Page 1
TechPro
™
Operation
Manual
Model AR1
MYRON L
COMPANY
10-02 (WEB-EG)
Page 2
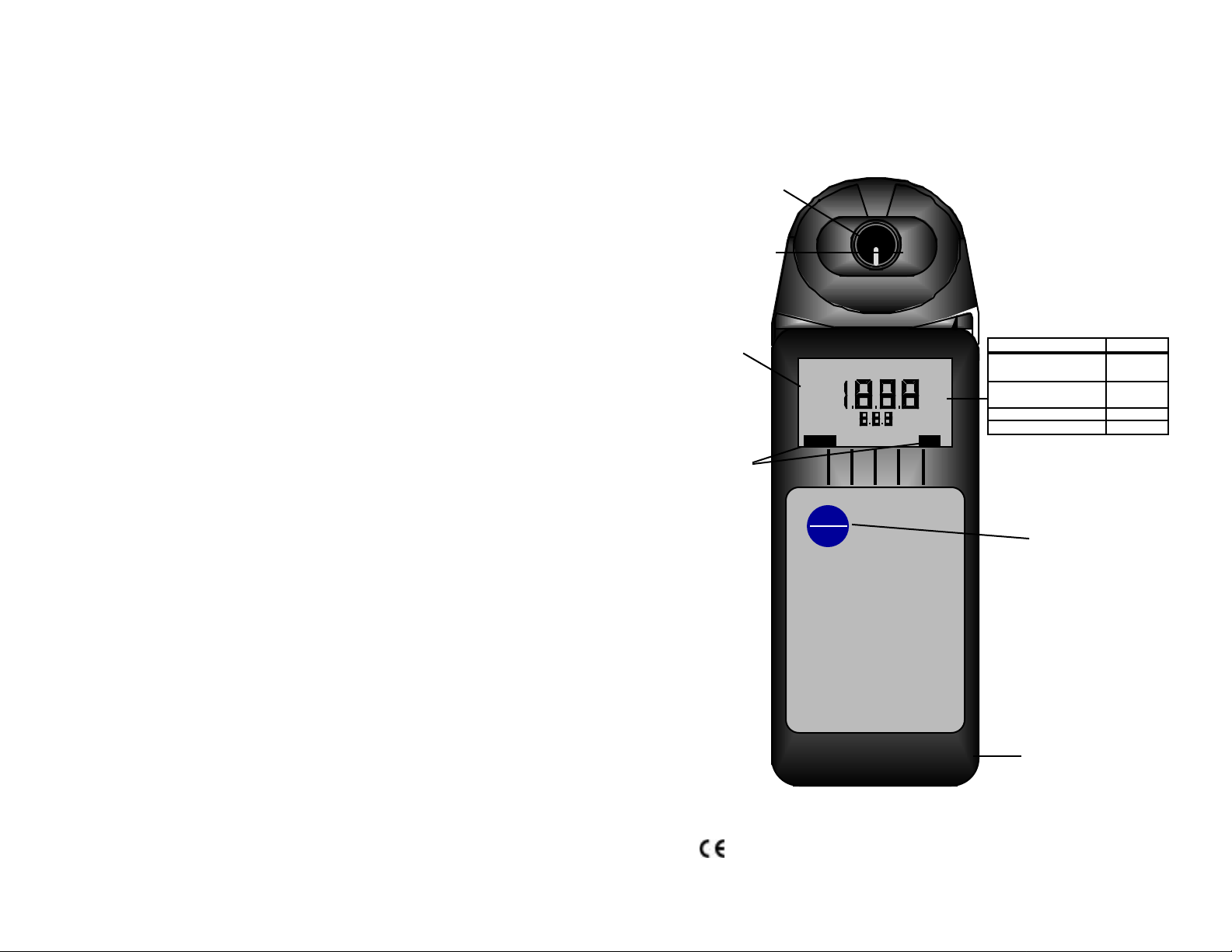
Instrument Illustration
CONDUCTIVITY
METER
Conductivity Cell
(Built-in
Electrodes)
Temperature
Sensor
User selectable
temperature
compensation
ratios (Solution
Selection)
KCl
NaCl
442
Icons for either
Conductivity or
Total Dissolved
Solids (TDS)
CONDUCTIVITY
LO BATT
COND
TDS
Units of Measurement
mS - millisiemens/cm
(millimhos/cm)
mS
µS
PPM
PPT
°C
TDSCOND
µS - microsiemens/cm
(micromhos/cm)
PPM - parts per million
PPT - parts per thousand
Parameter
Conductivity
Conductivity
TDS
TDS
Measurement key:
User selectable for either
Conductivity or TDS
(Switch Selectable)
AR1
MYRON L
COMPANY
For detailed explanations, see Table of Contents
Wrist/neck strap slot
(user supplied)
1-12-99
1
Page 3
FEATURES and SPECIFICATIONS
A. Features
• Superior resolution 3 1/2 digit LCD
• Accuracy of ±1% of full scale
• All electrodes are internal for maximum protection
• Latest electrode cell technology
• Water resistant
• Autoranging Conductivity/TDS
• Easy calibration
• User selectable Conductivity/TDS modes
• 3 “User Selectable” solution conversions (tempcos)
• Temperature Accuracy of ±1° C/F
B. General Specifications
Display 3 1/2 Digit LCD
Dimensions (LxWxH) 7.7x2.7x2.5 in.
196x68x64 mm
Weight 10.1oz./290g
Case Material ABS
Cond/TDS Cell Material ABS
Cond/TDS Cell Capacity 0.2 oz./5 ml
Power 9V Alkaline Battery
Battery Life >100 Hours/5000 Readings
Operating/Storage Temperature 32-132°F/0-55°C
Protection Ratings IP64/NEMA 3
D. Warranty/Service
The Myron L AR1 has a limited two year warranty. If an instrument fails to
operate properly, see Troubleshooting Chart, pg. 13. The battery is userreplaceable. For other service, return the instrument prepaid to the
Myron L Company.
MYRON L COMPANY
2450 Impala Drive
Carlsbad, CA 92010
USA
760-438-2021
If, in the opinion of the factory, failure was due to materials or
workmanship, repair or replacement will be made without charge. A
reasonable service charge will be made for diagnosis or repairs due to
normal wear, abuse or tampering. This warranty is limited to the repair or
replacement of the AR1 only. The Myron L Company assumes no other
responsibility or liability.
E. TechPro™ Series Models
TechPro Series Models pH1 AR1 ARH1
Parameters pH Conductivity or TDS, Conductivity or TDS,
& Temperature & Temperature pH & Temperature
C.Specification Chart
Additional information available on our web site at:
www.myronl.com
AR1 Conductivity TD S Temperature
Ranges 0-1999 µS 0-1999 ppm 0-71° C
2-19.99 mS 2-19.99 ppt 32 - 160° F
