Page 1

ULTRAMETER
Operation
Manual
MODELS 6PFCE & 4P
™
20 Sep. 2013
Page 2

Page 3
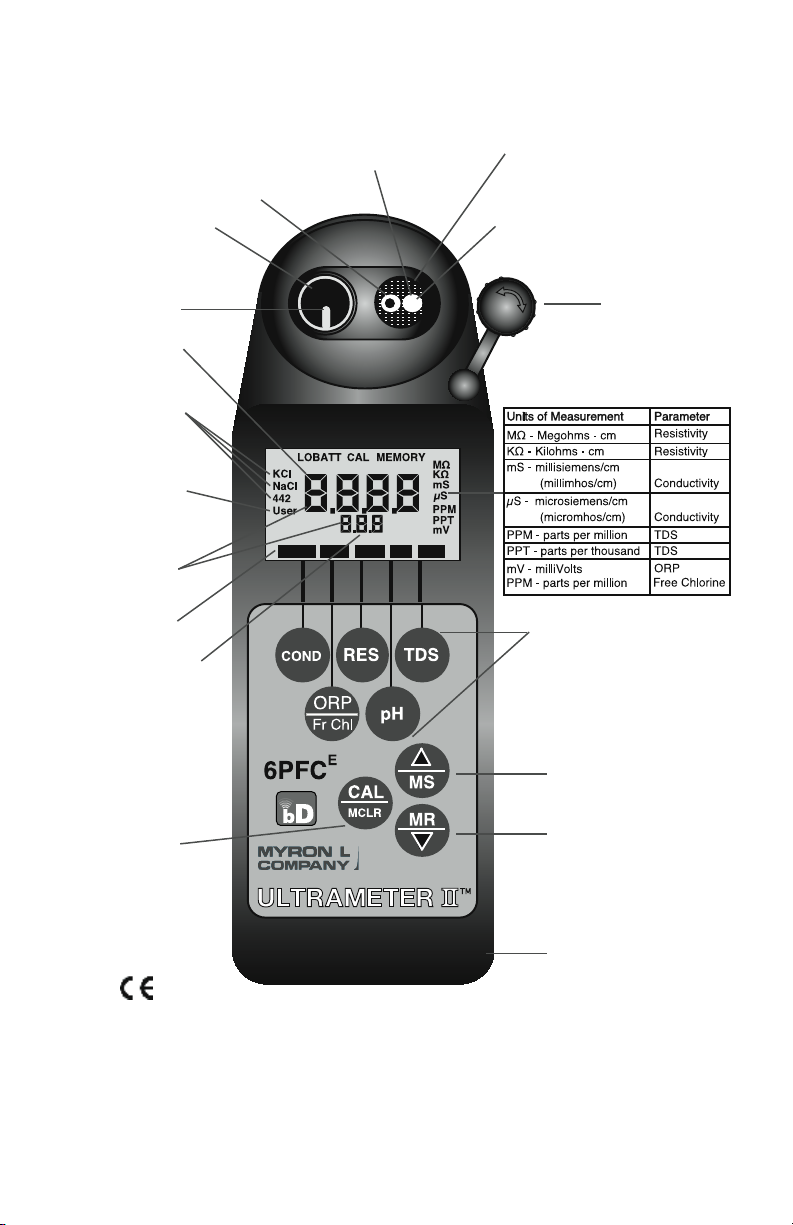
BUFFER
°C
RATIO
% /
ORP RES TDSpHCOND
22nov11
Reference
Junction under
Glass pH Bulb
These Measurement keys will:
• Turn instrument on
• Measure parameter
• Exit any function
(Built-in
Electrodes)
Preprogrammed
variable
conductivity/
TDS ratios
Parameters
Wrist/neck strap slot
(user supplied)
pH/ORP Sensor
Protective Cap
This key for:
• Calibration
• Memory Clear
• Solution Selection
• Confirmation
Up key/Memory Store
Down key/Memory Recall
Conductivity Cell
USER mode
for programming
special
temperature
compensation
factor and
conductivity/TDS
ratio
Displayed here:
• Temperature
readout
• USER temperature
compensation or
conductivity/TDS
ratio
• Memory Storage/
Recall
• pH Calibration
ORP
Electrode
pH Glass
Electrode
pH/ORP Sensor
(Replaceable)
For detailed explanations see Table of Contents
Temperature
Sensor
MODEL 6PFCE
Shown with bluDock™ option installed
Time & Date
displayed here
TEST Value
bluDock Enabled
i
Page 4

ii
Page 5

I. INTRODUCTION
Thank you for selecting the feature-packed Ultrameter II™, one of the
Myron L Company’s latest in an increasing line of instruments utilizing
advanced microprocessor-based circuitry and SMT manufacturing
processes. This circuitry makes the instrument extremely accurate,
reliable and very easy to use.
Model 6PIIFCE includes Myron L Company’s exclusive Free Chlorine
Equivalent (FCE) feature for making ORP-based free chlorine
measurements. Both Ultrameter IIs now also feature optional
wireless data transfer. Other features include a clock with time and date,
memory of up to 100 locations with time and date stamp, the ability of
the user to adjust the timeout “Auto oFF”, and enhanced performance.
See Features and Specications on pages 2 & 3.
The most exciting new feature is data logging with the ability to download
the memory or stored test data wirelessly with its corresponding time,
date and unit name. This feature allows the user to create spreadsheets
and graphs with ease and quickly and accurately manipulate data more
effectively. The optional bluDock™ and accompanying U2CI software is
compatible with most computers using either Microsoft Windows XP, Vista,
or 7™ or Macintosh OSX™. The data may be imported into a variety of
spreadsheet formats like Microsoft Excel CSV™.
Please Note: Although the Myron L Company has performed extensive
testing, we cannot guarantee compatibility of all applications and formats.
We suggest testing your application and format for compatibility before
relying on it.
Bluetooth®
For your convenience, a brief set of instructions is provided on the
bottom side of your Ultrameter II. A waterproof pocket-sized card with
abbreviated instructions is also included with the instrument.
Special note ... Conductivity, resistivity, and TDS require mathematical
correction to 25°C values (ref. Temperature Compensation, pg. 39). On
the left of the Ultrameter II’s liquid crystal display is shown an indicator of
the salt solution characteristic used to model temperature compensation of
conductivity and its TDS conversion. The indicator may be KCl, NaCl, 442™
or User. Selection affects the temperature correction of conductivity, and
the calculation of TDS from compensated conductivity (ref. Conductivity
Conversion to Total Dissolved Solids (TDS), pg. 41). The selection can
affect the reported conductivity of hot or cold solutions, and will change
the reported TDS of a solution. Generally, using KCl for conductivity, NaCl
for resistivity, and 442 (Natural Water characteristic) for TDS will reect
present industry practice for standardization. This is how your instrument,
as shipped from the factory, is set to operate. For use in sea water
desalination for example, both the conductivity and TDS may easily be
changed to NaCl.
1
Page 6
II. FEATURES and SPECIFICATIONS
A. Features
• ORP-based FCE free chlorine measurement, displayed as ppm
concentration (6PFCE)
• Superior resolution 4 digit LCD displays full 9999 µS/ppm
• Cond/TDS accuracy of ±1% of READING in a handheld instrument
±0.1% at calibration point
• All electrodes are internal for maximum protection
• Improved 4 electrode sensor technology
• Waterproof to 1 meter/3 feet
• Autoranging conductivity/TDS/resistivity
• Prompts for easy pH calibration (6PFCE)
• Factory calibrations stored in microprocessor
• 3 conductivity/TDS solution conversions preprogrammed into
microprocessor
• User mode feature allows:
Programming your own cond/TDS conversion factor
Programming your own temperature compensation factor
Disabling temperature compensation
• Real Time Clock with Time and Date
• Data Logging with TIME and DATE in memory
• Memory stores 100 readings
• User adjustable timeout “Auto oFF”
•
Bluetooth®
wireless download capability with optional bluDock™
B. General Specications
Display 4 Digit LCD
Dimensions (LxWxH) 196 x 68 x 64 mm/
7.7 x 2.7 x 2.5 in.
Weight 352 g/12.4 oz.
Case Material VALOX*
Cond/Res/TDS Cell Material VALOX*
Cond/TDS Electrodes (4) 316 Stainless Steel
Cond/Res/TDS Cell Capacity 5 ml/0.2 oz.
pH/ORP Sensor Well Capacity 1,2 ml/0.04 oz. (6PFCE)
Power 9V Alkaline Battery
Battery Life >100 Hours/5000 Readings
Operating/Storage Temperature 0-55°C/32-132°F
Protection Ratings IP67/NEMA 6 (waterproof to
1 meter/3 feet)
EMI/EMC Ratings EN61326-1: 2006 + Annex A: 2008
(hand-held devices)
(Conformité Européenne)
CISPR 11: 2003
IEC 61000-4-2: 2001 and,
IEC 61000-4-3: 2002
* ™ SABIC Innovative Plastics IP BV
Additional information is available on our website:
www.myronl.com
MADE IN USA
2
Page 7
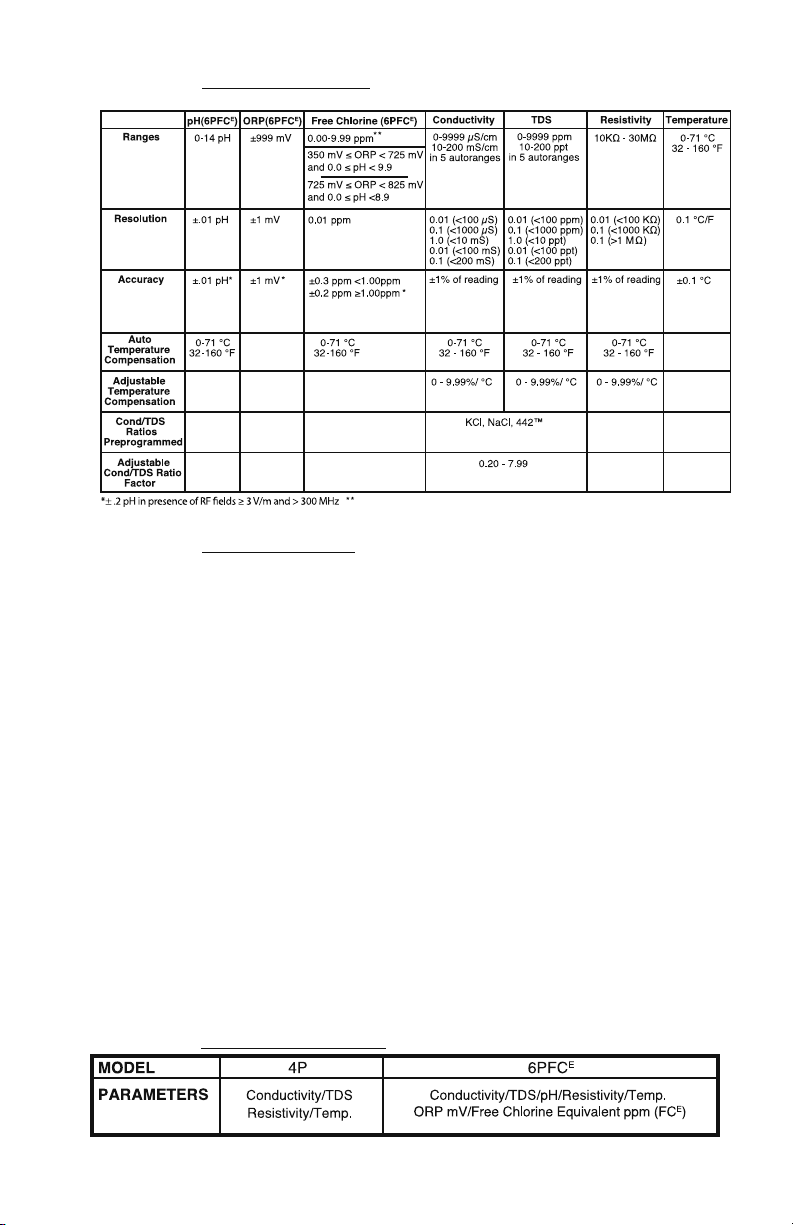
C. Specication Chart
If either ORP or pH is outside the specied limits, the instrument will display “-Or-”.
D. Warranty/Service
The Myron L Ultrameter II, excluding the pH/ORP sensor (6PFCE), has
a Two (2) Year Limited Warranty. The pH/ORP sensor (6PFCE) has a
Six (6) Month Limited Warranty for materials and workmanship. If an
instrument fails to operate properly, see Troubleshooting Chart, pg. 36.
The battery and pH/ORP sensor are user-replaceable. For other service,
return the instrument prepaid to the Myron L Company.
MYRON L COMPANY
2450 Impala Drive
Carlsbad, CA 92010-7226 USA
+1-760-438-2021
E-Mail: info@myronl.com
techquestions@myronl.com
www.myronl.com
If, in the opinion of the factory, failure was due to materials or
workmanship, repair or replacement will be made without charge. A
reasonable service charge will be made for diagnosis or repairs due to
normal wear, abuse or tampering. This warranty is limited to the repair or
replacement of the Ultrameter II only. The Myron L Company assumes
no other responsibility or liability.
E. Ultrameter II Models
3
Page 8

