Page 1

SpectraMax® M3, M4, M5, and M5e
Multi-Mode Microplate Readers
User Guide
0112-0115 F
July 2010
Page 2

This document is provided to customers who have purchased Molecular Devices, Inc.
(“Molecular Devices”) equipment, software, reagents, and consumables to use in the
operation of such Molecular Devices equipment, software, reagents, and
consumables. This document is copyright protected and any reproduction of this
document, in whole or any part, is strictly prohibited, except as Molecular Devices
may authorize in writing.
Software that may be described in this document is furnished under a license
agreement. It is against the law to copy, modify, or distribute the software on any
medium, except as specifically allowed in the license agreement. Furthermore, the
license agreement may prohibit the software from being disassembled, reverse
engineered, or decompiled for any purpose.
Portions of this document may make reference to other manufacturers and/or their
products, which may contain parts whose names are registered as trademarks and/or
function as trademarks of their respective owners. Any such usage is intended only
to designate those manufacturers' products as supplied by Molecular Devices for
incorporation into its equipment and does not imply any right and/or license to use
or permit others to use such manufacturers' and/or their product names as
trademarks.
Molecular Devices makes no warranties or representations as to the fitness of this
equipment for any particular purpose and assumes no responsibility or contingent
liability, including indirect or consequential damages, for any use to which the
purchaser may put the equipment described herein, or for any adverse circumstances
arising therefrom.
For research use only. Not for use in diagnostic procedures.
The trademarks mentioned herein are the property of Molecular Devices, Inc. or their
respective owners. These trademarks may not be used in any type of promotion or
advertising without the prior written permission of Molecular Devices, Inc.
Product manufactured by Molecular Devices, Inc.
1311 Orleans Drive, Sunnyvale, California, United States of America 94089.
Molecular Devices, Inc. is ISO 9001 registered.
© 2010 Molecular Devices, Inc.
All rights reserved.
Printed in the USA.
Page 3

Contents
Chapter 1 Description . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Applications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Certified SpectraMax® M5e-HTRF Readers . . . . . . . . . . . . 8
Optics. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Dynamic Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
PathCheck® Pathlength Measurement Technology . . . . . . 9
Automix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Temperature Control . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Supported Plates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Computer Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Instrument Control. . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Data Collection and Display . . . . . . . . . . . . . . . . . . . . . 10
Data Reduction and Plotting . . . . . . . . . . . . . . . . . . . . 10
Immediate Results Reporting and Analysis . . . . . . . . . . 10
Reader Components . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
The Control Panel. . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Temp On/Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Temp . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Wavelengths (
Ref . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Read Cuvette. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Drawer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
The Microplate Drawer . . . . . . . . . . . . . . . . . . . . . . . . 15
Microplates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
The Cuvette Chamber. . . . . . . . . . . . . . . . . . . . . . . . . 17
Cuvettes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
The Back Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
0112-0115 F 3
Page 4

Contents
Chapter 2 Principles of Operation . . . . . . . . . . . . . . . . . 19
Absorbance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Optical Density. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Transmittance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
PathCheck® Pathlength Measurement Technology . . . . . . 19
Water Constant or Cuvette Reference? . . . . . . . . . . . . . 22
Background Considerations . . . . . . . . . . . . . . . . . . . . . 22
PathCheck Pathlength Measurement Technology
and Interfering Substances . . . . . . . . . . . . . . . . . . . . . 23
Normalizing Absorbance Measurements. . . . . . . . . . . . . 24
Fluorescence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Time-resolved Fluorescence (M4, M5, and M5e only) . . . . 27
Fluorescence Polarization (M5 and M5e only) . . . . . . . . . . 28
Luminescence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Functional Description. . . . . . . . . . . . . . . . . . . . . . . . . . 29
Temperature Regulation . . . . . . . . . . . . . . . . . . . . . . . 29
Read Types . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Endpoint Read . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Kinetic Read. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Spectrum Read. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Well Scan Read . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Automix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Computer Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Chapter 3 Installation . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Unpacking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Setting up the Instrument . . . . . . . . . . . . . . . . . . . . . . . 35
Installing the Drawer Adapter . . . . . . . . . . . . . . . . . . . . 36
Removing the Drawer Adapter . . . . . . . . . . . . . . . . . . . . 37
4 0112-0115 F
Page 5

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Chapter 4 Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Cuvette Read—Quick Overview . . . . . . . . . . . . . . . . . . . 39
Microplate Read—Quick Overview . . . . . . . . . . . . . . . . . 40
Preparing for a Cuvette or Microplate Reading . . . . . . . . . 40
Turn the Instrument and Computer On . . . . . . . . . . . . . 40
Set the Temperature (Optional) . . . . . . . . . . . . . . . . . . 41
Select the Wavelength . . . . . . . . . . . . . . . . . . . . . . . . 42
Read the Cuvette . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
Read the Microplate . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
Optimizing Fluorescence Assays. . . . . . . . . . . . . . . . . . . 44
Optimizing Absorbance Assays . . . . . . . . . . . . . . . . . . . 44
Excitation and Emission Wavelengths . . . . . . . . . . . . . 44
Emission Cutoff Filter . . . . . . . . . . . . . . . . . . . . . . . . 45
Readings Per Well . . . . . . . . . . . . . . . . . . . . . . . . . . 45
PMT Voltage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Temperature Control . . . . . . . . . . . . . . . . . . . . . . . . 45
Using Spectral Scanning to Optimize Excitation
and Emission Wavelengths for Fluorescence Assays . . . . 46
Optimizing Time-resolved Fluorescence Assays . . . . . . . . 50
Optimizing Fluorescence Polarization Assays . . . . . . . . . . 51
Optimizing Luminescence Assays . . . . . . . . . . . . . . . . . . 52
Chapter 5 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . 53
Technical Support . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Moving a SpectraMax Multi-Mode Microplate Reader. . . . . 55
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Cleaning the Fan Filter . . . . . . . . . . . . . . . . . . . . . . . . . 57
Changing the Fuses . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
Chapter 6 Troubleshooting . . . . . . . . . . . . . . . . . . . . . . 61
Opening the Drawer Manually . . . . . . . . . . . . . . . . . . . . 61
Error Codes and Probable Causes. . . . . . . . . . . . . . . . . . 62
Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62
0112-0115 F 5
Page 6

Contents
Appendix A Specifications . . . . . . . . . . . . . . . . . . . . . . . 67
SpectraMax® Multi-Mode Microplate Reader
Performance Specifications . . . . . . . . . . . . . . . . . . . . . . 67
System Diagrams and Dimensions . . . . . . . . . . . . . . . . . 73
Common Fluorescence and Luminescence Wavelengths . . 74
Fluorescence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
Time-resolved Fluorescence . . . . . . . . . . . . . . . . . . . . . 75
Luminescence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
Appendix B Cables and Accessories . . . . . . . . . . . . . . . . 77
Cables. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Serial Interface Cable . . . . . . . . . . . . . . . . . . . . . . . . . 77
USB Adapter Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
Cuvettes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 78
Standard and Semi-micro Cuvettes. . . . . . . . . . . . . . . . 79
Ultra-micro Cuvettes (Hellma) . . . . . . . . . . . . . . . . . . . 79
Standard, Semi-micro, and Microcuvettes (Hellma) . . . . 80
Ultra-micro Cuvettes (Hellma) . . . . . . . . . . . . . . . . . . . 81
Index. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83
6 0112-0115 F
Page 7
Description
Introduction
The SpectraMax® M3, M4, M5, and M5e Microplate Readers are a series
of dual-monochromator, multidetection, multi-mode instruments with a
triple-mode cuvette port and 6-well to 384-well microplate reading
capability. Detection modalities are shown in Table 1-1.
Table 1-1 SpectraMax® Multi-Mode Microplate Readers and Applicable
Modes
1
Modes
Absorbance X X X X
Fluorescence intensity X X X X
Fluorescence
polarization
Time-resolved
fluorescence
Luminescence x x X X
Note: In this user guide, all references to SpectraMax Multi-Mode
Microplate Readers include the M3, M4, M5, and M5e models. When a
feature or capability applies to only certain readers, this exception is
noted.
The optical performance is comparable to a top-of-the-line dedicated
spectrophotometer or spectrofluorometer with no trade-off between
instrument performance and the number of read modes.
The built-in cuvette port can be used for absorbance, fluorescence and
luminescence readings. Dual monochromators allow selection of any
absorbance wavelength between 200 nm and 1000 nm, and any
excitation wavelength between 250 nm and 850 nm for readings in
fluorescence intensity, time-resolved fluorescence (M4, M5, M5e models
only) or wavelength-selectable luminescence modes, and 400–750 nm
for readings in fluorescence polarization mode (M5, M5e models only).
Assays requiring a read in two or more modes can be combined and run
on the SpectraMax Multi-Mode Microplate Readers by issuing a single
command in SoftMax® Pro Software, Molecular Devices’ leading
microplate data acquisition and analysis software platform.
SpectraMax M3SpectraMax M4SpectraMax M5SpectraMax
XX
XXX
M5
e
0112-0115 F 7
Page 8

Description
Applications
Endpoint, kinetic, spectrum, and multi-point well-scanning applications
combining absorbance and fluorescence in 6-well to 384-well
microplates, as well as endpoint, kinetic, and spectrum applications in
absorbance and fluorescence using cuvettes, can be run with little to no
optimization.
The extreme flexibility and high sensitivity of the SpectraMax Multi-
Mode Microplate Readers make them appropriate for applications within
the fields of biochemistry, cell biology, immunology, molecular biology,
and microbiology.
Typical applications include ELISA, nucleic acid, protein, enzymatic type
homogeneous and heterogeneous assays, microbial growth, endotoxin
testing, and pipettor calibration.
Certified SpectraMax® M5e-HTRF Readers
The SpectraMax M5e reader has the same performance specifications as
the M5 but is certified for use with Cisbio Bioassays’ HTRF
(Homogeneous Time-Resolved Fluorescence) technology. HTRF is a
proprietary time-resolved fluorescence technology that overcomes
many of the drawbacks of standard Fluorescence Resonance Energy
Transfer (FRET) techniques, such as the requirements to correct for
autofluorescence and the fluorescent contributions of unbound
fluorophores.
Optics
The use of two holographic diffraction grating monochromators allows
for individual optimization of wavelengths for both excitation and
emission in fluorescence readings. Mirrored optics focus the light into
the sample volume, and cutoff filters are used to reduce stray light and
minimize background interference. The light source is a high-powered
Xenon flash lamp. Sensitivity or read-speed can be optimized by
varying the number of lamp flashes per read.
Dynamic Range
The dynamic range of detection is from 10-6 to 10
Variations in measured fluorescence values are virtually eliminated by
internal compensation for detector sensitivity, photomultiplier tube
voltage and sensitivity, as well as excitation intensity. The photometric
range is 0–4 ODs with a resolution of 0.001 OD.
8 0112-0115 F
-12
molar fluorescein.
Page 9

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
PathCheck® Pathlength Measurement Technology
A SpectraMax Multi-Mode Microplate Reader with PathCheck Pathlength
Measurement Technology allows normalization of variable well volumes
to 1-cm cuvette readings. PathCheck Pathlength Measurement
Technology allows for multichannel pipettor validation and for
experiment comparison from different days.
Automix
Using the Automix feature of the SoftMax Pro Software, the contents of
the wells in a microplate can be mixed automatically by linear shaking
before each read cycle, making it possible to perform kinetic analysis of
solid-phase, enzyme-mediated reactions (mixing is not critical for
liquid-phase reactions).
Temperature Control
Temperature in the microplate chamber is isothermal, both at ambient
and when the incubator is turned on. When the incubator is on, the
temperature may be controlled from 2°C above ambient to 60°C.
Supported Plates
Microplates having 6, 12, 24, 48, 96, and 384 wells can be used in the
SpectraMax Multi-Mode Microplate Readers. Top and bottom reads are
available for fluorescence, time-resolved fluorescence and
luminescence detection. When reading optical density at wavelengths
below 340 nm, special UV-transparent, disposable or quartz
microplates and cuvettes that allow transmission of the far UV spectra
must be used.
One plate carrier adapter is provided with the instrument. The adapter
is required for optimum performance with standard 96-well and 384-
well format microplates for all top-read applications.
0112-0115 F 9
Page 10

Description
Computer Control
An external computer running SoftMax Pro Software, which provides
integrated instrument control, data display, and statistical data
analysis, controls the SpectraMax Multi-Mode Microplate Readers.
Cuvette port functionality can also be controlled using SoftMax Pro
Software.
SoftMax Pro Software provides the following functionality:
Instrument Control
SoftMax Pro Software allows you to set up and run a complete protocol
for the SpectraMax Multi-Mode Microplate Reader, as well as all other
Molecular Devices' microplate readers. Instrument settings can be
saved as a protocol file and used repeatedly for reading different
microplates or cuvettes. All stand-alone instrument functions can be
controlled using the software. In addition, SoftMax Pro Software
provides capabilities that are not available when using an instrument in
stand-alone mode such as user-defined kinetic run times, read
intervals, Automix parameters, etc.
Data Collection and Display
SoftMax Pro Software collects and stores all raw data received from the
instrument. Data is displayed in a grid format that corresponds to the
wells in a microplate or individual cuvettes.
SoftMax Pro Software can collect data from one or more microplates or
cuvettes and store it in a single data file, using the same or different
instrument settings for different microplates or cuvettes. For example,
microplates containing different samples can be read using the same or
different modes, all within the same experiment.
Data Reduction and Plotting
You can manipulate or “reduce” the raw data using dozens of built-in
formulas or define your own analysis structure to quickly and easily
summarize the raw data. More than one reduction can be shown, and
results from different microplates or cuvettes can be compared within
the same experiment.
Immediate Results Reporting and Analysis
Once you have defined instrument settings, and have customized a
SoftMax Pro Software data file with assay information, reduction
settings, custom columns in Group sections, and summary objects, you
can save this information to create an assay protocol. Protocols can be
used throughout a department or company for highly repeatable data
collection and analysis that is completed the second the plate read has
completed.
10 0112-0115 F
Page 11