in 3 autoranges in 3 autoranges
Resolution 0.1 (<200 µS) 0.1 (<200 ppm) 0.1° C/F
1 (<2000 µS) 1 (<2000 ppm)
0.01 (>2 mS) 0.01 (>2 ppt)
Accuracy ±1% of Full Scale ±1.0° C/F
Auto Temperature 0-71° C
Compensation 32 - 160° F
Conductivity or KCl, NaCl or 442™
TDS Ratios
2 3
Page 4

TABLE OF CONTENTS
Instrument Illustration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
FEATURES and SPECIFICATIONS. . . . . . . . . . . . . . . . . . . . . . . . . 2
A. Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
B. General Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . 2
C. Specification Chart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
D. Warranty/Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
E. TechPro Series Models . . . . . . . . . . . . . . . . . . . . . . . . .3
I. INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
II . RULES of OPERATION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
A. Operation in a Nutshell. . . . . . . . . . . . . . . . . . . . . . . . . . 6
B. Characteristics of the Keys . . . . . . . . . . . . . . . . . . . . . . 6
C. Operation of the Keys . . . . . . . . . . . . . . . . . . . . . . . . . . 6
1. Measurement Keys in General . . . . . . . . . . . . 6
2. COND/TDS Key . . . . . . . . . . . . . . . . . . . . . . . . .7
III. AFTER USING the AR1. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
IV. THE SPECIFIC RECOMMENDED MEASURING
PROCEDURES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
IX. CARE and MAINTENANCE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
A. Temperature Extremes . . . . . . . . . . . . . . . . . . . . . . . . 12
B. Battery Replacement (LO BATT) . . . . . . . . . . . . . . . . 12
C. Cleaning Cell Cup . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
X. TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
XI. ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
A. Conductivity/TDS Standard Solutions . . . . . . . . . . . 14
B. Soft Protective Case . . . . . . . . . . . . . . . . . . . . . . . . . . 14
C. pH Buffer Solutions . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
XII. TEMPERATURE COMPENSATION . . . . . . . . . . . . . . . . . . . . . . . 15
A. Standardized to 25°C . . . . . . . . . . . . . . . . . . . . . . . . . .15
B. Tempco Variation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
C. An Example . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
D. A Chart of Comparative Error . . . . . . . . . . . . . . . . . . . .16
E. Other Solutions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
XIII. CONDUCTIVITY CONVERSION to TOTAL DISSOLVED SOLIDS