TABLE OF CONTENTS
Instrument Illustration .......................................i
I. INTRODUCTION ................................... 1
II. FEATURES and SPECIFICATIONS .................... 2
A. Features ................................2
B. General Specications .....................2
C. Specication Chart ........................3
D. Warranty/Service..........................3
E. Ultrameter II Models ....................... 3
III. RULES of OPERATION..............................7
A. Operation ...............................7
B. Characteristics of the Keys ..................7
C. Operation of the Keys ......................7
1. Measurement Keys in General.........7
2. COND, RES and TDS Keys ........... 7
3. pH and ORP/Fr Chl Keys (6PFCE) ...... 8
4. CAL/MCLR Key ....................8
5. UP or DOWN Keys..................9
IV. AFTER USING THE ULTRAMETER II .................. 9
A. Maintenance of the Conductivity Cell ..........9
B. Maintenance of the pH/ORP Sensor (6PFCE)....9
V. SPECIFIC RECOMMENDED MEASURING
PROCEDURES .............................9
A. Measuring Conductivity &
Total Dissolved Solids (TDS)............. 9
B. Measuring Resistivity .....................10
C. Measuring pH (6PFCE) ....................10
D. Measuring ORP (6PFCE)................... 10
1. ORP/FCE Mode Selection............. 11
2. Measuring ORP .................... 11
E. Measuring Free Chlorine Using FCE..........12
1. Prepare for FCE Measurement ......... 12
2. FC
E
Flow Method ................... 12
3. FCE Immersion Method............... 13
4. FCE Best Practices .................. 14
VI. SOLUTION SELECTION............................14
A. Why Solution Selection is Available ..........14
B. The 4 Solution Types .....................14
C. Calibration of Each Solution Type ............14
D. Procedure to Select a Solution ..............14
E. Application of User Solution Type ............15
1. User Programmable Temperature
Compensation (Tempco) ........15
2. Disabling Temperature Compensation ... 15
4
Page 9

TABLE OF CONTENTS
3. User Programmable Conductivity to
TDS Ratio ....................16
VII. CALIBRATION .................................... 17
A. Calibration Intervals ......................17
B. Rules for Calibration of the Ultrameter II ...... 17
1. Calibration Steps .................. 17
2. Calibration Limits ..................18
C. Calibration Procedures ....................18
1. Conductivity or TDS Calibration ....... 18
2. User Calibration Conductivity/TDS.....18
3. Resistivity Calibration ............... 19
4. Reloading Factory Calibration
(Cond or TDS) ................19
5. pH Calibration (6PFCE) .............19
6. ORP Calibration (6PFCE) ............21
7. Temperature Calibration ............. 21
VIII. CALIBRATION INTERVALS ......................... 22
A. Suggested Intervals ......................22
B. Calibration Tracking Records ............... 22
C. Conductivity, RES, TDS Practices............22
D. pH and ORP Practices (6PFCE) .............23
IX. MEMORY........................................23
A. Memory Storage ......................... 23
B. Memory Recall ..........................23
C. Clearing a Record/Memory Clear ............24
X. TIME and DATE................................... 24
A. Setting TIME ............................24
B. Setting DATE............................25
C. DATE FORMAT “US & International (Int)” ...... 26
XI. TEMPERATURE FORMAT “Centigrade & Fahrenheit” ..... 26
XII. TOTAL RETURN to FACTORY SETTINGS..............27
XIII. CELL CHECK .................................... 28
XIV. AUTO OFF ......................................28
XV. USER Mode CALIBRATION LINC™ FUNCTION .........29
A. Calibration of Ultrameter II for use in
User mode .......................... 29
B. Setting User mode Calibration “Linc” .........30
C. Canceling User mode Calibration “Linc” .......31
XVI. bluDock™ Wireless Data Transfer Instructions........... 32
A. Software Installation ...................... 32
B. Hardware Setup .........................32
C. Memory Stack Download ..................33
XVII. CARE and MAINTENANCE .........................34
5
Page 10

TABLE OF CONTENTS
A. Temperature Extremes .................... 34
B. Battery Replacement...................... 34
C. pH/ORP Sensor Replacement (6PFCE) ....... 34
D. Cleaning Sensors ........................ 34
XVIII. TROUBLESHOOTING CHART ....................... 36
XIX. ACCESSORIES...................................38
A. Conductivity/TDS Standard Solutions .........38
B. pH Buffer Solutions (6PFCE) ................ 38
C. pH Sensor Storage Solution (6PFCE) .........38
D. ORP Sensor Conditioner Solution (6PFCE)..... 38
E. Soft Protective Carry Cases ................ 39
F. Hard Protective Carry Cases ...............39
G. Replacement pH/ORP Sensor (6PFCE) ....... 39
H. bluDock™ Wireless Data Transfer
Accessory Package ................... 39
XX. TEMPERATURE COMPENSATION (Tempco)
of Aqueous Solutions ........................ 39
A. Standardized to 25°C ..................... 39
B. Tempco Variation.........................39
C. An Example of 2 different solution selections and
the resulting compensation ............. 40
D. A Chart of Comparative Error ............... 40
E. Other Solutions ..........................41
XXI. CONDUCTIVITY CONVERSION to
TOTAL DISSOLVED SOLIDS (TDS) ............41
A. How it’s Done ...........................42
B. Solution Characteristics ...................42
C. When does it make a lot of difference?........ 42
XXII. TEMPERATURE COMPENSATION (Tempco)
and TDS DERIVATION....................... 43
A. Conductivity Characteristics ................ 43
B. Finding the Tempco of an Unknown Solution ... 43
C. Finding the TDS Ratio of an Unknown Solution ....43
XXIII. pH and ORP (6PFCE) ...............................44
A. pH (6PFCE) .............................44
B. ORP/Oxidation-Reduction Potential/
REDOX (6PFCE) .....................46
C. Free Chlorine Equivalent ..................47
1. FCE as an Indicator of Sanitizing
Strength .....................47
2. FCE Units ........................ 47
XXIV. SOFTWARE VERSION ............................. 48
XXV. GLOSSARY......................................49
6
Page 11
III. RULES of OPERATION
A. Operation
Using the instrument is simple:
• Individual or multiple parameter readings may be obtained by
lling individual sensors or entire cell cup area.
• Rinse the conductivity cell or pH/ORP sensor (6PFCE) well with
test solution 3 times and rell. Temperature and/or
measurement extremes will require additional rinses for
maximum accuracy.
• Press the desired measurement key to start measurement.
Pressing the key again restarts the 15 second “Auto oFF” timer.
• Note the value displayed or press the MS key to store the
reading (ref. Memory Storage, pg. 23). It’s that simple!
B. Characteristics of the Keys
• Though your Ultrameter II has a variety of sophisticated
options, it is designed to provide quick, easy, accurate
measurements by simply pressing one key.
• All functions are performed one key at a time.
• There is no “off” key. After 15 seconds of inactivity the
instrument turns itself off (60 seconds in CAL mode). User
adjustable up to 75 seconds (ref. Auto oFF, pg. 28).
• Rarely is it necessary to press and
to Select a Solution, pg. 14; or Conductivity or TDS Calibration,
pg. 18).
C. Operation of the Keys (See Instrument Illustration, pg. i)
1. Measurement Keys in General
Any of the 5 measurement keys in the upper part of the keypad turns on
the instrument in the mode selected. The mode is shown at the bottom
of the display, and the measurement units appear at the right. Pressing
a measurement key does this even if you are in a calibration sequence
and also serves to cancel a change (ref. Leaving Calibration, pg. 17).
hold
a key (as in Procedure
2. COND, RES and TDS Keys
These 3 keys are used with solution in the Conductivity Cell.
Precautions:
• While lling cell cup ensure no air bubbles cling on the cell wall.
• If the proper solution is not selected (KCl, NaCl, 442 or User),
refer to Why Solution Selection is Available, pg. 14 and
Procedure to Select a Solution, pg. 14.
a. COND Key
Solution to be tested is introduced into the conductivity cell and a press
of displays conductivity with units on the right. On the left is
shown the solution type selected for conductivity.
7
Page 12
b. RES Key
ORP
Fr Chl
ORP
Fr Chl
A press of displays resistivity with units on the right. On the left
is shown solution type selected for resistivity (ref. Solution Selection, pg.
14). The range of display of resistivity is limited to between 10 kilohms
(KΩ) and 30 megohms (MΩ). A solution outside that range will only show
[- - - -] in the display.
c. TDS Key
A press of displays Total Dissolved Solids with units on the right.
This is a display of the concentration of material calculated from
compensated conductivity using the characteristics of a known material.
On the left is shown solution type selected for TDS (ref. Solution
Selection, pg. 14).
3. pH and ORP/Fr Chl Keys (6PFCE)
Measurements are made on solution held in the pH/ORP sensor well
(ref. pH and ORP, pg. 44). The protective cap is removed and the sensor
well is lled and rinsed with the sample enough times to completely
replace the storage solution.
After use, the pH/ORP sensor well must be relled with Myron L Storage
Solution, and the protective cap reinstalled securely (ref. Maintenance
of the pH/ORP Sensor, pg. 9 and Cleaning Sensors, 2. pH/ORP, pg. 34).
a. pH Key (6PFCE)
A press of
b. ORP/Fr Chl Key (6PFCE)
displays pH readings. No units are displayed on the right.
In ORP mode, a press of
displays Oxidation-Reduction
Potential/REDOX reading in millivolts; “mV” is displayed.
When the FCE mode is activated, a press of
displays the Free
Chlorine Equivalent reading in “ppm” alternating with the FCE predictive
ORP reading in “mV”.
4. CAL/MCLR Key
A press of allows you to enter the calibration mode while
measuring conductivity, TDS or pH. Once in CAL mode, a press of this
key accepts the new value. If no more calibration options follow, the
instrument returns to measuring (ref. Leaving Calibration, pg. 17).
If
is held down for about 3 seconds when the ORP or FCE
functions are active, CAL mode is not entered. Instead either “OrP” or
“Chl” will appear depending on which mode is active. Change modes
by pressing the Up or Down buttons. Press any parameter key to
exit ORP unit preference selection or let the unit time out. ORP unit
preference will be saved.
8
Page 13

