Page 1
© 2016 Midmark Corp. | 60 Vista Drive Versailles, OH 45380 USA | 1-800-643-6275 | 1-937-526-3662 |
TP200 20-42-FO-00012 Rev A1 C2169
English
Español
Français
003-2302-99 Rev FA4 (12/17/18)
Style Q
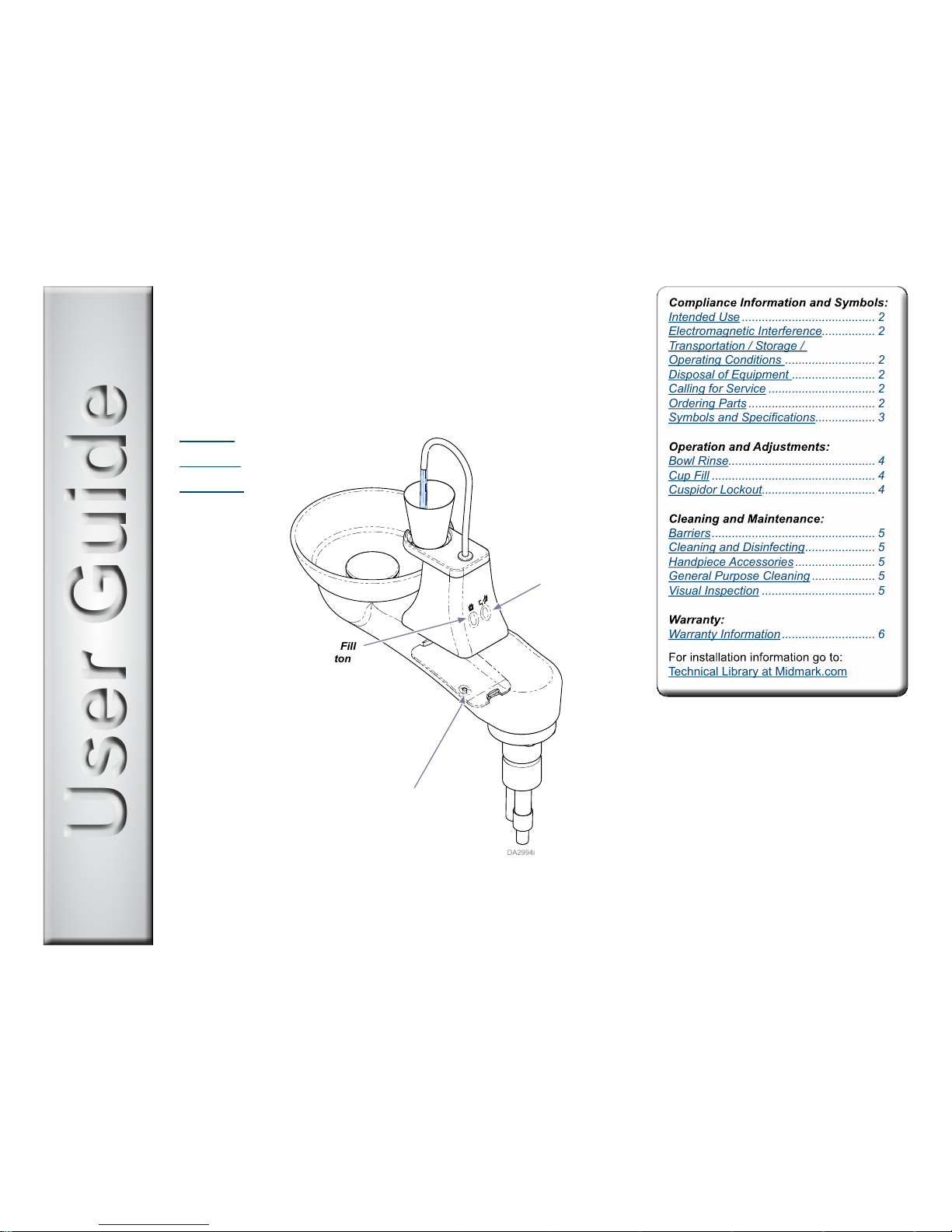
User GuideUser Guide
Elevance® Cuspidor
Cup Fill
Button
Bowl Rinse
Button
Program
Button
Intended Use ........................................ 2
Electromagnetic Interference................ 2
Transportation / Storage /
Operating Conditions ........................... 2
Disposal of Equipment ......................... 2
Calling for Service ................................ 2
Ordering Parts ...................................... 2
Symbols and Specications.................. 3
Bowl Rinse............................................ 4
Cup Fill ................................................. 4
Cuspidor Lockout.................................. 4
Barriers ................................................. 5
Cleaning and Disinfecting ..................... 5
Handpiece Accessories ........................ 5
General Purpose Cleaning ................... 5
Visual Inspection .................................. 5
Warranty Information ............................ 6
Technical Library at Midmark.com
Intended Use ........................................ 2
Electromagnetic Interference................ 2
Transportation / Storage /
Operating Conditions ........................... 2
Disposal of Equipment ......................... 2
Calling for Service ................................ 2
Ordering Parts ...................................... 2
Symbols and Specications.................. 3
Bowl Rinse............................................ 4
Cup Fill ................................................. 4
Cuspidor Lockout.................................. 4
Barriers ................................................. 5
Cleaning and Disinfecting ..................... 5
Handpiece Accessories ........................ 5
General Purpose Cleaning ................... 5
Visual Inspection .................................. 5
Warranty Information ............................ 6
Technical Library at Midmark.com
Page 2
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
English - 2
Disposal of Equipment
At the end of this product’s life, the unit, accessories and other consumable
goods may be contaminated from normal use. Consult local codes and ordinances for proper disposal of this equipment and other consumable goods.
Transportation / Storage / Operating Conditions
Transportation / Storage Temperature: ...........23°F to 100°F (-5°C to 38°C)
Relative Humidity:........................................ 10% to 90% (non-condensing)
Atmospheric Pressure: ................. 7.2 PSI to 15.3 PSI (50 kPa to 106 kPa)
Operating Temperature Range: ...................... 59°F to 95°F (15°C to 35°C)
Electromagnetic Interference
Midmark dental operatory components are designed and built to minimize electromagnetic interference with other devices. However, if interference is noticed
between another device and this operatory, remove the interfering device from
room and / or plug product into an isolated circuit.
Compliance Information and Symbols
Ordering Parts
The following information is required when ordering parts:
Serial number & model number
Part number for desired part
(Refer to Exploded Views & Parts at midmark.com)
Non-warranty parts orders may be faxed to Midmark using the Fax Order
Form in the back of this manual.
