MOWOOT II User Manual

Manufacturer:
usMIMA S.L.
Avinguda Cornellà 142, 08950 Esplugues de
Llobregat (Barcelona), Spain
Tel. (+34) 93 510 66 53
Mail. info@mowoot.com
Web. www.mowoot.com
Distributor (Country):
(Name)
(Address)
Tel. XXXX
Mail. XXXX@XXXX.XX
Web. XXXX
User Manual
MOWOOT II
Intestinal-Transit Management System
Pneumatic desktop device and exoperistaltic belt
MOWOOT II
User Manual
February 2019 Version 04 - English Page 2 of 18
TABLE OF CONTENTS
IMPORTANT INFORMATION - READ BEFORE OPERATING MOWOOT II SYSTEM 4
1 THE MOWOOT II SYSTEM 4
1.1 COMPONENTS OF THE MOWOOT II SYSTEM ........................................................................................ 4
1.2 LOCATIONS AND NUMBER OF SIMULTANEOUS USERS ..................................................................... 4
1.2.1
DAILY TIME OF USE IN THE HOME CARE AREA
4
1.2.2
DAILY TIME OF USE IN THE IN-PATIENT AREA
4
1.3 MAINTENANCE OF THE MOWOOT II SYSTEM ....................................................................................... 4
1.4 SAFETY WARNINGS .............................................................................................................................. 4
1.5 SAFETY PRECAUTIONS ....................................................................................................................... 5
1.6 LABELS ................................................................................................................................................................. 5
2 AREAS OF APPLICATION, CONTRAINDICATIONS, SIDE EFFECTS 6
2.1 APPLICATIONS .................................................................................................................................................. 6
2.2 CONTRAINDICATIONS .................................................................................................................................... 6
2.3 SIDE EFFECTS ................................................................................................................................................... 7
3 COMPONENTS OF THE MOWOOT II SYSTEM 7
3.1 PNEUMATIC CONSOLE (DESKTOP) AND ACCESSORIES ...................................................................... 7
3.2 EXOPERISTALTIC BELT ................................................................................................................................... 7
4 SETTING UP THE SYSTEM FOR THE APPLICATION 8
4.1 PLACING AND INSTALLING THE DESKTOP UNIT ................................................................................... 8
4.2 ATTACH EXOPERISTALTIC BELT ................................................................................................................. 8
4.3 ATTACH EXOPERISTALTIC BELT TO THE DESKTOP.............................................................................. 9
4.4 TREATMENT POSITION ................................................................................................................................. 9
5 OPERATE DESKTOP DEVICE 9
6 TREATMENT DURATION. SETTING TREATMENT FREQUENCY AND TIME 10
7 STARTING THE TREATMENT SESSION 10
8 ENDING THE TREATMENT SESSION 11
9 CLEANING, DISINFECTION, STORAGE, TRANSPORT 11
9.1 SPECIAL PRECAUTIONS – DESKTOP DEVICE BEFORE CLEANING........................................ 11
9.2 SPECIAL PRECAUTIONS – BELT BEFORE CLEANING ............................................................... 11

MOWOOT II
User Manual
February 2019 Version 04 - English Page 3 of 18
9.3 CLEANING AND DISINFECTION ................................................................................................................ 11
9.4 STORAGE ......................................................................................................................................................... 11
9.5 TRANSPORT ................................................................................................................................................... 12
10 TROUBLESHOOTING 12
11 ASSISTANCE 12
12 REPLACEMENT OF THE MOWOOT II SYSTEM 13
13 SPARE PARTS 13
14 DISPOSAL 14
ATTACHMENTS 14
ANNEX I – CLASSIFICATIONS 14
ANNEX II – TECHNICAL SPECIFICATIONS 14
ANNEX III – EMC MANUFACTURER’S DECLARATION 14

MOWOOT II
User Manual
February 2019 Version 04 - English Page 4 of 18
IMPORTANT INFORMATION - READ BEFORE OPERATING MOWOOT II SYSTEM
Be sure to read this user manual completely and carefully before operating the MOWOOT II system. The
user manual should be kept in a safe place so that later questions can be clarified.