If is held down for about 3 seconds at any other time, CAL mode
but “SEL” appears to allow Solution Selection (ref. pg. 14) with the Up
or is not entered, Down keys. As in calibration, the CAL key is now an
“accept” key.
While reviewing stored records, the MCLR side of the key is active to
allow clearing records (ref. Clearing a Record/Memory Clear, pg. 24).
5. UP or DOWN Keys
While measuring in any parameter, the or
the Memory Store and Memory Recall functions.
While in CAL mode, the keys step or scroll the displayed value up or
down. A single press steps the display and holding either key scrolls the
value rapidly.
While in Memory Recall, the keys scroll the display up and down through
the stack of records (ref. Memory Recall, pg. 23).
IV. AFTER USING THE ULTRAMETER II
A. Maintenance of the Conductivity Cell
Rinse out the cell cup with clean water. Do not scrub the cell. For oily
lms, squirt in a foaming non-abrasive cleaner and rinse (ref. Cleaning
Sensors, pg. 34). Even if a very active chemical discolors the electrodes,
this does not affect the accuracy; leave it alone.
keys activate
B. Maintenance of the pH/ORP Sensor (6PFCE)
The sensor well must be kept wet with a saline solution. Before replacing
the rubber cap, rinse and ll the sensor well with Myron L pH Sensor
Storage Solution. If unavailable, use an almost saturated KCl solution,
pH 4 buffer or a saturated solution of table salt and tap water (ref.
pH and ORP Practices to Maintain Calibration, pg. 23). NEVER USE
DISTILLED WATER.
V. SPECIFIC RECOMMENDED MEASURING PROCEDURES
If the proper solution is not selected (KCl, NaCl, 442 or User), see
Solution Selection, pg. 14.
NOTE: After sampling high concentration solutions or temperature
extremes, more rinsing may be required. When sampling low conductivity
solutions, be sure the pH cap is well seated so that no solution washes
into the conductivity cell from around the pH cap.
A. Measuring Conductivity & Total Dissolved Solids (TDS)
1. Rinse cell cup 3 times with sample to be measured. (This conditions
the temperature compensation network and prepares the cell.)
2. Rell cell cup with sample.
9
Page 14
3. Press or .
4. Take reading. A display of [- - - -] indicates an overrange condition.
B. Measuring Resistivity
Resistivity is for low conductivity solutions. In a cell cup the value may
drift from trace contaminants or absorption from atmospheric gasses, so
measuring a owing sample is recommended.
1. Ensure pH protective cap is secure to avoid contamination.
2. Hold instrument at 30° angle (cup sloping downward).
3. Let sample ow continuously into conductivity cell with no aeration.
4. Press
key; use best reading.
NOTE: If reading is lower than 10 kilohms display will be dashes:
[ - - - - ]. Use Conductivity.
C. Measuring pH (6PFCE)
1. Remove protective cap by rotating while grasping and pulling up.
2. Rinse pH/ORP sensor well and conductivity cell cup 3 times
with sample to be measured. Shake out each sample to
remove any residual liquid.
3. Rell both sensor well and cell cup with sample.
4. Press
5. Note value displayed.
6. IMPORTANT: After use, ll pH/ORP sensor well with Myron L
pH Sensor Storage Solution and replace protective cap.
If Myron L pH Sensor Storage Solution is unavailable, use a
strong KCl solution, a pH 4 buffer, or a saturated solution of
table salt and tap water (ref. Cleaning Sensors, 2. pH/ORP, pg.
34).
The Ultrameter II features the ability to measure the activity of oxidizing or
reducing chemicals in solution as ORP mV. The instrument also includes
an innovative Free Chlorine Equivalent (FCE) feature (Measuring Free
Chlorine Using FCE, pg. 12) that uses ORP and pH to measure free
available chlorine (FAC) concentration in ppm. ORP mV and ppm of
free available chlorine (FAC) are the two most commonly used sanitizer
units of measure in water quality management.
Do not allow pH/ORP sensor to dry out.
D. Measuring ORP
.
10
Page 15
1. ORP / FCE Mode Selection
PPM
The Ultrameter II allows the user to choose between measuring oxidizing
sanitizers using either ORP mV or as parts per million (ppm) of equivalent
free chlorine. Use ORP to directly measure the oxidizing power of all
sanitizers like ozone, bromine, peracetic acid or chlorine. Use FCE to
measure the strength of oxidizing sanitizers as ppm of equivalent free
chlorine. To select between ORP and Free Chlorine modes:
1. Press
.
2. Press and hold
for approximately 3 seconds.
The current preference for ORP units of measure is displayed. Factory
setting for this preference is ORP mV.
3. Press the or
keys to toggle between mV (standard
ORP mode) and FCE ppm. The setting chosen is displayed.
4. Press any parameter key to exit ORP unit preference selection
or let the unit time out. ORP unit preference will be saved.
2. Measuring ORP
1. Ensure the 6PFCE is in ORP mode (ref. ORP Mode Selection,
pg. 10).
2. Remove protective cap by rotating while grasping and pulling up.
3. Rinse sensor well and cell cup 3 times with sample to be
measured. Shake out each sample to remove any residual liquid.
11
Page 16
4. Rell both sensor well and cell cup with sample.
5. Press .
6. Take reading.
7. Press MS to store reading in memory, if desired.
IMPORTANT: After use, ll pH/ORP sensor well with Myron L
pH Sensor Storage Solution and replace protective cap. If
Myron L pH Sensor Storage Solution is unavailable, you can
use a strong KCl solution, a pH 4 buffer, or a saturated solution
of table salt and tap water (ref. Cleaning Sensors, 2. pH/ORP,
pg. 34). Do not allow pH/ORP sensor to dry out.
E. Measuring Free Chlorine Using FC
E
The FCE function can be used to measure discrete samples, owing
solution and bodies of water. Measurement technique is particular to the
type of sample. For accurate results, use the FCE Flow Method described
in section 2 below to measure discrete or owing samples. Use the FCE
Immersion Method described in section 3 below in situations where the
6PFCE can be dipped to obtain a sample. Read through section 4. FCE
Best Practices before you begin.
1. Prepare for FCE Measurement
1. For ease of measurement, set the instrument’s Auto oFF feature
to 75 sec (ref. Auto oFF, pg. 28).
2. Ensure the FCE mode has been activated (ref. ORP/FCE Mode
Selection, pg. 10).
3. Remove protective cap from the pH/ORP sensor by rotating
while grasping and pulling up.
2. FCE Flow Method
1. Empty the pH/ORP sensor well of all storage solution.
2. Hold the 6PFCE at a 30º angle (cup sloping downward).
3. Thoroughly ush the sensor well and cell cup with a steady
stream of the solution you intend to measure by allowing the
solution to ow into and out of the sensor well and cell cup for at
least 10 seconds.
4. Let sample ow continuously into conductivity cell with no
aeration.
5. Allow both the sensor well and cell cup to remain lled with
sample.
12
Page 17
6. Press
predicted nal ORP value and a free chlorine equivalent
concentration in ppm. Both readings will change rapidly at rst.
7. Wait for the readings to stabilize. When the mV and ppm values
are unchanging for 5 consecutive readings, the FCE reading
has reached a stable level. This may take 1 to 2 minutes.
NOTE: If the reading takes more than 1 minute to stabilize,
. The instrument will begin alternating between a
press the
from disturbing the measurement process. Annunciators will
alert you when either the pH or ORP of the nal FCE ppm value
are Out of Range (“-Or-”).
8. Press MS to store reading in memory if desired.
3. FCE Immersion Method
NOTE: Use this method for pools, spas and other large standing bodies
of water.
1. Hold instrument beneath the surface of the water to avoid
surface effects on the water’s chemistry.
2. Swirl the instrument around for at least 10 seconds to thoroughly
rinse the cell cup and sensor well.
3. Continue holding the instrument under the surface while taking
the reading.
4. Press .
5. The instrument will begin alternating between a predicted nal
ORP value and a free chlorine equivalent concentration in ppm.
Both readings will change rapidly at rst.
after 1 minute to prevent Auto oFF feature
6. Wait for the readings to stabilize. When the mV and ppm values
are unchanging for 5 consecutive readings, the FCE reading
has reached a stable level. This may take 1 to 2 minutes.
NOTE: If the reading takes longer than 1 minute to stabilize,
press after 1 minute to prevent Auto oFF feature from
disturbing the measurement process. Annunciators will alert
you when either the pH or ORP of the nal FCE ppm value are
Out of Range (“-Or-”).
13
Page 18

7. Press MS to store reading in memory if desired.
4. FCE Best Practices
For best results it is recommended that you:
1. Take 3 consecutive FCE measurements and record the
readings.
2. Calculate the average of the 3 measurements. Use this value.
3. Ignore measurements that are signicantly different from the
others. Ex: 3.20 ppm, 1.15 ppm, 3.10 ppm
IMPORTANT: After use, ll pH/ORP sensor well with Myron L
pH Sensor Storage Solution and replace protective cap. If
Myron L pH Sensor Storage Solution is unavailable, you can
use a strong KCl solution, a pH 4 buffer, or a saturated solution
of table salt and tap water (ref. Cleaning Sensors, 2. pH/ORP,
pg. 34). Do not allow pH/ORP sensor to dry out.
VI. SOLUTION SELECTION
A. Why Solution Selection is Available
Conductivity, resistivity, and TDS require temperature correction to
25°C values (ref. Standardized to 25°C, pg. 39). Selection determines
the temperature correction of conductivity and calculation of TDS from
compensated conductivity (ref. Cond. Conversion to TDS, pg. 41).
B. The 4 Solution Types
On the left side of the display is the salt solution characteristic used to
model temperature compensation of conductivity and its TDS conversion.
Generally, using KCl for conductivity, NaCl for resistivity, and 442 (Natural
Water characteristic) for TDS will reect present industry practice for
standardization. This is how your instrument is shipped from the factory
(ref. Solution Characteristics, pg. 42).
The User selection allows a custom value to be entered for the
temperature compensation of conductivity and also the conversion ratio
if measuring TDS.
C. Calibration of Each Solution Type
There is a separate calibration for each of the 4 solution types. Note
that calibration of a 442 solution does not affect the calibration of a NaCl
solution. For example: Calibration (ref. Conductivity or TDS Calibration,
pg. 18) is performed separately for each type of solution one wishes to
measure (ref. Conductivity/TDS Standard Solutions, pg. 38).
D. Procedure to Select a Solution
NOTE: Check display to see if solution displayed (KCl, NaCl, 442 or
14
Page 19

