Motus Pure-Vu System User Manual
Motus Pure-Vu® System
Instructions For Use
Error
Cleansing Mode
Low Medium
High
Warning System Test
Motus GI Medical Technologies LTD.
Address: Keren Hayesod 22,
Tirat Carmel, ZIP 3902638, Israel
Tel: +972-73-2627757
Fax: +972-4-6214442
This document is property of Motus GI Medical Technologies LTD, its contents are CONFIDENTIAL and shall not be disclosed, disseminated, copied or used,
without written permission. Pure-Vu® and Motus GI® are trademarks or registered trademarks of Motus GI Holdings, Inc. © 2018
Motus GI Holdings, Inc.
Address: 1301 E. Broward Blvd.
Suite 310
Fort Lauderdale, FL 33301
Tel: 954.541.8000
Fax: 954.541.8265
E-mail: mail@motusgi.com
EC REP
OBELIS S.A
Address: Bd. Général Wahis, 53,
1030 Brussels, Belgium
Tel: +32.2.732.59.54
Fax: +32.2.732.60.03
E-mail: mail@obelis.net
www.obelis.net

CONTENTS
1.0 WARNINGS AND PRECAUTIONS ................................................................................................ 3
1.1. Symbols Used ...................................................................................................................................
2.0 INTENDED USE
..............................................................................................................................................
3.0 PRODUCT DESCRIPTION ............................................................................................................... 5
3.1. System Workstation .....................................................................................................................
3.1.1. Workstation Components:...........................................................................................
3.1.2. Workstation Front Panel .............................................................................................
3.1.3. The Foot Pedal ...............................................................................................................
3.2. Pure-Vu Oversleeve .....................................................................................................................
3.2.1. The Oversleeve .............................................................................................................
3.3. Accessories for Use with Pure-Vu .............................................................................................
4.0 SETUP AND INTERCONNECTIONS ............................................................................................. 9
4.1. Unpacking .......................................................................................................................................
4.2. Assembly of the Oversleeve on the Workstation ...............................................................
4.2.1. Cleaning the Loading Fixture ....................................................................................
4.1.1. Unpacking the Oversleeve and Workstation Connector ...................................
4.1.2. Unpacking the Workstation and Loading Fixture ...............................................
4
4
6
7
7
8
9
9
9
9
9
9
10
10
4.2.2. Oversleeve Assembly ...................................................................................................
4.2.3. Attaching the Oversleeve to the Workstation .....................................................
10
13
5.0 SYSTEM OPERATION ...................................................................................................................... 16
5.1. Pre-procedure Operations .........................................................................................................
5.2. Operating the Pure-Vu Workstation .......................................................................................
5.3. Disassembling the WS Connector and Oversleeve ............................................................
5.4. Removing the Oversleeve from the Colonoscope..............................................................
5.5. Discarding the Oversleeve and WS Connector ...................................................................
16
16
17
18
21
6.0 CLEANSING WORKSTATION AND FOOT PEDAL ................................................................... 21
7.0 STORAGE AND TRANSPORTATION ........................................................................................... 21
8.0 TROUBLESHOOTING ....................................................................................................................... 22
9.0 TECHNICAL SPECIFICATIONS ...................................................................................................... 23
9.1. Environmental Standards ...........................................................................................................
9.2. Shipping and Storage ..................................................................................................................
9.3. Performance Limitations ............................................................................................................
9.4. Mechanical Properties and Sizes .............................................................................................
23
23
23
23

1. WARNINGS AND PRECAUTIONS
Please read this manual and follow its instructions carefully.
Warnings:
• FDA (USA) law restricts this device to sale by or on order of a physician.
• The Pure-Vu System should only be used according to the instruction and operating conditions
described in this manual. Failure to do so could result in compromised safety, equipment malfunction
or instrument damage.
• The Pure-Vu System should only be used under the supervision of a licensed physician. Do not use for
any purpose other than the intended application.
• The Pure-Vu System is to be used only by trained medical personnel.
• The Pure-Vu Oversleeve and WS Connector are provided clean and intended for single patient use. Do
not sterilize, clean or re-use the Oversleeve or WS Connector. Discard both devices properly after use.
