Millar Mikro-Tip Catheter Pressure Transducer User Manual

World Headquarters
Millar Instruments, Inc.
6001-A Gulf Freeway
Houston, Texas 77023-5417 USA
Phone: 832-667-7000 or 800-669-2343 (in the USA)
Fax: 832-667-7001
Email: info@millarmail.com
Web site: www.millarinstruments.com
Millar Worldwide Distribution
Millar Instruments, Inc. has a network of Authorized Distributors in most countries around the world.
For information on the Millar distributor in your country, please contact the Millar Customer Service
Department at our headquarters in Houston.
European Authorized Representative
Harald Hellmann
DeMeTec GmbH
Co: FMI
Lützelwiesen 5
35428 Langgöns
+49-6403-7874-0
Fax -30
GF: Dipl. Phys. Harald Hellmann
AG Gießen: HRB-Nr. 2604
Email: info@fmigmbh.de
© 2008 Millar Instruments, Inc. All rights reserved
Millar, Mikro-Tip and Sensors.Systems.Solutions. are registered trademarks of Millar Instruments,
Inc.
Products and company names used are the trademarks or trade names of their respective companies.
Models referred to herein are protected by USA and International patents.
M.I. P/N: 004-2184 Rev. --
0086
OBSERVE PRECAUTIONS
FOR HANDLING
ELECTROSTATIC
SENSITIVE DEVICES
Sensors.Systems.Solutions.®
Mikro-Tip
®
Catheter Pressure Transducer
Model SPC-721
Instructions for Use
NONSTERILE PRODUCT
Cardiology catheters MUST be cleaned and sterilized with ethylene oxide gas prior to every use.
Follow directions in this IFU.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.

12
Service Provision
Consult web site below for service information:
www.millarinstruments.com
Millar Limited Warranty
Millar Instruments, Inc. (Millar) warrants that at the time of sale to the original purchaser, the device
was free from defects in both materials and workmanship. For a period of 365 days (1-year) from the
date of original shipment to the original purchaser, Millar will, at no charge and at its option, either
repair or replace any Mikro-Tip transducer found to have been shipped with defects in either
materials or workmanship. Our warranty does not cover damage to the product from alterations,
misuse, abuse, negligence, or accident.
Millar hereby excludes all warranties not herein stated, whether express or implied by operation of
law or course of dealing or trade usage or otherwise, including but not limited to any implied
warranties of fitness or merchantability.
Since handling, storage, cleaning and sterilization of the product, as well as factors relating to patient
diagnosis, treatment, catheterization procedures, and other matters beyond Millar’s control, directly
affect the product and the results obtained from its use, Millar shall not be liable for any incidental or
consequential loss, damage, or expense arising directly or indirectly from the use of this product.
The user shall determine suitability for use of these medical devices for any surgical or clinical
procedure. Therefore, the user accepts these devices subject to all the terms hereof. Further, Millar
makes no warranty regarding device efficacy after three (3) years from the date of manufacture.
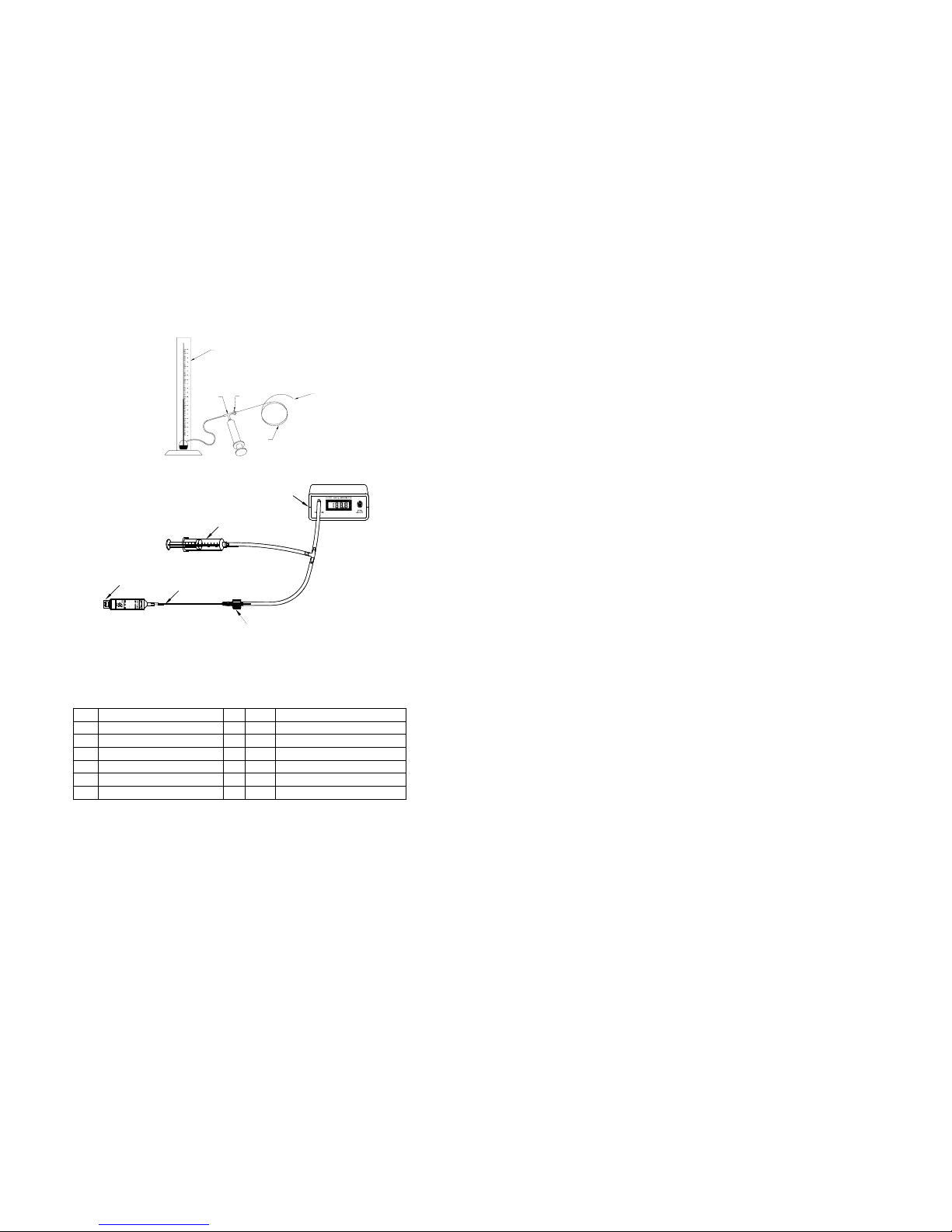
11
14
4
13
8
10
Figure 3b
1. Pressure Sensing Area 8. Manometer
2. Wires to Connector 9. Tee Fitting
3. Vent to Connector 10. Plastic Dome
4. Catheter 11. Pressure Transducer Catheter
5. Silicone Rubber Diaphragm 12. To Recorder
6. Pressure Sensor 13. Connector
7. Adapter 14. Syringe
8
9
11
12
10
Figure 3a
Table of Contents
RECOMMENDED ACCESSORIES ................................................................................................... 1
DEVICE DESCRIPTION ..................................................................................................................... 1
INTENDED USE / INDICATIONS ..................................................................................................... 1
