Page 1

pH electrodes are constructed from a special composition
glass which senses the hydrogen ion concentration. This
glass is typically composed of alkali metal ions. The alkali
metal ions of the glass and the hydrogen ions in solution
undergo an ion exchange reaction, generating a potential
difference. In a combination pH electrode, the most widely
used variety, there are actually two electrodes in one body.
One portion is called the measuring electrode, the other the
reference electrode. The potential generated at the junction
site of the measuring portion is due to the free hydrogen
ions present in solution.
The potential of the reference portion is produced by the
internal element in contact with the reference fill solution.
This potential is always constant. In summary, the measuring electrode delivers a varying voltage and the reference
electrode delivers a constant voltage to the meter. The voltage signal produced by the pH electrode is a very small,
high impedance signal. The input impedance requires that it
be interfaced only with equipment with high impedance circuits.
pH Electrode
basics
pH Electrodes
6
www.milwaukeeinst.com
Milwaukee has a wide assortment of pH and ORP electrodes to meet all your specific requirements. Finding
the right electrode for a specific application is a very
important task and in order to solve this selection problem it is important to consider the following:
• Glass body electrode versus Epoxy (plastic)
body electrode: Glass body electrodes stand
higher temperatures (typically 100°C against 80°C
for plastic) and are more resistant to corrosive
chemicals and solvents. They are easier to clean
and are available in different shapes depending on
the application. On the other hand plastic body
electrodes are more rugged and the glass bulb is
better protected.
• Gel filled electrodes versus refillable electrodes:
refillable electrodes last longer since electrolyte
can be changed for repeated usage. The
response is faster due to a greater outflow of electrolyte into the sample and therefore less likely to
clog. Gel filled electrodes require less maintenance and resist to higher pressure.
• Double reference junction versus Single junction reference: Double junction reference elec-