• Confirm that the other devices used with The Pure-Vu System function properly and that these other
devices will not adversely aect the operation or safety of this equipment. If any component of the
endoscopic system is not functioning properly, the procedure should not be performed.
• The Pure-Vu System should only be used with approved medical devices, complying with IEC 60601-1.
• The Pure-Vu System must only be connected to a supply main with protective earth.
• Check and confirm that all cords or cables are connected correctly and securely.
• Continually monitor The Pure-Vu System and the patient for any signs of irregularity.
• The Pure-Vu System may cause radio interference or may disrupt the operation of nearby equipment.
• If the system does not function properly, turn the Workstation o, abort the procedure and follow the
instructions noted in section “Operating the Pure-Vu Workstation”.
• The Pure-Vu System should never be stored in areas where the unit could get wet or be exposed to
high temperature, humidity, direct sunlight, dust, salt, which could adversely aect the equipment.
• The Pure-Vu System should never be installed, used or transported in an inclined position nor should it
be subjected to impact.
• A minimum of 2 people are required to transport the Pure-Vu Workstation.
• The Pure-Vu Workstation should be maintained in a clean condition during storage and be ready for
subsequent use.
• Electrical safety checks shall be performed annually (or as required by institutional procedures). The
check must be performed only by Motus GI authorized technical personnel.
• The Oversleeve, WS Connector, Loading fixture, as well as the Workstation (WS), are intended to be
used only with Motus Pure-Vu System and its accessories.