CONTRAINDICATIONS ..................................................................................................................... 2
COMPLICATIONS ............................................................................................................................... 2
WARNINGS ........................................................................................................................................... 2
PRECAUTIONS .................................................................................................................................... 3
ADVERSE EVENTS ............................................................................................................................. 3
MAINTAINING DEVICE EFFECTIVENESS .................................................................................. 3
S
TORAGE .............................................................................................................................................. 3
P
LASTIC DOME FITTING ....................................................................................................................... 3
ROUTINE INSPECTION AND TESTING ........................................................................................ 3
C
ATHETER ............................................................................................................................................ 3
P
RESSURE SENSOR(S) ........................................................................................................................... 3
C
ONNECTOR(S) AND CABLE(S) ............................................................................................................ 4
T
RANSDUCER VERIFICATION AND SETUP ............................................................................................ 4
OPERATING INSTRUCTIONS WHEN USING A MILLAR PRESSURE CONTROL UNIT .. 4
OPERATIONAL NOTES ..................................................................................................................... 5
P
HONOCARDIOGRAM RECORDING ....................................................................................................... 5
H
ANDLING PRECAUTIONS FOR MIKRO-TIP CATHETERS ..................................................................... 6
T
ROUBLESHOOTING AND CORRECTIVE MAINTENANCE ...................................................................... 6
CLEANING ............................................................................................................................................ 6
A
PPROVED CLEANERS .......................................................................................................................... 6
W
ATER RESISTANT CONNECTOR CAPS ............................................................................................... 7
C
LEANING PROCEDURE ........................................................................................................................ 7
M
ETHOD OF STERILIZATION FOR CATHETERS AND EXTENSION CABLES ........................................... 8
E
THYLENE OXIDE STERILIZATION CYCLE PARAMETERS .................................................................... 8
SENSOR SPECIFICATIONS .............................................................................................................. 9
SCHEMATICS ..................................................................................................................................... 10
FIGURES .............................................................................................................................................. 10
SERVICE PROVISION ...................................................................................................................... 12
MILLAR LIMITED WARRANTY ................................................................................................... 12
 Loading...
Loading...