Reader Components
Control Panel Back PanelCuvette Chamber
Microplate Drawer
The main components of the SpectraMax Multi-Mode Microplate
Readers are:
• Control panel: for cuvette chamber control.
• Microplate drawer: used for all five read modes and four read
types.
• Cuvette chamber: used for absorbance, fluorescence intensity,
and luminescence read modes for endpoint, kinetic, and
spectrum scanning.
• Back panel: connections and power switch.
SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Figure 1-1 SpectraMax® components.
0112-0115 F 11
Page 12

Description
MODE
The Control Panel
Figure 1-2 The control panel.
The control panel consists of a 2-x-20-character LCD and eleven
pressure-sensitive membrane keys that can be used to control some
functions of the instrument. When you press a control panel key, the
instrument performs the associated action.
Note: Settings made in SoftMax Pro Software override control panel
settings.
The left side of the display shows the temperature inside the cuvette
chamber, both actual and set point, and whether or not the
temperature is at the set point (the enunciator blinks if it is not at set
point). The temperature of the microplate chamber lags slightly behind
the temperature in the cuvette chamber. The temperature in the
microplate chamber is reported in the SoftMax Pro Software interface
display.
The middle of the display shows the wavelengths for
absorbance/excitation and emission.
The right side of the display shows the data received from the reading
as absorbance, percent transmission, fluorescence emission or
excitation, or luminescence, and indicates whether or not a reference
measurement was made (enunciator blinks if no reference reading was
taken).
To change the contrast of the display, press
and the temperature
up () or down () setting keys.
12 0112-0115 F
Page 13

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
TEMP on/off
TEMP
Temp On/Off
The key enables and disables the incubator that controls the
temperature within both the microplate chamber and the cuvette port.
• When the incubator is on, the set temperature and actual
temperature (cuvette chamber only) are shown on the front
panel LCD display.
• When the instrument is performing a kinetic or spectral scan,
the temperature keys on the front panel are disabled.
Temp
The keys allow you to enter a set point at which to regulate the
cuvette and microplate chamber temperature. However, remember that
the cuvette temperature only is reported on the LCD display, while the
microplate chamber temperature is reported in the SoftMax Pro
Software interface display.
Pressing this key scrolls the temperature up or down, starting at the
previous temperature setting (or the default of 37.0°C, if no setting had
been made):
• Pressing the up () or down () arrow once increments or
decrements the displayed temperature by 0.1°C.
• Pressing and holding either arrow increments or decrements the
displayed temperature by 1°C until it is released.
You cannot set a temperature beyond the upper (60°C) or lower (15°C)
instrument limits.
Wavelengths ()
Selects the wavelength to be used for reading the cuvette manually.
Two s et s o f up or down arrow keys are available for setting
absorbance/excitation (fluorescence) wavelengths and emission
(fluorescence) wavelengths.
The control panel does not display the wavelength selected through the
SoftMax Pro application.
Pressing the up or down arrow key scrolls up or down through the
available wavelengths, starting at the previous setting:
• Pressing the up () or down () arrow once increments or
decrements the displayed wavelength by 1 nm.
• Pressing and holding either arrow increments or decrements the
displayed wavelength by 10 nm until it is released.
0112-0115 F 13
Page 14

Description
DRAWER
Ref
A reading of buffer, water, or air taken in the cuvette that is used as I0
to calculate Absorbance or % Transmittance. If no reference reading is
taken, the instrument uses the I0 values stored in the NVRAM (non-
volatile memory) of the instrument.
This key is disabled during a computer-controlled run.
Read Cuvette
Initiates the sample reading of the cuvette.
This key is disabled during a computer-controlled run.
Mode
A toggle switch used to display cuvette data as percent transmittance
(%T), absorbance (A), relative fluorescence units (RFU), or relative
luminescence units (RLU).
Drawer
The key opens and closes (toggles) the microplate drawer.
14 0112-0115 F
Page 15

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
The Microplate Drawer
The microplate drawer is located on the right side of the instrument and
slides in and out of the reading chamber. An internal latch positions the
microplate in the drawer as it closes (allowing for better robot
integration-no springs or clips are used).
The drawer remains in the reading chamber during read cycles.
Figure 1-3 The microplate drawer.
Microplate drawer operation varies, depending on the incubator setting:
• If the incubator is off, the drawer remains open.
• If the incubator is on, the drawer closes after approximately 10
seconds to assist in maintaining temperature control within the
microplate chamber.
Do not obstruct the movement of the drawer. If you must retrieve a
plate after an error condition or power outage and the drawer does not
open, it is possible to open it manually (see
page 61
0112-0115 F 15
).
Troubleshooting on
Page 16

Description
Microplates
The SpectraMax Multi-Mode Microplate Reader can accommodate SBS-
standard 6-well to 384-well microplates and strip wells. When reading
optical density at wavelengths below 340 nm, special UV-transparent,
disposable or quartz microplates allowing transmission of the deep UV
spectra must be used.
Not all manufacturers' microplates are the same with regard to design,
materials, or configuration. Temperature uniformity within the
microplate may vary depending on the type of microplate used.
Microplates currently supported by the SoftMax Pro Software for use in
this instrument are:
• 96-well Standard, 96 Costar, 96 Greiner Black, 96 Bottom
Offset, 96 Falcon, 96 BD Optilux/Biocoat, 96 BD Fluoroblok MW
Insert, 96 Corning Half Area, 96 MDC HE PS
• 384-well Standard, 384 Costar, 384 Greiner, 384 Falcon, 384
Corning, 384 MDC HE PS
• 48 Costar
• 24 Costar
• 12 Costar, 12 Falcon
• 6 Costar, 6 Falcon.
The SoftMax Pro Software plate list also includes half area and low-
volume plates. SoftMax Pro can always be used to define a new plate
type using the manufacturer's specifications for well size, spacing and
distance from the plate edge.
16 0112-0115 F
Page 17

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
The Cuvette Chamber
Figure 1-4 The cuvette chamber.
Located at the right front of the SpectraMax instrument, the cuvette
chamber has a lid that lifts up, allowing you to insert or remove a
cuvette. The chamber contains springs that automatically position the
cuvette in the proper alignment for a reading. The cuvette door must
be closed before initiating a reading.
Cuvettes
The SpectraMax Multi-Mode Microplate Reader can accommodate
standard-height (45 mm), 1 cm cuvettes and 12 x 75 mm test tubes
when used with the test tube cover.
Not all manufacturers' cuvettes are the same with regard to design,
materials, or configuration. Temperature uniformity within the cuvette
may vary depending on the type of cuvette used.
Cuvettes used for absorbance readings are frosted on two sides. Be
sure to handle cuvettes on the frosted sides only. Place the cuvette into
the chamber so that the “reading” (clear) sides face left and right.
Fluorescence cuvettes are clear on all four sides and should be handled
carefully. Place a frosted cuvette into the chamber so that the “reading”
(clear) sides face left and right. Semi-Micro and Ultra-Micro cuvettes
can also be used with an adapter. See
information about supported cuvettes.
0112-0115 F 17
Cuvettes on page 78 for more
Page 18
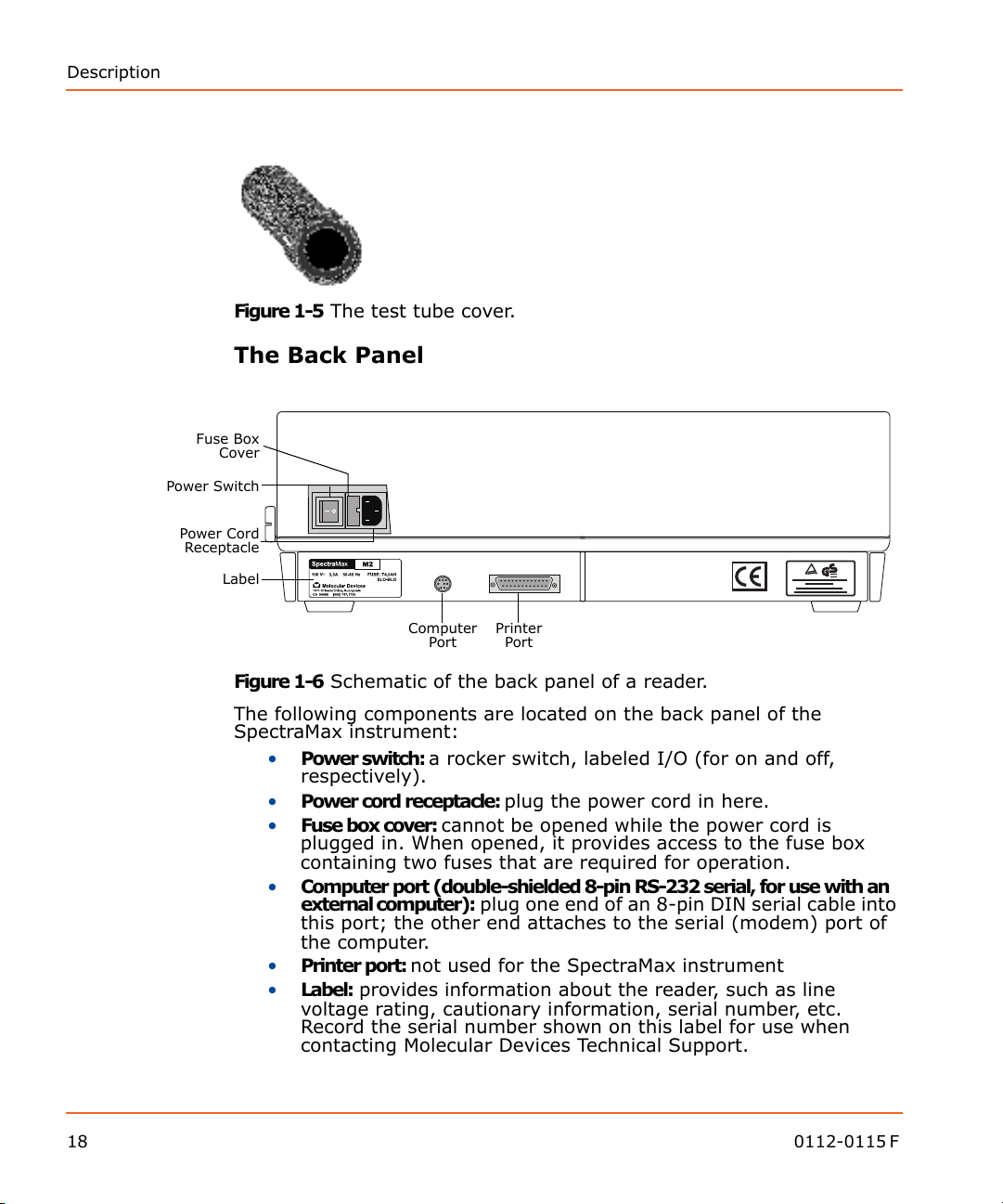
Description
Power Switch
Power Cord
Receptacle
Label
Fuse Box
Cover
Computer
Port
Printer
Port
Figure 1-5 The test tube cover.
The Back Panel
18 0112-0115 F
Figure 1-6 Schematic of the back panel of a reader.
The following components are located on the back panel of the
SpectraMax instrument:
• Power switch: a rocker switch, labeled I/O (for on and off,
respectively).
• Power cord receptacle: plug the power cord in here.
• Fuse box cover: cannot be opened while the power cord is
plugged in. When opened, it provides access to the fuse box
containing two fuses that are required for operation.
• Computer port (double-shielded 8-pin RS-232 serial, for use with an
external computer): plug one end of an 8-pin DIN serial cable into
this port; the other end attaches to the serial (modem) port of
the computer.
• Printer port: not used for the SpectraMax instrument
• Label: provides information about the reader, such as line
voltage rating, cautionary information, serial number, etc.
Record the serial number shown on this label for use when
contacting Molecular Devices Technical Support.
Page 19
Principles of Operation
Absorbance
Note: In this user guide, references to the SpectraMax® readers
include the M3, M4, M5, and M5e models. When a feature or capability
applies to only certain readers, this exception is noted.
Absorbance is the amount of light absorbed by a solution. To measure
absorbance accurately, it is necessary to eliminate light scatter. In the
absence of turbidity, absorbance = optical density:
where I is transmitted light, and IO is incident light.
In this manual, we use the terms absorbance and optical density
interchangeably.
Optical Density
Optical density is the amount of light passing through a sample to a
detector relative to the total amount of light available. Optical density
includes absorbance of the sample plus light scatter from turbidity.
2
A = –log(I/IO)
Transmittance
Transmittance is the ratio of transmitted light to the incident light.
T = (I/IO)
%T = 100T
where I is transmitted light, and IO is incident light.
PathCheck® Pathlength Measurement Technology
The Beer-Lambert law states that absorbance is proportional to the
distance that light travels through the sample:
A =
bc
where A is the absorbance,
is the pathlength, and c is the concentration of the sample. In short,
the longer the pathlength, the higher the absorbance.
0112-0115 F 19
is the molar absorptivity of the sample, b
Page 20

Principles of Operation
Horizontal
light path
Vertical light path
Cuvette Microplate wells
Microplate readers use a vertical light path so the distance of the light
through the sample depends on the volume. This variable pathlength
makes it difficult to perform extinction-based assays and also makes it
confusing to compare results between microplate readers and
spectrophotometers.
The standard pathlength of a cuvette is the conventional basis for
quantifying the unique absorbtivity properties of compounds in
solution. Quantitative analyses can be performed on the basis of
extinction coefficients, without standard curves (for example, NADH-
based enzyme assays). When using a cuvette, the pathlength is known
and is independent of sample volume, so absorbance is proportional to
concentration.
In a microplate, pathlength is dependent on the liquid volume, so
absorbance is proportional to both the concentration and the
pathlength of the sample. Standard curves are often used to determine
analyte concentrations in vertical-beam photometry of unknowns, yet
errors can still arise from pipetting the samples and standards. The
PathCheck
determines the pathlength of aqueous samples in the microplate and
normalizes the absorbance in each well to a pathlength of 1 cm. This
novel approach to correcting the microwell absorbance values is
accurate to within 2.5% of the values obtained directly in a 1 cm
cuvette.
®
Pathlength Measurement Technology feature automatically
Figure 2-1 Cuvette and microwell light paths.
Reference measurements made by reading the cuvette (Cuvette
Reference) or using factory-stored values derived from deionized water
(Water Constant) can be used to normalize the optical density data for
microplate wells.
Pathlength correction is accomplished only when using the PathCheck
Pathlength Measurement Technology with SoftMax
®
Pro Software.
PathCheck Pathlength Measurement Technology is patented by
Molecular Devices and can be performed only on an Molecular Devices
plate reader.
20 0112-0115 F
Page 21