(TDS) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
A. How it’s Done . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
B. Solution Characteristics . . . . . . . . . . . . . . . . . . . . . . . .17
C. When does it make a lot of difference? . . . . . . . . . . . 18
V. SOLUTION SELECTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
A. Why Solution Selection is Available . . . . . . . . . . . . . . .8
B. The 3 Solution Types . . . . . . . . . . . . . . . . . . . . . . . . . . .8
C. Procedure to Select a Solution . . . . . . . . . . . . . . . . . . 8
D. Procedure to Select the Units of Measurement . . . . .9
VI. CALIBRATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
A. Calibration Intervals . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
B. Rules for Calibration in the AR1. . . . . . . . . . . . . . . . . . .9
1. Calibration Steps . . . . . . . . . . . . . . . . . . . . . . . .9
2. Calibration Limits . . . . . . . . . . . . . . . . . . . . . . . .9
C. Calibration Procedures. . . . . . . . . . . . . . . . . . . . . . . . .10
VII. CALIBRATION INTERVALS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
A. Suggested Intervals . . . . . . . . . . . . . . . . . . . . . . . . . . .10
B. Calibration Tracking Records . . . . . . . . . . . . . . . . . . . 10
C. Conductivity or TDS Practices . . . . . . . . . . . . . . . . . . 11
VIII. CHANGING from CENTIGRADE to FAHRENHEIT. . . . . . . . . . . . 11
4 5
XIV. TEMPERATURE COMPENSATION (Tempco)
and TDS DERIVATION. . . . . . . . . . . . . . . . . . . . . . . . . . . .18
XV. GLOSSARY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20
NOTES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . 21
Page 5

I. INTRODUCTION
Thank you for selecting the TechPro™ Series, Model AR1, one of the
Myron L Company’s latest in a new line of digital instruments utilizing
advanced circuitry. This circuitry makes it very accurate and easy to use
(see pages 2 & 3 for Features and Specifications on this and other
models). For your convenience, on the bottom side of your AR1 is a brief
set of instructions.
Special note ...... Conductivity/TDS require mathematical correction to
25°C values (ref. Temperature Compensation, pg. 15).
On the left side of the AR1 liquid crystal display is shown an indicator of
the salt solution characteristic used to model temperature compensation
(tempco) of conductivity or its TDS conversion. The indicator can be KCl,
NaCl or 442. Internal selection affects the temperature correction of
conductivity, and the calculation of TDS from compensated conductivity
(ref. Conductivity Conversion to TDS, pg. 17).