User) is already the type desired. If not:
Figure 1
KCl
442
NaCl
User
Figure 2
°C% /
User
COND
1. Press , or to select the parameter on which
you wish to change the solution type.
2. Press and hold key
for 3 seconds to make “SEL”
appear (see Figure 1). For
demonstration purposes, all
4 solution types are shown
simultaneously.
3. Use the or key to select type of solution desired
(ref. Solution Characteristics, pg. 42). The selected solution
type will be displayed: KCl, NaCl, 442 or User.
4. Press to accept new solution type.
E. Application of User Solution Type
1. User Programmable Temperature Compensation
(Tempco)
This feature allows you to change your Ultrameter II’s temperature
compensating factor to another factor between 0-9.99%/°C (ref.
Temperature Compensation, pg. 39). This feature does not apply to pH
or ORP.
a. As in D. Procedure to Select a Solution above, select “User” mode.
b. With User mode now selected, press . You may now
adjust a temperature compensation from .00%/°C to 9.99%/°C,
by pressing or .
See example in Figure 2.
c. Press twice to skip
calibration adjustment and
accept the new tempco (3
times if in TDS mode). You
are now ready to measure samples with your new temperature
compensation factor.
2. Disabling Temperature Compensation
a. Select User mode (ref. Procedure to Select a Solution, pg. 14).
15
Page 20

b. With “User” selected, press . If the display does not
Figure 3
°C% /
User
COND
Figure 4
RATIO
User
TDS
In these first six sections, you have learned
all you need to make accurate measurements.
The following sections contain calibration,
advanced operations and technical information.
show .00%/°C, hold long enough to bring the tempco to
.00%/°C (see Figure 3).
c. Press twice
(3 times if in TDS mode).
Temperature compensation
is now disabled (=0) for
measurements in User mode.
3. User Programmable Conductivity to TDS Ratio
This feature allows you to select a custom conductivity to TDS conversion
ratio within the range of 0.20-7.99 for User mode measurements.
To determine the conversion ratio for a custom solution of known
TDS ppm value, measure the solution conductivity at 25ºC with the
Ultrameter II and divide the ppm value by the µS value. For example,
a solution of known 75 ppm TDS and measured 100 µS conductivity at
25ºC would have a conversion ratio of 75/100 or 0.75. Enter the new
conversion ratio as follows:
a. While in User mode, press .
b. Press twice (to skip
over tempco adjustment) and
“RATIO” will appear (see
Figure 4).
c. Adjust with or
key until new conversion
ratio is displayed.
d. Press twice (to skip over calibration adjustment) to
accept new conversion ratio. You are now ready to measure
samples with the new conductivity/TDS ratio.
16
Page 21
VII. CALIBRATION
KCl, NaCl or 442 User
Cond Gain only Tempco, then Gain
Res Done in conductivity Done in conductivity or TDS
TDS Gain only Tempco, Ratio, then Gain
pH 7, acid and/or base (6PFC
E
)
ORP Zero set with pH 7 automatically (6PFC
E
)
A. Calibration Intervals
Generally, calibration is recommended about once per month with
Conductivity or TDS solutions. Calibration with pH solutions should
be checked twice a month. Calibration of ORP is not necessary (ref.
CALIBRATION INTERVALS, pg. 22).
B. Rules for Calibration of the Ultrameter II
1. Calibration Steps
a. Starting Calibration
Calibration is begun by pressing while measuring Conductivity, TDS
or pH. Measuring continues, but the “CAL” icon is on, indicating
calibration is now changeable.
The reading is changed with the and keys to match the known value.
The calibration for each of the 4 solution types may be performed in
either conductivity or TDS mode.
Depending on what is being calibrated, there may be 1, 2 or 3 steps to
the calibration procedures.
Once in CAL mode, the key becomes an “ACCEPT” key. At
each point, pressing accepts the new calibration value and steps
you to the next adjustment (or out of CAL mode if there are no more
steps).
To bypass a calibration step, simply press to accept the present
value as is.
b. Leaving Calibration
Calibration is complete when the “CAL” icon goes out. Pressing any
measurement key cancels changes not yet accepted and exits calibration
mode.
Leaving pH after the 2nd buffer results in the same gain being entered
in place of the 3rd buffer.
17
Page 22

2. Calibration Limits
Figure 5
°C
KCl
COND
CAL
There are calibration limits. A nominal “FAC” value is an ideal value
stored by the factory. Attempts to calibrate too far, up or down, from
there will cause the displayed value to be replaced with “FAC”. If
you accept it (press the “Cal” key), you will have the original default
factory calibration for this measurement. The need to calibrate so
far out that “FAC” appears indicates a procedural problem, incorrect
standard solution, a very dirty cell cup or an aging pH/ORP sensor (ref.
Troubleshooting Chart, pg. 36).
C. Calibration Procedures
1. Conductivity or TDS Calibration
a. Rinse conductivity cell three times with proper standard (KCl,
NaCl, or 442) (ref. Cond/TDS Standard Solutions, pg. 38). For
user calibration see User Calibration Conductivity/TDS below.
b. Rell conductivity cell with same standard. KCl-7000 shown.
c. Press or
, then
press ; “CAL” icon will
appear on the display
(see Figure 5).
d. Press or to
step the displayed value toward the standard’s value (7032 >
7000) or hold a key down to scroll rapidly through the reading.
e. Press once to conrm new value and end the
calibration sequence for this particular solution type. If another
solution type is also to be measured, change solution type now
and repeat this procedure.
2. User Calibration Conductivity/TDS
Instrument must be in User mode, see Solution Selection, pg. 14.
a. Rinse conductivity cell three times with your standard.
b. Rell conductivity cell with same standard.
c. Press or , then press twice in COND/
three times in TDS. The “CAL” icon will appear on the display.
d. Press or to step the displayed value toward the
standard’s value or hold a key down to scroll rapidly through
the reading.
18
Page 23

e. Press once to conrm new value and end the
Figure 6
BUFFER
pH
CAL
calibration sequence for this particular solution type.
3. Resistivity Calibration
Resistivity is the reciprocal of conductivity. To calibrate resistivity,
calibrate conductivity for the solution type you wish to measure (ref.
Conductivity or TDS Calibration, pg. 18).
4. Reloading Factory Calibration (Cond or TDS)
If calibration is suspect or known to be incorrect, and no standard solution
is available, the calibration value can be replaced with the original factory
value for that solution. This “FAC” value is the same for all Ultrameter IIs,
and returns you to a known state without solution in the cell. The “FAC”
internal electronics calibration (which bypasses the electrodes and cell)
is not intended to replace calibration with conductivity/TDS standard
solutions. If another solution type requires resetting, change solution
type and repeat this procedure.
a. Press or
.
b. Press . (If in User solution mode. Press CAL key
twice if in Conductivity, and three times if in TDS to skip over
tempco and ratio adjustments.)
c. Press key until “FAC” appears and release.
d. Press to accept the factory calibration setting.
5. pH Calibration (6PFCE)
IMPORTANT: Always “zero” your Ultrameter II with a pH 7 buffer solution
before adjusting the gain with acid or base buffers, i.e., 4 and/or 10, etc.
a. pH Zero Calibration (6PFCE)
1. Rinse sensor well and cell cup 3 times with 7 buffer solution.
2. Rell both sensor well and cell cup with 7 buffer solution.
3. Press to verify the
pH calibration. If the display
shows 7.00, skip the pH
Zero Calibration and
proceed to section b. pH
Gain Calibration.
19
Page 24

4. Press
and “7” annunciators will appear (see Figure 6, page 19).
Displayed value will be the uncalibrated sensor.
to enter calibration mode. The “CAL”, “BUFFER”
NOTES: If a wrong buffer is added (outside of 6-8 pH),“7” and “BUFFER”
will ash, and the Ultrameter II will not adjust.
The uncalibrated pH value displayed in step 4 will assist in determining
the accuracy of the pH sensor. If the pH reading is above 8 with pH 7
buffer solution, the sensor well needs additional rinsing or the pH sensor
is defective and needs to be replaced
5. Press or until the display reads 7.00.
.
NOTE: Attempted calibration of >1 pH point from factory calibration will
cause “FAC” to appear. This indicates the need for sensor replacement
(ref. Troubleshooting Chart pg. 36) or fresh buffer solution. The “FAC”
internal electronic calibration is not intended to replace calibration with
pH buffers. It assumes an ideal pH sensor. Each “FAC” indicates a
factory setting for that calibration step (i.e., 7, acid, base).
You may press to accept the preset factory value, or you may
reduce your variation from factory setting by pressing or
6. Press to accept the new value. The pH Zero Calibration
is now complete. You may continue with pH Gain Calibration or
exit by pressing any measurement key.
b. pH Gain Calibration (6PFCE)
IMPORTANT: Always calibrate or verify your Ultrameter II with a pH 7
buffer solution before adjusting the gain with acid or base buffers, i.e.,
4 and/or 10, etc. Either acid or base solution can be used for the 2nd
point “Gain” calibration and then the opposite for the 3rd point. The
display will verify that a buffer is in the sensor well by displaying either
“Acd” or “bAS”.
1. The pH calibration mode is initiated by either completion of the
pH Zero Calibration, or verifying 7 buffer and pressing the
key twice while in pH measurement mode.
2. At this point the “CAL”, “BUFFER” and “Acd” or “bAS”
annunciators will be displayed (see Figures 7 and 8).
.
20
Page 25

Figure 7
BUFFER
pH
CAL
Figure 8
pH
BUFFER
CAL
NOTE: If the “Acd” and “bAS” indicators are blinking, the unit is indicating
an error and needs either an acid or base solution present in the sensor
well
.
3. Rinse sensor well 3 times with acid or base buffer solution.
4. Rell sensor well again with same buffer solution.
5. Press or
until display agrees with buffer value.
6. Press to accept 2nd point of calibration. Now the
display indicates the next type of buffer to be used.
Single point Gain Calibration is complete. You may continue for the 3rd
point of Calibration (2nd Gain) or exit by pressing any measurement key.
Exiting causes the value accepted for the buffer to be used for both acid
and base measurements.
To continue with 3rd point calibration, use basic buffer if acidic buffer
was used in the 2nd point, or vice-versa. Again, match the display to the
known buffer value as in step 2 and continue with the following steps:
7. Repeat steps 3 through 6 using opposite buffer solution.
8. Press to accept 3rd point of calibration, which
completes the Calibration procedure. Fill sensor well with
Myron L Storage Solution and replace protective cap.
6. ORP/FCE Calibration (6PFCE)
ORP electrodes rarely give false readings without problems in the
reference electrode. For this reason, and because calibration solutions
for ORP are highly reactive and potentially hazardous, your Ultrameter II
has an electronic ORP calibration. This causes the zero point on the
reference electrode to be set whenever pH 7 calibration is done.
7. Temperature Calibration
Temperature calibration is not necessary in the Ultrameter II.
21
Page 26