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
dcm
Sample OD
1000
OD
900
–
k
-----------------------------------------------------------------=
k
Cuvette OD
1000
OD
900
–
=
OD
cm
---------
OD
Sample
dcm
------------------------=
The SpectraMax Multi-Mode Microplate Reader offers both the Cuvette
Reference and the Water Constant methods.
The actual pathlength, d, of a solvent is found from the following
equation:
When a Cuvette Reference is used for pathlength correction, the value
of k is obtained by taking optical density measurements on the fluid in
the cuvette at two wavelengths, 1000 and 900 nm:
When the Water Constant is used for pathlength correction, the value of
k is obtained from the instrument. This constant is saved in the
instrument in the factory and may differ slightly from instrument to
instrument.
Once the pathlength d is found, the following equation is used for the
pathlength correction:
PathCheck Pathlength Measurement Technology is applicable to almost
all biological/pharmaceutical molecules in aqueous solution because
they have little or no absorbance between 900 nm and 1000 nm at
concentrations normally used. PathCheck Pathlength Measurement
Technology can also be used with samples containing small amounts of
organics or high buffer concentrations by using the Cuvette Reference.
See
Water Constant or Cuvette Reference? on page 22.
0112-0115 F 21
Page 22

Principles of Operation
Water Constant or Cuvette Reference?
The PathCheck Pathlength Measurement is based on the absorbance of
water in the near infrared region (between 900 nm and 1000 nm). If
the sample is completely aqueous, has no turbidity and has a low salt
concentration (less than 0.5 M), the Water Constant is adequate. The
Water Constant is determined during manufacture and is stored in the
instrument.
If the sample contains an organic solvent such as ethanol or methanol,
we recommend using the cuvette reference. It is important that the
solvent does not absorb in the 900 nm to 1000 nm range (to determine
whether or not a given solvent would interfere, see the discussion of
interfering substances below). When a non-interference solvent is
added to the aqueous sample, the water absorbance decreases
proportionally to the percentage of organic solvent present. For
example, 5% ethanol decreases the water absorbance by 5% and
results in a 5% underestimation of the pathlength. You can avoid the
error by putting the same water/solvent mixture in a cuvette and using
the Cuvette Reference.
To use the Cuvette Reference, place into the cuvette port a standard
1 cm cuvette containing the aqueous/solvent mixture that is used for
the samples in the microplate. The cuvette must be in place when you
read the microplate. When you click the Read button in the SoftMax Pro
program, the instrument first makes the 900 nm and 1000 nm
measurements in the cuvette, and then makes the designated
measurements in the microplate. The cuvette values are stored
temporarily and used in the PathCheck Pathlength Measurement
Technology calculations for the microplate samples.
Use of Cuvette Reference with PathCheck Pathlength Measurement
Technology is different from a reference reading of a cuvette in a
CuvetteSet section (by clicking the Ref button in the CuvetteSet section
tool bar in the SoftMax Pro program). The cuvette reference used for
PathCheck Pathlength Measurement Technology calculations
(measurements at 900 nm and 1000 nm) does not produce data that
can be viewed in a CuvetteSet section and is used only with data in
microplates, not cuvettes.
Background Considerations
Raw optical density measurements of microplate samples include both
pathlength-dependent components (sample and solvent) and a
pathlength-independent component (OD of microplate material). The
latter must be eliminated from the PathCheck Pathlength Measurement
Technology calculation in order to obtain PathCheck Technology-
normalized results. There are 3 ways to accomplish this: plate blanks,
plate background constants, and plate pre-reads, all of which are
described in the PathCheck Pathlength Measurement Technology
section of the SoftMax Pro User Guide.
22 0112-0115 F
Page 23

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
PathCheck Pathlength Measurement Technology and Interfering Substances
Any material that absorbs in the 900 nm to 1000 nm spectral region
could interfere with PathCheck Pathlength Measurement Technology
measurements. Fortunately, there are few materials that do interfere at
the concentrations typically used.
Turbidity is the most common interference: if you can detect any
turbidity in your sample, you should not use the PathCheck Technology
feature. Turbidity elevates the 900 nm measurement more than the
1000 nm measurement and causes an erroneously low estimate of
pathlength. Using Cuvette Reference does not reliably correct for
turbidity.
Samples that are highly colored in the upper visible spectrum may have
absorbance extending into the near infrared (NIR) and can interfere
with the PathCheck Pathlength Measurement Technology. Examples
include Lowry assays, molybdate-based assays and samples containing
hemoglobins or porphyrins. In general, if the sample is distinctly red or
purple, you should check for interference before using the PathCheck
Pathlength Measurement Technology.
To determine possible color interference, do the following:
• Measure the optical density at 900 nm and 1000 nm (both
measured with air reference).
• Subtract the 900 nm value from the 1000 nm value.
• Do the same for pure water.
If the delta OD for the sample differs significantly from the delta OD for
water, then it is advisable not to use the PathCheck Technology feature.
Use of Cuvette Reference does not correct for the interference with the
current calculation scheme in the SoftMax Pro program. Currently,
Cuvette Reference involves a single (automated) read at 900 nm and
1000 nm and the automated calculations in the SoftMax Pro program
do not compensate for color or solvent interference. However, you
could correct for such interference by taking two cuvette
measurements and using a different set of calculations. For further
information, contact Molecular Devices Technical Support.
Organic solvents could interfere with the PathCheck Technology feature
if they have absorbance in the region of the NIR water peak. Solvents
such as ethanol and methanol do not absorb in the NIR region, so they
do not interfere, except for causing a decrease in the water absorbance
to the extent of their presence in the solution. Their passive
interference can be avoided by using the Cuvette Reference. If,
however, the solvent absorbs between 900 and 1000 nm, the
interference would be similar to the interference of highly colored
samples described above. If you are considering adding an organic
solvent other than ethanol or methanol, you are advised to run a
spectral scan between 900 nm and 1000 nm to determine if the solvent
would interfere with the PathCheck Technology feature.
0112-0115 F 23
Page 24
Principles of Operation
Normalizing Absorbance Measurements
SoftMax Pro Software automatically reports absorbance values
normalized to a 1-cm pathlength. SoftMax Pro Software automatically
reports absorbance values normalized to a 1-cm pathlength. The table
below shows results obtained with 75 µL to 300 µL yellow reagent.
Table 2-1 Yellow reagent results.
Well Volume
Optical pathlengths and raw absorbance values were directly
proportional to well columns. After normalization to a 1-cm pathlength,
all absorbance values, regardless of the volume in the wells, were
within 1% of the value obtained by measuring the same solution in a 1-
cm cuvette.
Fluorescence
Fluorescent materials absorb light energy of a characteristic wavelength
(excitation), undergo an electronic state change, and instantaneously
emit light of a longer wavelength (emission). Most common fluorescent
materials have well-characterized excitation and emission spectra.
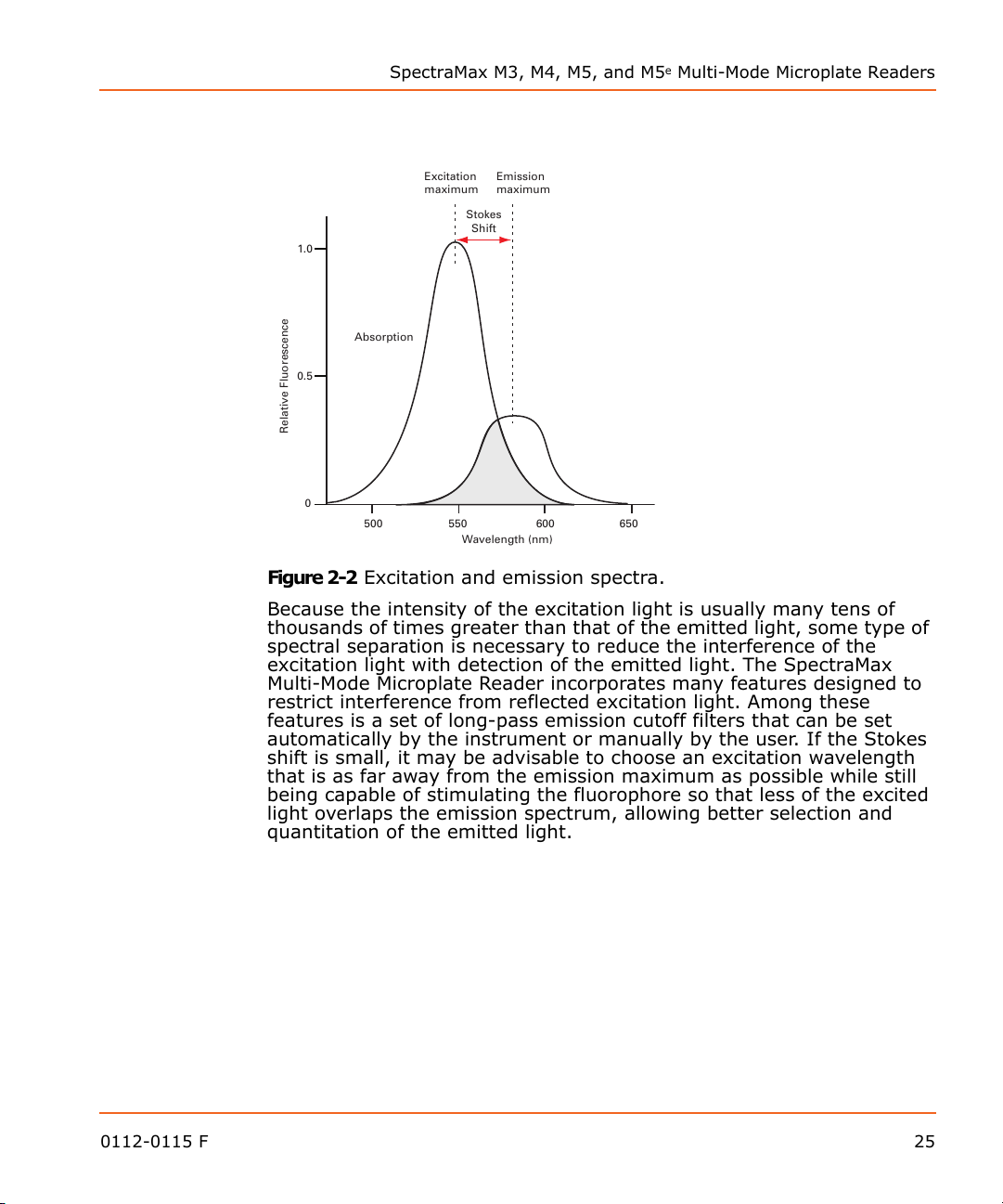
Figure 2-2 shows an example of excitation and emission spectra for a
fluorophore. The excitation and emission bands are each fairly broad,
with half-bandwidths of approximately 40 nm, and the wavelength
difference between the excitation and emission maxima (the Stokes
shift) is typically fairly small, about 30 nm. There is considerable
overlap between the excitation and emission spectra (gray area) when
a small Stokes shift is present.
Pathlength
(µL)
75 0.231 0.090 0.390 0.006 1.6
100 0.300 0.116 0.387 0.005 1.2
150 0.446 0.172 0.385 0.003 0.8
200 0.596 0.228 0.383 0.002 0.4
250 0.735 0.283 0.384 0.002 0.5
300 0.874 0.336 0.384 0.001 0.3
(cm)
Absorbance in 1-cm cuvette = 0.386
Raw
Absorbance
Absorbance/
cm
SD CV%
24 0112-0115 F
Page 25
Wavelength (nm)
Relative Fluorescence
Absorption
Excitation
maximum
Emission
maximum
Stokes
Shift
500 550 600 650
0
0.5
1.0
SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Figure 2-2 Excitation and emission spectra.
Because the intensity of the excitation light is usually many tens of
thousands of times greater than that of the emitted light, some type of
spectral separation is necessary to reduce the interference of the
excitation light with detection of the emitted light. The SpectraMax
Multi-Mode Microplate Reader incorporates many features designed to
restrict interference from reflected excitation light. Among these
features is a set of long-pass emission cutoff filters that can be set
automatically by the instrument or manually by the user. If the Stokes
shift is small, it may be advisable to choose an excitation wavelength
that is as far away from the emission maximum as possible while still
being capable of stimulating the fluorophore so that less of the excited
light overlaps the emission spectrum, allowing better selection and
quantitation of the emitted light.
0112-0115 F 25
Page 26
Principles of Operation
Wavelength (nm)
Relative Fluorescence
Excitation
reading
wavelength
Emission
reading
wavelength
Fluorophore’s
excitation
maximim
Fluorophore’s
emission
maximim
500 550 600 650
0
0.5
1.0
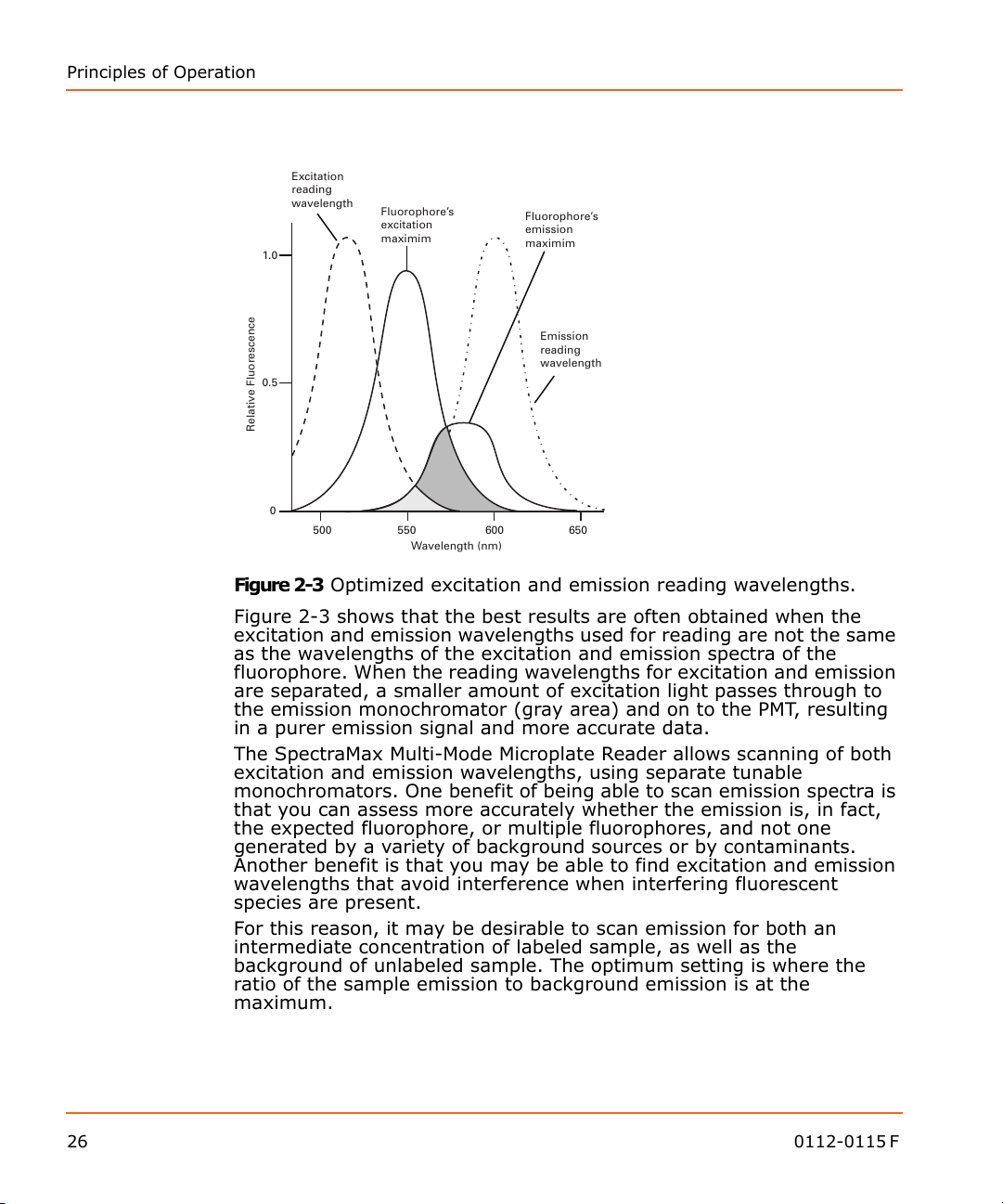
Figure 2-3 Optimized excitation and emission reading wavelengths.
Figure 2-3 shows that the best results are often obtained when the
excitation and emission wavelengths used for reading are not the same
as the wavelengths of the excitation and emission spectra of the
fluorophore. When the reading wavelengths for excitation and emission
are separated, a smaller amount of excitation light passes through to
the emission monochromator (gray area) and on to the PMT, resulting
in a purer emission signal and more accurate data.
The SpectraMax Multi-Mode Microplate Reader allows scanning of both
excitation and emission wavelengths, using separate tunable
monochromators. One benefit of being able to scan emission spectra is
that you can assess more accurately whether the emission is, in fact,
the expected fluorophore, or multiple fluorophores, and not one
generated by a variety of background sources or by contaminants.
Another benefit is that you may be able to find excitation and emission
wavelengths that avoid interference when interfering fluorescent
species are present.
For this reason, it may be desirable to scan emission for both an
intermediate concentration of labeled sample, as well as the
background of unlabeled sample. The optimum setting is where the
ratio of the sample emission to background emission is at the
maximum.
26 0112-0115 F
Page 27