The selection can affect the reported conductivity of hot or cold
solutions, and will change the reported TDS of a solution. Generally,
using KCl for conductivity and 442™ (Natural Water characteristic) for TDS
will reflect present industry practice for standardization. NaCl may also be
selected for either conductivity or TDS as is needed.
Your instrument, as shipped from the factory, is set for conductivity with
the KCl tempco. However, if you are measuring natural waters and wish to
have maximum accuracy, you may want to change it to the 442 tempco
setting. To change the Tempco or to read in TDS/ppm, see Solution
Selection on pg. 8.
2. COND/TDS Key
This key is used with solution in the Conductivity Cell.
Precautions:
• While filling cell cup ensure no air bubbles cling on the cell wall.
• If the proper solution is not selected (KCl, NaCl or 442) refer to
Why Solution Selection is Available, pg. 8.
Solution to be tested is introduced into the conductivity cell and a press
COND
of displays conductivity or Total Dissolved Solids (TDS) with units
TDS
on the right. On the left is shown the solution type selected for
conductivity. An overrange condition will show only [- - - -].
III. AFTER USING the AR1
Rinse out the cell cup with clean water. Do not scrub the cell. For oily
films, squirt in a foaming non-abrasive cleaner, and rinse. Even if a very
active chemical discolors the electrodes, this does not affect the
accuracy; leave it alone (ref. Cleaning Cell Cup, pg. 12).
IV. THE SPECIFIC RECOMMENDED MEASURING
PROCEDURES
If the proper solution is not selected (KCl, NaCl or 442), see Solution
Selection, Pg. 8.
II. RULES of OPERATION
NOTE: After sampling high concentration solutions or temperature
A. Operation
extremes, more rinsing may be required.
Using the instrument is simple:
• Rinse the Conductivity cell with test solution 3 times and Refill.
• Pressing COND/TDS key starts a 20 second timer.
• Note the value displayed. It’s that simple!
1. Rinse cell cup 3 times with sample to be measured. (This
Measuring Conductivity/Total Dissolved Solids (TDS)
conditions the temperature compensation network and prepares
B. Characteristics of the Key
the cell).
• Your AR1 is designed to provide quick, easy, accurate
measurements by simply pressing one key.
C. Operation of the Key (See Instrument Illustration on page 1)
1. Measurement Keys in General
The measurement key turns on the instrument in the mode selected.
The mode is shown at the bottom of the display, and the measurement
2. Refill cell cup with sample.
3. Press .
COND
TDS
4. Take reading. A display of [- - - -] indicates an overrange
condition.
units appear at the right.
6 7
Page 6

V. SOLUTION SELECTION
A. Why Solution Selection is Available
Conductivity and TDS require temperature correction to 25°C values (ref.
Standardized to 25°C, pg. 15). Selection determines the temperature
correction of conductivity and calculation of TDS from compensated
conductivity (ref. Conductivity Conversion to TDS, pg. 17).
D. Procedure to Select the Units of Measurement
i.e., µS to ppm
1. Dry Instrument THOROUGHLY.
2. Remove the 4 bottom screws and open Instrument.
B. The 3 Solution Types
On the left side of the display is the salt solution characteristic used to
model temperature compensation of conductivity and its TDS
conversion. Generally, using KCl for conductivity and 442 (Natural Water
characteristic) for TDS will reflect present industry practice for
standardization. Your instrument as shipped from the factory is set for
conductivity with the KCl tempco. If you are measuring natural waters and
wish to have maximum accuracy, it may be better to change it to the 442
setting. However, selecting NaCl for either conductivity or TDS may best
reflect your specific specialized needs (ref. Solution Characteristics, pg.
17).
C. Procedure to Select a Solution
NOTE: Check display to see if solution displayed (KCl, NaCl or 442) is
already the type desired. If not:
1. Dry Instrument THOROUGHLY.
2. Remove the 4 bottom screws and carefully open Instrument.
3. Locate dip switch labeled “TEMP COMP” on the right side of the
circuit board. Switch positions are 1-4 (left to right).