VIII. CALIBRATION INTERVALS
There is no simple answer as to how often one should calibrate an
instrument. The Ultrameter II is designed to not require frequent
recalibration. The most common sources of error were eliminated in
the design, and there are no mechanical adjustments. Still, to ensure
specied accuracy, any instrument must be checked against chemical
standards occasionally.
A. Suggested Intervals
On the average, we expect calibration need only be checked monthly
for the Conductivity, RES or TDS functions. The pH (6PFCE) function
should be checked every 2 weeks to ensure accuracy. Measuring some
solutions will require more frequent intervals.
B. Calibration Tracking Records
To minimize your calibration effort, keep records. If adjustments you
are making are minimal for your application, you can check less often.
Changes in conductivity calibration should be recorded in percent.
Changes in pH calibration (6PFCE) are best recorded in pH units.
Calibration is purposely limited in the Ultrameter II to ±10% for the
conductivity cell, as any change beyond that indicates damage, not
drift. Likewise, calibration changes are limited to ±1 pH unit (6PFCE), as
any change beyond that indicates the end of the sensor’s lifetime and
replacement is recommended.
C. Conductivity, RES, TDS Practices to Maintain Calibration
1. Clean oily lms or organic material from the cell electrodes
with foaming cleaner or mild acid. Do not scrub inside the cell.
2. Calibrate with solutions close to the measurements you make.
Readings are compensated for temperature based on the type
of solution. If you choose to measure tap water with a KCl
compensation, which is often done (ref. An Example of 2
different solution selections and the resulting compensation,
pg. 40), and you calibrate with 442 solution because it is
handy, the further away from 25°C you are, the more error you
have. Your records of calibration changes will reect
temperature changes more than the instrument’s accuracy.
3. Rinse out the cell with pure water after taking measurements.
Allowing slow dissolving crystals to form in the cell
contaminates future samples.
4. For maximum accuracy, keep the pH sensor cap on tight so
that no uid washes into the conductivity cell.
22
Page 27

D. pH and ORP Practices to Maintain Calibration (6PFCE)
Figure 9
°C
KCl
COND
MEMORY
1. Keep the sensor wet with Myron L Storage Solution.
2. Rinse away caustic solutions immediately after use.
ORP calibration solutions are caustic, and ±5% is considered very
accurate. By using the pH zero setting (0 mV = 7 pH) for ORP and
precision electronics for detection, the Ultrameter II delivers better
accuracy without calibration than a simpler instrument could using
calibration solutions.
IX. MEMORY
This feature allows up to 100 readings with their temperatures to be
stored simultaneously for later recall. At the same time, the TIME and
DATE are also recorded. To download the memory to a computer, (ref.
bluDock™ WIRELESS DATA TRANSFER INSTRUCTIONS, pg. 32).
A. Memory Storage
1. While displaying a
measurement, press
to record the
displayed value.
2. “MEMORY” will appear
and the temperature
display will be momentarily replaced by a number (1-100)
showing the position of the record. Figure 9 shows a reading
of 1806 µS stored in memory record #4.
B. Memory Recall
1. Press any measurement key.
2. Press
, “MEMORY” will appear, and the display will
show the last record stored.
3. Press or
to scroll to the record location desired
(the temperature display alternates between temperature
recorded and location number).
4. Press to display time and date stamp.
5. Press any measurement key to leave memory recall or allow to
automatically turn off.
23
Page 28

C. Clearing a Record/Memory Clear
Figure 10
MEMORY
Figure 11
CAL
After recalling a certain record location, press and HOLD to
clear that memory. This space will be the place for the next memory
record, unless you scroll to another empty position before ending the
recall sequence. The next memory stored will go into the next highest
available memory location.
Example:
You have locations 1-7 lled and wish to clear the conductivity
reading stored in record location #3 and replace it with a pH reading.
1. Press
and scroll to location #3.
2. Press and HOLD
to clear old record #3.
3. Fill pH/ORP sensor well with sample.
4. Press
to measure sample and press to store
reading in location #3.
5. The next memory stored will go into location #8.
6. To clear all records: After
pressing
, scroll down.
“CLr ALL” will be displayed
(see Figure 10).
7. Press
. All records will
be cleared.
X. TIME and DATE
The Time and Date may easily be changed as you travel.
A. Setting TIME
Time is always displayed in 24 hour
time.
Example shown in Figure 11, 16:05
equals 4:05 PM.
1. Press
.
2. Press until the time is displayed (scrolling through
24
Page 29

stored readings, PC OFF, and CLr ALL to time, e.g., “16:05”).
Figure 12
Figure 13
CAL
Figure 14
CAL
3. Press to initiate.
“CAL” will be displayed along with the time (see Figure 11).
4. Press or to change the time.
5. Press to accept the change (new time).
B. Setting DATE
Example shown in Figure 12
is in US format, i.e., mo/dy/yr.
NOTE: The default format is US.
Date format may be changed
(ref. Date Format “US and
International (Int)”, pg. 26).
1. Press
.
2. Press repeatedly until the date is displayed (scrolling
through stored readings, PC OFF, CLr ALL and time to the
date, e.g., “11.18 11” (Figure 12), November 18, 2011).
3. Press to initiate. “CAL” will be displayed along with the
YEAR (see Figure 13).
4. Press or to
change the YEAR.
5. Press to accept the
change (new year).
6. Press or to
change the month.
7. Press to accept the
25
Page 30

change (new month),
Figure 15
CAL
Figure 16
Figure 17
(see Figure 14).
8. Press
or
to change the day.
9. Press to accept
the change (new day)
(see Figure 15).
C. DATE FORMAT “US & International (Int)”
1. Press .
2. Press repeatedly until the format is displayed (scrolling
through stored readings, PC OFF, CLr ALL, time and date to
date format).
3. Press to change. Display will now indicate other format
(see Figures 16 & 17).
4. Press any measurement key or allow to automatically turn off.
XI. TEMPERATURE FORMAT “Centigrade & Fahrenheit”
1. Press .
2. Press to display the stored memory records.
3. Press repeatedly until you pass the “US” or “Int” date
26
Page 31

format location. The display will show a “C” or “F”
Figure 18
Figure 19
Figure 20
(see Figures 18 and 19).
4. Press to switch units.
5. Press to accept unit preference for all temperature
readings.
NOTE: Tempco will still be shown in %/°C
.
XII. TOTAL RETURN to FACTORY SETTINGS “FAC SEL”
There may come a time when it would be desirable to quickly reset
all the recorded calibration values in the instrument back to the factory
settings. This might be to ensure all calibrations are set to a known
value, or to give the instrument to someone else free of adjustments or
recorded data for a particular application.
NOTE: All stored data will be lost.
1. Press
.
2. Press to display the stored memory records.
3. Press repeatedly
until you pass the CLr ALL
and the C-F locations. The
display will show a “FAC
SEL” (see Figure 20).
4. Press to accept the resetting. Display will return to
Conductivity.
27
Page 32

XIII. CELL CHECK
Figure 21
Figure 22
Figure 23
Figure 24
The cell check veries the cleanliness of the conductivity/TDS/resistivity
sensor. In normal use the cell may become dirty or coated and require
cleaning. If the display is showing “.00” when the cell cup is dry, the
sensor is probably clean. However, when testing high purity water in
resistivity (“RES”) mode improved accuracy may be desired. No matter
what a manufacturer claims, a sensor can and will become contaminated
or coated and, therefore, require cleaning. A true 4-wire sensor, as in
the Ultrameter II, helps to mitigate contamination, but NO SENSOR IS
100% IMMUNE.
1. Press .
2. Press to display the
stored memory records.
3. Press repeatedly until
you pass the FAC SEL
location. The display will
show a “CELL ch”
(see Figure 21).
4. Press to test.
If cell is clean, “Good” will
momentarily be displayed
(see Figure 22). If cell is
dirty, “CELL cLn” will be
displayed (see Figure 23)
(ref. Cleaning Sensors,
pg. 34).
XIV. AUTO oFF
Auto oFF allows the user to adjust the
time the instrument is ON (up to 75
seconds) after each press of a key.
Default time is 15 seconds with 60
seconds in CAL (calibration) mode.
1. Press .
28
Page 33

2. Press to display the stored memory records.
Figure 25
CAL
Figure 26
CAL
3. Press repeatedly until you pass the CELL ch location.
The display will show “Auto oFF” (Figure 24).
4. Press
to initiate. “CAL”
will be displayed along with
“15 SEC” or current Auto
oFF value (see Figure 25).
5. Press or to
change the amount of time
(see Figure 26). Maximum
time of 75 seconds is shown.
6. Press to accept the
change (new time).
XV. USER MODE CALIBRATION LINC™ FUNCTION
The Linc™ function allows easy calibration when in User mode and the
user does not have a user standard solution to calibrate the instrument.
This function will ensure more repeatable and accurate measurements
than many other calibration methods. It is recommended that this
function be used to provide the highest degree of condence when the
Ultrameter II is used in User mode. When Linc is used, the User mode
is linked to another standard, i.e., if User and KCl are linked, a KCI
standard solution is used to calibrate the instrument. It is that simple.
A. Calibration of Ultrameter II for use in User mode
1. Press
or key.
2. Calibrate the unit using a Standard Solution (ref.
CALIBRATION, pg. 17).
3. Place the Ultrameter II in User mode (ref. SOLUTION
SELECTION, pg. 14).
4. Verify/Set the calibration linc. (See B. Setting User
Mode Calibration “Linc”, pg. 30.)
29
Page 34

B. Setting User mode Calibration “Linc”
Figure 27
Figure 28
User
Figure 29
KCl
User
The Linc function sets or “links” the calibration gain factor of a Standard
Solution to the User solution mode. Once set, the “Linc” will stay
intact with future calibrations unless the Linc has been canceled. For
more information on canceling the User mode Calibration Linc refer to
C. “Canceling User mode Calibration “Linc”.
Follow the steps below to set either the KCl, NaCl or 442 calibration
factor to the User solution mode.
1. Press measurement key desired to be “Linked”, i.e., ,
.
or
2. Place the Ultrameter II in
User mode (ref. SOLUTION
SELECTION, pg. 14, for
selecting the User mode).
3. Press arrow key until
the menu “Linc” appears
(see Figure 27).
4. Press key. The
instrument will display “SEL”
and the “User” Icon (see
Figure 28).
Any additional display of KCl,
NaCl or 442 icons indicates a “Linc”
between the User solution and the
other solution displayed.
5. Press or
keys
to select a Standard
Solution to be linked to the
User mode calibration
constant. In Figure 29 the display indicates that “User” is
linked to “KCl”.
If none of the Solution Selection icons are displayed (i.e., KCl, NaCl or
442), nothing has been linked to User mode.
6. Press key to accept the setting. Pressing any of the
30
Page 35

measurement keys will exit without changing the setting. User
mode “Linc” is now complete. The User mode will now use the
calibration gain constant used for the calibration of the
Standard Solution as outlined above.
C. Canceling User mode Calibration “Linc”
The Ultrameter II must be in “User” linked mode in order to cancel the
“Linc” (ref. SOLUTION SELECTION, pg. 14).
1. Press “Linked” measurement key ,
Two solution icons will be shown in the left side of display “User” and another, e.g., “KCl”.
2. Press key until the menu “Linc” appears (see Figure 27).
3. Press key; the instrument will display both “SEL” and
the “User” Icon.
4. Press key until “User” is the only solution icon being
displayed.
5. Press key.
6. The User mode calibration “Linc” has now been canceled.
NOTES:
1. To maintain repeatability, use the same standard solutions for
future calibrations.
2. Calibration of the Ultrameter II Gain Factor for User mode is
not available when the calibration linc has been established.
The other calibration functions (i.e., Temperature Compensation
%/C settings and TDS Ratio settings) are still intact. To
perform a calibration of the User mode as described in User
Calibration Conductivity/TDS, pg. 18, the User mode Linc should
be canceled. See above, “Canceling User mode calibration
“Linc””.
or .
3. Once a “Linc” has been established for User mode , the “Linc”
will apply to all measurement modes using User solution
selection (i.e., TDS/User, Cond/User or Res/User).
31
Page 36