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
For more information regarding optimizing excitation and emission
wavelengths using the spectral scanning capabilities of the SpectraMax
series readers, see
Optimizing Absorbance Assays on page 44.
Time-resolved Fluorescence (M4, M5, and M5e only)
In normal fluorescence mode, the SpectraMax readings are taken while
the lamp is on. The most common limitation to sensitivity in normal
fluorescence is excitation energy or background fluorescence that
cannot be eliminated from the emission signal. Since the lamp is the
source of excitation energy, turning it off provides the best means of
eliminating background excitation. The elimination of background
excitation is the critical difference between fluorescence intensity
measurements and TRF measurements.
Time-resolved fluorescence is performed by flashing the excitation
lamp and, after it is off, collecting the delayed emission for a period of
time before the lamp is flashed again. Long-lifetime rare-earth
lanthanide dyes are typically used to provide a long-lived fluorescent
signal that persists after the lamp is turned off. Background
fluorescence usually fades after 50 µs, while lanthanide chelates and
cryptates have fluorescent lifetimes between 100 µs and 2 ms.
To optimize data collection for a particular assay, the user can select
when to start and end data acquisition-the minimum is 50 µs after the
lamp has been turned off, and the maximum is 1450 µs, in 50- or 200-
µs steps.
Some examples of TRF assays are:
• IMAP
• Cisbio HTRF
• LanthaScreen TR-FRET
• LANCE TR-FRET
• DELFIA TRF
®
TR-FRET
0112-0115 F 27
Page 28

Principles of Operation
mP
parallel G perpendicular–
parallel G perpendicular+
------------------------------------------------------------------------=
r
parallel G perpendicular–
parallel 2G perpendicular+
----------------------------------------------------------------------------=
Fluorescence Polarization (M5 and M5e only)
By using a fluorescent dye to label a small molecule, its binding to a
large molecule can be monitored through its speed of rotation.
Fluorescence Polarization mode returns two sets of data: one for
fluorescence intensity parallel (P) to the excitation plane, and the other
for fluorescence intensity perpendicular (S) to the excitation plane.
These S and P values are used to calculate the polarization (mP) and
anisotropy (r) values in SoftMax Pro Software. Although the Raw S&P
value is the true raw data returned from the instrument, the calculated
polarization (mP) and anisotropy (r) values are treated as the raw data,
and these values become the basis for further reduction calculations in
SoftMax Pro Software.
Polarization (mP) is calculated as follows:
Anisotropy (r) is calculated as follows:
Luminescence
Luminescence is the emission of light by processes that derive energy
from essentially non-thermal changes, the motion of subatomic
particles, or the excitation of an atomic system by radiation.
When the SpectraMax Multi-Mode Microplate Reader is in luminescence
mode, no excitation is necessary as the species being measured emit
light naturally. For this reason, the lamp does not flash, so no
background interference occurs. A dark estimate is done over a dark
reference, and multiple readings are averaged together into one
reading per well.
The default setting for luminescence is the “zero order” position where
the grating monochromator acts as a mirror that reflects all light to the
PMT detector. If wavelength selection is desired, you can choose the
wavelength where peak emission is expected to occur. In addition,
multiple wavelength choices allow species with multiple components to
be differentiated and measured easily. In luminescence read mode, no
emission cutoff filter is used.
28 0112-0115 F
Page 29

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
TEMP on/off
TEMP on/off
Functional Description
The full power of a SpectraMax Multi-Mode Microplate Reader can only
be harnessed when the instrument is controlled using SoftMax® Pro
Software running on a computer connected to the instrument. For a
complete description of the modes of operation, how to choose
instrument settings, etc. refer to the SoftMax® Pro Software User
Guide.
However, some functionality is available directly on the instrument
without having to use SoftMax Pro Software:
• Temperature control
• Wavelength control
• Fixed-point cuvette readings
Temperature Regulation
The SpectraMax Multi-Mode Microplate Readers have been designed to
regulate the temperature of the cuvette and microplate chamber from
2°C above ambient to 60°C. Upon power up, when the incubator is off,
the temperature in the chambers is ambient and isothermal. Turning on
the incubator by pressing the key causes the instrument to
begin warming the cuvette and microplate chambers. The temperature
set point defaults to 37.0°C at start-up.
Accuracy of the temperature set point is guaranteed only if the set
point is at least 2°C above ambient. If the temperature set point is
lower than the ambient temperature, the chamber temperature
remains at ambient. Temperature regulation is controlled by heaters
only and, therefore, cannot cool the temperature to a setting lower
than ambient. Additionally, the highest setting (60°C) can be achieved
only if the ambient temperature is greater than 20°C.
Typically, the cuvette and microplate chambers reach 37.0°C in less
than 30 minutes. The temperature is maintained at the set point until
you press the incubator key again, turning temperature
regulation off.
If you turn the incubator back on after a momentary shutdown, allow
about ten minutes for the control algorithm to fully stabilize the
temperature.
Temperature regulation and control is achieved through electric
heaters, a fan, efficient insulation, and temperature sensors. The
heaters are located in the microplate chamber, which is insulated to
maintain the temperature set point. The sensors are mounted inside
the chamber and measure the air temperature.
0112-0115 F 29
Page 30

Principles of Operation
The temperature feedback closed-loop control algorithms measure the
chamber air temperature, compare it to the temperature set point, and
use the difference to calculate the regulation of the heating cycles. This
technique results in accurate, precise control of the chamber
temperature with a temperature variation of the air inside the chamber
of less than 1.0°C. The temperature uniformity within the microplate
depends on its design and composition.
Read Types
The SpectraMax Multi-Mode Microplate Reader can perform four types
of read: endpoint, kinetic, spectrum and well scan. Instrument setup
parameters for each read type are discussed in the SoftMax® Pro
Software User Guide.
Endpoint Read
In an endpoint read, a reading of each microplate well is taken at a
single or multiple wavelengths.
Depending on the data mode selected in the Reduction window, values
can be reported as optical density or % Transmittance.
Kinetic Read
In a kinetic read the data are collected over time with multiple readings
taken at regular intervals. To achieve the shortest possible interval for
kinetic readings, choose wavelengths in ascending order.
Kinetic analysis can be performed for up to 99 hours. The kinetic read
interval depends upon the instrument setup parameters chosen in
SoftMax Pro Software.
Kinetic analysis has many advantages when determining the relative
activity of an enzyme in different types of microplate assays, including
ELISAs and the purification and characterization of enzymes and
enzyme conjugates. Kinetic analysis is capable of providing improved
dynamic range, precision, and sensitivity relative to endpoint analysis.
Spectrum Read
Spectral analysis measures optical density or % Transmittance across a
spectrum of wavelengths 200 nm to 1000 nm. For fluorescence or
luminescence mode, relative fluorescence units (RFU) or relative
luminescence units (RLU) values are reported.
All spectrum readings are made using the scanning monochromators of
the instrument.
30 0112-0115 F
Page 31

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Well Scan Read
A well scan read takes one or more readings of a single well of a
microplate at single or multiple wavelengths. Every option available for
endpoint reads is available for well scans.
Some applications involve the detection of whole cells in large-area
tissue culture plates. Well scan mode can be used with such microplates
to allow maximum surface area detection in whole-cell assays. Since
many cell lines tend to grow as clumps or in the corners of microplate
wells, you can choose from several patterns and define the number of
points to be scanned to work best with your particular application.
Values reported are optical density, % Transmittance, relative
fluorescence units (RFU), or relative luminescence units (RLU).
Automix
The Automix function permits automatic linear shaking along the long
axis of the microplate at preset intervals, thereby mixing the contents
within each well. Automix must be selected before beginning a reading.
The actions associated with the Automix setting depend on the read
mode chosen:
• Endpoint mode: Automix shakes the plate for a definable number
of seconds and then reads at all selected wavelengths.
• Kinetic mode: two types of Automix can be enabled: Automix can
shake the plate for a definable number of seconds before the
initial reading, and/or for a definable number of seconds before
each subsequent reading.
Use of Automix is strongly recommended for ELISAs and other solid-
phase, enzyme-mediated reactions to enhance accuracy.
Computer Control
The SpectraMax Multi-Mode Microplate Readers are equipped with an
8-pin DIN RS-232 serial port through which the computer
communicates with the instrument using the SoftMax Pro Software.
Different types of cables are available for connecting to different types
of computers. See
0112-0115 F 31
Appendix B: Cables and Accessories on page 77.
Page 32

Principles of Operation
32 0112-0115 F
Page 33

Installation
WARNING! Always make sure the power switch on the
instrument is in the OFF position and remove the power cord
from the back of the instrument prior to any installation or
relocation of the instrument.
WARNING! Do not operate the instrument in an environment
where potentially damaging liquids or gases are present.
CAUTION! Do not operate the instrument in a cold room with a
temperature below 15°C.
3
CAUTION! Do not touch or loosen any screws or parts other than those
specifically designated in the instructions. Doing so might cause
misalignment and voids the instrument warranty.
0112-0115 F 33
Page 34

Installation
Unpacking
Note: In this user guide, references to the SpectraMax® readers
include the M3, M4, M5, and M5e models. When a feature or capability
applies to only certain readers, this exception is noted.
The SpectraMax Multi-Mode Microplate Readers are packed in a
specially designed carton. Please retain the carton and the packing
materials. If the unit should need to be returned for repair, you must
use the original packing materials and carton for shipping. If the carton
has been damaged in transit, it is particularly important that you retain
it for inspection by the carrier in case there has also been damage to
the instrument.
WARNING! The SpectraMax Multi-Mode Microplate Reader
weighs approximately 36 pounds (16.4 kg) and should be lifted
with care. It is recommended that two people lift the
instrument together, taking the proper precautions to avoid
injury.
After examining the carton, place it on a flat surface in the upright
position. Open the top of the box and lift the accessory kit out. Open
the accessory kit box and check that all parts are accounted for:
• Plate adapter (purple)
• Reader dustcover
• Test tube cover
• Hex wrench, 3/32˝, ball drive, L
• Mouse pad, SpectraMax instrument
• Cable, PC–SpectraMax, 9 pin–8 pin mini
• Country-specific Power cord
• Fuses, 4-amp (2 ea.)
• SpectraMax User Guide
• Applications guide to microplate systems
Make sure all these items are present before proceeding.
Remove the cardboard divider from the top of the SpectraMax. Lift the
reader up and out of the shipping box and set it down carefully.
34 0112-0115 F
Page 35