4. Set switch numbers 1 and 2 to the desired position.
Note: Factory setting is for KCl - both switches UP or ON.
3. Locate dip switch labeled “TEMP COMP” located on the right
side of the circuit board. Switch positions are 1-4 (left to right).
4. Set switch number 3 to the desired position - COND or TDS.
Note: Factory setting is for COND - DOWN or OFF.
5. Carefully turn instrument over and press the key. The
COND
TDS
correct icon “µS” or “PPM” should be shown on the right side of
the display.
6. Replace bottom, ensuring the sealing gasket is installed in the groove
of the top half of case. Tighten screws securely. (Do NOT overtighten)
7. Recalibrate as necessary. See Calibration Procedures, pg. 10.
In the first five sections, you have learned all
you need to make accurate measurements.
The following sections contain calibration,
advanced operations, and technical information.
VI. CALIBRATION
A. Calibration Intervals
Generally, calibration is recommended about once per month with
Conductivity or TDS solutions.
5. Carefully turn instrument over and press the key. The
correct icon “KCl”, NaCl” or “442” should be shown on the left
side of the display.
COND
TDS
B. Rules for Calibration in the AR1
1. Calibration Steps
The calibration is accomplished by a Calibration Control located under the
respective cap plug located on the bottom of the instrument.
After pressing the COND/TDS key, the reading is changed/adjusted to
6. Replace bottom, ensuring the sealing gasket is installed in the groove
match the known standard value.
of the top half of case. Tighten screws securely. (Do NOT overtighten)
2. Calibration Limits
7. Recalibrate as necessary. See Calibration, pg. 9.
In Conductivity or TDS, the inability to calibrate may indicate improper or
contaminated calibration solution, or a damaged conductivity cell.
8 9
Page 7

C. Calibration Procedures
C. Conductivity or TDS Practices to Maintain Calibration
a. Rinse conductivity cell three times with proper standard (KCl,
NaCl or 442) (ref. Conductivity/TDS Standard Solutions, pg. 14).
b. Refill conductivity cell with same standard solution.
c. Press key. If reading is acceptable, end procedure. If
COND
TDS
reading is unacceptable, continue.
d. Remove cap plug labeled COND CAL on bottom of Instrument.
e. Refill conductivity cell with same standard solution.
f. While pressing the key, adjust COND CAL Control with
COND
TDS
finger until the display agrees with the value on the standard
solution bottle.
g. Repeat steps b. & c. to verify the setting.
h. Replace bottom cap plug securely to maintain water resistance.
The COND/TDS Calibration procedure is now complete.
VII. CALIBRATION INTERVALS
There is no simple answer as to how often one should calibrate an
instrument. The AR1 is designed to not require frequent recalibration.
The most common sources of error were eliminated in the design, and
there are simple electromechanical adjustments. Still, to ensure specified
accuracy, any instrument has to be checked against chemical standards
occasionally.
1. Clean oily films or organic material from the cell electrodes with
foaming cleaner or mild acid. Do not scrub inside the cell.
2. Calibrate with solutions close to the measurements you make.
Readings are compensated for temperature based on the
type of solution. If you choose to measure tap water with a
KCl compensation, which is often done (ref. Temperature
Compensation, pg. 15), and you calibrate with 442 solution
because it is handy, the further away from 25°C you are, the
more error you have. Your records of calibration changes
will reflect temperature changes more than the instrument’s
accuracy.
3. Rinse out the cell with pure water after making measurements.
Allowing crystals to form in the cell contaminates future samples.
VIII. CHANGING from CENTIGRADE to FAHRENHEIT
(Note: °F to °C is the reverse)
1. Dry Instrument THOROUGHLY.
2. Remove the 4 bottom screws and carefully open Instrument.
3. Locate dip switch labeled “TEMP COMP” on the right side of the
circuit board. Note: Factory setting is degrees “C”.
4. Set switch number 4 to the down position.
5. Carefully turn instrument over and press the key. The
COND
TDS
displayed reading will be in Fahrenheit “°F”.