trodes have a longer live and protects the sample
measured from silver contamination from the electrolyte. The Silver wire is more protected and
therefore gets less contaminated. The single junction electrodes normally costs less and are ideal
for general purpose applications
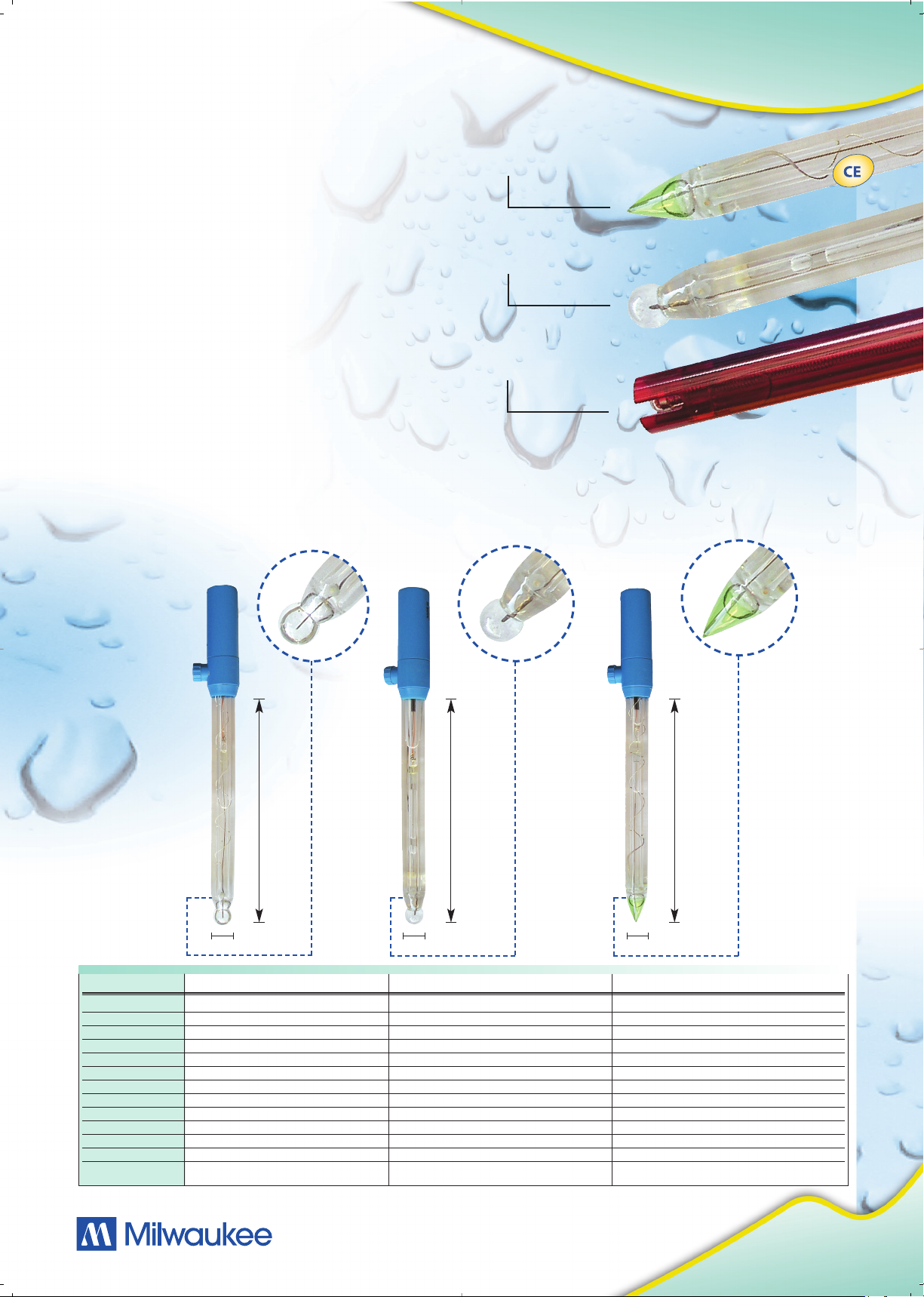
• Conic shaped versus Sphere shaped: The
conic-shaped electrode is easier to clean and to
maintain (ideal for applications such as dairy). Has
a more rugged tip and therefore ideal for penetration. The sphere-shaped has a faster response
time due to the larger surface area on the bulb.
Model
MA919B/1 MA924B/1
Measuring Range 0 to 13 pH ±2000 mV
Temperature Range -5 to 80 °C -5 to 80 °C
Shaft material glass glass
Reference Electrolyte KCL 3.5M KCL 3.5M
Reference Type double Ag/AgCl double Ag/AgCl
Reference Junction open open
Shape of membrane spheric Platinum ring
Max. Pressure 0,1 bar 0,1 bar
Connector type BNC BNC
Cable length coaxial 1 meter coaxial 1 meter
Shaft length 120 mm 120 mm
Diameter 8 mm 8 mm
Application food laboratory food laboratory
8 mm
M
A
9
2
4
B
/1 =1 m
M
A
9
1
9
B
/1 =1 m
8 mm
120 mm
120 mm
Page 2
The pH electrode, due to the nature of its construction,
needs to be kept moist at all times. In order to operate properly, glass needs to be hydrated. Hydration is required for
the ion exchange process to occur. If an electrode should
become dry, it is best to place it in some tap water for a half
hour to condition the glass.
pH electrodes are like batteries; they run down with time and
use. As an electrode ages, its glass changes resistance.
This resistance change alters the electrode potential. For
this reason, electrodes need to be calibrated on a regular
basis. Calibration in pH buffer solution corrects for this
change. Calibration of any pH equipment should always
begin with buffer 7.0 as this is the "zero point." The pH scale
has an equivalent mV scale. The mV scale ranges from
+420 to -420 mV. At a pH of 7.0 the mV value is 0. Each pH
change corresponds to a change of approx. ±60 mV. As pH
values become more acidic the mV values become greater.
pH electrodes have junctions which allow the internal electrolyte solution of the measuring electrode to leak out into
the solution being measured.