• Avoid using the cleansing mode on the same area of tissue for an extended period of time.
• A new, single use waste receptacle should be used for each patient.
• A new, single use irrigation bag (IB) must be used for each patient.
Precautions:
• Use only new irrigation bag (IB).
• Advance cautiously when performing anal retroflex or/and entering the ileocecal valve.
• For optimal operation of the system, refrain from using the colonoscope suction and insuation
when The Pure-Vu System is activated.
• Care should be taken to avoid pinching the tubing during advancement through the colon and/or
when applying torque to the colonoscope.
• Only Motus GI personnel or a trained designee should service The Pure-Vu System.
MOTUS GI PURE-VU SYSTEM INSTRUCTIONS FOR USE
3
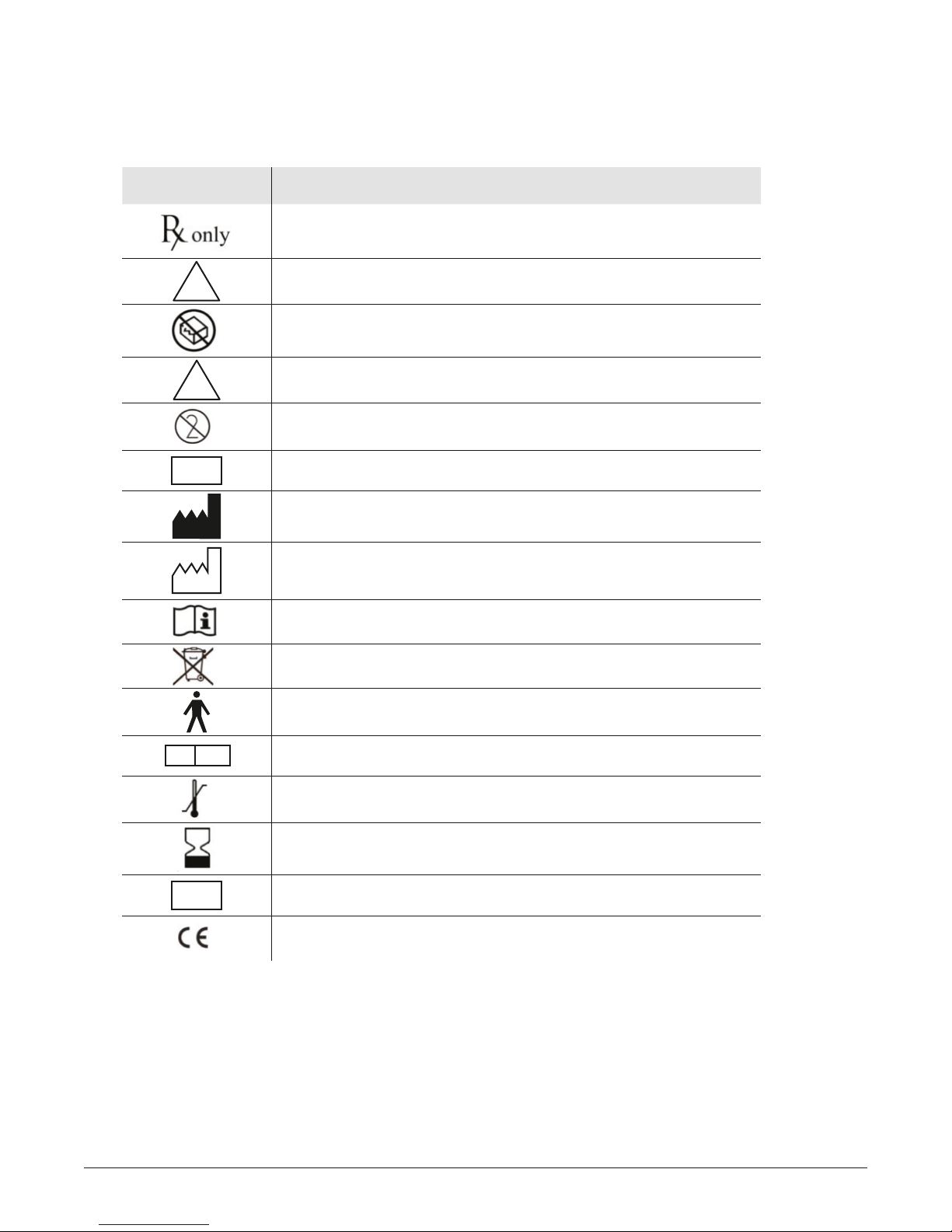
1.1. Symbols Used
The Pure-Vu System has a safety warning and compliance label located at the rear of the WS. For a
defi nition of individual symbols, see table below:
SYMBOL DESCRIPTION
FDA (USA) law restricts this device to sale by or on order
of a physician
NON
STERILE
!
LOT
Non-sterile
Do not use if package is damaged
Caution! Consult accompanying documents for
safety instructions
Do not re-use
Batch code (adjacent to the lot symbol)
Manufacturer
Date of manufacture
Consult Instructions For Use, symbols are meant to support, not
replace, operating instructions
Must not be treated as unsorted municipal waste
Type BF equipment
EC REP
SN
2.0 INTENDED USE
The Pure-Vu System is intended to connect to standard colonoscopes to help facilitate
intraprocedural cleaning of a poorly prepared colon by irrigating or cleaning the colon and
evacuating the irrigation fl uid (water), feces and other bodily fl uids and matter, e.g. blood.
It is for use only by trained medical personnel located in hospitals, clinics and doctor offi ces.