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Setting up the Instrument
1. Place the instrument on a level surface, away from direct
sunlight, dust, drafts, vibration, and moisture.
2. Turn the instrument around so that the back of the instrument is
facing you as shown in
of a reader. on page 18
3. Insert the round end of the serial cable into the RS-232 serial
port on the back panel of the instrument. (A Keyspan USB
adapter is necessary for a Macintosh computer or a Windows
computer without a serial port; see
information on adapter cables.) Attach the other end to your
computer.
4. Insert the female end of the power cord into the power
receptacle at the rear of the instrument. Connect the male end
to a grounded power outlet of the appropriate voltage. Molecular
Devices recommends that you use a surge protector between
the power cord and the grounded power outlet.
5. Turn the instrument around so that the control panel now faces
you. Ensure no cables run beneath the instrument. Leave at
least three inches between the back of the instrument and the
nearest objects or surfaces to ensure proper ventilation and
cooling.
6. Remove the tape from the cuvette door.
7. Turn on the power to the instrument, wait for the microplate
drawer to open, and remove the tape and protective covering
from the drawer subplate.
Figure 1-6 Schematic of the back panel
.
Appendix B for more
0112-0115 F 35
Page 36

Installation
DRAWER
Installing the Drawer Adapter
CAUTION! Incorrect insertion or removal of the adapter may cause
damage to the microplate drawer of the SpectraMax instrument. The
corner cutout must be in the lower left corner where the plate pusher is
located.
If you are reading standard 96-well or 384-well microplates from the
top, you need to install the drawer adapter.
1. Power on the instrument using the switch on the back panel.
2. Press the button on the front panel or select the
Control > Open Drawer command in SoftMax® Pro Software.
3. Hold the adapter so that the label is on the front side facing up.
4. Place the top back (Row A) portion of the adapter into the
drawer first. The corner cutout must be in the lower left corner
where the plate pusher is located. While pushing against the
back edge of the adapter, lower the front of the adapter into the
drawer.
Figure 3-1 Adapter inserted in microplate drawer.
36 0112-0115 F
Page 37

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
DRAWER
Removing the Drawer Adapter
If the adapter is in the drawer and you are either reading from the
bottom or using “high profile” (6-well, 12-well, 24-well, or 48-well)
plates, you need to remove the adapter.
Incorrect insertion or removal of the adapter may cause damage to the
microplate drawer of the SpectraMax instrument.
1. Power on the instrument using the switch on the back panel.
2. Press the button on the front panel or select the
Control > Open Drawer command in SoftMax Pro Software.
3. Remove the adapter plate.
Figure 3-2 The microplate drawer without adapter.
0112-0115 F 37
Page 38

Installation
38 0112-0115 F
Page 39

Operation
TEMP on/off
REF
READ CUVETTE
Cuvette Read—Quick Overview
If you are an experienced user of this instrument, the following steps
provide a quick reminder of the basic operating procedures required to
read a cuvette using a SpectraMax Multi-Mode Microplate Reader:
1. Turn on the power switch (located on the back panel). The
microplate drawer opens automatically.
2. If you want to regulate the temperature inside the chamber,
touch the (incubator) key to turn the incubator on
and bring the chamber to the default temperature of 37.0°C.
The microplate drawer closes.
3. If the incubator is on, the LCD shows the current temperature of
the cuvette chamber along with the temperature set point. To
change the set point (to any setting from ambient +2°C to
60°C), press the up or down arrow keys.
4. Select the desired measurement wavelength by pressing the up
or down arrow near
5. Load the prepared cuvette into the chamber, being sure that the
clear sides are left and right (when facing the instrument).
6. Press the or key.
.
4
0112-0115 F 39
Note: In this user guide, references to the SpectraMax® readers
include the M3, M4, M5, and M5e models. When a feature or capability
applies to only certain readers, this exception is noted.
Page 40

Operation
TEMP on/off
Microplate Read—Quick Overview
If you are an experienced user of this instrument, the following steps
provide a quick reminder of the basic operating procedures required to
read a microplate using a SpectraMax Multi-Mode Microplate Reader:
1. Turn on the power switch (located on the back panel). The
microplate drawer opens automatically.
2. If you want to regulate the temperature inside the chamber,
touch the (incubator) key to turn the incubator on
and bring the chamber to the default temperature of 37.0°C.
The microplate drawer closes.
3. If the incubator is on, the LCD shows the temperature set point,
but the current temperature in the microplate chamber is
reported in SoftMax® Pro only—the LCD reports the cuvette
chamber temperature. To change the set point to any setting
from ambient +2°C to 60°C, press the up or down arrow keys.
4. Select the desired instrument settings (read mode, type of
analysis, template, etc.) using SoftMax Pro Software on the
external computer.
5. If you are performing kinetic analysis, add substrate at this
time.
6. Load the prepared microplate into the drawer, being sure to
match well A1 with the A1 mark on the upper left-hand corner of
the drawer.
7. Using SoftMax Pro Software, start the reading by selecting the
Control > Read command or clicking the Read button on the Plate
section tool bar.
Preparing for a Cuvette or Microplate Reading
40 0112-0115 F
Turn the Instrument and Computer On
The power switch is located on the back panel. Press the rocker switch
to the ON position.
The instrument automatically performs diagnostic checks to ensure that
it is functioning correctly. Turn the computer on at this time also and
start the SoftMax Pro Software program.
Page 41

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
TEMP on/off
Set the Temperature (Optional)
To set the temperature within the microplate or cuvette chamber, you
should turn on the incubator first, allowing enough time for the
temperature to reach the set point before performing a reading. When
you first turn the instrument on, up to 60 minutes may be required for
the temperature within the chamber to reach the set point. Turning on
the incubator and choosing a temperature set point can be done using
the software or the front panel of the instrument (described below).
However, only the cuvette temperature is reported on the front panel;
the SoftMax Pro program reports the current microplate chamber
temperature, which lags very slightly behind the cuvette temperature.
Temperature cannot be regulated at a set point that is lower than 2°C
above the ambient temperature.
To enable the incubator
1. Press the incubator key.
2. The LCD display indicates that temperature control is on and
shows the set point, and current temperature of the cuvette
chamber only.
To change the temperature set point, press the up or down arrow keys
until the desired temperature set point is shown in the display.
To control the temperature from the SoftMax Pro Software, use the
Control > Incubator dialog box both to enable the incubator and to set
the temperature.
The chamber temperature is maintained at the set point until you
disable temperature control by touching the incubator key again. When
the incubator is off, the temperature within the chamber gradually
returns to ambient.
Should you turn the incubator back on after a momentary shutdown,
allow about ten minutes for the control algorithm to fully stabilize the
chamber temperature.
0112-0115 F 41
Page 42

Operation
Select the Wavelength
The absorbance wavelength, or the excitation or emission wavelengths
for a fluorescence or luminescence read, can be selected for cuvette
reading using either the control panel or the SoftMax Pro Software. The
wavelengths must be selected using the software when reading
microplates.
To select the wavelength using the control panel
1. Select the desired measurement wavelength by pressing the up
or down arrow near .
2. Scroll through the wavelengths shown on the LCD to increment
or decrement the wavelength setting (in1-nm increments) until
the desired measurement wavelength is reached.
3. If A or %T type is chosen, only the Abs/Ex wavelength can be
set.
To select the wavelength using SoftMax Pro Software
1. Select the Plate > Settings command, or click the Settings
button in the appropriate Plate or CuvetteSet section.
2. In the Settings dialog box, select Wavelengths from the list of
settings on the left-hand side.
3. Specify the number of wavelengths to be read in each well or
cuvette.
4. Specify the excitation and emission wavelengths to use.
5. Select Auto-cutoff if you would like the reader software to
automatically select the emission cut-off filter.
42 0112-0115 F
Page 43

Read the Cuvette
REF
READ CUVETTE
1. Insert the cuvette into the chamber, making sure that the clear
sides are to the left and right (facing the instrument). Do not
touch the clear surfaces of the cuvette.
2. Make sure the cuvette is completely seated in the chamber and
close the cuvette door.
3. If the cuvette contains a blank (typically this solvent contains
everything that the samples contain except for analyte), press
the key to acquire the reference reading from the cuvette.
The instrument automatically calibrates in less than two
seconds, closes the microplate drawer (if it is open), and reads
the cuvette according to the selected instrument settings.
4. If the cuvette contains a sample, touch the key to
acquire the sample reading from the cuvette.
5. When the reading is complete, remove the cuvette.
Read the Microplate
Note: The underside of the microplate must be dry prior to placing it in
the drawer. If the microplate has fluid on the underside, dry it using a
paper towel (or equivalent) before placing it in the drawer.
SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
0112-0115 F 43
1. Insert the filled microplate into the drawer, matching well A1
with position A1 in the drawer. Make sure the microplate is flat
against the drawer bottom (for 6-, 12-, 24-, or 48-well
microplates) or against the adapter (if using top read for 96- or
386-well plates-see
for more information).
2. You must have SoftMax Pro Software running on a computer
connected to the instrument.
Open a SoftMax Pro data file or protocol file that contains the
appropriate experiment settings for the plate read. Alternatively,
create new settings by selecting the Plate section in the SoftMax
Pro program and configuring the instrument using the Plate
>Settings dialog box.
Installing the Drawer Adapter on page 36
Page 44

Operation
DRAWER
3. Select the Control > Read command or press the Read button in
SoftMax Pro Software to start the plate read.
4. When reading is complete, the drawer of the instrument opens,
allowing you to remove the microplate. If the incubator is on,
the drawer closes again after approximately 10 seconds.
5. If you return to the SpectraMax instrument and find the drawer
closed after a reading has finished, press the
When the drawer opens, you can remove the microplate.
For more information about configuring the software for plate reading,
please consult the SoftMax® Pro Software User Guide.
Optimizing Fluorescence Assays
The wavelength of the transmitted light can be adjusted in 1-nm
increments between 200 nm and 1000 nm. Guke also allows reading up
to four wavelengths per plate. This enables reference wavelength
readings such as A260 and A280 for nucleic determination.
An appropriate plate blank should be applied. Unless the user suspects
that there is significant well-to-well variability due to the thickness and
optical properties of the plate, the use of Pre-Read Plate in the SoftMax
Pro program is not required. Instead, we recommend using appropriate
plate blanks or group blanks in the Template dialog box of the Plate
section in the SoftMax Pro program. For discussion of the different
types of blanking, please refer to the SoftMax® Pro Software User
Guide.
If desired, the PathCheck Technology feature in SoftMax Pro program
can be activated to normalize the data to a 1-cm pathlength.
key.
Optimizing Absorbance Assays
The optimum instrument settings for detection of a particular
fluorophore depend on a number of different factors. Settings that can
be adjusted for assay optimization include the excitation and emission
wavelengths, emission cutoff filter, readings per well, the PMT voltage,
and the temperature of the reading chamber.
Another important factor that is independent of the instrument but
which affect assays optimization is the Stokes’ shift. When the Stokes'
shift is very small, optimizing the excitation and emission wavelengths
and correct cutoff filter choices are very important.
Excitation and Emission Wavelengths
The excitation and emission wavelengths may be set in 1-nm
increments between 250 nm and 850 nm. A procedure to optimize
excitation and emission wavelengths for a given assay is outlined
below.
44 0112-0115 F
Page 45

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Emission Cutoff Filter
The emission cutoff filters assist in reducing background. Sources of
background include stray excitation light and native fluorescence of
plate materials, sample constituents, and solvents (including water).
The default setting allows the instrument and SoftMax Pro Software to
determine which cutoff filter should be used (see Table for default
settings) in endpoint and kinetic modes. The spectral scan mode
default uses no cutoff filter.
Readings Per Well
The number of readings per well may vary between 1 (used for a quick
estimate) and 100 (for very precise measurements). The default
number of readings per well varies with the read mode: for
fluorescence, the default is 6, and for luminescence the display shows 1
read per well.
PMT Voltage
The voltage of the photomultiplier tube may be set to low (for higher
concentration samples), medium, or high (for lower concentration
samples) in all read modes. In endpoint and spectrum mode, there is
an additional setting, automatic, in which the instrument automatically
adjusts the PMT voltage for varying concentrations of sample in the
plate.
Temperature Control
The chamber of the SpectraMax Multi-Mode Microplate Reader is
isothermal at ambient as well as at elevated temperatures. The
temperature in the reading chamber may be adjusted from 2°C above
ambient to 60°C.
Note that assay optimization requires the use of a computer and
SoftMax Pro Software.
0112-0115 F 45
Page 46

Operation
Using Spectral Scanning to Optimize Excitation and Emission Wavelengths for Fluorescence Assays
Put 200 µL of sample that includes the fluorophore and 200 µL of a
buffer control into separate wells of a microplate.
1. Perform the excitation scan:
Using SoftMax Pro Software, set up a Plate section for a
fluorescence read, spectrum mode, Em Fixed/Ex Scan, with
no cutoff filter (default), and medium PMT.
Set the emission wavelength based on the tentative value
from the literature (or from a customary filter set used to
measure your fluorophore). If the emission wavelength is
not known, select a tentative emission wavelength about 50
nanometers greater than the absorbance maximum of the
fluorophore. If necessary, the absorbance maximum can be
determined by performing an optical density spectral scan
first.
Set the excitation scan to start/stop approximately 50 nm
below/above the tentative excitation value obtained from the
literature (or the customary excitation filter).
Set the step increment to 2 or 3 nm. (You may choose to do
a preliminary scan with a 10-nm increment to determine the
approximate peak location, and then repeat the scan over a
narrower wavelength range with a 2-nm or 3-nm
increment.)
Perform the scan and view the results as a plot of emission
fluorescence vs. excitation wavelength. Note the excitation
wavelength at the emission peak and the maximum RFU
value.
If an error message reporting missing data points occurs, it may
be due to possible saturation reported by the SoftMax Pro
program at the end of the spectral scan. Reset the PMT to “low”
and rescan the sample (scan the buffer blank with the PMT set
to “medium” or “high”). If the error occurs after scanning with
the PMT set to “low,” it may be necessary to dilute the sample.
46 0112-0115 F
Page 47