pH Electrode
basics
pH Electrodes
7
www.milwaukeeinst.com
Model MA916B/1 - MA916B/3 MA917B/1 MA918B/1
Measuring Range 0 to 13 pH 0 to 14 pH 0 to 12 pH
Temperature Range -5 to 100°C (23 to 212°F) 0 to 100°C (32 to 212°F) -5 to 100°C (23 to 212°F)
Shaft Material glass glass glass
Reference Electrolyte KCl 3.5M + AgCl KCl 3.5M KCl 3.5M + AgCl
Reference Junction ceramic, single ceramic, single ceramic, triple
Reference Type single, Ag/AgCl double, Ag/AgCl single, Ag/AgCl
Shape of membrane spheric spheric conic
Max pressure 0.1 bar 0.1 bar 0.1 bar
Connector Type BNC BNC BNC
Cable length coaxial, 1 or 3 m coaxial, 1 m coaxial, 1 m
Shaft length 120 mm 120 mm 120 mm
Diameter 12 mm 12 mm 12 mm
Application laboratory applications laboratory applications laboratory applications
Glass Conic Tip Sensor
Glass Spheric Sensor
Epoxy Electrode
M
A
9
1
8
B
/1 =1 m
M
A
9
1
7
B
/1 =1 m
M
A
9
1
6
B
/1 =1 m
/3 =3 m
12 mm
120 mm
12 mm
120 mm
12 mm
120 mm
MA916B/1 (will be
replaced by SE100)
Page 3

This junction can become clogged by particulates in the
solution and can also facilitate poisoning by metal ions present in the solution. If a clogged junction is suspected it is
best to soak the electrode in tap water to dissolve the material and clear the junction. When not in use it is best to store
the electrode in either buffer 4.0 or buffer 7.0. Never store an
electrode in distilled or deionized water as this will cause
migration of the electrolyte solution from the electrode.
How long a pH electrode will last will depend on how it is
cared for and the solutions it is used to measure. Typically, a
gel-filled combination pH electrode will last six months to 1
year depending on the care and application.
How long an electrode will last is determined by how well the
probe is maintained and the pH application. The harsher the
system, the shorter the lifespan. For this reason it is always
a good idea to have a back-up electrode on hand to avoid
any system down time. Calibration is also an important part
of electrode maintenance. This assures not only that the
electrode is behaving properly but that the system is operating correctly.
pH Electrode
basics
pH Electrodes
8
www.milwaukeeinst.com
Model MA915B/2 - MA915B/3 MA920B/1 MA991B/1
Measuring Range 0 to 13 pH 0 to 12 pH 0 to 13 pH
Temperature Range -5 to 95°C 0 to 50°C (32 to 122°F) -5 to 100°C (23 to 212°F)
Shaft Material glass PVDF glass
Reference Electrolyte polymer Viscolene KCl 3.5M
Reference Junction ground glass open ceramic, single
Reference Type double, ground glass single, Ag/AgCl single, Ag/AgCl
Shape of membrane spheric conic spheric
Max pressure 3 bar 0.1 bar 0.1 bar
Connector Type BNC BNC BNC
Cable length 2 or 3 m coaxial, 1 m coaxial, 1 m
Shaft length 75 mm 75 mm più di 120 mm
Diameter 12 mm 6 mm 12 mm
Application industrial applications laboratory applications laboratory applications
12 mm
6 mm
75 mm
12 mm
120 mm
M
A
9
9
1
B
/1 =1 m
M
A
9
2
0
B
/1 =1 m
M
A
9
1
5
B
/2 =2 m
/3 =3 m
75 mm
Silver-Free
Electrolyte
Ceramic
Junction
Silver
Silver/Chloride
Reference
Wire
Double Junction
Electrode
Single Junction
Electrode
Outer
Ceramic
Junction
Electrolyte
Containing
Silver
Inner
Ceramic
Junction
Inner Tube
Housing the
Membrane
Sensing Wire
MA991B/1 (will be
replaced by SE120)
Page 4

Temperature compensation: When measuring pH using a
pH electrode the temperature error from the electrode varies
based on the Nernst Equation as 0.03pH/10C/unit of pH
away from pH7. The error due to temperature is a function
of both temperature and the pH being measured.
Temperature compensation can be achieved manually or
automatically. Manual temperature compensation is usually
achieved by entering the temperature of the fluid being
measured into the instruments menu and then the instrument will display a "Temperature Compensated" pH reading.
This means that the temperature is corrected to the value
expected at 25 Deg C. Automatic temperature compensation requires input from a temperature sensor and constantly sends a compensated pH signal to the display. Automatic
temperature compensation is useful for measuring pH in
systems with wide variations in temperature.