For warranty parts orders, call Midmark’s Technical Service Department
with the required information.
Hours: 8:00 am to 5:00 p.m. EST (Monday thru Friday)
Phone: 1-800-Midmark
Calling For Service
Direct all service inquiries to your authorized Midmark dealer. When calling for service, you must provide the following information:
Model / serial number
Date of purchase
Symptom(s) of malfunction
Intended Use
Midmark instrument delivery systems are intended to provide dental professionals with air, water, and suction along with low-voltage electricity to operate
dental handpieces, syringes, and accessoreis during dental examinations and
treatments.
Caution
Federal law restricts this device to sale by or on
the order of a licensed dental practitioner.
WARNING
Do not modify this equipment without authorization
of the manufacturer.
Page 3
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
English - 3
Specications
Electrical Rating for Elevance
Chair:
See power supply output (153941 ...)
Complies to the applicable
requirements of:
ES/IEC/EN 60601-1, CAN/CSA C22.2 No. 60601-1
(Safety Standards), EN/IEC 60601-1-2
(EMC Standards)
Air Supply Requirements: Maximum 80-100 PSI (5.52 - 6.9 bar)
Water Supply Requirements: Maximum 30 - 50 PSI (2.1 -3.4 bar)
Classications:
Class I, Type B Applied Part, Ordinary Equipment
(IPX0), Continuous Operation
Certications: Midmark Corporation..........ISO-9001
Equipment Alert
Indicates a potentially hazardous situation which
could result in equipment damage if not avoided.
Caution
Indicates a potentially hazardous situation which may
result in minor or moderate injury if not avoided. It may
also be used to alert against unsafe practices
NOTE
Amplifies a procedure, practice, or condition.
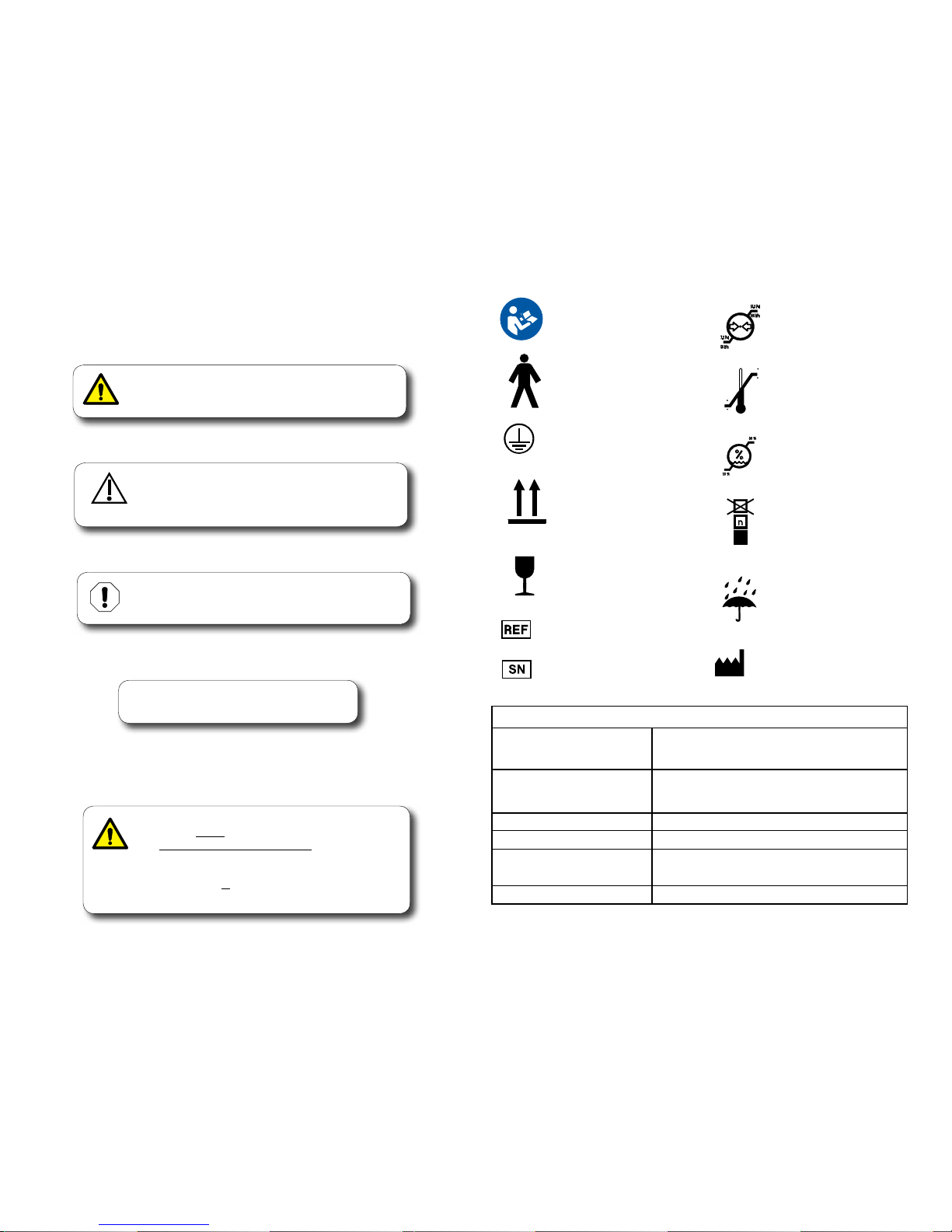
Symbols and Specications
These symbols may appear on your equipment and/or in the manuals.
Warnings and cautions are provided in the manuals where applicable.
WARNING
Indicates a potentially hazardous situation which
could result in serious injury if not avoided.
Temperature
Limit
Pressure
Limit
Humidity
Limit
Refer to instruction
manual/booklet
Keep Dry
Maximum stacking
height (Refer to “n”
number on package.)
Fragile
Proper Shipping
Orientation
Protective
Earth Ground
Type B,
Applied Part
100 F
38 C
23 F
-5 C
WARNING
Equipment is not suitable for use in the presence
of a flammable anesthetic mixture with oxygen, air,
or nitrous oxide.
Clarification: Equipment is suitable for use in the presence
of oxygen, air, or nitrous oxide.
Manufacturer
Catalogue Number
Serial Number
Page 4
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
English - 4
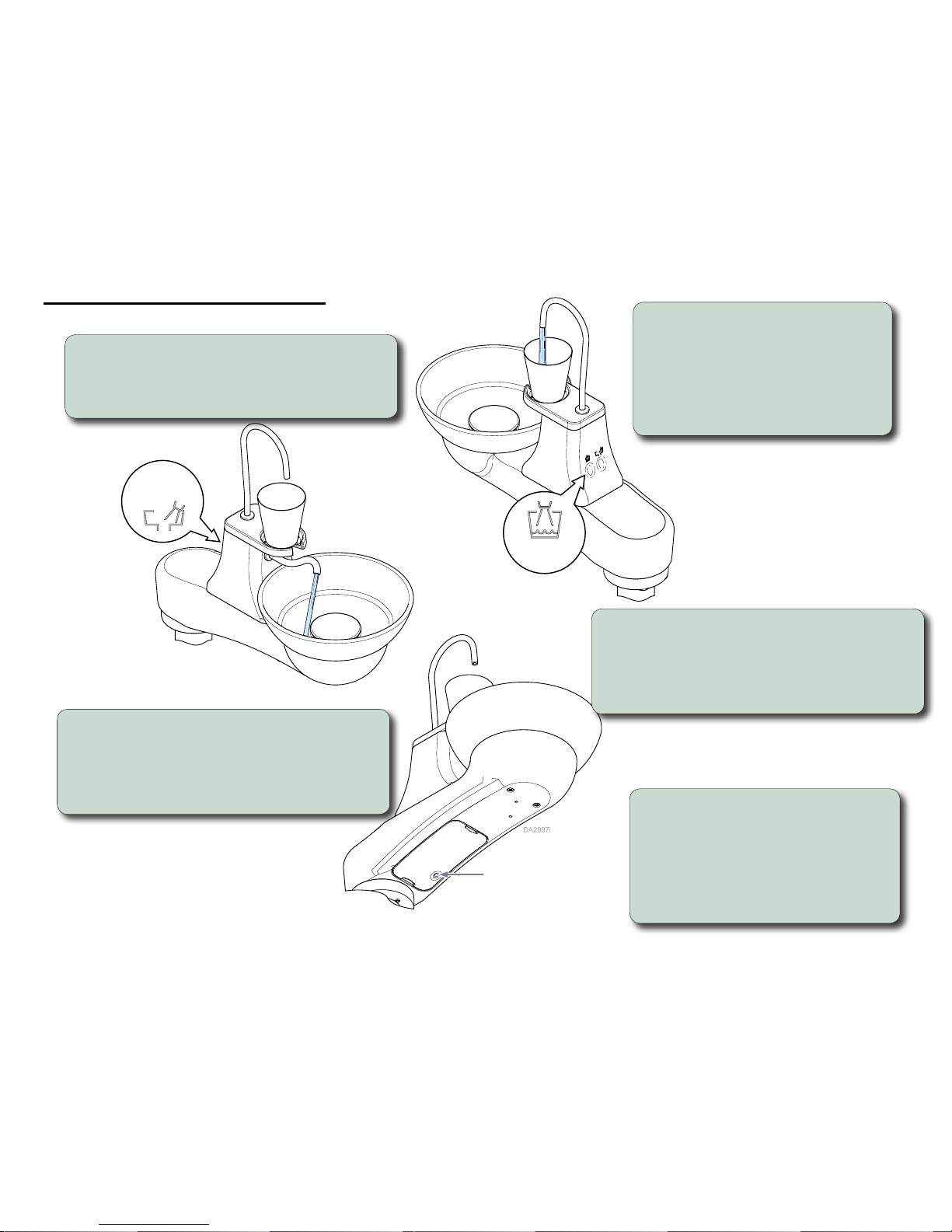
Operation and Adjustments
Cup Fill
Button
Bowl Rinse
Button
Program
Button
Program a new time for the cup fill function...
A) Press and release program button.
Wait to hear a beep to confirm it is in program mode.
B) Press and hold cup fill button for the desired amount
of cup fill time.
C) Listen for two beeps to confirm the program.
Cup fill...
Press cup fill button to activate the cup fill
function. Press and hold button after a cup
fill to “top off” cup.
Note: The default cup fill function allows water to run
for 5 seconds then stops.
Note: The “top off” function remains active for only 60
seconds after the cup fill function.
Program a new time for the bowl rinse function...
A) Press and release program button.
Wait to hear a beep to confirm it is in program mode.
