MYLAN WIXELA INHUB User Manual
(
doses
00000000
)
do
s
e
s
0000000
0
Rx
o
n
ly
Pouch opene
d
:
Use by:
Disc
a
r
d 1
month aft
e
r
rem
oval
f
ro
m
t
h
e f
oil
p
o
u
ch
.
MDR:9320
:60D:R2
-A
N
D
C
0
3
78-
9320
-3
2
(flut
icasone
p
ropionate and
salmeterol inhalation powder, USP
)
100/
5
0
doses
0000000
0
Rx only
Pouch opened:
Use by:
Discar
d
1 month after
A-2R:D06:0239:RDM
NDC 0378-
9
3
20
-32
(
fluticasone
propionate and
salmeterol inhalation powder, USP
)
250
/
50
fluticasone propionate
and salmeterol inhalation powder, USP
100/50 mcg
250/50 mcg
500/50 mcg
)
HOW TO USE WIXELATM INHUB
TM
Wixela Inhub was designed with patients in mind to help you make
®
a seamless transition from ADVAIR DISKUS
(fluticasone propionate
and salmeterol inhalation powder).
Take Wixela Inhub out of the foil pouch just before you use it for the first time. Write the “Pouch opened” and “Use by” dates on the label.
The “Use by” date is one month from the date you open the pouch for your first dose.
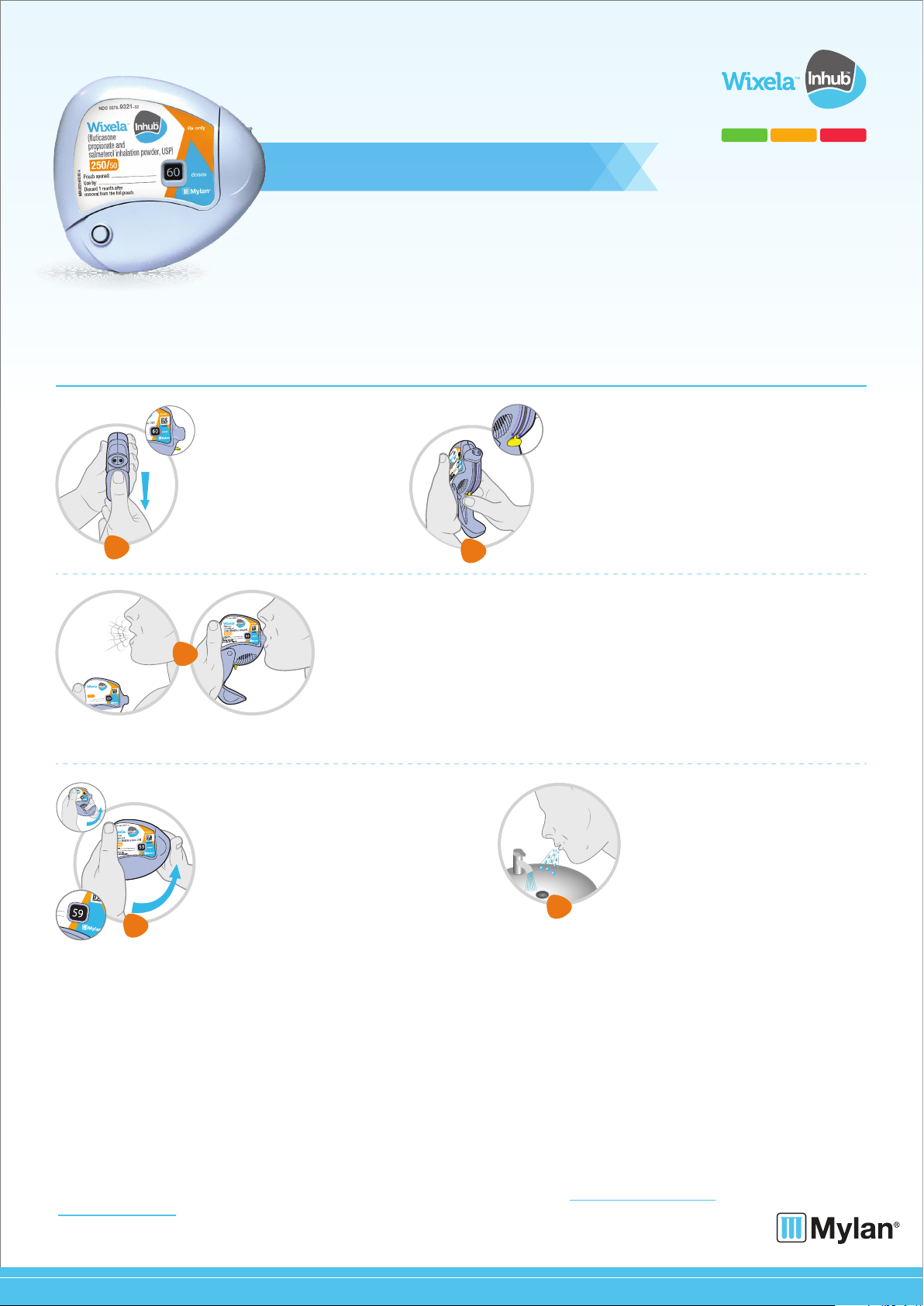
Follow the 5 steps below each time you take a dose.