1
THE MOWOOT II SYSTEM
1.1
COMPONENTS OF THE MOWOOT II SYSTEM
The MOWOOT II system consists of the MOWOOT II Desktop unit (see 3.1.) and one or two MOWOOT II
belts (see 3.2). The MOWOOT II system can only be used if both components are present.
1.2
LOCATIONS AND NUMBER OF SIMULTANEOUS USERS
The MOWOOT II system is intended for use at home as well as for inpatient use (eg hospitals, nursing
homes and retirement homes).
When used in the in-patient area, the MOWOOT II system can be used simultaneously by two users due
to the second air outlet on the MOWOOT II desktop. However, this is only possible if the application
time and application speed are identical for both users.
For domestic applications, the second air outlet on the MOWOOT II desktop should always be kept
sealed with the supplied plug (see chapter 4.3).
1.2.1
DAILY TIME OF USE IN THE HOME CARE AREA
The MOWOOT II desktop device and the MOWOOT II exoperistaltic belt should not exceed the
application time of a maximum of 2x20 minutes at maximum speed (see 6).
1.2.2
DAILY TIME OF USE IN THE IN-PATIENT AREA
The MOWOOT II desktop device and the MOWOOT II exoperistaltic belt can be used up to 15 times 20
minutes at maximum speed in the in-patient area.
1.3
MAINTENANCE OF THE MOWOOT II SYSTEM
The MOWOOT II system is maintenance-free when used as intended. However, it should be noted that,
if necessary, tests must be carried out on the basis of statutory provisions (e.g. in Germany electrical
testing in accordance with DGUV regulation 3, safety checks according to § 11 MPBetreibV).
If malfunctions occur, see Chapter 10.
1.4
SAFETY WARNINGS
Electric shock hazard. Do not immerse the MOWOOT II desktop device in liquid and never bring it into
contact with liquids, even partially. For cleaning, follow the instructions in sections 9.1 to 9.3. The
desktop device must not be opened. Only connect original MOWOOT II components to the desktop unit.