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
If the excitation scan shows no apparent peak, change the PMT
setting to “high” and rescan the sample. If the spectral scan still
shows no apparent peak, adjust the Y-scale of the zoom plot so
that the plot fills the graph.
Select the optimal excitation wavelength. If the excitation
peak wavelength and emission wavelength are separated by
more than 80 nm, use the excitation peak wavelength value.
If the excitation and emission wavelengths are less than
80 nm apart, use the shortest excitation wavelength that
gives 90% maximal emission. (Follow the plot to the left of
the peak until the RFU value falls to approximately 90% of
the maximum, and then drop a line from the 90% point on
the plot to the x-axis—see Figure 4-1.)
RFU at 90% of max λ
max λ
RFU
Wavelength
90% of max λ
Figure 4-1 Plot of RFU vs. wavelength.
2. Perform emission scan #1:
In the SoftMax Pro program, set up a second plate section
for a fluorescence read, spectrum mode, Ex Fixed/Em Scan,
with no cutoff filter (default), and medium PMT.
Set the excitation wavelength to the value determined in
Step 1w above.
Set the emission scan to start/stop approximately 50 nm
below or above the tentative emission value obtained from
the literature (or existing filter pair). Note: If the Stokes shift
is less than 50 nm, then start the emission scan above the
excitation wavelength.
Set the step increment to 2–3 nm (or do a preliminary scan
with a 10-nm increment to determine the approximate peak
location and then repeat the scan over a narrower
wavelength range using a 2–3 nm increment.)
Perform the scan and view the results as a plot of
fluorescence vs. emission wavelength.
0112-0115 F 47
Page 48

Operation
3. Choose the emission filter:
Select an emission cutoff filter that blocks as much of the
residual excitation light as possible without unduly reducing
the fluorescence signal. The cutoff wavelength choices are
325, 420, 435, 475, 495, 515, 530, 550, 570, 590, 610,
630, 665, or 695 nm. The cutoff value should be near the
maximum emission wavelength (preferably between the
excitation wavelength and the maximal emission
wavelength) but at least 10 nm less than the emission
wavelength. If you have questions about this procedure
please contact MDC Technical Support and ask to speak to
an applications scientist.
4. Perform emission scan #2:
In the SoftMax Pro Software, set up a third plate section for
an emission scan as specified in Step 2 above, except
selecting Manual Cutoff Filter and setting the wavelength to
that determined in Step 3.
Perform the scan and view the results as a plot of
fluorescence vs. emission wavelength. Note the wavelength
giving the maximum emission (the optimal emission
wavelength).
Compare the spectra of the sample containing the
fluorophore to the spectra of the buffer blank to get an
estimate of the signal-to-noise ratio. If there is significant
background interference, repeat Steps 3 and 4 with another
choice of cutoff filter.
5. Results
The optimal excitation and emission wavelengths are those
determined in Steps 1 and 4, above.
6. Comments
In endpoint or kinetic fluorescence modes, the “Autofilter”
feature generally selects the same cutoff filter wavelength as
the above optimization method. If desired, however, you
may specify the cutoff filters manually.
For emission wavelengths less than 325 nanometers,
experimental iteration is usually the best method of
determining the optimal emission and excitation
wavelengths. Begin optimization by performing Steps 1–4
above. Try emission and excitation wavelength combinations
with the 325 cutoff or with no cutoff filter. Similarly, for
excitation wavelengths greater than 660 nanometers, try
emission and excitation wavelength combinations with the
695 cutoff or with no cutoff filter.
48 0112-0115 F
Page 49

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Figure 4-2 Effects of cutoff filters on fluorescein. Emission was scanned
from 490 to 560 nm; excitation was fixed at 485 nm.
Figure 4-2 shows the effects of different cutoff filters on a scan of
fluorescein where excitation was fixed at 485 nm and emission was
scanned from 490 nm to 560 nm (buffer blanks are not shown in this
plot). Table following lists default settings for the emission cutoff
filters.
Table 4-1 Emission cutoff filter default settings.
#
1 None < 415
2 420 415–434
3 435 435–454
4 455 455–474
5 475 475–494
6 495 495–514
7 515 515–529
0112-0115 F 49
Automatic Cutoff Selection Endpoint and Kinetic Modes
Wavelength (nm) Emission Wavelength (nm)
Page 50

Operation
Table 4-1 Emission cutoff filter default settings.
#
8 530 530–549
9 550 550–569
10 570 570–589
11 590 590–609
12 610 610–629
13 630 630–664
14 665 665–694
15 695 695–850
Automatic Cutoff Selection Endpoint and Kinetic Modes
Wavelength (nm) Emission Wavelength (nm)
For spectrum mode, the default is “manual” (no automatic cutoff).
Optimizing Time-resolved Fluorescence Assays
Time-resolved fluorescence assays on a SpectraMax M4, M5, and M5e
reader may be read from the top or bottom of a microplate. Solid white
plates are recommended for top time-resolved fluorescence reads, and
white plates with clear bottoms are recommended for bottom reads.
If the time-resolved fluorescence assay you are using has low signal or
gives results with high %CV, use 100 readings per well. If a faster read
speed is required, be sure Settling Time is “Off” in the SoftMax Pro
Plate Settings dialog box, and experiment with fewer flashes per well
until acceptable precision and speed are achieved.
Important settings for obtaining the best results in TRF assays are
integration delay and integration time:
• The integration delay is the amount of time that elapses
between the flash of the lamp (excitation) and the beginning of
data acquisition from the well.
• The integration time is the amount of time the well is read.
Delay and integration time are usually specified in the package insert of
commercially available TRF reagent kits. If a kit is not used, start with a
delay of 50 µs and try different delays up to 400 µs with a fixed
integration time of 400 µs. Once the optimum delay is chosen (based
on the highest ratio of a well containing a fluorophore divided by wells
containing only buffer) optimize the integration time, which is usually
between 400 µs and 1000 µs.
50 0112-0115 F
Page 51

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Optimizing Fluorescence Polarization Assays
Fluorescence polarization for SpectraMax M5 and M5e readers may only
be read from the top of a microplate. The plastic from a microplate will
affect the light polarization, precluding bottom reads and reading a
covered plate.
Solid black plates are recommended for fluorescence polarization
reads. If the assay components seem to bind to the microplate, as
evidenced by poor mP dynamic range (small difference between bound
and unbound tracer), we suggest using plates treated to minimize
binding, or polypropylene plates and/or adding a very small amount of
detergent, such as Tween-20, to the assay buffer.
Background wells, containing all assay components minus the
fluorophore, should be tested. If the signal in the background wells is
more than 1/10 the signal in the wells containing fluorophore, then
background wells should be run on each assay plate. The average raw
signal from the background's parallel and perpendicular readings
should be subtracted from the raw parallel and perpendicular readings
of each sample well before the mP calculation is performed. See the
SoftMax
in fluorescence polarization.
For best precision in assays using a low amount of fluorophore (for
example, <5 nm fluorescein), set the PMT sensitivity to High and the
number of readings to 100. If faster read speed is required, be sure
Settling Time is “Off” in the SoftMax Pro Plate Settings dialog box, and
experiment with fewer flashes per well until acceptable precision and
speed are achieved.
®
Pro Software User Guide for set-up of background subtraction
0112-0115 F 51
Page 52

Operation
Optimizing Luminescence Assays
Luminescence may be read from the top or the bottom of a microplate
or the cuvette. Solid white plates or white plates with clear bottoms are
recommended for luminescence reads.
For standard luminescence a separate light path without
monochromators carries the emitted light to a dedicated PMT. The
optimum emission wavelength is between 360 and 630 nm. Under
reader set-up the emission says “All”.
For wavelength-selectable luminescence, the emission monochromator
is used to differentiate the wavelengths being emitted from the well. Up
to four emission wavelengths between 250 nm and 850 nm may be
specified. If reading only one luminescent event in the well, best
sensitivity should be achieved using the standard luminescence
measurement, without a wavelength selected.
Luminescence read times are not designated by multiple reads per well,
but rather by choosing the total integration time desired between 1 ms
and 1,500 ms. Typical luminescence assays require between 500 ms
and 1,000 ms integration.
If wells have been incubating for a long period of time, it is a good idea
to mix the plate before reading. This can be done using Automix in the
reader.
If it appears that the signal is always higher in the first wells read (for
example, column A), the plate may need to be “dark adapted” to
reduce the auto-luminescence of the white plastic. The auto-
luminescence decreases quickly, so manually load the plate from the
control panel and wait for 1–2 minutes before initiating the read and
determine if the read-out is more consistent across the plate.
52 0112-0115 F
Page 53

Maintenance
Technical Support
Molecular Devices is a leading worldwide manufacturer and distributor
of analytical instrumentation. We are committed to the quality of our
products and to fully supporting our customers with the highest
possible level of technical service. In order to fully benefit from our
technical services, please complete the registration card and return it to
the address printed on the card.
Note: In this user guide, all references to SpectraMax Multi-Mode
Microplate Readers include the M3, M4, M5, and M5e models. When a
feature or capability applies to only certain readers, this exception is
noted.
If you have any problems using your SpectraMax Multi-Mode Microplate
Reader, in the U.S., contact our Technical Services group at 1-800-635-
5577; elsewhere contact your local representative.
WARNING! BIOHAZARD: It is your responsibility to
decontaminate the instrument, as well as any accessories,
before requesting service by Molecular Devices representatives
and before returning the instrument or any components to
Molecular Devices.
5
WARNING! All maintenance procedures described in this
manual can be safely performed by qualified personnel.
Maintenance not covered in this manual should be performed
only by an Molecular Devices representative.
WARNING! Removal of protective covers that are marked with
the High Voltage warning symbol shown below can result in a
safety hazard.
0112-0115 F 53
Page 54

Maintenance
WARNING! Always turn the power switch off and disconnect
the power cord from the main power source before performing
any maintenance procedure that requires removal of any panel
or cover or disassembly of any interior instrument component.
WARNING! Never perform any operation on the instrument in
an environment where liquids or potentially damaging gases
are present.
WARNING! Risk of electrical shock. Refer servicing to qualified
personnel.
CAUTION! Use of organic solvents (such as dichloromethane) may
cause harm to the optics in the instrument. Extreme caution is advised
when using organic solvents. Always use a plate lid and avoid placing a
plate containing these materials in the reading chamber for prolonged
periods of time. Damage caused by the use of incompatible or
aggressive solvents is NOT covered by the instrument warranty
CAUTION! Never touch any of the optic mirrors, filters, or cables or
their housing, or manifold. The optics are extremely delicate, and
critical to the function of the instrument.
CAUTION! Do not touch or loosen any screws or parts other than those
specifically designated in the instructions. Doing so could cause
misalignment and possibly void warranty.
54 0112-0115 F
Page 55

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
DRAWER
Moving a SpectraMax Multi-Mode Microplate Reader
If you need to relocate a SpectraMax Multi-Mode Microplate Reader,
follow these steps.
WARNING! The instrument weighs approximately 36 pounds
(16.4 kilograms). To avoid injury, it is recommended that two
people lift the instrument together, using proper lifting
techniques.
1. Remove any microplate from the drawer and then close the
drawer.
2. Turn off the power switch and unplug the power cord from the
source and from the receptacle on the back of the instrument.
3. Depending on the distance that you are moving the instrument,
you may want to repackage the instrument in its original
shipping carton. Otherwise, carry the instrument or place it on a
rolling cart to transport it.
4. Ensure that the new location meets the proper specifications as
described in “Setting Up the Instrument”.
General
Keep the drawer closed when the instrument is not in use. The drawer
can be opened by pressing the button. Always close the
drawer immediately prior to switching the instrument off.
0112-0115 F 55
Page 56

Maintenance
Cleaning
WARNING! BIOHAZARD: Wear gloves during any cleaning
procedure that could involve contact with either hazardous or
biohazardous materials or fluids.
CAUTION! Never clean the inside of the instrument. Cleaning the
interior may cause damage to the instrument.
Periodically, you should clean the outside surfaces of the instrument
using a cloth or sponge that has been dampened with water:
• Do not use abrasive cleaners.
• If required, clean the surfaces using a mild soap solution diluted
with water or a glass cleaner and then wipe with a damp cloth or
sponge to remove any residue.
• Do not spray cleaner directly onto the instrument.
If needed, clean the microplate drawer using a cloth or sponge that has
been dampened with water.
Should fluids spill in the drawer area (when the drawer is out), they are
directed to a tray at the bottom of the instrument, from which they exit
to the bench or counter beneath the instrument. Wipe up any spills
immediately.
Do not allow excess water or other fluids to drip inside the instrument.
56 0112-0115 F
Page 57

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Cleaning the Fan Filter
The fan filter on the bottom of the instrument requires periodic
cleaning. The frequency of cleaning depends on how dusty your
particular lab is and could range from once a month to once every six
months.
1. Turn power to the instrument OFF and then remove the power
cord and cables from the back of the instrument.
2. Remove any plate or adapter from the instrument drawer. Turn
the instrument over so that it rests flat on the bench.
3. Pop the black fan cover off and remove the filter.
4. Clean the filter by blowing clean, canned air through it or by
rinsing it—first with water and then with alcohol—and allowing it
to dry completely.
5. Place the clean, dry filter over the fan and replace the black
cover.
6. Turn the instrument back over. Reconnect the power cord and
cables to the instrument.
Changing the Fuses
Fuses burn out occasionally and must be replaced.
If the instrument does not seem to be getting power after switching it
on (the LCD shows no display):
• Check to see whether the power cord is securely plugged in to a
functioning power outlet and to the receptacle at the rear of the
instrument.
If power failed while the instrument was already on:
• Check that the power cord is not loose or disconnected and that
power to the power outlet is functioning properly.
If these checks fail to remedy the loss of power, follow the steps listed
below to replace the fuses. Spare fuses (two U.S. and two metric) are
shipped with the instrument. The U.S. and metric fuses are identical
except for physical size. They may be taped to the back of the
instrument.
If you no longer have spare fuses, you may obtain new ones from
Molecular Devices (part numbers: 4601-0013 for U.S., 4601-0014 for
metric) or from a local hardware store. Make sure fuses are rated
SLOWBLOW (U.S.: 4-amp time-delay; metric: 4-amp, 5 x 20 mm,
time-delay).
0112-0115 F 57
Page 58

Maintenance
To change fuses
1. Switch power to the instrument off and then remove the power
cord from the outlet and from the instrument power cord
receptacle.
2. Remove the computer cable (if connected) from the back of the
instrument.
3. Turn the instrument around for easy access to the rear panel.
4. On the left-hand side of the rear panel (viewed from the back) is
the power switch, fuse box, and power cord receptacle. As
shown in the figures below, press to the left of the black plastic
cover of the fuse box to release it. Pull the fuse box cover away
from the instrument. The fuse box will begin to slide forward.
5. Continue gently pulling the fuse box forward until it is free of the
instrument.
Figure 5-1 Prying open the fuse box cover.
6. When removed, the fuse assembly contains two fuses. Once the
fuse box is out, you will see a holder inside containing two fuses.
Pull the fuse holder out of the box (see Figure 5-2).
58 0112-0115 F
Page 59