B) Press and hold the bowl rinse button for the desired
amount of bowl rinse time.
C) Listen for two beeps to confirm the program.
Cuspidor Lockout...
Tap program button three times to activate
cuspidor lockout. Listen for a beep to confirm
that the lockout is active.
Tap program button three times to inactivate
cuspidor lockout. Listen for two beeps to
confirm the lockout is inactive.
Bowl rinse...
Press bowl rinse button to activate the bowl rinse function.
Note: The default bowl rinse function allows water to run for 15 seconds
then stops.
Page 5
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
English - 5
Barriers
Midmark recommends the use of disposable barriers on all clinician controls
that may be in contact with clinician hands and ngers during dental procedures. The use of barriers signicantly reduces the need for chemical clean-
ers, thus prolonging the life of the equipment.
Only use barrier material that is intended for use with dental equipment.
Midmark recommends the use of an FDA market-cleared barrier such as Pinnacle Cover-all™. Follow barrier manufacturer instructions for proper use of
these products.
Cleaning and Disinfecting
In addition to the use of barriers Midmark recommends the use of an EPA registered and FDA market-cleared cleaner/disinfectant such as Cavicide™ to be
used on all clinician controls or surfaces that may come in contact with dental
instruments during dental procedures.
Follow cleaner/disinfectant manufacturer
instructions for proper use of the product.
Care should be taken to avoid excessive
application and pooling of liquids.
Handpiece Accessories
Only use dental handpiece accessories with the delivery system that are FDA
market-cleared and refer to manufacturer’s instructions for proper cleaning
and disinfecting. Either an autoclavable syringe tip or a single use disposable
syringe tip may be used.
Cleaning, Disinfecting and Maintenance
Equipment Alert
AUTOCLAVABLE SYRINGE TIP STERILIZATION
The autoclavable syringe tips supplied with the delivery system
must be sterilized prior to use with each patient, including initial use. Be sure to
thoroughly rinse and clean syringe tips prior to sterilization, any debris may reduce
the effectiveness of the sterilization. Recommended sterilization process is steam
autoclave. Recommended parameters are 125°C (250°F) and 106 kPa (15 PSI) for 40
minutes at temperature and pressure.
General Purpose Cleaning
For general purpose cleaning, use cleaners that are appropriate for the
situation, such as warm water and mild detergents, or a 10% solution of
bleach with water.
Visual Inspection
After cleaning, visually inspect the product for deterioration of covers and
touch pads. Do not use the delivery system if excessive discoloration,
cracking, or other signs of wear are noticeable (see Calling for Service
instructions).
Cleaning and Disinfecting Assistance
For assistance with cleaning and disinfecting instructions contact the
Midmark Technical Service Department at 1-800-Midmark, it is helpful to
provide the delivery system model number and serial number when asking
for assistance.
Additional information is available from the organizations listed below:
• Organization for Safety & Asepsis Procedures:
http://www.osap.org
• American Dental Association:
http://www.ada.org
• Dept. of Health & Human Resources
Centers for Disease Control & Prevention (CDC):
http://www.cdc.gov
• European Dental Association:
http://www.eda-eu.org
Read all labels
carefully!
Page 6

© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
English - 6
Warranty Information
Midmark Limited Warranty - Dental Products
SCOPE OF WARRANTY
Midmark Corpor ation (“ Midmark”) warrants to the original retail purchaser that it will at Midmark's
option repair or replace componentsof the dental productsmanufactured byMidmark (except for
componentsnot warranted under "Exclu sions" ) that are defectivein materialor workmanship under
normal use andse rvice. Midmark'sobligation under this limited warr anty is limited to the repair or
replacement of the applicable components. This limited warranty shallonly apply to defectsthat are
reported to Midmark within the applicablewarranty period and which, uponexamination byMidmark,
prove tobe defective. This warranty extendsonly to the fir st retail purchaser of a product and isnot
transferable or assignable. Replacement components or products may beused and/or refurbished
componentsor products,pr ovided they are of like qualityand specificationsas new components or
products.
APPLICABLE WARRANTY PERIOD
The applicable warranty period, measured from thedate ofdelivery to the original user, shall be as
follo ws: EffectiveM ar ch 1, 2018 these applicable warranty periods are measured from thedate of
invoice to the original user, shall be asf ollows:
1. OPERATORY PRODUCTS
a. Five (5) years for all products(except for the items in (b) through (e)).
b. Two (2) years for upholstery (chairs andst ools) .
c. “KIN K-VALVE” module carriesa ten (10) year warranty.
d. The originalligh t bulb on a new light carries a one(1) year warranty.
e. Accessor ies not manufactured byMidmark are excluded includingbut not limited to Bien Air
handpiecesystems, Dentsply Cavitron scaler, Satelecsca ler and curinglight , and Sopro
cameras.
2. ORAL SURGERY PRODUCTS are warranted for aperiod ofone (1) year.
3. STERILIZER PRODUCTSar e warranted for aper iod of one (1) year.
4. ULTRASONIC CLEANER PRODUCTSare warranted for a period of two (2) years.
5. AIR AND VACUUM PRODUCTS
a. Power Air® oil-le ss compressors – Five (5) years or 3,500 hoursof use, whichever occurs first.
b. PowerVac® andPower Vac® G dry vacuums– Five (5) years or 10,000 hoursof use,
whichever occur s first (except thatthe vacuum pump warranty term is ten (10) years or 20,000
hours ofuse, whichever occurs first).
c. Cla ssic Series®wet-r ing vacuums– Five (5) years or 10,000 hoursof use, whichever occurs
first.
d. PowerMax surgical suctio n – Two (2) years.
e. Hg5 SeriesAmalgam Separator - One (1) year.
(f) Midmark manufactured accessor ies - One
(1) year.
6. SYNTH ESIS™ DENT AL CASEWORK AND ARTIZAN® EXPRESSIONS PRODUCT
a. Five (5) years for all productsand components includingdoor and drawer fronts, casters and
slides, except for the items in (b), (c) and (d).
b. Three (3) yearsfor electrical components such as ta sk lights/LED light s, cord s, controlsand
accessories.
c. Two (2) years for slidin g tra ck monitor mount and componentsand upholstery.( d) One (1)
year for countertops andr esin, including accessorie s.
7. IMAGING PRODUCTSar e warranted for aper iod of two (2) years except for the Clear Visio n CR
reader which is warranted for aper iod ofone (1) year.
8. MIDMARK Replacement Parts and Accessor ies carrya ninety (90) day warranty
EXCLU SIONS
Th is warranty does not cover and Midmark shallnot be liable for thefollowing;
1.
defects,damage or other conditions caused, in whole or in part, by misuse, abuse,
negligence, alteration, accident, freight damage,negligentstorage, tamperingor fa ilur e to
seekand obtain repair or replacement in a tim ely manner;
2.
products wh ich are not installed, used, andpr operly cleaned andmaintained asrequired or
recommended in the Midmark"Installation" and/or "Installation/Operation Manual" for the
applicable product, including the specifiedstructural and operationalenvironment conditions
and electricalpower requirements;
3.
products considered to be of a consumableor ste rile nature;
4.
accessories or parts notmanufactured byMidmark;
5.
charges by anyone for adjustments, repairs, replacement parts,in sta llatio n or other work
performed upon or in connection with such productsw hich are not exp re ssly authorized in
wr iting in advance by Midmark
6.
costs and expenses of routinemaintenance and cleaning;
7.
representations and warranties made byany person or entity other than Midmark;
8.
matchingof color, grainor texture exceptto comm er cially acceptable standards;
9.
changesin color caused bynatur al or ar tificia l light;
10.
custom manufactured products;
11.
alterationsor modifications tothe product byany person or entityother than Midmark;and
12.
Products that wouldother wise by covered under Sections1 and 2 of this limited warranty, but
are acquired: (i) from a person or entity thatis not Midmark or one of its authorizeddealers;
or (ii) from a Midmark dealer that is not authorized tose ll the product at issue in the
geographic territorywher e the purchaser islocated,or is not authorized to sell the productat
issue with in the medical, animal
health or dental market, asthe case may be, in which
purchaser intends to use the product.
EXCLU SIVE REM EDY; CONSEQUENTIAL DAMAGES DISCLAIMER
MIDMAR K'S ONLY OBLIGAT ION UNDER TH IS LIMITED WARRANTYIS THEREPAIR OR
REPLACEMENT OF DEFEC TIVE PARTS. MIDMARK SHALL N OT BE LIABLE FOR AND HER EBY
DISCLAIMS ANY DIRECT, SPECIAL, INDIRECT, INCIDENTAL,EXEMPLARY OR
CONSEQUEN TIAL DAMAGES OR DELAYS, INCLUDING,BU T NOT LIMITED TO, DAMAGES
FOR LOSS OF PROFITSOR INCOME,LOS S OF USE, DOWNTIME,COVER AND EMPLOYEE
OR INDEPENDENT CONTRACTORWAGES, PAYMENTS AND BENEFITS.
WARRANTY DISCLAIMER
THIS LIMITED WARRANTY IS MIDMARK'S ONLY WARRANTY AND IS IN LIEU OF ALL OTHER
WARRAN TIES, EXPRESS OR IMPLIED.MIDMARK MAKES NOI MPLIED WARRAN TIES OF ANY
KIND INCLUDINGANY IMPLIED WARRAN TIES OF MERCH ANT ABILITY OR FITNESS FOR A
PARTICULAR PUR POSE. THIS WARRANTYIS LIMITED TOTHE REPAIR OR REPLACEMENT
OF DEFECTIVE PARTS.
STATUE OF LIMIT ATIONS
No action my bebrought against Midmark for breach of th is limited warranty, or implied warranty, if
any, or for anyother claim ar ising out of or relatingto theproducts, more than ninety (90) days
follo wing expiration ofthe limited warranty period.
Page 7

© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
English - 7
Page 8

© Midmark Corporation 2011
Midmark Corporation
60 Vista Drive
Versailles, OH 45380 USA
1-800-643-6275
1-937-526-3662
Page 9

Botón de
llenado
del vaso
Botón de
enjuague
de la taza
Botón de
programación
Guía del usuarioGuía del usuario
© 2016 Midmark Corp. | 60 Vista Drive Versailles, OH 45380 USA | 1-800-643-6275 | 1-937-526-3662 | midmark.com
TP200 20-42-FO-00012 Rev A1 C2169
Escupidera Elevance
®
Indicaciones de uso .............................. 2
Interferencias electromagnéticas.......... 2
Condiciones de transporte,
almacenamiento y funcionamiento .... 2
Cómo deshacerse del equipo ............... 2
Contactar con el Servicio Técnico ........ 2
Cómo pedir piezas de repuesto............ 2
Símbolos............................................... 3
Especicaciones ................................... 3
Enjuague de la taza .............................. 4
Llenado del vaso .................................. 4
Bloqueo de la escupidera ..................... 4
Barreras ................................................ 5
Limpieza y desinfección ....................... 5
Accesorios del mango .......................... 5
Limpieza general .................................. 5
Inspección visual .................................. 5
Ayuda sobre limpieza y desinfección ... 5
Alcance de la Garantía ......................... 6
“Technical Library” en Midmark.com
Indicaciones de uso .............................. 2
Interferencias electromagnéticas.......... 2
Condiciones de transporte,
almacenamiento y funcionamiento .... 2
Cómo deshacerse del equipo ............... 2
Contactar con el Servicio Técnico ........ 2
Cómo pedir piezas de repuesto............ 2
Símbolos............................................... 3
Especicaciones ................................... 3
Enjuague de la taza .............................. 4
Llenado del vaso .................................. 4
Bloqueo de la escupidera ..................... 4
Barreras ................................................ 5
Limpieza y desinfección ....................... 5
Accesorios del mango .......................... 5
Limpieza general .................................. 5
Inspección visual .................................. 5
Ayuda sobre limpieza y desinfección ... 5
Alcance de la Garantía ......................... 6
“Technical Library” en Midmark.com
English
Español
Français
003-2302-99
Page 10

Cómo deshacerse del equipo
Al nal del ciclo de vida del producto, tanto la unidad como sus accesorios y
otros consumibles podrían estar contaminados por efecto de su uso habitual.
Consulte la normativa local sobre eliminación de residuos para saber cómo
deshacerse del equipo y otros consumibles.
Condiciones de transporte, almacenamiento
y funcionamiento
Temperatura de transporte
y almacenamiento: ......................................de -5 °C a 38 °C (de 23 °F a 100 °F)
Humedad relativa: ........................................ del 10% al 90% (sin condensación)
Presión atmosférica: ...................... de 50 kPa a 106 kPa (de 7,2 PSI a 15,3 PSI)
Límites de temperatura ambiente: ...............de 15 °C a 35 °C (de 59 °F a 95 °F)
Interferencias electromagnéticas
Los componentes del equipo dental de Midmark están diseñados y construidos
para reducir al mínimo las interferencias electromagnéticas con otros
dispositivos. Sin embargo, si se observan interferencias entre este equipo y otros
dispositivos, saque de la habitación el dispositivo que produce la interferencia
y/o enchufe el producto en un circuito aislado.
Información y símbolos sobre cumplimiento
Cómo pedir piezas de repuesto
Al pedir piezas de repuesto se necesita la siguiente información:
Número de modelo y de serie
Número de parte de la pieza deseada
(Consulte las Partes y vistas desglosadas en midmark.com)
Los pedidos de piezas fuera de garantía pueden hacerse por fax enviando
a Midmark el Formulario de pedido por fax que está en el dorso de este
manual.
Para pedidos de piezas en garantía, llame al Departamento de servicio
técnico de Midmark con la información necesaria.
Horario: de 8:00 am a 5:00 pm, hora de la costa este (de lunes a viernes)
Teléfono: 1-800-Midmark
Contactar con el Servicio Técnico
Todas las solicitudes de servicio técnico se deben dirigir a un distribuidor
autorizado de Midmark. Al solicitar servicio técnico, debe proporcionar la
información siguiente:
Modelo, número de serie
Fecha de compra
Descripción del fallo
Precaución
Las leyes federales de los EE. UU. sólo autorizan la venta de este
dispositivo a odontólogos debidamente certificados o por orden suya.
ADVERTENCIA
No modifique este equipo sin tener autorización del fabricante.
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Español - 2
Indicaciones de uso
Los sistemas de instrumental dental de Midmark suministran a los
profesionales de odontología aire, agua y succión, junto con electricidad de
bajo voltaje para que puedan utilizar las piezas de mano, jeringas y accesorios
durante los exámenes y tratamientos dentales.
Page 11

Símbolos
Estos símbolos pueden aparecer en su equipo o en los manuales. Los avisos de
advertencia y precaución se indicarán donde correspondan en el manual
Alerta del equipo
Indica una situación potencialmente peligrosa que, de
no evitarse, podría provocar daños al equipo.
Precaución
Indica una situación potencialmente peligrosa que, de
no evitarse, puede dar lugar a lesiones leves o moderadas. Podría también usarse para avisar de prácticas riesgosas.
ADVERTENCIA
Indica una situación potencialmente peligrosa que,
de no evitarse, podría ocasionar lesiones graves.
Límite de
humedad
Límite de
presión
Límite de
temperatura
100 F
38 C
23 F
-5 C
Altura máxima de apilamiento (se reere
a “n” número de bultos)
Manténgase seco
Nota
Amplía la información sobre un procedimiento,
una práctica o un error de funcionamiento.
Especicaciones
Clasicación eléctrica del sillón
Elevance:
Nº de modelo de la fuente de alimentación:
153808-001, -002
Cumple con los requerimientos
vigentes de:
ES/IEC/EN 60601-1, CAN/CSA C22.2 Nº 60601-1
(Estándares de seguridad), EN/IEC 60601-1-2
(Estándares de CEM)
Requisitos del suministro
de aire:
Máximo 5,52 - 6,9 bares (80 - 100 PSI)
Requisitos del suministro
de agua:
Máximo 2,1 - 3,4 bares (30 - 50 PSI)
Clasicaciones:
Clase I, parte aplicada Tipo B, equipo ordinario
[IPX0], funcionamiento continuo
Certicaciones: Midmark Corporation..........ISO-9001
ADVERTENCIA
El equipo no se debe usar en presencia de mezclas
anestésicas inflamables que contengan oxígeno,
aire, u óxido nitroso.
Aclaración: el equipo puede utilizarse en presencia de
oxígeno, aire u óxido nitroso.
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Español - 3
Tipo B,
pieza aplicada
Orientación correcta
para el transporte
Frágil
Toma de tierra
de protección
Consulte el manual
de instrucciones o
folleto
Fabricante
Número de serie
Número del catálogo
Page 12