A. Suggested Intervals
On the average, we expect calibration need only be checked monthly.
Measuring some solutions will require more frequent intervals.
B. Calibration Tracking Records
6. Replace bottom, ensuring the sealing gasket is installed in the
groove of the top half of case. Tighten screws securely.
IX. CARE and MAINTENANCE
To minimize your calibration effort, keep records. If adjustments you are
making are minimal for your application, you can check less often.
Changes in conductivity calibration should be recorded in percent.
The AR1 should be rinsed with clean water after each use. Solvents
should be avoided. Shock damage from a fall may cause instrument
failure.
Calibration is purposely limited in the AR1 to approximately ±8% for the
conductivity cell because more than that indicates damage, not drift.
10 11
Page 8

A. Temperature Extremes
Solutions in excess of 160°F/71°C should not be placed in the cell cup
area; this may cause damage. Care should be exercised not to exceed
rated operating temperature. Leaving the AR1 in a vehicle or storage
shed on a hot day can easily subject the instrument to over 150°F. This
will void the warranty.
B. Battery Replacement (LO BATT)
Dry Instrument THOROUGHLY. Remove the four bottom screws.
Open instrument. Carefully detach battery from circuit board. Replace
with 9 volt alkaline battery. Replace bottom, ensuring the sealing gasket
is installed in the groove of the top half of case. Tighten screws evenly
and securely. (Do NOT overtighten)
C. Cleaning Cell Cup
The cell cup should be kept as clean as possible. Flushing with clean
water following use will prevent buildup on electrodes. However, if very
dirty samples — particularly scaling types — are allowed to dry in the cell
cup, a film will form. This film reduces accuracy. When there are visible
films of oil, dirt, or scale in the cell cup or on the electrodes, use a foaming
non-abrasive household cleaner. Rinse out the cleaner, and your AR1 is
ready for accurate measurements.
X. TROUBLESHOOTING CHART
Check connections or replace battery.
(ref. Battery Replacement, pg. 12).
Clean cell cup and electrodes.
Corrective ActionSymptom
(ref. Cleaning Cell Cup, pg. 12)
Clean cell cup and electrodes (ref.
Cleaning Cell Cup, pg. 12).
Possible Cause
Battery weak or not connected.
No display, even though
12 13
Film or deposits on electrodes.
Unstable Conductivity or
measurement key pressed.
Film or deposits on electrodes.
TDS readings.
Unable to calibrate
Conductivity or TDS.
Page 9

XI. ACCESSORIES
A. Conductivity/TDS Standard Solutions
Your AR1 has been factory calibrated with the appropriate Myron L
Company NIST traceable standard solution. Most Myron L conductivity
standard solution bottles show three values referenced at 25°C:
Conductivity in microsiemens/micromhos and the ppm/TDS equivalents
based on our 442 Natural Water™ and NaCl standards. All standards are
within ±1.0% of reference solutions.
1. Potassium Chloride (KCl)
The concentrations of these reference solutions are calculated from data
in the International Critical Tables, Vol. 6. The 1800 µS or 18,000 µS are
the recommended standards. Order KCl-1800 or KCl-18,000.
2. 442 Natural Water™
442 Natural Water Standard Solutions are based on the following salt
proportions: 40% sodium sulfate, 40% sodium bicarbonate, and 20%
sodium chloride which represent the three predominant components
“anions” in freshwater). This salt ratio has conductivity characteristics
approximating fresh natural waters and was developed by the Myron L
Company over three decades ago. It is used around the world for
measuring both conductivity and TDS in drinking water, ground water,
lakes, streams, etc. The 1500 ppm or 15,000 ppm are the recommended
standards. Order 442-1500 or 442-15,000.
3. Sodium Chloride (NaCl)
This is especially useful in sea water mix applications, as sodium chloride
is its major salt component. Most Myron L standard solution labels show
the ppm NaCl equivalent to the conductivity and to ppm 442 values. The
14.0 mS is the recommended standard. Order NaCl-14.0.
