUFFERED ACID X X X
NEUTRAL X X X
ALKALINE X X X
MIXING ON PRESS
COND. pH COND. pH
Page 4

Because contaminants in water are often ionized,
conductivity has long been recognized as a good
overall indicator of water quality. Portable conductivity
instruments and/or in-line conductivity monitor/
controllers are normally included in industrial reverse
osmosis and deionization water treatment systems.
If the system in your plant did not include a Myron L
portable instrument, the instrument in your Litho-Kit™
can be used to check:
1. System Efciency
2. Tap Water Quality Fluctuations
Other models designed specically for water
treatment equipment testing can be found in the
REPLACEMENTS/OPTIONS section of this Guide.
ACCESSORIES IN YOUR LITHO-KIT
Calibration solutions ensure continued instrument
accuracy. Information on the procedures and frequency
of recalibration will be found in your instrument
Operating Instructions booklet. The small bottles
included in the kit can be relled with the 1 L/32 oz.
solutions found in REPLACEMENTS/OPTIONS.
™
provide is a solution concentration graph. After plotting
various mixtures of etch with your water, it will be very
easy to later check the strength of your dampening
solution on the press. Then, you can see which
concentration results in the best quality printing.
The following procedure can be accomplished using the
blank graph form at the end of this guide. This form may
be copied for future use.
1. Measure the conductivity and pH
of water normally used to make the
dampening solution. Fill a clean
3,8 L /1 gallon bottle with water.
2. Add 29,6 mL/1 oz. of fountain solution
concentrate. Remeasure both conductivity
and pH. Record these values.
3. Add another ounce (59,15 mL/2 oz. total) of
fountain solution concentrate and
remeasure both conductivity and pH.
Repeat this process (in 14,79 mL/1/2 oz.
additions, if preferred) until the amount
of fountain solution added exceeds the
manufacturer's recommendations.
The syringe included in your kit is the easiest way to take
a dampening solution sample. This is especially true
when the sample is drawn from the fountain pan (the
most representative source). Carefully transfer solution
directly into the meter's cell cup for testing. Make sure to
always rinse the syringe with clean water after use.
Using the thermometer to check dampening solution
temperature regularly during a run is important for
continued quality printing. Dampening solutions,
especially alcohol-free types are more viscous (therefore,
more effective) when chilled to 10-13º C/ 50-55°F.
Since it is normal for the temperature to increase slightly in
the fountain pan, it is preferable to check temperature there,
rather than in the recirculator. The temperature difference
between the recirculator and pan usually should not be
more than + 1 °C/2 °F. If the difference is greater, and pan
solution temperature is not within the ideal range, steps can
be taken to reduce the differential. These include insulating
solution supply and return lines and moving the recirculator.
For advice on ideal solution and how to maintain it, see
your local supplier.
RECOMMENDATIONS FOR USING YOUR
LITHO-KIT
PLOTTING A CONCENTRATION GRAPH
One of the most useful benets your Litho-Kit™ can
™
4. Plot these values on the graph form
provided.
5. Make new charts when changing
brands of dampening solution, or tap
water quality changes more than
+ 50 microsiemens (micromhos).
ADDITIONAL RECOMMENDATIONS
1. Discuss your printing requirements with your
chemical supplier to obtain the best dampening
solution for your local water and other conditions.
2. Carefully follow the manufacturer's mixing
instructions.
3. Run the recommended mixture of dampening
solution and monitor its printability. Communicate
this information back to the dampening solution
manufacturer.
4. Check dampening solutions regularly with your
Myron L instrument and thermometer. Paper
coating, ink bleed, and blanket or roller cleaners
can contaminate dampening solution. Take
temperature, pH and conductivity readings after
every 1-3 hours of press operation. Record these
readings in the press log book. Keep the solution
at the concentration you have found works best.
Page 5

5. As the press run continues, observe the
changes in pH and conductivity. When printing
problems such as plugging or scumming
begin, the dampening solution is probably
contaminated. Record your ndings in the press
log book and remix a fresh batch of solution.
6. If alcohol is used in your fountain solution, it
should be added after the solution has been
mixed to the desired conductivity range. Alcohol
has no conductivity, and it dilutes the solution
conductivity value. To properly monitor a
solution containing alcohol while running, the
reading after dilution should be used as the
"standard”.
7. Drain and clean your dampening system
weekly.
M6/pH
512M5
6PII
REPLACEMENT/OPTIONS
These items can be obtained from your local dealer:
Item Description
M6/PH Conductivity/pH meter (Ranges:
0-5000 µS; 2-12 pH)
512M5 Conductivity meter (Range: 0-5000 µS)
EP Conductivity meter for DI water system
testing
(5 ranges: 0-0.5, 5, 50, 500, 5000 µS)
EP11/PH Conductivity/pH meter for checking
reverse osmosis water treatment
systems
(4 conductivity ranges: 0-10, 100, 1000,
10,000 µS; pH range 2-12)
6PII Conductivity, TDS, Resistivity, pH, ORP
& Temperature
CLK Litho-Kit only (without instrument) for
512M5 or other conductivity model
PLK Litho-Kit only (without instrument) for
M6/PH or other conductivity/pH model
ULK Litho-Kit only (without instrument) for
Ultrameter 6PII instruments
pH 4 Buffer* pH calibration solution
pH 7 Buffer* pH calibration solution
pH 10 Buffer* pH calibration solution
442-3000* 3900 microsiemens Conductivity
Standard Solution
KCl-7000* 7000 microsiemens Conductivity
Standard Solution
CSFP Calibrating Solution Four-Pack (1 ea., 2oz
bottles pH 4, 7, 10 & 442-3000)
PS35 Plastic Syringe (35 CC)
TM Thermometer
*Available in 2oz., quart and gallon sizes
Page 6

FOUNTAIN CONCENTRATE CONTROL WORKSHEET
FOR
DATE
OPERATOR
WATER pH
WATER CONDUCTIVITY
FOUNTAIN BRAND
3500
Concentration
(oz. per gallon)
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
4.5
5.0
5.5
6.0
Conductivity
(Microsiemens)
pH
9
Conductivity
(microsiemens)
3000
2500
2000
1500
1000
500
0
8
7
6
pH
5
4
3
2
0 1 2 3 4 5 6
Concentration
(oz. per gallon)
Page 7

Page 8

MYRON L COMPANY
2450 Impala Drive
Carlsbad, CA 92010-7226
USA
Tel: +1-760-438-2021
Fax: +1-760-931-9189
E-Mail: info@myronl.com
techquestions@myronl.com
www.myronl.com
Made in USA
ACCURACY • RELIABILITY • SIMPLICITY
© Myron L Company 2008 LKG 10-08
 Loading...
Loading...