Enjuague de la taza...
Pulse el botón de enjuague de la taza para activar la
función de enjuague de la misma.
Nota: El ajuste predeterminado de la función de enjuague de la taza deja
que el agua corra durante 15 segundos y después se detiene.
Botón de
llenado
del vaso
Botón de
enjuague de
la taza
Botón de
programación
Programa un nuevo tiempo para la función de
llenado del vaso...
A) Pulse y suelte el botón de programación.
Espere hasta que oiga un pitido que confirma que
está en el modo de programación.
B) Mantenga pulsado el botón de llenado del vaso
durante el tiempo deseado de llenado del vaso.
C) Dos pitidos confirman la programación.
Llenado del vaso...
Pulse el botón de llenado del vaso para
activar la función de llenado del vaso.
Mantenga pulsado el botón después del
llenado del vaso para “llenar más” el vaso.
Nota: El ajuste predeterminado de la función de
llenado del vaso deja que el agua corra durante
5 segundos y después se detiene.
Nota: La función de “llenar más” permanece activa
durante solo 60 segundos después de la función
de llenado del vaso.
Programa un nuevo tiempo para la función de
llenado de la taza...
A) Pulse y suelte el botón de programación.
Espere hasta que oiga un pitido que confirma que
está en el modo de programación.
B) Mantenga pulsado el botón de enjuague de la taza
durante el tiempo deseado de enjuague de la taza.
C) Dos pitidos confirman la programación.
Bloqueo de la escupidera...
Presione el botón de programación tres veces
para activar el bloqueo de la escupidera. Un
pitido confirmará que el bloqueo se ha activado.
Presione el botón de programación tres veces
para desactivar el bloqueo de la escupidera.
Dos pitidos confirmarán que el bloqueo se ha
desactivado.
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Español - 4
Funcionamiento y ajustes
Page 13

Limpieza y mantenimiento
Barreras
Midmark recomienda el uso de barreras desechables en todos los controles
clínicos que puedan estar en contacto con las manos y los dedos del doctor
durante los procedimientos dentales. El uso de barreras reduce signicativamente la necesidad de usar limpiadores químicos, y por tanto prolongan la
vida del equipo.
Use sólo barreras de un material indicado para ser usado con equipos dentales. Midmark recomienda el uso de una barrera autorizada por la FDA, como
por ejemplo Cover-all™ de Pinnacle. Siga las instrucciones del fabricante de
las barreras para un uso correcto de estos productos.
Limpieza y desinfección
Además del uso de barreras, Midmark recomienda el uso de un desinfectante/
limpiador registrado por la EPA y aprobado por la FDA, como Cavicide™ para
todos los controles clínicos y supercies que puedan entrar en contacto con
instrumentos dentales durante los procedi-
mientos relacionados.
Siga las instrucciones del fabricante del limpiador/desinfectante para un uso correcto
de estos productos. Hay que tener cuidado
de evitar la aplicación excesiva y la formación de charcas.
Accesorios del mango
Use con el sistema dental únicamente los accesorios del mango aprobados
por la FDA y consulte las instrucciones del fabricante para su adecuada limpieza y desinfección. Puede usar una punta de jeringa desinfectable al autoclave
o una punta de jeringa desechable.
Alerta del equipo
ESTERILIZACIÓN DE LA PUNTA DE LA JERINGA DESINFECTABLE AL
AUTOCLAVE
Las puntas de jeringa desinfectables al autoclave que se adjuntan con el sistema dental
deben esterilizarse antes de su uso con cada paciente, incluido su primer uso. Asegúrese
de enjuagar y limpiar cuidadosamente las puntas de jeringa antes de esterilizarlas, ya
que cualquier suciedad puede reducir la eficacia de la esterilización. El proceso de
esterilización recomendado es el de autoclave de vapor. Los parámetros de temperatura y
presión recomendados son 125 °C (250 °F) y 106 kPa (15 PSI) durante 40 minutos.
Limpieza general
Para limpieza de tipo general, utilice limpiadores adecuados para la situación, como agua templada y detergentes suaves o una solución de agua y
lejía al 10%.
Inspección visual
Después de la limpieza, inspeccione visualmente el producto en busca de
deterioro de cubiertas y paneles de control. No use el sistema dental si
nota decoloración excesiva, grietas u otros signos de desgaste (consulte
las instrucciones en Contactar con el servicio técnico).
Ayuda sobre limpieza y desinfección
Para obtener ayuda con las instrucciones de limpieza y desinfección,
póngase en contacto con el Departamento de servicio técnico de Midmark
llamando al 1-937-526-3662. Es útil proporcionar el número de modelo y el
número de serie del sistema dental al pedir ayuda.
Puede encontrar información adicional en las siguientes organizaciones:
• Organización de Seguridad y Procedimientos de Asepsia:
http://www.osap.org
• Asociación Dental Americana:
http://www.ada.org
• Departamento de Salud y Recursos Humanos de los
Centros para el Control y la Prevención de Enfermedades (CDC):
http://www.cdc.gov
• Asociación Dental Europea:
http://www.eda-eu.org
¡Lea cuidadosa-
mente
todas las eti-
quetas!
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Español - 5
Page 14