XVI. bluDock™ WIRELESS DATA TRANSFER INSTRUCTIONS
Figure 30
Figure 31
NOTE:
Bluetooth®
is a registered trademark of Bluetooth SIG. The
bluDock Bluetooth module is a registered Bluetooth device.
Requires Myron L bluDock™ accessory package, Model # BLUDOCK.
Package includes Ultrameter II hardware modication that allows the
unit to communicate wirelessly with a personal computer congured for
wireless device communication. Package also includes U2CI software
application that will operate on Windows XP, Vista and 7*, and Macintosh
OSX** based computer systems and Bluetooth USB adapter (dongle)
for computers that do not have Bluetooth capability.
A. Software Installation
Follow the instructions in the “U2CI Software Installation Guide” that
was shipped with your blueDock equipped instrument or download it
from the Myron L Company website.
http://www.myronl.com/main/U2CI_Application_DL.htm
B. Hardware Setup
For a computer without
Bluetooth®
capability:
If you don’t have the dongle that came with the BLUDOCK, one can be
ordered separately from the Myron L Company. Order Model # BDDO.
Plug in your dongle and install per manufacturer’s instructions.
For computers with Bluetooth
capability/Bluetooth dongle installed:
First time use of the bluDock:
1. Press any parameter button
to turn the Ultrameter II on.
2. Put the Ultrameter II in “PC
On” mode by pressing the
key until “PC OFF”
appears (see Figure 30).
3. Then press the key.
“PC On” will be displayed
(see Figure 31).
32
Page 37

NOTE: “PC Ini” may momentarily be
Figure 32
displayed while initializing (see
Figure 32).
4. Add bluDock to your
Bluetooth devices per your
operating system procedure.
THE BLUDOCK DEVICE
PASSKEY IS 1234.
5. After pairing, note the number
of the COM port assigned
by the computer. In Windows XP, note the number of the
outgoing COM port assigned by the computer.
NOTE: The unit will automatically power down after 60 sec. If the unit
powers down during pairing, repeat steps 1-3 above and continue.
C. Memory Stack Download
1. With the Ultrameter II in “PC On” mode, open the U2CI
software application.
2. Verify that the port selected matches the COM port number
noted (rst time only). This is the outgoing COM port on
Windows XP.
3. In the U2CI application, click on the data download button. A
data transfer bar will appear while the data is being downloaded.
Once downloaded, the data may be manipulated, printed or
stored within the Myron L U2CI application, or the data may
be exported to another more powerful spreadsheet
***,
such as
Excel*.
Additional features such as assigning a name to the unit,
setting time and date and erasing data are available. See U2CI
Operation Manual or visit our website for the latest instructions:
http://myronl.com/main/U2CI_Application_DL.htm
4. Upon completion, click on the “disconnect” icon.
5. Turn off Ultrameter II PC download mode by selecting any
measurement function. Failure to do so will reduce battery life.
* Windows 2000, 2007, XP & Vista and Excel are registered trademarks of Microsoft
Corporation.
** Macintosh OSX is a registered trademark of Apple Computer Inc.
*** Please Note: Although the Myron L Company has performed extensive testing, we
cannot guarantee compatibility of all applications and formats. We suggest testing your
application and format for compatibility before relying on it.
33
Page 38

XVII. CARE and MAINTENANCE
Ultrameter IIs should be rinsed with clean water after use. Solvents should
be avoided. Shock damage from a fall may cause instrument failure.
A. Temperature Extremes
Solutions in excess of 71°C/160°F should not be placed in the cell
cup area; this may cause damage. The pH sensor may fracture if the
Ultrameter II temperature is allowed to go below 0°C/32°F. Care should
be exercised not to exceed rated operating temperature.
Leaving the Ultrameter II in a vehicle or storage shed on a hot day
can easily subject the instrument to over 66°C/150°F. This will void the
warranty.
B. Battery Replacement
Dry Instrument THOROUGHLY. Remove the four (4) bottom screws.
Open instrument carefully. Carefully detach battery from circuit board.
Replace with 9 volt alkaline battery. Replace bottom, ensuring the
sealing gasket is installed in the groove of the top half of case. Re-install
screws, tighten evenly and securely.
NOTE: Because of nonvolatile EEPROM circuitry, all data stored in
memory and all calibration settings are protected even during power
loss or battery replacement. However, loss of time and date may occur if
battery is removed for longer than 3 minutes (180 seconds).
C. pH/ORP Sensor Replacement (6PFCE)
Order model RPR. When ordering, be sure to include the model and
serial number of your instrument to ensure receipt of the proper type.
Complete installation instructions are provided with each replacement
sensor.
D. Cleaning Sensors
1. Conductivity/TDS/Resistivity
The conductivity cell cup should be kept as clean as possible. Flushing
with clean water following use will prevent buildup on electrodes.
However, if very dirty samples — particularly scaling types — are
allowed to dry in the cell cup, a lm will form. This lm reduces accuracy.
When there are visible lms of oil, dirt, or scale in the cell cup or on the
electrodes, use isopropyl alcohol or a foaming non-abrasive household
cleaner. Rinse out the cleaner and your Ultrameter II is again ready for
accurate measurements.
2. pH/ORP (6PFCE)
The unique pH/ORP sensor in your Ultrameter II is a nonrellable
combination type that features a porous liquid junction.
34
It should not be
Page 39

allowed to dry out.
pH/ORP SENSOR
Top View
ORP
Electrode
pH Glass
Electrode
Sensor
Body
Reference
Junction
under Glass
pH Bulb
be rejuvenated by rst cleaning the sensor well with Isopropyl alcohol or
a liquid spray cleaner such as Windex™ or Fantastic™ and rinsing well.
Do not scrub or wipe the pH/ORP sensor.
Then use one of the following methods:
1. Pour a HOT salt solution ~60°C/140°F — a potassium chloride
(KCI) solution such as Myron L pH/ORP Sensor Storage
Solution is preferable, but HOT tap water with table salt (NaCl)
will work ne — in the sensor well and allow to cool. Retest.
or
2. Pour DI water in the sensor well and allow to stand for no more
than 4 hours (longer can deplete the reference solution
and damage the glass bulb). Retest.
If neither method is successful, the sensor must be replaced.
“Drifting” can be caused by a lm on the pH sensor bulb and/or reference.
Use isopropyl alcohol (IPA) or spray a liquid cleaner such as Windex™
or Fantastic™ into the sensor well to clean it. The sensor bulb is very
thin and delicate. Do not scrub or wipe the pH/ORP sensor.
However, if this occurs, the sensor may sometimes
Leaving high pH (alkaline) solutions in contact with the pH sensor for
long periods of time is harmful and will cause damage. Rinsing such
liquids from the pH/ORP sensor well and relling it with Myron L Storage
Solution, a saturated KCl solution, pH 4 buffer, or a saturated solution of
table salt and tap water, will extend the useful life.
Samples containing chlorine, sulfur, or ammonia can “poison” any pH
electrode. If it is necessary to measure the pH of any such sample,
thoroughly rinse the sensor well with clean water immediately after
taking the measurement. Any sample element that reduces (adds an
electron to) silver, such as cyanide, will attack the reference electrode.
Replacement sensors are available only from the Myron L Company or its
authorized distributors (see Replacement pH/ORP Sensor (6PFCE), pg. 39).
35
Page 40

XVIII. TROUBLESHOOTING CHART
Symptom Possible Cause Corrective Action
No display, even though
measurement key pressed
Inaccurate pH readings (6PFCE) 1. pH calibration needed. Ref. pH Cal.,
No response to pH changes (6PFCE) Sensor bulb is cracked or an
Will not adjust down to pH 7 (6PFCE) pH/ORP sensor has lost KCl. Clean and rejuvenate sensor (ref. Cleaning Sensors, pg. 34) and recalibrate. If no
pH readings drift or respond slowly to
changes in buffers/samples or “FAC”
is displayed repeatedly (6PFCE)
Unstable Conductivity/TDS/
Resistivity readings
Unable to calibrate Conductivity/TDS Film or deposits on electrodes. Clean cell cup and electrodes. Ref. Cleaning Sensors, pg. 34.
Resistivity readings much lower than
expected
Battery weak or not connected. Check connections or replace battery. Ref. Battery Replacement, pg. 34.
pg. 19.
2. Cross-contamination from residual pH
buffers or samples in sensor well.
3. Calibration with expired pH buffers.
electromechanical short caused by an
internal crack.
1. Temporary condition due to memory” of
solution in pH sensor well for long periods.
2. Bulb dirty or dried out.
3. Reference junction clogged or coated.
1. Dirty electrodes.
2. Actual resistance is changing due to
atmospheric contamination.
1. Contamination from previous sample or
from pH sensor well.
2. Carbon dioxide in test sample.
Low ORP Reading
Slow or no response to ORP changes
(6PFCE)
FCE responds very slowly or returns an
atypically high Predictive ORP value
(6PFCE).
36
ORP platinum electrode is dirty. Check the ORP sensor functioning. Take an ORP reading of Myron L pH/ORP
1. Dirty platinum electrode (see above).
2. ORP sensor memory/battery effect.
Some ORP sensors exhibit a residual
charge when measuring LOW Free
Chlorine concentrations soon after
measuring a HIGH Free Chlorine
concentration.
Page 41

1. Recalibrate instrument.
2. Thoroughly rinse sensor well.
3. Recalibrate using fresh buffers. Ref. pH Buffer Solutions, pg. 38.
Replace pH/ORP sensor. Ref. Replacement pH/ORP Sensor, pg. 39.
improvement, replace pH/ORP sensor (ref. Replacement pH/ORP Sensor, pg. 39).
Clean and rejuvenate sensor (ref. Cleaning Sensors, pg. 34) and recalibrate. If no
improvement, replace pH/ORP sensor (ref. Replacement pH/ORP Sensor, pg. 39).
1. Clean cell cup and electrodes. Ref. Cleaning Sensors, pg. 34.
2. Minimize test sample exposure to air by taking a owing sample.
Ref. Measuring Resistivity, pg. 10.
1. Rinse cell cup more thoroughly before measurement. Ensure pH cap is snugly in
place.
2. See Measuring Resistivity, pg. 10.
Sensor Storage Solution (ref. pH Sensor Storage Solution (6PFCE), pg. 38). If the
reading is outside the range of 350-400 mV, clean ONLY the platinum ORP
electrode with Myron L ORP Conditioner solution-soaked cotton swab (ref. ORP
Sensor Conditioner Solution (6PFCE), pg. 38), being careful not to touch the swab to
the glass bulb of the pH sensor.
1. Rinse the pH/ORP sensor well briey with a small amount of ORP Sensor
Conditioner Solution. DO NOT leave the conditioning solution in the sensor well
for more than 10 seconds.
2. Rinse the pH/ORP sensor 3 times with Sensor Storage Solution.
3. Fill the sensor well with Sensor Storage Solution and let rest for 5 minutes.
37
Page 42