4
Authorized representative in the European Community
Temperature limit
Use-by-date
Serial number
CE compliance symbol
MOTUS GI PURE-VU SYSTEM INSTRUCTIONS FOR USE

Error
Low Medium
Cleansing Mode
High
Warning System Test
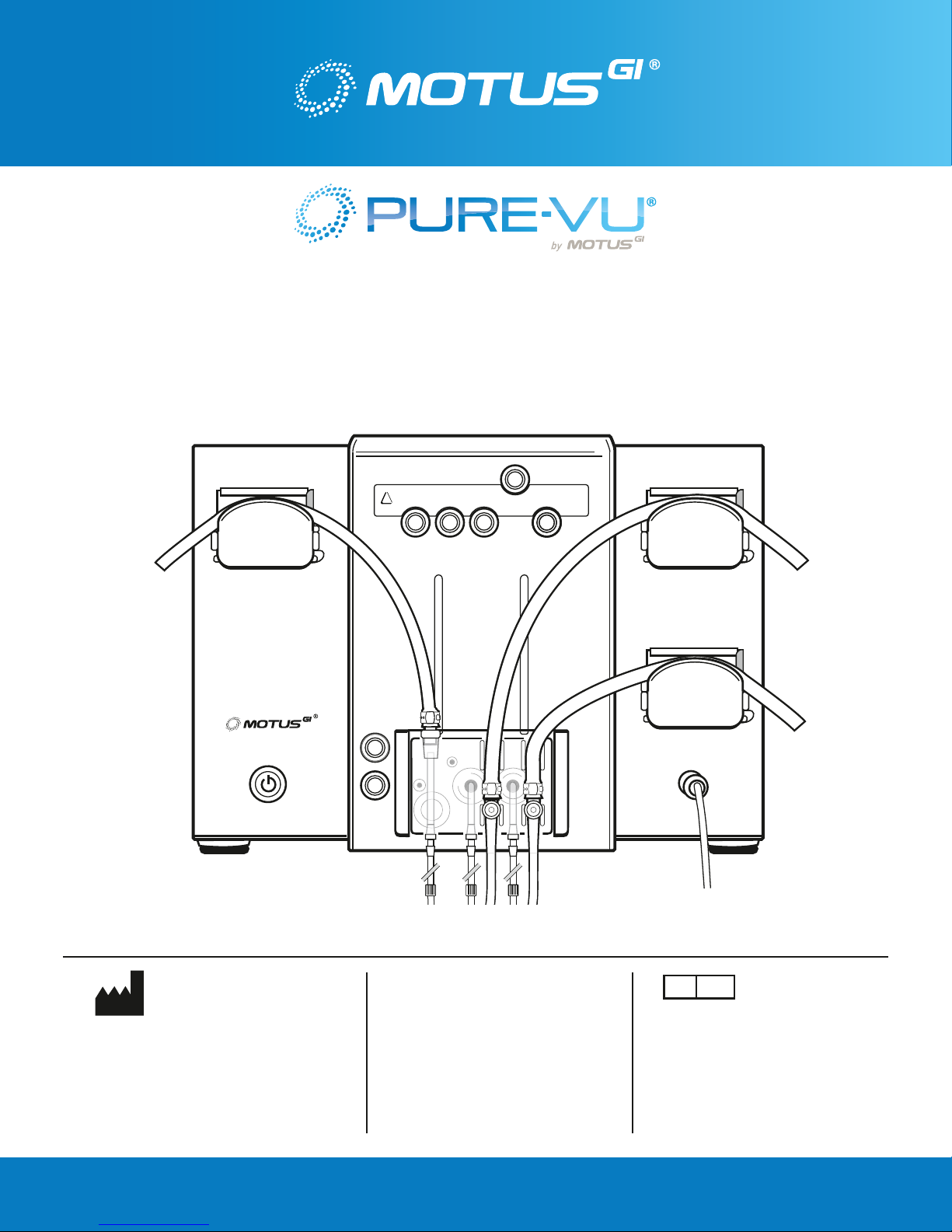
3.0 PRODUCT DESCRIPTION
The Pure-Vu System enables colon cleansing during colonoscopy using a standard or slim colonoscope
with a length of 1630mm – 1710mm and a slim colonoscope outer diameter range of 11.7mm – 13.3mm
and a standard colonoscope outer diameter range of 12.8mm – 13.7mm. The Oversleeve, which fi ts over
the colonoscope and is connected to an external Workstation, generates fl uid to break up feces. The
fecal matter & fl uids are removed through the evacuation channels of the Oversleeve into an external
waste receptacle.
The Pure-Vu System consists of the following main components:
• The Oversleeve [O] is a disposable device that fi ts over the colonoscope and is connected to the
external Workstation., using the WS Connector [WSC].
• The Workstation [WS] is reusable and supplies an irrigation mixture of water/saline and gas, and
evacuates fecal material & fl uids.
• WS Connector [WSC] is disposable and serves as an interface between the Oversleeve and the
Workstation.
• The loading fi xture is reusable and aids in assembling the Oversleeve onto the colonoscope.
Below is a drawing showing the various components of the system and where they connect to each other.