Información de la garantía
Midmark Garantía limitada - Productos dentales
ALCANCE DE LA GARANTÍA
Midmark Corporation («Midmark») garantiza al comprador minorista original que reparará o
reemplazará los componentes de los productos dentales fabricados por Midmark (excepto los
componentes no garantizados en «Exclusiones») que contengan materiales defectuosos o fallos de
mano de obra en condiciones normales de uso y servicio. La obligación de Midmark en virtud de
esta garantía limitada se limita a la reparación o la sustitución de los componentes aplicables. Esta
garantía limitada solo se aplicará a los defectos que se notifiquen a Midmark dentro del período de
garantía y que, después de un examen efectuado por Midmark, se compruebe que son
improcedentes. Esta garantía se expide únicamente al primer comprador minorista de un producto y
no es transferible ni asignable. Se pueden utilizar componentes o productos de repuesto o
reacondicionados, siempre y cuando tengan la misma calidad y especificaciones que los
componentes o productos nuevos.
PERÍODO DE VIGENCIA DE LA GARANTÍA
El período de validez de la garantía, a partir de la fecha de entrega al usuario original, será el
siguiente: A partir del 1 de marzo de 2018, estos períodos de garantía aplicables se calcularán a
partir de la fecha de facturación al usuario original y serán los siguientes:
1. PRODUCTOS ODONTOLÓGICOS
a. Cinco (5) años para todos los productos, excepto los artículos indicados en las letras b) a e).
b. Dos (2) años para tapicería (sillas y taburetes).
c. El módulo «KINK-VALVE» tiene una garantía de diez (10) años.
d. La bombilla original de una luz nueva tiene una garantía de un (1) año.
e. Quedan excluidos los accesorios no fabricados por Midmark incluidos, sin limitación, los
sistemas manuales Bien Air, el raspador Dentsply Cavitron, el raspador y la lámpara de
fotopolimerización Satelec y las cámaras Sopro.
2. Los PRODUCTOS DE CIRUGÍA ORAL tienen una garantía de un (1) año.
3. Los PRODUCTOS DE ESTERILIZACIÓN tienen una garantía de un (1) año.
4. Los PRODUCTOS DE LIMPIEZA DE ULTRASONIDOS tienen una garantía de dos (2) años.
5. PRODUCTOS DE AIRE Y VACÍO
a. Los compresores PowerAir
®
sin aceite tienen una garantía de cinco (5) años o 3500 horas
de uso, lo que suceda primero.
b. Los sistemas de vacío PowerVac
®
y PowerVac®G para limpieza en seco tienen una
garantía (5) años o 10 000 horas de uso, lo que suceda primero (excepto para la garantía
de la bomba de aspiración, que es de (10) años o 20 000 horas de uso, lo que ocurra
antes).
c. Las bombas de vacío con funcionamiento por agua Classic Series
®
tienen una garantía de
cinco (5) años o 10 000 horas de uso, lo que suceda primero.
d. Los sistemas de succión quirúrgica PowerMax tienen una garantía de (2) años.
e. Los separadores de amalgama de la serie Hg5 tienen una garantía de (1) año. f) Los
accesorios fabricados por Midmark tienen una garantía de un (1) año.
6. CASOS DENTALES SYNTHESIS™ Y PRODUCTOS ARTIZAN
®
EXPRESSIONS
a. Garantía de cinco (5) años para todos los productos y componentes, incluidas las puertas
y los frontales de los cajones, ruedas y deslizadores, excepto los artículos incluidos en las
letras b), c) y d).
b. Garantía de tres (3) años para los componentes eléctricos, como luces LED y luces para
tareas visuales, cables, controles y accesorios.
c. Garantía de dos (2) años para los montajes deslizantes para monitores, componentes y
tapicería. d) Garantía de un (1) año para encimeras y resinas, incluidos los accesorios.
7. Los PRODUCTOS DE RESONANCIA tienen una garantía de dos (2) años excepto el lector
ClearVision CR, que tiene una garantía de un (1) año.
8. Las piezas de recambio y los accesorios MIDMARK tienen una garantía de noventa (90) días.
EXCEPCIONES
Esta garantía no cubre (y Midmark no es responsable de) lo siguiente:
1. defectos, daños u otras condiciones causadas, en su totalidad o en parte, por mal uso,
abuso, negligencia, alteración, accidente, daños durante el transporte, almacenamiento
negligente, manipulación o incapacidad de solicitar y lograr la reparación o la sustitución
dentro del plazo estipulado;
2. productos que no se hayan instalado, utilizado o limpiado y mantenido adecuadamente tal
y como se indica o se recomienda en la «Instalación» de Midmark y/o el «Manual de
instalación/uso» del producto en cuestión, incluidas las condiciones de entorno estructural
y operativo y los requisitos de alimentación eléctrica;
3. productos considerados de naturaleza consumible o estéril;
4. accesorios o piezas no fabricados por Midmark;
5. facturas de terceros en concepto de ajustes, reparaciones, piezas de recambio,
instalaciones o cualquier otra modificación del producto o relacionada con el mismo que se
hayan realizado sin la autorización previa por escrito de Midmark;
6. costes y gastos de mantenimiento y limpieza rutinarios;
7. declaraciones y garantías hechas por cualquier persona o entidad que no sea Midmark;
8. coincidencia de color, grano o textura, a excepción de las normas comercialmente
aceptables;
9. cambios en el color causados por la luz natural o artificial;
10. productos fabricados a medida;
11. alteraciones o modificaciones del producto efectuadas por cualquier persona o enti dad que
no sea Midmark; y
12. productos que estarían cubiertos, de acuerdo con las secciones 1 y 2, por esta garantía
limitada pero que se hayan adquirido: i) a través de una persona o entidad distinta
a Midmark o sus distribuidores autorizados; o ii) a través de un distribuidor de Midmark que
no tenga la autorización para vender el producto en cuestión en el territorio donde se
encuentre el comprador, o que no tenga la autorización para vender el producto en el
sector médico, veterinario o dental en el que el comprador pretenda usarlo.
RECURSO EXCLUSIVO; DAÑOS EMERGENTES; EXENCIÓN DE RESPONSABILIDAD:
LA ÚNICA OBLIGACIÓN DE MIDMARK EN VIRTUD DE ESTA GARANTÍA LIMITADA ES LA DE
REPARAR O CAMBIAR LAS PIEZAS DEFECTUOSAS. MIDMARK NO SE HACE RESPONSABLE, Y
POR LA PRESENTE, RENUNCIA A CUALESQUIERA DAÑOS DIRECTOS, ESPECIALES,
INDIRECTOS, ACCIDENTALES, EJEMPLARES, CONSECUENTES O DEMORAS, INCLUIDOS, SIN
LIMITACIÓN, DAÑOS POR PÉRDIDA DE GANANCIAS O INGRESOS, PÉRDIDA DE USO, TIEMPO
MUERTO, COBERTURA Y SALARIOS DE EMPLEADOS O DE CONTRATISTAS INDEPENDIENTES,
PAGOS Y BENEFICIOS.
EXENCIÓN DE RESPONSABILIDAD DE GARANTÍA
ESTA GARANTÍA LIMITADA ES LA ÚNICA GARANTÍA DE MIDMARK Y SUSTITUYE A TODAS LAS
DEMÁS GARANTÍAS, EXPRESAS O IMPLÍCITAS. MIDMARK NO OFRECE NINGUNA GARANTÍA
IMPLÍCITA DE NINGÚN TIPO, INCLUIDAS LAS GARANTÍAS IMPLÍCITAS DE COMERCIABILIDAD O
IDONEIDAD PARA UN PROPÓSITO PARTICULAR. ESTA GARANTÍA SE LIMITA A LA REPARACIÓN
O SUSTITUCIÓN DE PIEZAS DEFECTUOSAS.
ESTATUTO DE LIMITACIONES
No podrá interponerse ninguna acción contra Midmark por incumplimiento de esta garantía limitada, de una
garantía implícita, si las hubiere, o por cualquier otra reclamación que surja de o en relación con los
productos, tras los noventa (90) días siguientes al vencimiento del período de garantía limitada.
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Español - 6
Page 15

© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Español - 7
Page 16

003-2302-99
Midmark Corporation
60 Vista Drive
Versailles, OH 45380 USA
1-800-643-6275
1-937-526-3662
www.midmark.com
TP200 20-42-FO-00012 Rev A1 C2169
Page 17