Open your INHUB
• Hold the INHUB in one hand
and with your other hand on the
grip lower the mouthpiece
cover from top to bottom.
1
2
Inhale your medicine.
• Before you breathe in your dose from the INHUB, breathe out (exhale) as long as you
can while you hold the INHUB away from your mouth. Do not breathe into the mouthpiece.
3
• Put the mouthpiece to your lips. Breathe in quickly and deeply through the INHUB.
Do not breathe in through your nose.
• Remove the INHUB from your mouth and hold your breath for about 10 seconds,
or for as long as is comfortable for you.
• Breathe out slowly for as long as you can.
• The INHUB delivers your dose of medicine as a very fine powder that you may or may not taste
or feel. Do not take an extra dose from the INHUB even if you do not taste or feel the medicine.
Push down the lever.
• Hold the INHUB in the vertical position. Push the
yellow lever down to the end of the purple arrows (you
may hear a click).
• The INHUB is now ready to use.
Follow the instructions below so you will not accidentally
waste a dose:
• Do not close the INHUB.
• Do not move the lever on the INHUB once pushed down.
Close the INHUB
• Push the mouthpiece cover up to the closed
position, this will automatically return the yellow
lever to the start position. Make sure the INHUB
is shut and you cannot see the mouthpiece.
Rinse your mouth
• Rinse your mouth with water after
breathing in the medicine. Spit out the
water. Do not swallow it.
• The dose counter will count down one dose as
you close the mouthpiece cover. This will now
tell you how many doses are left.
• The INHUB is now ready for you to take your
next scheduled dose in about 12 hours.
4
• When you are ready to take your next dose,
5
repeat Steps 1 through 4.
Indications
WIXELA INHUB is a twice-daily prescription medicine used to control symptoms of asthma and to prevent symptoms such as
wheezing in patients 4 years and older. WIXELA INHUB is for adults and children with asthma who are not well controlled with an
asthma control medicine, such as an inhaled corticosteroid (ICS) medicine and need both an ICS and long-acting beta
-adrenergic
2
agonist (LABA) medicine.
WIXELA INHUB 250/50 is a twice-daily prescription medicine used long term to treat chronic obstructive pulmonary disease
(COPD), including chronic bronchitis, emphysema, or both, for better breathing and fewer flare-ups.
Important Limitation of Use: WIXELA INHUB is not used to relieve sudden breathing problems from asthma or COPD and won't
replace a rescue inhaler.
Please see additional Important Safety Information on next page. Click here for full Prescribing Information and
Patient Information for full Instructions for Use.
When using your Wixela
doses
00000000
Rx only
)
50
60
doses
00000000
Rx only
)
50