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
7. It is possible that only one of the fuses may have blown.
However, Molecular Devices recommends that you replace both
fuses to ensure continued proper operation. Pull both fuses out
of the holder and discard them.
Figure 5-2 The fuse box and holder with fuses removed.
8. Insert new SLOWBLOW-rated fuses into the fuse holder. Either
end of the fuse may be forward.
9. Insert the fuse holder into the fuse box, making sure that the
fuses face toward the right (toward the tongue on the cover) as
you insert it. Slide the fuse holder all the way into the box.
10. Insert the fuse box into the opening in the instrument, making
sure that the fuses are on the left side (toward the power
receptacle). Press the fuse box into place, making sure the
cover snaps closed.
11. Reconnect the power cord to the instrument and to the wall
outlet and reconnect other cables previously disconnected.
0112-0115 F 59
Page 60

Maintenance
60 0112-0115 F
Page 61

Troubleshooting
This chapter lists error codes that may occur while using the
instrument, followed by their most likely causes and remedies.
Maintenance procedures are described in Chapter 1.
Note: In this user guide, SpectraMax® refers to several SpectraMax
Multi-Mode Microplate Readers including the M3, M4, M5, and M5e.
When a feature or capability applies to only certain readers, this
exception is noted.
For problems with SpectraMax Multi-Mode Microplate Readers that are
not listed here, in the U.S., contact Molecular Devices Technical
Services group at
1-800-635-5577; elsewhere, call your local representative.
WARNING! BIOHAZARD: It is your responsibility to
decontaminate the instrument, as well as any accessories,
before requesting service by Molecular Devices representatives
and before returning the instrument or any components to
Molecular Devices.
6
Opening the Drawer Manually
If an error occurs while the drawer is closed and you need to remove a
microplate, press the DRAWER key.
If the drawer does not open, turn power to the instrument off and then
on again. If the drawer still remains closed, turn the power off and
using your thumbnail, locate the groove in the upper left side wall of
the door. Open the door, and with your index finger, pull the microplate
drawer out of the instrument (do not force the drawer) and remove the
microplate. This action will not harm the instrument, but should only be
taken if the first two options have failed to open the drawer.
If you are still unable to open the drawer, contact your local Molecular
Devices representative.
0112-0115 F 61
Page 62

Troubleshooting
Error Codes and Probable Causes
If a problem occurs during operation that causes an unrecoverable
error, the instrument will stop and an error code number will be shown
in the display on the front panel. To correct the problem, call your local
Molecular Devices representative for assistance.
Error Messages
The LCD displays Fatal Error codes when a situation arises that requires
attention. Any reading in progress will stop.
Warning messages do not stop a reading but are logged in the error
buffer; they indicate a situation that requires attention but is not
sufficient to stop or prevent a reading. Examples of situations that
might cause warning messages are low memory, entries being out of
range, or operations that could result in loss of data. These messages
are generally self-explanatory.
For assistance regarding warning messages, contact your local
Molecular Devices representative.
Table 6-1 SpectraMax
®
error code ranges.
Error Code
Numbers
100 -199 Errors possibly caused by unrecognized commands
200-299 Errors probably due to a main board failure or an error
300-399 Instrument errors due to either a main board failure or
400-499 Errors caused by a motor motion failure. Most of these
500-599 Errors due to failure or improper initialization of the
Possible Causes
being sent from the computer to the instrument.
in the firmware code. Most of these errors require the
assistance of Technical Support.
other system failure. Most of these errors require the
assistance of Technical Support.
errors require the assistance of Technical Support.
instruments non-volatile memory (NVRAM). All of these
errors require the assistance of Technical Support.
Some errors are considered fatal in that if they are detected during
power up, the instrument aborts the power up sequence and displays
“FATAL ERROR” on the LCD panel.
Check the following list to see if there is something that you can do to
change the condition of the instrument to prevent the fatal error. (for
example, closing the cuvette door during the power up sequence
prevents errors 111, 219, 302, and 310).
62 0112-0115 F
Page 63

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
After correcting the problem, leave the instrument on for about five
minutes, turn it off and then back on.
If you continue to get the fatal error message on power up, record the
error message number and contact Molecular Devices Technical
Support or your local representative for assistance.
If the instrument is functioning normally when using SoftMax Pro
Software, no errors should be in the buffer (except error number 100).
For all other error messages (codes not listed here), please contact
your local Molecular Devices representative for assistance.
Table 6-2 Error codes, error messages, and notes about the errors
ERROR
CODE
ERROR MESSAGE NOTES
100-199: Operator Errors
100 command not found Command string not recognized.
101 invalid argument Command Argument not recognized.
102 too many arguments Too many arguments after command.
103 not enough arguments Missing arguments.
104 input line too long Too many characters in the input line.
105 command invalid,
system busy
106 command invalid,
measurement in
progress
107 no data to transfer Inputting transfer when there's no data
108 data buffer full Too many data sets in the buffer. Can
109 error buffer overflow More than 65 errors in the buffer, clear
110 stray light cuvette, door
open?
111 invalid read settings
200 assert failed Firmware error.
Instrument could not perform the give
command because it was busy doing
another task. Example: Request a
wavelength while the monochromator
is in motion.
Instrument could not perform
command because a measurement was
in progress
in the buffer
be caused by setting up a long kinetic
and disconnecting computer, or
SoftMax Pro is preempted by another
application.
the buffer.
Cuvette door open while doing a read.
0112-0115 F 63
Page 64

Troubleshooting
Table 6-2 Error codes, error messages, and notes about the errors
ERROR
CODE
ERROR MESSAGE NOTES
200-299: Firmware Errors
201 bad error number Firmware error.
202 receive queue overflow Caused by external device sending too
203 serial port parity error Parity bit error detected with incoming
204 serial port overrun error Caused by host computer sending too
205 serial port framing error
206 cmd generated too much
output
207 fatal trap Instrument error. Instrument locks up.
208 RTOS error Firmware error.
209 stack overflow Firmware error.
210 unknown interrupt Firmware error.
211 bootup fpga check failure Firmware error.
much data over serial port and ignoring
flow control.
serial data.
much data and ignoring the flow
control signal.
Firmware error.
300-399: Hardware Errors
300 thermistor faulty Unable to read a reasonable thermistor
301 safe temperature limit
exceeded
302 low ref light Not enough light detected to make an
303 unable to cal dark
current
304 signal level saturation During a cuvette read, could be due to
value. Thermistor faulty or
disconnected, Main board problem, or
ambient temperature out of range.
A temperature of over 60°C detected
on one or more of the 4 thermistors.
Temperature will be shut off and
remain off until a successful
completion of power-up reset.
accurate measurement. If doing a
cuvette read, the cuvette door may be
open.
Too much stray light detected on
power-up, faulty or disconnected pre-
amp boards.
cuvette door being open.
64 0112-0115 F
Page 65

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Table 6-2 Error codes, error messages, and notes about the errors
ERROR
CODE
305 reference level
306 plate air cal fail Minimum signal/reference ratio not
307 cuv air ref fail Measurement error during cuvette air
308 stray light Light leak in reading chamber or
309 front panel not
310 PMT over current PMT pre-flash value is outside of
311 PMT auto-range fail When using auto PMT-A setting, PMT
312 gain calibration failed Power-up calibration and check of
313 reference gain check fail Power-up check of the Reference
314 low lamp level warning Lamp check upon power-up failed.
315 can't find zero order On power-up, grating motor could not
316 grating motor driver
317 monitor ADC faulty Error found during periodic check of
318 PMT cal coef check failed PMT calibration measurement is >20%
320 Absorbance boot check
ERROR MESSAGE NOTES
saturation
responding
faulty
failed
During a cuvette read, could be due to
cuvette door being open.
met during plate air calibration.
reference read.
cuvette door open. Could also be a
faulty pre-amp board.
LCD front panel bad or disconnected.
tolerated range.
voltage limit was reached during read.
When using auto PMT-C, even at the
lowest PMT voltage levels, the read had
saturated wells.
signal path gain is out of tolerance.
Could be due to bad or disconnected
pre-amp or excessive stray light.
amplifier's gain out of tolerance. Could
be due to bad or disconnected pre-amp
board or excessive stray light.
find zero-order home position.
Grating motor didn't move to where it
was commanded to in a reasonable
time.
ADC system.
different from previous good value.
Plate or cuvette check failed.
0112-0115 F 65
Page 66

Troubleshooting
Table 6-2 Error codes, error messages, and notes about the errors
ERROR
CODE
ERROR MESSAGE NOTES
400-499: Motion Errors
400 carriage motion error Carriage did not move to either of its
401 filter wheel error Filter wheel did not move to its photo
402 grating error Grating did not move to its photo
403 stage error Stage did not move to its photo
404 shutter error One of the shutters has problems
405 polarization filter error Problem with actuating the polarization
photo interrupts in a reasonable time,
or can't find its photo interrupt.
interrupt in a reasonable time, or can't
find its photo interrupt.
interrupt in a reasonable time, or can't
find its photo interrupt.
interrupt in a reasonable time, or can't
find its photo interrupt.
performing its action.
filter mechanism.
500-599: NVRAM Errors
500 NVRAM CRC corrupt The CRC for the NVRAM data is
501 NVRAM Grating cal data
bad
502 NVRAM Cuvette air cal
data error
503 NVRAM Plate air cal data
error
504 NVRAM Carriage offset
error
505 NVRAM Stage offset
error
506 NVRAM Battery Time to replace the NVRAM battery
507 NVRAM PMT Cal Volts
bad
508 NVRAM PMT Sensitivity
Cal error
corrupt.
Grating calibration data is
unreasonable.
Cuvette air calibration data is
unreasonable.
Plate air calibration data is
unreasonable.
Carriage offset data is unreasonable.
Stage offset data is unreasonable.
(U3).
66 0112-0115 F
Page 67

Specifications
A
SpectraMax® Multi-Mode Microplate Reader Performance Specifications
Thermal specifications for microplates used in the SpectraMax®
Multimode Microplate Reader apply to flat-bottom microplates with
isolated wells. All other microplate specifications apply to standard 96-
well polystyrene flat-bottom microplates.
Note: In this user guide, all references to SpectraMax Multi-Mode
Microplate Readers include the M3, M4, M5, and M5e models. When a
feature or capability applies to only certain readers, this exception is
noted.
Performance specifications for cuvette readings apply only to aqueous
solutions having solute molal concentrations less than 0.4 M.
When pathlength compensation is applied to microplate absorbance
measurements, agreement with cuvette absorbance measurements for
the same solution requires that the solution volume in the microplate
well is between 100 µL and 300 µL.
Technical specifications are subject to change without notice.
Table A-1 Technical Specifications
ABSORBANCE PHOTOMETRIC PERFORMANCE
Wavelength range 200–1000 nm
Wavelength selection Monochromator tunable in 1-nm
Wavelength bandwidth 4.0 nm full width half maximum
Wavelength accuracy ± 2.0 nm across wavelength range
Wavelength repeatability ± 0.2 nm
Photometric range 0.0 to 4.0 OD
Photometric resolution 0.001 OD
Photometric accuracy/linearity,
0–2.0 OD
Photometric precision
(repeatability), 0–2.0 OD
0112-0115 F 67
increments
< ± 1.0% and ± 0.006 OD
< ± 1.0% and ± 0.003 OD
Page 68

Specifications
Table A-1 Technical Specifications (cont’d)
Stray light 0.05% at 230 nm
Photometric stabilization Instantaneous
Photometric drift None — continuous referencing of
monochromatic input
Calibration Automatic before first kinetic read and
before every endpoint reading
Optical alignment None required
Light source Xenon flash lamp (50 Watts)
Average lamp lifetime 1 billion flashes
Photodetectors Silicon photodiode
Endpoint baseline noise (cuvette) ± 0.003 OD @190, 405, 850 nm
Endpoint kinetic noise (cuvette) ± 0.003 OD @190, 405, 850 nm
0.2 mOD/min and 0.2 mOD/min
FLUORESCENCE INTENSITY PERFORMANCE
Sensitivity Top Read
< 5 pM FITC, 1 fmol/200 µL(96),
< 20 pM, 2 fmol/100 µL(384)
Bottom Read
< 20 pM FITC(96)
Sensitivity (cuvette) < 15 pM fluorescein
Wavelength range 250–850 nm
Wavelength selection Monochromators, tunable in 1-nm
increments
Bandwidth (excitation, emission) 9 nm, 15 nm
Number of excitation/emission pairs
4
per plate
6
Dynamic range 10
in 96-well black plates: auto gain
circuitry
System validation Self-calibrating with built-in
fluorescence calibrators
Light source Xenon flash lamp (1 joule/flash)
Average lamp lifetime 2 years normal operation
Detector Photomultiplier (R3896)
68 0112-0115 F
Page 69

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Table A-1 Technical Specifications (cont’d)
FLUORESCENCE POLARIZATION PERFORMANCE
Wavelength range
(M5 and M5e models only)
Wavelength selection Monochromators, tunable in 1-nm
Bandwidth (excitation, emission) 9 nm, 15 nm
Precision < 5 mP standard deviation at 1 nM
400–750 nm
increments
fluorescein in 96 and 384 wells
TIME-RESOLVED FLUORESCENCE PERFORMANCE
Sensitivity (M4, M5, M5e models
only)
Wavelength range 250–850 nm
Bandwidth (excitation, emission) 9 nm, 15 nm
Precision data collection 1–100 flashes; delay of 0–600 µs before
100 fM europium in 96 or 384 wells
(top read)
read; integration time selectable
50–1500 µs
LUMINESCENCE PHOTOMETRIC PERFORMANCE
Sensitivity < 2 fg/well for firefly luciferase in
Wavelength range 250–850 nm
Crosstalk < 0.5% in 96- and 384-well microplates
96- and 384-well top read
PHOTOMETRIC ANALYSIS MODES
Front Panel Operation Single wavelength Absorbance,
Using SoftMax Pro Express data as Absorbance,
%Transmittance, Fluorescence reading
of the cuvette (or test tube)
%Transmittance, Fluorescence,
Luminescence
Single wavelength reading of
microplate and/or cuvette
Multiple wavelength (up to four)
reading of microplate or cuvette
Kinetic and kinetic graphics of
microplate and/or cuvette
Spectral scan (190–1000 nm) of
microplate and/or cuvette
Well scan of microplate using
absorbance or fluorescence intensity
0112-0115 F 69
Page 70