pH Electrode
basics
pH Electrodes
9
www.milwaukeeinst.com
Model
MA905B/3 MA913B/3 MA923B/3
Measuring Range 0 to 13 pH 0 to 13 pH ±1999 mV
Temperature Range -5 to 95°C 0 to 60°C (32 to 140°F) 0 to 80°C (32 to 176°F)
Shaft Material Epoxy Epoxy
Reference Electrolyte polymer gel gel
Reference Junction double, Teflon ceramic, single cloth
Reference Type single, Ag/AgCl single, Ag/AgCl
Shape of membrane spheric spheric pH: conic / ORP: Platinum sensor
Max pressure 6 bar 2 bar 3 bar
Connector Type 3/4” NPT - BNC BNC DIN
Cable length 3 m coaxial, 3 m 7-pole, 1 m
Shaft length 120 mm 120 mm 120 mm
Diameter 22 mm 12 mm 14 mm
Application industrial applications water, waste water water, waste water
12 mm
120 mm
12 mm
120 mm
22 mm
120 mm
M
A
9
2
3
B
/3 =3 m
M
A
9
1
3
B
/3 =3 m
M
A
9
0
5
B
/3 =3 m
DIN Connector
BNC Connector
Page 5

Electrodes & Probes
pH, ORP, Conductivity, Dissolved Oxygen
Milwaukee has a wide assortment of pH, ORP, Conductivity
and other specialty sensors to meet all your specific
requirements.
Finding the right electrode for a specific application
is a very important task and in order to solve this
selection problem it is important to consider the
following: electrode body, reference construction
and junction.
Below you will find a list of Milwaukee electrodes and probes with corresponding instruments they are supplied with.
OTHERS ELECTRODES & PROBES
MA811D/1 Conductivity/TDS probe with DIN connector and 1 meter cable
(for SM301 & SM401)
MA811/2 Conductivity/TDS probe with 2 meter cable
(for SMS310)
MA812D/1 Conductivity/TDS probe with DIN connector and 1 meter cable
(for SM302 & SM402)
MA812/2 Conductivity/TDS probe with 2 meter cable
(for SMS410)
MA814DB/1 4-ring Conductivity/TDS/NaCl/Temperature probe
with DIN connector and 1 meter cable
(for Mi170 & Mi180)
MA814D/1 4-ring Conductivity/TDS/NaCl/Temperature probe
with DIN connector and 1 meter cable
(for Mi306)
MA815/2 Conductivity probe with 2 meter cable
(for SMS315)
MA816/2 TDS probe with 2 meter cable
(for SMS415)
MA818/5 In line 4-pin Conductivity probe
with pipe threads at both end
with NTC sensor and 5 meter cable
MA831R Stainless steel Temperature probe
MA840 Polarographic D.O. probe with 3 meter cable
(for SMS600 & Mi605)
MA850 Combination spare probe for pH/Conductivity/TDS
with 1 meter cable
(for SM801 & SM802)
MA851D/1 pH/Conductivity/TDS/Temperature amplified probe
with DIN connector and 1 meter cable
(for Mi805 & Mi806)
SE230 (*) Double junction, gel filled pH electrode with BNC connector,
SE230/2 (*) with 1 or 2 meter cable
SE240 (*) pH/Temperature amplified probe with BNC & RCA connectors
with 1 meter cable
SE310 (*) Double junction, gel filled ORP electrode with platinum sensor,
SE310/2 (*) with BNC connector and 1 or 2 meter cable
SE260 pH/ORP/Temperature amplified probe with DIN connector
and 1 meter cable (for Mi106)
Electrodes
48
www.milwaukeeinst.com
(*) Available from the 1stof September 2010
Page 6

Milwaukee has a wide assortment of pH, ORP,
Conductivity and other specialty sensors to
meet all your specific requirements.
Before selecting an electrode, please consult the table below. The recommended
electrodes are the ones best suited to
each application, however we also ask
you to verify the specifications on pages
6-7-8-9
Special electrodes for specific applications
can also be manufactured upon request.
Electrode Selection Guide
pH, ORP, Conductivity, Dissolved Oxygen
Electrodes
pH
MA905B/3
MA911B/1
SE230
MA913B/3
MA914BR/1
SE240
MA915B/2
MA916B/1
SE100
MA916B/3
MA917B/1
MA918B/1
MA919B/1
MA920B/1
MA923D/1
SE260
MA991B/1
SE120
ORP
MA921B/1
SE310
MA923B/3
MA924B/1
Conductivity
MA818/5
MA813D/1
D.O.
MA840
49
www.milwaukeeinst.com
Applications
Agriculture / Soil testing
Aquarium
Cheese
Dairy products
Emulsions
Environmental, Pollution
Fish farming
Food and beverage (general use)
Galvanizing waste solution
Hi purity water
Heavy duty applications
In-line applications
Laboratory (general use)
Meat
Paints
Paper
Photographic chemicals
Strong acid
Swimming pools
Water supply
Wine processing
 Loading...
Loading...