Do not use the MOWOOT II Desktop if the system is wet or in the presence of flammable materials. Stop
the treatment session (see 7.) if a change in the performance of the MOWOOT II system (on the desktop
or belt) is detected. The MOWOOT II system may only be used by children or mentally handicapped
persons under close and continuous surveillance. Danger of choking due to small parts.
MOWOOT II
User Manual
February 2019 Version 04 - English Page 5 of 18
1.5
SAFETY PRECAUTIONS
Keep MOWOOT II desktop and belt away from pets, sources of heat and moisture, and protect it from
dust, lint and dirt. Do not use the desktop unit outside the temperature range of 10-30 °C or outside the
humidity range of 30-85% RH. Do not use the device during transport. Do not operate the desktop unit
at altitudes above 3,000 m above sea level. The desktop unit must stand securely on a horizontal firm
surface during operation. Do not cover the desktop unit, not even partially.
1.6
LABELS
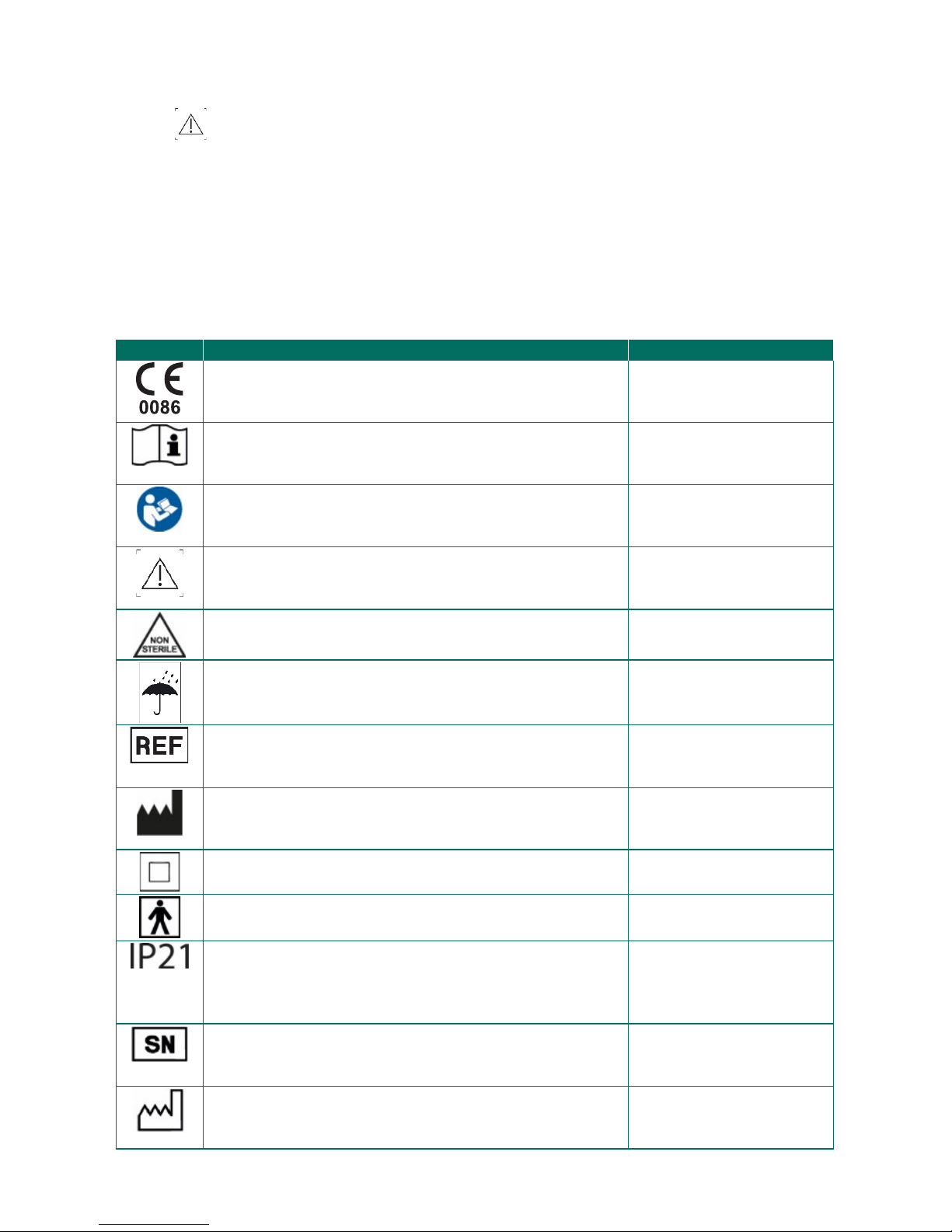
Symbol
Description
Position
CE label showing compliance with European Directive
2007/47/EC amending Directive 93/42/EEC, concerning
medical devices.
Desktop device
Belt
Packaging
Consult instructions for use.
Desktop device
Belt
Packaging
Read the user manual before use.
Desktop device
Belt
Packaging
Caution
Desktop device
Belt
Packaging
Non-sterile product
Desktop device
Packaging
Keep dry
Desktop device
Belt
Packaging
Item Reference number
Desktop device
Belt
Packaging
Name and address of manufacturer.
Desktop device
Belt
Packaging
Protected housing (
protection class II).
Desktop device
Packaging
Degree of protection against leakage currents (type BF)
Desktop device
Packaging
Protection against penetration of foreign bodies
Ø> 12.5 mm.
Protection against dripping water falling vertically
(dripping water).
Desktop device
Packaging
Serial number.
Next to this symbol, the serial number of
the product must be added.