B. Soft Protective Case
Padded Cordura® Nylon carrying case features a belt clip for hands-free
mobility. Order Model: UCC ® Registered trade mark of DuPont
C. pH Buffer Solutions
For your Myron L pH instruments; pH buffers are available in pH values of
4, 7 and 10. Myron L Company buffer solutions are traceable to NIST
certified pH references and are color-coded for instant identification.
They are also mold inhibited and accurate to within ±0.01 pH units @
25°C. Order 4, 7 or 10 buffer.
XII. TEMPERATURE COMPENSATION
(Tempco) of Aqueous Solutions
Electrical conductivity indicates solution concentration and ionization of
the dissolved material. Since temperature greatly affects ionization,
conductivity measurements are temperature dependent and are normally
corrected to read what they would be at 25°C.
A. Standardized to 25°C
Conductivity is very accurately measured in the AR1 by a method that
ignores fill level, electrolysis, electrode characteristics, etc., and uses a
unique circuit to perform temperature compensation. In simpler
instruments, conductivity values are usually assigned an average
correction similar to KCl solutions for correction to 25°C. The correction to
an equivalent KCl solution is a standard set by chemists. It standardizes
the measurements and allows calibration with precise KCl solutions
recognized for stability. In the AR1, this correction can be set to either
KCl, NaCl or 442 to best match your applications.
B. Tempco Variation
Most conductivity instruments use an approximation of the temperature
characteristics of solutions, perhaps even assuming a constant value.
The value for KCl is often quoted simply as 2%/°C. In fact, KCl tempco
2.500%
2.400%
2.300%
2.200%
2.100%
2.000%
1.900%
1.800%
1.700%
1.600%
1.500%
% / °C
KCl % / °C
Temperature
0 5 10 15 20 25 30 35 40 45 50 55 60
Chart 1
varies with concentration and temperature in a non-linear fashion. Other
solutions have more variation still. The AR1 uses corrections that
14 15
Page 10

change with concentration and temperature instead of single average
values.
C. An Example of 2 different solution selections and the
resulting compensation:
How much error results from treating natural water as if it were KCl at
15°C?
A tap water solution should be compensated as 442 with a tempco of
1.68 %/°C, where the KCl value used would be 1.90 %/°C.
Suppose a measurement at 15°C (or 59°F) is 900 microsiemens of true
uncompensated conductivity.
Users wanting to measure natural water based solutions to 1% would
have to alter the internal compensation to the more suitable preloaded
“442” values or stay close to 25°C. Some who have standardized to KCl
based compensation may want to stick with it, regardless of increasing
error as you get further from 25°C. The AR1 will provide the repeatability
and convertibility of data needed for relative values for process control.
E. Other Solutions
A salt solution like sea water or liquid fertilizer acts like NaCl. An internal
correction for NaCl can be selected for greatest accuracy with such
solutions. Many solutions are not at all similar to KCl, NaCl or 442. A sugar
solution, or a silicate, or a calcium salt at a high or low temperature may
require a value peculiar to the application to provide readings close to the
true compensated conductivity.
Using a 442 correction of 10 (degrees below 25) x 1.68% indicates the
solution is reading 16.8% low. For correction, dividing by (.832) yields
1082 microsiemens as a compensated reading.
A KCl correction of 10 (degrees below 25) x 1.9% indicates the solution
is reading 19% low. Dividing by (.81) yields 1111 microsiemens for a
compensated reading. The difference is 29 out of 1082 = 2.7%.
D. A Chart of Comparative Error
In the range of 1000 µS, the error using KCl on a solution that should be
compensated as NaCl or as 442, is shown in the graph below.
7%
442 error with KCl tempco
6%
NaCl error with KCl tempco
5%
4%
3%
2%
1%
0%
(1)%
Temperature
(2)%
0 5 10 15 20 25 30 35 40 45 50 55
Chart 2
16 17
Clearly, the solution characteristics should be chosen to truly represent
the actual water under test for rated accuracy of ±1% of full scale. Many
industrial applications have always been relative measurements seeking a
number to indicate a certain setpoint or minimum concentration or trend.