XIX. ACCESSORIES
NOTE: MSDSs are available on the Myron L website for all solutions:
http://www.myronl.com/main/Material_Safety_DS_DL.htm
A. Conductivity/TDS Standard Solutions
Your Ultrameter II has been factory calibrated with the appropriate
Myron L Company NIST traceable KCl, NaCl, and our own 442™
standard solutions. Most Myron L conductivity standard solution bottles
show three values referenced at 25°C: Conductivity in microsiemens/
micromhos, the ppm/TDS equivalents (based on our 442 Natural
Water™) and NaCl standards. All standards are within ±1.0% of reference
solutions.
1. Potassium Chloride (KCl)
The concentrations of these reference solutions are calculated from
data in the International Critical Tables, Vol. 6. The 7000 µS is the
recommended standard.
2. 442 Natural Water™
442 Natural Water Standard Solutions are based on the following salt
proportions: 40% sodium sulfate, 40% sodium bicarbonate, and 20%
sodium chloride, which represent the three predominant components
(anions) in freshwater. This salt ratio has conductivity characteristics
approximating fresh natural waters and was developed by the Myron L
Company over four decades ago. It is used around the world for
measuring both conductivity and TDS in drinking water, ground water,
lakes, streams, etc. 3000 ppm is the recommended standard.
Available in 2 oz., quarts/liters, and gallon/~3.8 liter bottles.
Order KCL-7000
Order 442-3000
3. Sodium Chloride (NaCl)
This is especially useful in sea water mix applications, as sodium chloride
is the major salt component. Most Myron L standard solution labels show
the ppm NaCl equivalent to the conductivity and to ppm 442 values. The
14.0 mS is the recommended standard.
B. pH Buffer Solutions (6PFCE)
pH buffers are available in pH values of 4, 7 and 10. Myron L Company
buffer solutions are traceable to NIST certied pH references and are
color-coded for instant identication. They are also mold inhibited and
accurate to within ±0.01 pH units @ 25°C. Order 4, 7 or 10 Buffer.
Order NACL-14.0
Available in 2 oz., quarts/liters, and gallon/~3.8 liter bottles. Order SS.
C. pH Sensor Storage Solution (6PFCE)
Myron L pH Sensor Storage Solution prolongs the life of the pH sensor.
Available in 2 oz., quarts/liters, and gallon/~3.8 liter bottles.
D. ORP Sensor Conditioner Solution (6PFCE)
Myron L ORP Conditioner Solution removes contaminants and conditions
the ORP electrode.
38
Available in 1 oz. Order ORPCOND1OZ
.
Page 43

E. Soft Protective Carry Cases
Padded Nylon carrying case features a belt clip for hands-free mobility.
Two colors to choose from:
Blue - Model #: UCC
Desert Tan - Model #: UCCDT
F. Hard Protective Carry Cases
Large case with 2 oz. bottles of calibration standard solutions (KCl-7000,
442-3000, 4, 7, & 10 pH buffers and pH storage solution).
Small case (no calibration standard solutions) -
G. Replacement pH/ORP Sensor (6PFCE)
pH/ORP sensor is gel lled and features a unique porous liquid junction.
It is user-replaceable and comes with easy to follow instructions.
Model #: UPP
Model #: PKUU
Model #: RPR
H. bluDock™ Wireless Data Transfer Accessory Package
This accessory allows the operator to download the Ultrameter II
memory stack to a spreadsheet on a computer. The package includes
bluDock modied circuit board in the unit, software CD, installation and
operating instructions, and dongle.
XX. TEMPERATURE COMPENSATION (Tempco)
of Aqueous Solutions
Electrical conductivity indicates solution concentration and ionization
of the dissolved material. Since temperature greatly affects ionization,
conductivity measurements are temperature dependent and are normally
corrected to read what they would be at 25°C.
Model #: BLUDOCK
A. Standardized to 25°C
Conductivity is measured with great accuracy in the Ultrameter II using a
method that ignores ll level, electrolysis, electrode characteristics, etc.,
and features a microprocessor to perform temperature compensation. In
simpler instruments, conductivity values are usually assigned an average
correction similar to that of KCl solutions for correction to 25°C. The
correction to an equivalent KCl solution is a standard set by chemists
that standardizes the measurements and allows calibration with precise
KCl solutions. In the Ultrameter II, this correction can be set to other
solutions or tailored for special measurements or applications.
B. Tempco Variation
Most conductivity instruments use an approximation of the temperature
characteristics of solutions, perhaps even assuming a constant value.
The value for KCl is often quoted simply as 2%/°C. In fact, KCl tempco
varies with concentration and temperature in a non-linear fashion. Other
solutions have more variation still. The Ultrameter II uses corrections
that change with concentration and temperature instead of single
average values. See Chart 1, pg. 40.
39
Page 44

Chart 1
0 5 10 15 20 25 30 35 40 45 50 55 60
1.500%
1.600%
1.700%
1.800%
1.900%
2.000%
2.100%
2.200%
2.300%
2.400%
2.500%
KCl % / °C
% / °C
Temperature
C. An Example of 2 different solution selections and the
resulting compensation
How much error results from treating natural water as if it were KCl at
15°C?
A tap water solution should be compensated as 442 with a tempco of
1.68 %/°C, where the KCl value used would be 1.90 %/°C.
Suppose a measurement at 15°C/59°F is 900 microsiemens of true
uncompensated conductivity.
Using a 442 correction of 10 (degrees below 25) x 1.68% indicates the
solution is reading 16.8% low. For correction, dividing by (.832) yields
1082 microsiemens as a compensated reading.
A KCl correction of 10 (degrees below 25) x 1.9% indicates the solution
is reading 19% low. Dividing by (.81) yields 1111 microsiemens for a
compensated reading. The difference is 29 out of 1082 = 2.7%.
D. A Chart of Comparative Error
In the range of 1000 µS, the error using KCl on a solution that should
be compensated as NaCl or as 442, is illustrated in Chart 2 on pg. 41.
Users wanting to measure natural water based solutions to 1% would have
to alter the internal compensation to the more suitable preloaded “442”
values or stay close to 25°C. Users who have standardized to KCl- based
compensation may want to stick with it, regardless of increasing error as
you get further from 25°C. The Ultrameter II will provide the repeatability
and convertibility of data necessary for relative values for process control.
40
Page 45

7%
Chart 2
55
(1)%
(2)%
0%
1%
2%
3%
4%
5%
6%
0 5 10 15 20
25
30 35 40 45 50
Temperature
NaCl error with KCl tempco
442 error with KCl tempco
E. Other Solutions
A salt solution like sea water or liquid fertilizer acts like NaCl. An internal
correction for NaCl can be selected for greatest accuracy with such
solutions. Many solutions are not at all similar to KCl, NaCl or 442. A
sugar solution, or a silicate, or a calcium salt at a high or low temperature
may require a User value peculiar to the application to provide readings
close to the true compensated conductivity.
Clearly, the solution characteristics should be chosen to truly represent
the actual water under test for rated accuracy of ±1%. Many industrial
applications have historically used relative measurements seeking a
number to indicate a certain setpoint or minimum concentration or trend.
The Ultrameter II gives the user the capability to collect data in “KCl
conductivity units” to compare to older published data, in terms of NaCl
or 442, or as appropriate. The Ultrameter II can be used to reconcile
data taken with other compensation assumptions, especially with its
ability to allow custom characteristics through the User mode.
XXI. CONDUCTIVITY CONVERSION to TOTAL
DISSOLVED SOLIDS (TDS)
Electrical conductivity indicates solution concentration and ionization
of the dissolved material. Since temperature greatly affects ionization,
conductivity measurements are temperature dependent and are
normally corrected to read what they would be at 25°C (ref. Temperature
Compensation, pg. 39).
41
Page 46

A. How it’s Done
Once the effect of temperature is removed, the compensated conductivity
is a function of the concentration (TDS). Temperature compensation of
the conductivity of a solution is performed automatically by the internal
processor with data derived from chemical tables. Any dissolved salt at
a known temperature has a known ratio of conductivity to concentration.
Tables of conversion ratios referenced to 25°C have been published by
chemists for decades.
B. Solution Characteristics
Real world applications have to measure a wide range of materials and
mixtures of electrolyte solutions. To address this problem, industrial
users commonly use the characteristics of a standard material as a
model for their solution, such as KCl, which is favored by chemists for
its stability.
Users dealing with sea water, etc., use NaCl as the model for their
concentration calculations. Users dealing with freshwater work with
mixtures including sulfates, carbonates and chlorides, the three
predominant components (anions) in freshwater that the Myron L
Company calls “Natural Water”. These are modeled in a mixture called
“442™” which the Myron L Company markets for use as a calibration
standard, as it does standard KCl and NaCl solutions.
The Ultrameter II contains algorithms for these 3 most commonly
referenced compounds. The solution type in use is displayed on the
left. Besides KCl, NaCl, and 442, there is the User choice. The benet
of the User solution type is that one may enter the temperature
compensation and TDS ratio by hand, greatly increasing accuracy of
readings for a specic solution. That value remains a constant for all
measurements and should be reset for different dilutions or temperatures.
C. When does it make a lot of difference?
First, the accuracy of temperature compensation to 25°C determines the
accuracy of any TDS conversion. Assume we have industrial process
water to be pretreated by RO. Assume it is 45°C and reads 1500 µS
uncompensated.
1. If NaCl compensation is used, an instrument would report 1035
µS compensated, which corresponds to 510 ppm NaCl.
2. If 442 compensation is used, an instrument would report 1024
µS compensated, which corresponds to 713 ppm 442.
The difference in values is 40%.
In spite of such large error, some users will continue to take data in
the NaCl mode because their previous data gathering and process
monitoring was done with an older NaCl referenced device.
Selecting the correct Solution Type on the Ultrameter II will allow the
user to attain true TDS readings that correspond to evaporated weight.
42
Page 47

If none of the 3 standard solutions apply, the User mode must be used.
Temperature Compensation (Tempco) and TDS Derivation below, details
the User mode.
XXII. TEMPERATURE COMPENSATION (Tempco)
and TDS DERIVATION
The Ultrameter II contains internal algorithms for characteristics of the
3 most commonly referenced compounds. The solution type in use is
displayed on the left. Besides KCl, NaCl, and 442, there is the User
choice. The benet of User mode is that one may enter the tempco and
TDS conversion values of a unique solution via the keypad.
A. Conductivity Characteristics
When taking conductivity measurements, the Solution Selection determines
the characteristic assumed as the instrument reports what a measured
conductivity would be if it were at 25°C. The characteristic is represented
by the tempco, expressed in %/°C. If a solution of 100 µS at 25°C increases
to 122 µS at 35°C, then a 22% increase has occurred over this change of
10°C. The solution is then said to have a tempco of 2.2 %/°C.
Tempco always varies among solutions because it is dependent on their
individual ionization activity, temperature and concentration. This is why
the Ultrameter II features mathematically generated models for known
salt characteristics that also vary with concentration and temperature.
B. Finding the Tempco of an Unknown Solution
One may need to measure compensated conductivity of some solution
unlike any of the 3 standard salts. In order to enter a custom xed tempco
for a limited measurement range, enter a specic value through the
User function. The tempco can be determined by 2 different methods:
1. Heat or cool a sample of the solution to 25°C, and measure its
conductivity. Heat or cool the solution to a typical temperature
where it is normally measured. After selecting User function, set
the tempco to 0 %/°C as in Disabling Temperature Compensation,
pg. 15 (No compensation). Measure the new conductivity and the
new temperature. Divide the % decrease or increase by the 25°C
value. Divide that difference by the temperature difference.
2. Heat or cool a sample of the solution to 25°C, and measure its
conductivity. Change the temperature to a typical measuring
temperature. Set the tempco to an expected value as in User
Programmable Temperature Compensation, pg. 15. See if the
compensated value is the same as the 25°C value. If not, raise or
lower the tempco and measure again until the 25°C value is read.
C. Finding the TDS Ratio of an Unknown Solution
Once the effect of temperature is removed, the compensated conductivity
is a function of the concentration (TDS).
43
Page 48