Desktop device
Belt
Packaging
Date or year of manufacture
Desktop device
Belt
Packaging
MOWOOT II
User Manual
February 2019 Version 04 - English Page 6 of 18
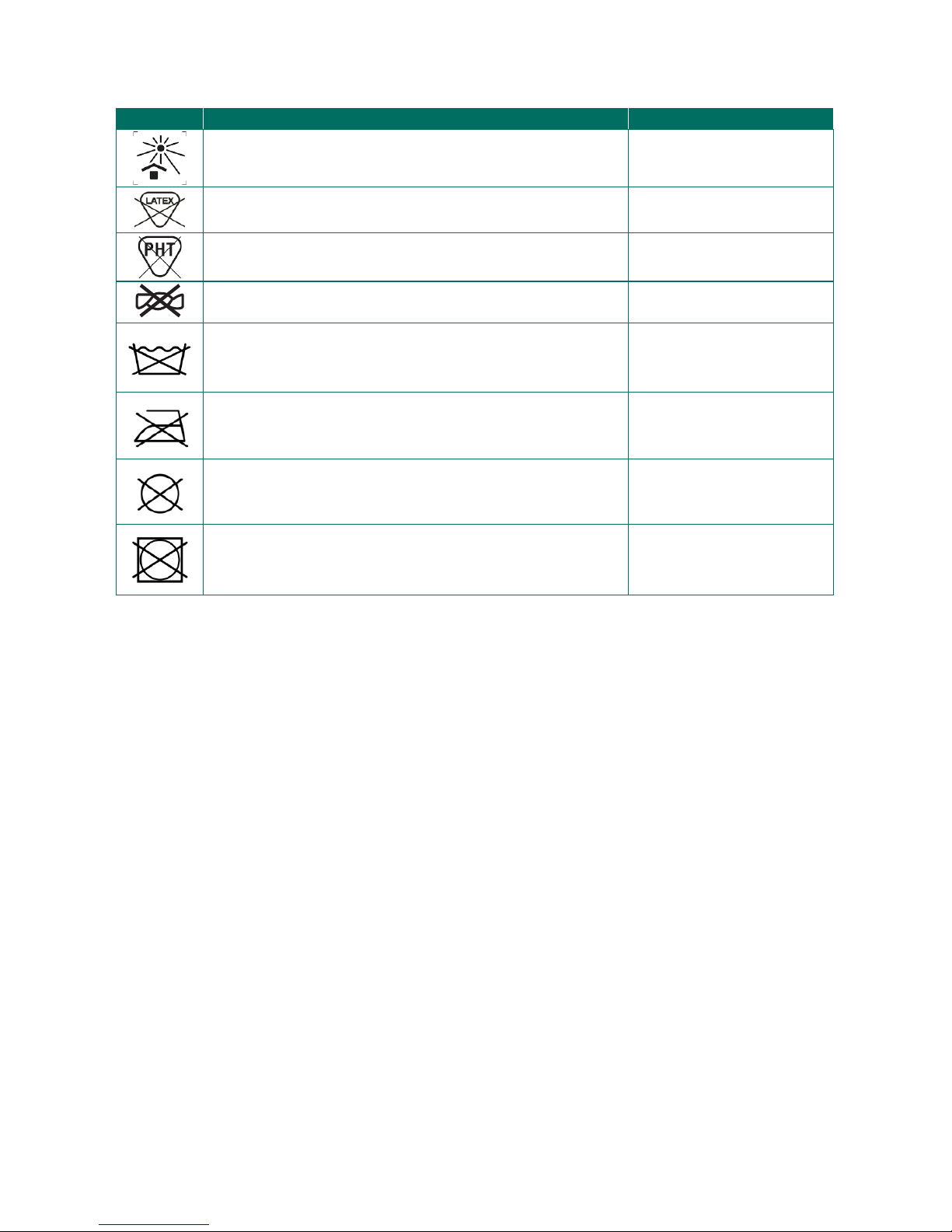
Symbol
Description
Position
Protect from sunlight, heat.
Belt
Packaging
Latex free
Belt
Packaging
Phthalate free
Belt
Packaging
Do not wring
Belt
Packaging
Do not wash
Belt
Packaging
Do not iron
Belt
Packaging
Do not dry clean.
Belt
Packaging
Do not tumble dry
Belt
Packaging
2
AREAS OF APPLICATION, CONTRAINDICATIONS, SIDE EFFECTS
2.1
APPLICATIONS
The MOWOOT II system has been developed for the treatment of chronic constipation, in particular slow
intestinal transit.
• due to neurogenic bowel disease: as in spinal cord injuries, spina bifida (meningomyelocele),
multiple sclerosis, Parkinson's disease, and others
• as side effects of medications, for example, medication for neurological disorders, opioids,
anticholinergics, diuretics, analgesics, calcium supplements, and others
• due to endocrine disorders, for example, hypothyroidism, hypercalcemia, and others
• due to systemic diseases, for example, collagenosis, amyloidosis, and others
• due to other disorders of the colonic passage (idiopathic)
2.2
CONTRAINDICATIONS
The treatment is contraindicated in the following cases:
• pregnancy,
• acute active abdominal tumour disease,
• unstable vertebral fracture,
• acute inflammatory processes in the abdomen.
 Loading...
Loading...