9
doses
00000000
Rx only
d:
nth after
the foil pouch.
sone
)
50
0
™
Inhub
™
please be
aware of these important components
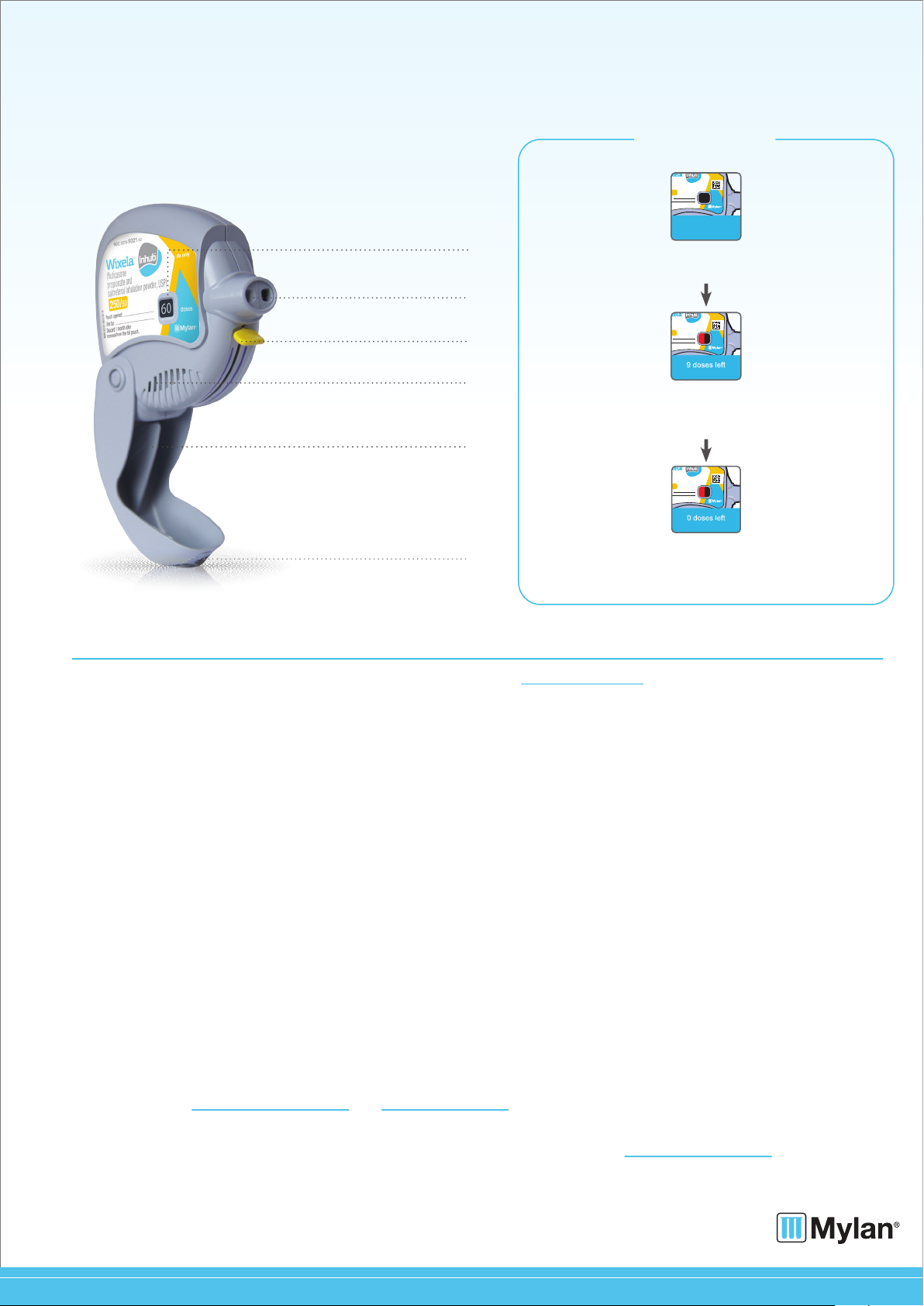
DOSE COUNTER
Dose counter
Mouthpiece
Yellow lever
Air vents
Mouthpiece cover
Grip
60 doses left
Your dose counter will be set at 60
when you first receive your INHUB.
9 doses left
After you have taken 51 doses, a red indicator will be
present. This indicator warns you there are 9 or fewer
doses left and is a reminder to get a refill.
0 doses left
The dose counter will read 0 and the lever will not
reach the end of the purple arrows when
there are no doses remaining.
For more information and to watch the How to Use video, visit www.wixela.com
Important Safety Information
®
• WIXELA INHUB contains salmeterol, the same medicine found in SEREVENT
LABA medicines such as salmeterol when used alone increase the risk of hospitalizations and death from asthma
problems. WIXELA INHUB contains an ICS and a LABA. When an ICS and LABA are used together, there is not a
significant increased risk in hospitalizations and death from asthma problems.
• Do not use WIXELA INHUB to treat sudden breathing problems from asthma or COPD. Always have a rescue inhaler
with you to treat sudden symptoms.
• Do not use WIXELA INHUB if you have a severe allergy to milk proteins. Do not use WIXELA INHUB if you are allergic
to any of the ingredients in the products. Ask your healthcare provider if you are not sure.
• Do not use WIXELA INHUB more often than prescribed.
• Do not take WIXELA INHUB with other medicines that contain a LABA for any reason.
• Tell your healthcare provider about all the medicines you take and about all of your health conditions.
• Common side effects of WIXELA INHUB for asthma include upper respiratory tract infection, throat irritation,
hoarseness and voice changes, thrush in your mouth or throat, bronchitis, cough, headache, and nausea and vomiting. In
children with asthma, infections in the ear, nose, and throat are common.
• Common side effects of WIXELA INHUB for COPD include thrush in your mouth or throat, throat irritation, hoarseness
and voice changes, viral respiratory infections, headache, and muscle and bone pain.
Click Here for full Prescribing Information and Patient Information for full Instructions For Use.
For additional information please contact us at 800-395-3376.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call
1-800-FDA-1088.
(salmeterol xinafoate inhalation powder).
WIXELA and INHUB are trademarks of Mylan Pharmaceuticals Inc.
ADVAIR DISKUS and SEREVENT are registered trademarks of the GSK group of companies.
The Mylan logo is a registered trademark of Mylan Inc.
© 2019 Mylan Pharmaceuticals Inc. All rights reserved. WIX-2018-0017
 Loading...
Loading...