Specifications
Table A-1 Technical Specifications (cont’d)
MEASUREMENT TIME (CALIBRATION OFF)
Microplate read time (endpoint),
Standard read
Microplate read time (endpoint),
Standard read with PathCheck
Pathlength Measurement
Technology
Microplate read time (endpoint,
Speed read
96 wells in 24 seconds (single
wavelength, absorbance)
96 wells in 15 seconds (single
wavelength, fluorescence intensity)
384 wells in 1:57 minutes (single
wavelength, absorbance)
384 wells in 45 seconds (single
wavelength, fluorescence intensity)
96 wells in 2:07 minutes (single
wavelength, absorbance)
384 wells in 7:19 minutes (single
wavelength, absorbance)
96 wells in 18 seconds (single
wavelength, absorbance)
384 wells in 49 seconds (single
wavelength, absorbance)
SCAN SPEED
Cuvette: Normal scan 45*K nm/min (K = wavelength interval)
Cuvette: Speed scan 130*K nm/min
Wavelength repeatability ± 0.2 nm
TEMPERATURE REGULATION
Reading chamber Isothermal when temperature regulation
Range 4°C above ambient to 60°C when
Resolution ± 0.1°C
Accuracy ± 1.0°C for microplate and cuvette
Temperature uniformity at
equilibrium
Chamber warm-up time 15–30 minutes (measured on air) after
Temperature regulation 4 sensors
Drift ± 0.2°C (regulated)
is not enabled
temperature regulation enabled. The
ambient temperature must be > 20°C to
achieve temperature regulation at 60°C.
chamber
± 0.5°C at 37°C
initiation of temperature regulation
70 0112-0115 F
Page 71

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Table A-1 Technical Specifications (cont’d)
Temperature regulation diagnostics Temperature regulation system is
Evaporation Plate lid required to minimize
Recommended microplate Flat-bottom microplates with isolated
Control Front panel reports cuvette chamber
continuously monitored and updated
evaporative cooling
wells and lid
temperature only (temperature for
microplate chamber reported in
SoftMax® Pro Software)
AUTOMIX WITH SOFTMAX PRO
Plate mixing modes Selectable: off, once prior to any
Plate mixing duration Selectable: 0 to 999 seconds
reading, and once prior to and between
kinetic readings
(three-second default)
COMPATIBILITY
Microplates Standard 6- to 384-well flat-bottomed
Cuvettes Standard height (45 mm) cells with
Test tubes 12 x 75 mm test tubes can be used in
microplates. Polystyrene plates for
absorbance wavelengths above 340 nm;
UV-transparent plates for absorbance
readings above 220 nm; quartz plates
for absorbance readings above 200 nm;
low-volume 384-well plates. Use purple
adapter plate only with 96- and 384-well
plates.
10 mm pathlength (12.5 mm x
12.5 mm outside) with minimum inside
width of 4mm (typical for 3mL volume
cells). See
more information.
the cuvette chamber with the test tube
cover.
Cuvettes on page 78 for
GENERAL INSTRUMENT
Display 2-x-20-character backlit LCD
Operating panel 11-key membrane keypad
Self-diagnosis Continuous on-board diagnostics
0112-0115 F 71
Page 72

Specifications
Table A-1 Technical Specifications (cont’d)
Spill control Drawer mechanism and reading
Computer interface 8-pin DIN RS-232 serial
Printer interface Parallel 25-pin to Centronics
Microplates supported All 6- to 384-well and strip-well
chamber assembly protected from
accidental spillage by drainage ports
(double shielding required)
(double shielding required)
microplates, including lids
ROBOTICS AND AUTOMATION
Robot compatible drawer Positioning and plate gripping
Integrated automation interface SoftMax® Pro Software automation
as drawer closes
interface integrated with robot partners.
SpectraMax® Multi-Mode Microplate
Readers and SoftMax Pro Software are
the #1 choice of robotic partners and
robots. Please visit the Molecular
Devices web site for more information:
www.moleculardevices.com.
ENVIRONMENTAL
Operating temperature 15°C to 60°C
Operating humidity 0 to 70%, non-condensing
Storage temperature -20°C to 65°C
PHYSICAL
Size (h x w x d) 8.6" (220 mm) x 22.8" (580 mm)
Weight 36 lb (16.4 kg)
Power consumption < 420 W
Line voltage and frequency 100–240 VAC autoranging, 3.5 A, 50/60
72 0112-0115 F
x 15.3" (390 mm)
Hz
Page 73

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
System Diagrams and Dimensions
Dimensions are shown in inches (millimeters).
Figure A-1 Front view of a SpectraMax ® reader
Figure A-2 Side view of a SpectraMax ® reader
0112-0115 F 73
Page 74

Specifications
Figure A-3 Top view of a SpectraMax ® reader
Common Fluorescence and Luminescence Wavelengths
Values in the following tables are based on the literature. You must
scan the fluorochrome of interest in the SpectraMax M5 or M5e reader
to determine the optimal excitation and emission wavelengths for your
application. Excitation and emission wavelengths listed by fluorochrome
manufacturers are generally determined in methanol and do not reflect
actual values, due to changes in pH, salt content, etc.
74 0112-0115 F
Page 75

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Fluorescence
Fluorophore
HPPA 320 405
4-MeU, NADH, NADPH 355 460
Biotinidinase 355 544
PKU 390 485
Green Fluorescent Protein 390 510
Attophos /Attofluor 444 555
FITC 485 538
Ethidium Homodmer
(DNA)
TRITC, Ethidium Bromide 544 590
Texas Red 584 612
TAMRA 547 580
Tryptophan 280 340
Excitation
Wavelength (nm)
530 620
Emission
Wavelength (nm)
Time-resolved Fluorescence
Fluorophore
Eu-Chelate 360 610
Excitation Wavelength
(nm)
Emission
Wavelength (nm)
Luminescence
Probe
Emerald and Emerald II 542
Sapphire and Sapphire II 461
Ruby 620
0112-0115 F 75
Wavelength
(nm)
Page 76

Specifications
76 0112-0115 F
Page 77

Cables and Accessories
Cables
Molecular Devices recommends that you use high-quality, double-
shielded cables to connect your SpectraMax® reader to the computer.
Choose cables that meet the requirement described in this appendix.
B
Note: In this user guide, all references to SpectraMax Multi-Mode
Microplate Readers include the M3, M4, M5, and M5
a feature or capability applies to only certain readers, this
exception is noted.
e
models. When
Serial Interface Cable
The serial interface cable used to connect the instrument to the
computer is a custom cable designed and built by Molecular Devices.
Please use the cable supplied by Molecular Devices, or contact
Molecular Devices for specific pin-out requirements:
Male DB8 to Female DB9 (custom cable made by Molecular Devices, PN
9000-0149)
USB Adapter Cable
Macintosh computers, and many newer Windows-based computers do
not have a serial port. You can connect a serial cable between these
computers and the instrument using a USB-to-serial adapter.
Molecular Devices has tested many third-party serial-to-USB adapter
cables and has found the Keyspan USA-19HS (Molecular Devices, PN
9000-0938) to be the most reliable. It is the only one we recommend.
Figure B-1 Molecular Devices’ custom serial cable (left) and a serial-to-
USB converter (right).
0112-0115 F 77
Page 78

Cables and Accessories
Accessories
Description Part #
SpectraTest ABS1 Absorbance Validation Test Plate 0200-6117
SpectraTest FL1 Fluorescence Validation Test Plate 0200-5060
Cuvette Absorbance Validation Kit 9000-0161
SpectraPlate—Quartz UV-transparent microplate R8024
Fuse, 4-amp Time Delay 4601-0013
Fuse, 4-amp (5 x 20 mm) Time Delay 4601-0014
Power Cord (US, Canada, Japan, Mexico, India) 4400-0002
Power Cord, EC1 (Germany, France, Scandinavia, Italy,
Korea)
Power Cord, EC2 (UK, Indonesia, Singapore, Malaysia) 4400-0037
Power Cord, AP1 (Australia, Hong Kong, China) 4400-0038
SpectraMax Mouse Pad 9000-0133
Cable, RS-232, 8-pin DIN to 8-pin DIN (instrument to
pre-G3 Macintosh)
Cable, RS-232, 9-pin DIN to 8-pin DIN (instrument to
PC serial port)
Adapter USB-Serial High-Speed (KeySpan adapter;
instrument to USB-only instrument)
Test Tube Cover 2300-0277
4400-0036
9000-0091
9000-0149
9000-0938
Cuvettes
The guidelines for cuvette use in the SpectraMax Multi-Mode Microplate
Readers are the same that apply to any high-quality
spectrophotometer. The user must ensure that the meniscus is
comfortably above the light beam in standard cuvettes and that the
sample chamber in a microcuvette is aligned properly with the beam.
The light beam is 0.625 in (15.87 mm) above the cuvette bottom.
Below are some cuvettes that have been tested. All have an optical
pathlength of 1 cm (10 mm) and standard external dimensions
(12.5 mm x 12.5 mm). Their fill volumes differ only because of their
different internal width and chamber height dimensions.
78 0112-0115 F
Page 79

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Standard and Semi-micro Cuvettes
Note: Several brands are available at Hellma: http://www.hellma-
worldwide.com.)
.
Internal Width Minimum Volume Maximum Volume
10 mm ~ 1.80 mL 4.0 mL
4 mm ~ 0.75 mL 1.4 mL
2 mm ~ 0.40 mL 0.7 mL
Ultra-micro Cuvettes (Hellma)
When ordering, specify the Z-dimension to be 15 mm.
Hellma Cat. No.
Window
Size
Chamber
Volume
Fill
Volume
105.201-QS 2.0 x 5.0 mm 100 µL 120 µL
105.202-QS
105.210-QS
2
2.0 x 2.5 mm 50 µL 70 µL
0.8 mm
5µL 10µL
1
diameter
1.You must put a riser (0.8–1 mm) on cuvette bottom to match the cuvette window to
the beam.
2.You must put a riser (0.8–1 mm) on cuvette bottom to match the cuvette window to
the beam. Gives good qualitative results (i.e. spectral scans), but quantitative results
are impractical because the window is smaller than the beam.
0112-0115 F 79
Page 80

Cables and Accessories
Standard, Semi-micro, and Microcuvettes (Hellma)
Figure B-2 Standard, semi-micro, and microcuvettes.
Standard Semi-micro Micro
Hellma Cat.
No.
Internal
Dimensions
Fill Volume
100 104 105.004 104.002108.002105
10 x 10 4 x 10 4 x 10 2 x 10 2 x 10 2 x 10
4 mL 1.4 mL 600 µL 700 µL 500 µL 300 µL
80 0112-0115 F
Page 81

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
Ultra-micro Cuvettes (Hellma)
Figure B-3 Ultra-microcuvettes.
Hellma Cat. No.
Optical Pathlength
Fill Volume
105.200 105.201 105.202 105.210
10 mm 10 mm 10 mm 10 mm
180 µL 120 µL 70 µL 10 µL
0112-0115 F 81
Page 82

Cables and Accessories
82 0112-0115 F
Page 83

Index
A
absorbance 19
cuvettes for 17
normalized measurements 24
optimizing assays 44
selecting wavelength 42
accessories 78
accessory kit 34
adapter plate, see drawer adapter
anisotropy 28
applications 8
assays
optimizing absorbance 44
optimizing fluorescence 44
Automix 9, 31
B
back panel 18
background constant subtraction 22
background wells 51
Beer-Lambert law 19
computer control 10, 31
computer port 18
control panel 12
cuvette chamber 17
Cuvette Reference 20, 21
cuvettes 17
absorbance 17
fluorescence 17
handling 17
microcuvettes 80
reading 14, 39, 43
semi-micro 78
tested 78
ultra-micro 79
D
data display 10
drawer adapter
installing 36
removing 37
Drawer key 14
drawer, opening manually 61
dynamic range 8
C
E
cables
serial interface 77
USB adapter 77
cleaning fan filter 57
cleaning instrument 56
components 11
0112-0115 F 83
emission cutoff filters 45
selecting 48
emission wavelength, optimizing 48
endpoint reads 30
error codes 62
excitation wavelength, optimizing 47
Page 84

Index
F
fan filter, cleaning 57
fluorescence 24
cuvettes for 17
optimizing assays 44
readings per well 45
selecting wavelengths 42
fluorescence polarization 28
optimizing 51
fuse box cover 18
fuses, changing 57
H
HTRF 8
I
incubator 41
installation 33
instrument
accessories 78
accessory kit components 34
changing fuses 57
cleaning 56
moving 55
setting up 35
troubleshooting 61
K
kinetic reads 30
L
(lambda) keys 13
luminescence 28
dye wavelengths 75
optimizing 52
read times 52
M
maintenance 53
microplates, see plates
MODE key 14
monochromators 8
O
optical density 19
optics 8
organic solvents 54
P
PathCheck Technology
Choosing Water Constant or
Cuvette Reference 22
Cuvette Reference 21
interfering substances 23
Water Constant 21
plates 16
background constants 22
reading 40, 43
supported 9
PMT voltage 45
polarization 28
84 0112-0115 F
Page 85

SpectraMax M3, M4, M5, and M5e Multi-Mode Microplate Readers
power cord 18
power switch 18
printer port 18
R
READ cuvette key 14
read types 30
readings per well 45
REF key 14
reference measurements 20
S
serial interface cable 77
setting up instrument 35
SoftMax Pro 10
spectrum reads 30
Stokes shift 24
system parts 34
U
USB adapter cables 77
W
Water Constant 20, 21
wavelengths
selecting for absorbance 42
selecting for fluorescence 42
well scan reads 31
T
technical support 53
TEMP keys 13
temperature
changing set point 41
controlling from SoftMax Pro 41
regulation 29
test tube cover 18
time-resolved fluorescence 27
fluorophore wavelengths 75
optimizing 27, 50
transmittance 19
troubleshooting 61
turbidity 23
0112-0115 F 85
Page 86

Index
86 0112-0115 F
 Loading...
Loading...