2
5
1
3
7
4
6
FIGURE 1: Pure-Vu Workstation – General Design & Components
[WS] Workstation
1
[IB] Irrigation Bag
2
[C] Colonoscope
3
MOTUS GI PURE-VU SYSTEM INSTRUCTIONS FOR USE
[O] Oversleeve
4
[V] Evacuation Canisters
5
[FP] Foot Pedal
6
[C] Connector
7
5

3.1. System Workstation
The Workstation is intended to provide irrigating water or saline & gas, to evacuate fecal matter &
fl uids out of the body during the colonoscopy procedure. Care should be taken when removing the
Workstation from the packaging so as not to damage any components.
2
1
Error
53
Cleansing Mode
Low Medium
64
High
Warning System Test
7
8
9
10
12
FIGURE 2: Pure-Vu Workstation
[IT] Irrigation Tube
1
[IP] Irrigation Pump
2
[EL] Error Light
3
[LM] Cleansing Mode (LOW)
4
[MM] Cleansing Mode (MED)
5
13
14
15
[ET1] Evacuation Tube 1
10
[EP2] Evacuation Pump 2
11
[PW] Power
12
[CLU] Lock and Unlock
13
[D] Drain
14
11
16
17
[HM] Cleansing Mode (HIGH)
6
[WL] Warning Light
7
[ST] System Test
8
[EP1] Evacuation Pump 1
9
6
[WSC] Workstation Connector
15
[ET2] Evacuation Tube 2
16
[PC] Foot Pedal Connector
17
MOTUS GI PURE-VU SYSTEM INSTRUCTIONS FOR USE

3.1.1. Workstation Components:
The Workstation [WS] includes the following components:
• A monitoring & Control Unit that controls the delivery of irrigation fluids and gas into the colon, and
evacuation of fluids and feces from the colon.
• An external receptacle containing irrigation liquid (saline or water) which is connected to the
irrigation line (please note this is a consumable not supplied by Motus GI).
• An external waste receptacle for collecting the fecal material & fluids that are evacuated from the
colon through the evacuation line (please note this is a consumable not supplied by Motus GI).
• External foot pedals that activate the cleansing, evacuation and purging functions, and switch
between cleansing modes used by the physician.
3.1.2. The Workstation Front Panel:
The WS contains the following main components: On/O power button [PW], Irrigation pump (IP) that
pumps room temperature water or saline (not shown in this figure) and gas into the Pure-Vu Oversleeve
(not shown in this figure) via the irrigation tubing [IT]. The fecal material & fluids are removed from
the colon, using the evacuation pumps [EP1, EP2] via the evacuation tubing [ET1, ET2], into the waste
receptacle (not shown in this figure). The WS Connector [WSC] connects the Oversleeve to the
Workstation. The WS Connector slides into place in the front of the Workstation and has a lock/unlock
button [CLU] to lock and unlock the connector and a drain button [D] to drain the Oversleeve and
WSC lines. The cleansing/evacuation mode is controlled by the foot pedal (not shown in this figure)
which connects to the pedal connector [PC]. The Pure-Vu System undergoes a system test when
the System Test button [ST] is activated. The system test ensures The Pure-Vu System is functioning
properly prior to the start of the procedure. The system can be operated in three cleansing modes; low,
medium (default mode) and high. The mode selection is activated by using the cleansing mode selector
button [CMS] on the foot pedal. The selected mode is indicated by the cleansing mode lights [LM, MM,
HM] on the WS. The warning indication light [WL] will indicate an assembly related issue or when a
system malfunction occurs. This warning indication is both visual and audible. For warning indication
instructions see troubleshooting section. The error light (EL) will indicate when a non-recoverable
error occurs.