There is a ratio of TDS to compensated conductivity for any solution,
which varies with concentration. The ratio is set during calibration in User
mode as in User Programmable Conductivity to TDS Ratio, pg. 16.
A truly unknown solution has to have its TDS determined by evaporation
and weighing. Then the solution whose TDS is now known can be
measured for conductivity and the ratio calculated. Next time the same
solution is to be measured, the ratio is known.
XXIII. pH and ORP (6PFCE)
A. pH (6PFCE)
1. pH as an Indicator (6PFCE)
pH is the measurement of Acidity or Alkalinity of an aqueous solution. It
is also stated as the Hydrogen Ion activity of a solution. pH measures
the effective, not the total, acidity of a solution.
A 4% solution of acetic acid (pH 4, vinegar) can be quite palatable, but
a 4% solution of sulfuric acid (pH 0) is a violent poison. pH provides the
needed quantitative information by expressing the degree of activity of
an acid or base.
In a solution of one known component, pH will indicate concentration
indirectly. However, very dilute solutions may be very slow reading, just
because the very few ions take time to accumulate.
2. pH Units (6PFCE)
The acidity or alkalinity of a solution is a measurement of the relative
availabilities of hydrogen (H+) and hydroxide (OH-) ions. An increase
in (H+) ions increases acidity, while an increase in (OH-) ions increases
alkalinity. The total concentration of ions is xed as a characteristic
of water, and balance would be 10
-
7
mol/liter (H+) and (OH-) ions in a
neutral solution (where pH sensors give 0 voltage).
pH is dened as the negative logarithm of hydrogen ion concentration.
Where (H+) concentration falls below 10-7, solutions are less acidic than
neutral, and therefore are alkaline. A concentration of 10-9 mol/liter of
(H+) would have 100 times less (H+) ions than (OH-) ions and be called
an alkaline solution of pH 9.
3. The pH Sensor (6PFCE)
The active part of the pH sensor is a thin glass surface that is selectively
receptive to hydrogen ions. Available hydrogen ions in a solution will
accumulate on this surface and a charge will build up across the glass
interface. The voltage can be measured with a very high impedance
voltmeter circuit; the dilemma is how to connect the voltmeter to solution
on each side.
The glass surface encloses a captured solution of potassium chloride
holding an electrode of silver wire coated with silver chloride. This is
the most inert connection possible from a metal to an electrolyte. It can
44
Page 49

Glass surface
Figure 33
KCl solution
Electrode wire
Electrode
wire
H
+
ions
Junction
Plug
KCl solution
Figure 34
Junction plug
Platinum button
H+ ions
Electrode wires
Glass
Glass
Surface
still produce an offset voltage, but using the same materials to connect
to the solution on the other side of the membrane causes the 2 equal
offsets to cancel.
The problem is, on the other side of the membrane is an unknown test
solution, not potassium chloride. The outside electrode, also called the
Reference Junction, is of the same construction with a porous plug in place
of a glass barrier to allow
the junction uid to contact the test
solution without signicant migration
of liquids through the plug material.
Figure 33 shows a typical 2
component pair. Migration does
occur, and this limits the lifetime of a
pH junction from depletion of solution
inside the reference junction or from
contamination. The junction may be
damaged if dried out because
insoluble crystals may form in a
layer, obstructing contact with test
solutions. See pH and ORP, pg. 44.
4. The Myron L Integral
pH Sensor (6PFCE)
The sensor in the Ultrameter II
(see Figure 34) is a single
construction in an easily replaceable
package. The sensor body holds
an oversize solution supply for long
life. The reference junction “wick” is
porous to provide a very stable, low
permeable interface, and is located
under the glass pH sensing
electrode. This construction
combines all the best features of
any pH sensor known.
5. Sources of Error (6PFCE)
The basics are presented in pH and ORP, pg. 44.
a. Reference Junction
The most common sensor problem will be a clogged junction because a
sensor was allowed to dry out. The symptom is a drift in the “zero” setting
at 7 pH. This is why the Ultrameter II 6PFCE does not allow more than 1
pH unit of offset during calibration. At that point the junction is unreliable.
b. Sensitivity Problems
Sensitivity is the receptiveness of the glass surface. A lm on the surface
can diminish sensitivity and cause a long response time.
45
Page 50

c. Temperature Compensation
pH sensor glass changes its sensitivity slightly with temperature, so the
further from pH 7 one is, the more effect will be seen. A pH of 11 at 40°C
would be off by 0.2 units. The Ultrameter II 6PFCE senses the sensor
well temperature and compensates the reading.
B. ORP/Oxidation-Reduction Potential/REDOX (6PFCE)
1. ORP as an Indicator (6PFCE)
ORP is the measurement of the ratio of oxidizing activity to reducing
activity in a solution. It is the potential of a solution to give up electrons
(oxidize other things) or gain electrons (reduce).
Like acidity and alkalinity, the increase of one is at the expense of the
other, so a single voltage is called the Oxidation-Reduction Potential,
with a positive voltage showing, a solution wants to steal electrons
(oxidizing agent). For instance, chlorinated water will show a positive
ORP value.
2. ORP Units (6PFCE)
ORP is measured in millivolts, with no correction for solution temperature.
Like pH, it is not a measurement of concentration directly, but of activity
level. In a solution of only one active component, ORP indicates
concentration. Also, as with pH, a very dilute solution will take time to
accumulate a readable charge.
3. The ORP Sensor (6PFCE)
An ORP sensor uses a small platinum surface to accumulate charge
without reacting chemically. That charge is measured relative to the
solution, so the solution “ground” voltage comes from a reference
junction - same as the pH sensor uses.
4. The Myron L ORP Sensor (6PFCE)
Figure 34, pg. 45, shows the platinum button in a glass sleeve. The
same reference is used for both the pH and the ORP sensors. Both
pH and ORP will indicate 0 for a neutral solution. Calibration at zero
compensates for error in the reference junction.
A zero calibration solution for ORP is not practical, so the Ultrameter II
6PFCE uses the offset value determined during calibration to 7 in pH
calibration (pH 7 = 0 mV). Sensitivity of the ORP surface is xed, so
there is no gain adjustment either.
5. Sources of Error (6PFCE)
The basics are presented in pH and ORP, pg. 44, because sources of
error are much the same as for pH. The junction side is the same, and
though the platinum surface will not break like the glass pH surface,
its protective glass sleeve can be broken. A surface lm will slow
the response time and diminish sensitivity. It can be cleaned off with
detergent or acid, as with the pH glass.
46
Page 51

C. Free Chlorine
1. Free Chlorine as an Indicator of Sanitizing Strength
Chlorine, which kills bacteria by way of its power as an oxidizing agent,
is the most popular germicide used in water treatment. Chlorine is
not only used as a primary disinfectant, but also to establish a
sufcient residual level of Free Available Chlorine (FAC) for ongoing
disinfection.
FAC is the chlorine that remains after a certain amount is consumed by
killing bacteria or reacting with other organic (ammonia, fecal matter) or
inorganic (metals, dissolved CO2, Carbonates, etc) chemicals in solution.
Measuring the amount of residual free chlorine in treated water is a well
accepted method for determining its effectiveness in microbial control.
The Myron L Company FCE method for measuring residual disinfecting
power is based on ORP, the specic chemical attribute of chlorine (and
other oxidizing germicides) that kills bacteria and microbes.
2. FCE Free Chlorine Units
The 6PIIFCE is the rst handheld device to detect free chlorine directly,
by measuring ORP. The ORP value is converted to a concentration
reading (ppm) using a conversion table developed by Myron L Company
through a series of experiments that precisely controlled chlorine levels
and excluded interferants.
Other test methods typically rely on the user visually or digitally
interpreting a color change resulting from an added reagent-dye. The
reagent used radically alters the sample’s pH and converts the various
chlorine species present into a single, easily measured species. This
ignores the effect of changing pH on free chlorine effectiveness and
disregards the fact that some chlorine species are better or worse
sanitizers than others.
The Myron L Company 6PIIFCE avoids these pitfalls. The chemistry of
the test sample is left unchanged from the source water. It accounts for
the effect of pH on chlorine effectiveness by including pH in its calculation.
For these reasons, the Ultrameter II’s FCE feature provides the best
reading-to-reading picture of the rise and fall in sanitizing effectivity of
free available chlorine.
The 6PIIFCE also avoids a common undesirable characteristic of other
ORP-based methods by including a unique Predictive ORP value in its
FCE calculation. This feature, based on a proprietary model for ORP
sensor behavior, calculates a nal stabilized ORP value in 1 to 2 minutes
rather than the 10 to 15 minutes or more that is typically required for an
ORP measurement.
47
Page 52

XXIV. SOFTWARE VERSION
Figure 35
Contact the Myron L Company to see if a software upgrade is available.
1. Press
key.
2. Press key until three
numbers are displayed as
shown in Figure 35.
3. Press key, instrument
will time out in ~15 seconds.
48
Page 53

XXV. GLOSSARY
Anions Negatively charged ions.
See Solution Characteristics, pg. 42.
Algorithm A procedure for solving a mathematical problem.
See Temperature Compensation (Tempco) and
TDS Derivation, pg. 43.
FAC Free Available Chlorine. The amount of chlorine that
remains active in solution and is available for ongoing
disinfection. See Free Chlorine as an Indicator, pg. 47.
FCE FCE™ directly measures ORP, the germ killing
property of chlorine and other oxidizing germicides.
It displays both the ORP reading (in mVDC) as well
as an equivalent free chlorine concentration (in
familiar ppm). For more information see FCE™:
Groundbreaking Measurement of Free Chlorine
Disinfecting Power in a Hand-Held Instrument on
the Myron L Company website.
Logarithm An arithmetic function. The inverse of an exponential
function. See pH Units, pg. 44.
ORP Oxidation-Reduction Potential or REDOX, See ORP/
Oxidation-Reduction Potential/REDOX, pg. 46.
REDOX An abbreviation for Reduction-Oxidation reactions.
Reaction This is the basic electrochemical process by which
chlorine destroys microbes by grabbing electrons from
the microbe’s proteins, denaturing the protein and
killing the organism. ORP directly measures the
strength of a solutions’ REDOX potential and,
therefore, sanitizing strength.
TDS Total Dissolved Solids or the Total Conductive Ions
in a solution. See Conductivity Conversion to
Total Dissolved Solids (TDS), pg. 41.
Tempco Temperature Compensation
See Temperature Compensation of Aqueous
Solutions, pg. 39.
User A mode of operation that allows the instrument user
(operator) to set a tempco and/or a TDS factor for
their specic solution type. See Temperature
Compensation of Aqueous Solutions, pg. 39
and Temperature Compensation (Tempco) and
TDS Derivation, pg. 43.
For details on specic areas of interest refer to the Table of Contents.
49
Page 54

50
Page 55

HigH Performance features:
• Accuracy of ±1% of
READING
±.2% at Calibration Point
• Reliable Repeatable
Results
• KCl, NaCl and 442™
Natural Water Modes
• Automatic Temperature
Compensation
• Autoranging
• Durable, Fully
Encapsulated
Electronics
• Waterproof
• Powered by 1 N Type
Battery
(included)
Page 56

MYRON L COMPANY
2450 Impala Drive
Carlsbad, CA 92010-7226
USA
Tel: +1-760-438-2021
Fax: +1-760-931-9189
E-Mail: info@myronl.com
techquestions@myronl.com
www.myronl.com
Made In USA
UMIIFCEOM 20SEP13
 Loading...
Loading...