The AR1 gives the user the capability to take data in “KCl conductivity
units” to compare to older published data, as in terms of NaCl or 442, or
as may be appropriate.
XIII. CONDUCTIVITY CONVERSION to TOTAL
DISSOLVED SOLIDS (TDS)
Electrical conductivity indicates solution concentration and ionization of
the dissolved material. Since temperature greatly affects ionization,
conductivity measurements are temperature dependent and are normally
corrected to read what they would be at 25°C (ref. Temperature
Compensation, pg. 15).
A. How it’s Done
Once the effect of temperature is removed, the compensated
conductivity is a function of the concentration (TDS). Temperature
compensation of the conductivity of a solution is performed automatically
by the internal processor, using data derived from chemical tables. Any
dissolved salt at a known temperature has a known ratio of conductivity to
concentration. Tables of conversion ratios referenced to 25°C have been
published by chemists for decades.
B. Solution Characteristics
Real world applications have to measure a wide range of materials and
mixtures of electrolyte solutions. To solve this problem, industrial users
commonly use the characteristics of a standard material as a model for
Page 11

their solution, like the KCl favored by chemists for its stability.
Users dealing with sea water, etc., use NaCl as the model for their
concentration calculations. Users dealing with freshwater work with
mixtures including sulfates, carbonates and chlorides, the three
predominant components (anions) in freshwater that Myron L Company
calls “natural water”. These are modeled in a mixture called “442” which
the Myron L Company markets for use as a calibration standard, as it does
standard KCl and NaCl solutions.
C. When does it make a lot of difference?
First, the accuracy of temperature compensation to 25°C determines the
accuracy of any TDS conversion. Assume we have industrial process
water to be pretreated by R.O. Assume it is 45°C and reads 1500 µS
uncompensated.
1. If NaCl compensation is used, an instrument would report 1035
µS compensated, which corresponds to 510 ppm NaCl.
2. If 442 compensation is used, an instrument would report 1024
µS compensated, which corresponds to 713 ppm 442.
The difference in values is 40%.
In spite of such large error, some users will continue to take data in the
NaCl mode because their previous data gathering and process
monitoring was done with an older NaCl referenced device.
Another solution would have a different tempco because of its ionization
activity. And, that tempco may be a little different at a different
concentration or temperature. This is why the AR1 uses mathematically
generated models for known salt characteristics that vary with
concentration and temperature.
The AR1 contains circuitry for characteristics of the 3 most commonly
referenced compounds — KCl, NaCl and 442. In the display, the solution
type being used is shown on the left.
Those who want true TDS readings that will correspond to evaporated
weight will select the correct solution type.
The AR1 contains circuitry for the 3 most commonly referenced
compounds — KCl, NaCl and 442. In the LCD display, the solution type
being used is shown on the left.
XIV. TEMPERATURE COMPENSATION (Tempco)
and TDS DERIVATION
When making conductivity measurements, the Solution Selection
determines the characteristic assumed as the instrument reports what a
measured conductivity would be if it were at 25°C. The characteristic is
represented by the tempco, expressed in %/°C. If a solution of 100 µS at
25°C increases to 122 µS at 35°C, then a 22% increase has happened
over this change of 10°C. The solution is said to have a tempco of 2.2
%/°C.
18 19
Page 12

XV. GLOSSARY
Anions - Negatively charged ions.
See Solution Characteristics, pg. 17.
Algorithm - A procedure for solving a mathematical problem.
See Temperature Compensation and TDS Derivation,
pg. 18.
TDS - Total Dissolved Solids or the Total Conductive Ions
in a solution. See Conductivity Conversion to TDS,
pg. 17.
Tempco - Temperature Compensation
See Temperature Compensation, pg. 15.
For details on specific areas of interest refer to Table of Contents.
NOTES
20 21
 Loading...
Loading...