MOTUS GI PURE-VU SYSTEM INSTRUCTIONS FOR USE
7
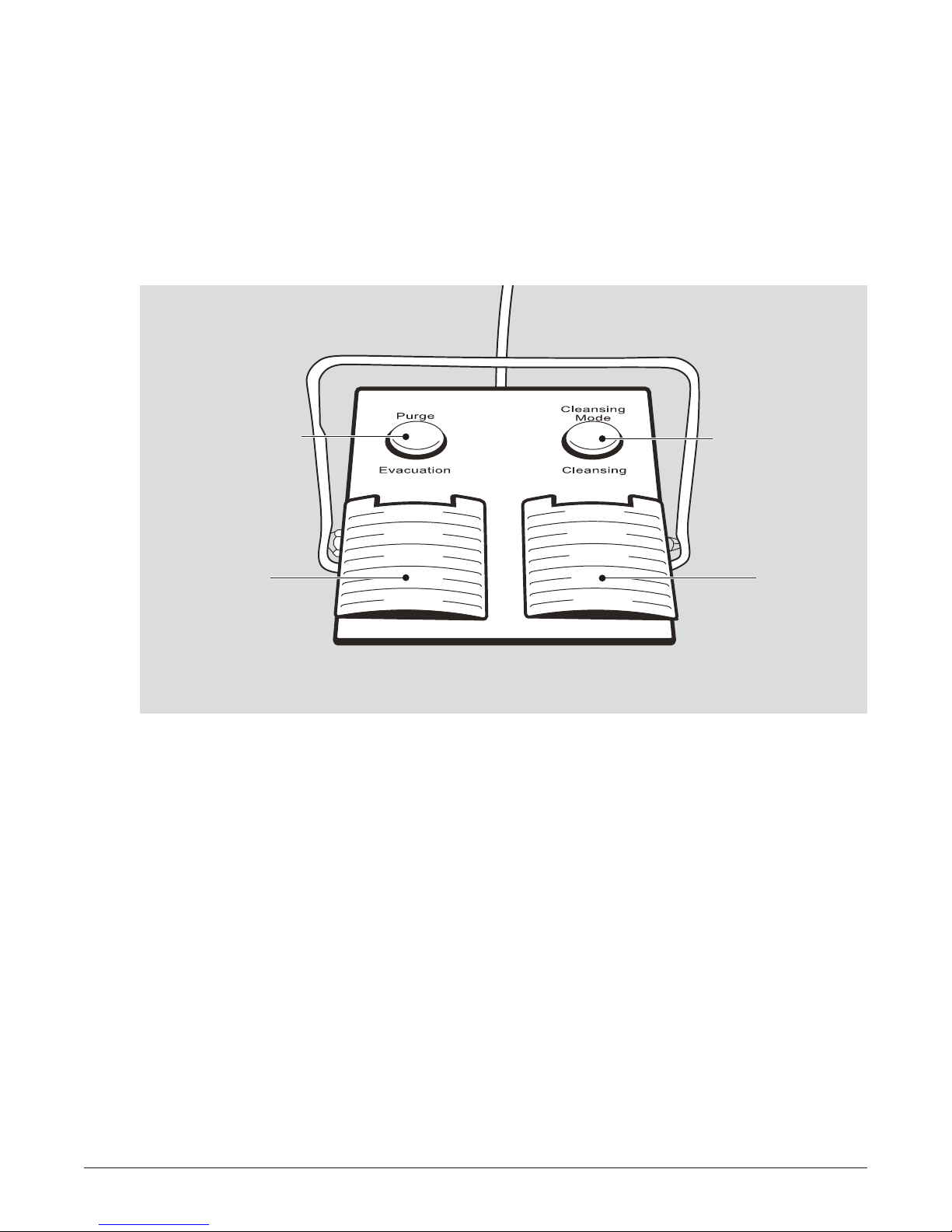
3.1.3. The Foot Pedal Unit
The foot pedal allows the physician to choose each of the operating modes during the procedure. The
operating modes are as follows (Figure 3: Pure-Vu Foot Pedal Unit):
• Cleansing – simultaneous irrigation and evacuation, activated by the right foot pedal [CP].
• Evacuation only mode – activated by the left pedal [EP].
• Manual Purge – activated by the left button [MP].
• Cleansing Mode Selector – activated by the right button [CMS]. User can switch between three
cleansing modes; low, medium (default mode) and high.
Manual Purging
[MP]
Evacuation
[EP]
FIGURE 3: Pure-Vu Foot Pedal Unit
Cleansing Mode
Selector
[CMS]
Cleansing
[CP]
8
MOTUS GI PURE-VU SYSTEM INSTRUCTIONS FOR USE
 Loading...
Loading...