Bouton de
remplis-
sage du
gobelet
Bouton de
rinçage de
la cuvette
Bouton
de programme
Guide de l’utilisateurGuide de l’utilisateur
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
© 2016 Midmark Corp. | 60 Vista Drive Versailles, OH 45380 USA | 1-800-643-6275 | 1-937-526-3662 |
Crachoir Elevance
®
Indications ............................................ 2
Interférences électromagnétiques ........ 2
Conditions de transport, de stockage
et d’utilisation .................................... 2
Mise au rebut de l’équipement ............ 2
Service après-vente.............................. 2
Commande de pièces........................... 2
Symboles .............................................. 3
Rinçage de la cuvette ........................... 4
Remplissage du gobelet ....................... 4
Verrouillage du crachoir ........................ 4
Nettoyage ............................................. 5
Protections............................................ 5
Accessoires de pièce à main ................ 5
Nettoyage général ................................ 5
Contrôle visuel ...................................... 5
Aide au nettoyage et à la désinfection.. 5
Limitation de Garantie .......................... 6
« Technical Library » du site Midmark.com
Indications ............................................ 2
Interférences électromagnétiques ........ 2
Conditions de transport, de stockage
et d’utilisation .................................... 2
Mise au rebut de l’équipement ............ 2
Service après-vente.............................. 2
Commande de pièces........................... 2
Symboles .............................................. 3
Rinçage de la cuvette ........................... 4
Remplissage du gobelet ....................... 4
Verrouillage du crachoir ........................ 4
Nettoyage ............................................. 5
Protections............................................ 5
Accessoires de pièce à main ................ 5
Nettoyage général ................................ 5
Contrôle visuel ...................................... 5
Aide au nettoyage et à la désinfection.. 5
Limitation de Garantie .......................... 6
« Technical Library » du site Midmark.com
English
Español
Français
Page 18

Mise au rebut de l’équipement
À l’issue de la durée de vie du produit, l’unité, les accessoires et d’autres
fournitures peuvent être contaminés à la suite d’une utilisation normale.
Consultez les codes et les réglementations locaux pour vous renseigner sur la
mise au rebut appropriée de l’équipement et autres fournitures.
Conditions de transport, de stockage et d’utilisation
Température de transport et de stockage : ............-5 °C à 38 °C (23 °F à 100 °F)
Humidité relative : ............................................10 % à 90 % (sans condensation)
Pression atmosphérique : ........................ 50 kPa à 106 kPa (7,2 PSI à 15,3 PSI)
Plage de température de fonctionnement : ...........15 °C à 35 °C (59 °F à 95 °F)
Interférences électromagnétiques
Les composants des cabinets dentaires Midmark sont conçus et construits de
façon à minimiser les interférences électromagnétiques avec d’autres dispositifs.
Si des interférences sont néanmoins constatées, il sera nécessaire de retirer de
la pièce l’appareil causant les interférences et/ou de brancher le produit sur un
circuit isolé.
Informations sur la conformité et symboles
Commande de pièces
Les informations suivantes sont requises pour toute commande de pièces :
numéro de série et numéro de modèle
référence de la pièce souhaitée
(reportez-vous aux Vues éclatées et Pièces sur le site midmark.com)
Les commandes de pièces hors garantie peuvent être faxées à Midmark
à l’aide du formulaire de commande par fax qui gure à la n du présent
manuel.
Pour les commandes de pièces garanties, contactez le Service technique
de Midmark en vous munissant des informations requises.
Heures : de 8h00 à 17h00, du lundi au vendredi (heure normale de
l’Est des États-Unis) Téléphone : 1-937-526-3662
Service après-vente
Adressez toutes vos demandes de réparation à votre revendeur Midmark
agréé. Lorsque vous demandez une réparation, vous devez fournir les
informations suivantes :
numéro de modèle / de série
date d’achat
symptôme(s) du dysfonctionnement
Attention
Selon la loi fédérale des États-Unis, ce dispositif ne
peut être vendu que par un dentiste autorisé à exercer
sa profession ou sur son ordonnance.
AVERTISSEMENT
Ne modifiez pas cet équipement sans l’autorisation
du fabricant.
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Français - 2
Indications
Les systèmes de distribution d’instruments Midmark fournissent aux dentistes
de l’air, de l’eau, un système de succion et une alimentation à basse tension
pour utiliser des pièces à main dentaires, des seringues et des accessoires
pendant les procédures d’examens et de soins dentaires.
Page 19

Symboles
Les symboles suivants peuvent apparaître sur votre équipement et/ou dans
les manuels. Des avertissements et mises en gardes sont fournis dans les
manuels, le cas échéant.
Avertissement relatif à l’équipement
Signale un danger potentiel qui, s’il n’est pas évité,
risque d’entraîner des dommages matériels.
Attention
Signale un danger potentiel qui, s’il n’est pas évité,
risque d’entraîner des blessures mineures ou modérées. Il peut
également servir à signaler à l’utilisateur des pratiques dangereuses.
AVERTISSEMENT
Signale un danger potentiel qui, s’il n’est pas évité,
risque d’entraîner des blessures graves.
Limite
d’humidité
Limite de
pression
Limite de
température
100 F
38 C
23 F
-5 C
Hauteur d’empilage
maximale (reportezvous au nombre « n »
sur l’emballage)
Conserver au sec
Remarque
Attire l’attention sur une procédure, une pratique
ou une situation.
Caractéristiques techniques
Caractéristiques électriques du
fauteuil Elevance :
Bloc d’alimentation, n° de modèle :
153808-001, -002
Conforme aux exigences en
vigueur de :
ES/CEI/EN 60601-1, CAN/CSA C22.2 n° 60601-1
(normes de sécurité), EN/CEI 60601-1-2 (normes
CEM)
Exigences relatives à
l’alimentation en air :
5,52 à 6,9 bar maximum (80 à 100 PSI)
Exigences relatives à
l’alimentation en eau :
2,1 à 3,4 bar maximum (30 à 50 PSI)
Classications :
Classe I, partie appliquée sur le patient de type
B, équipement ordinaire (IPX0), fonctionnement
continu
Certications : Midmark Corporation..........ISO-9001
Numéro de série
Numéro de catalogue
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Français - 3
AVERTISSEMENT
Cet équipement ne doit pas être utilisé en présence d’un
mélange anesthésique inflammable à l’oxygène, à l’air ou au
protoxyde d’azote.
Clarification : cet équipement peut être utilisé en présence d’oxygène,
d’air ou de protoxyde d’azote.
Type B,
partie appliquée
sur le patient
Orientation
correcte pour l’expédition
Fragile
Mise à la terre
de protection
Se reporter au manuel/
livret d’instructions
Fabricant
Page 20

Rinçage de la cuvette...
La fonction de rinçage de la cuvette est activée lorsque le
bouton correspondant est pressé.
Remarque : la fonction de rinçage de la cuvette par défaut permet à
l’eau de s’écouler pendant 15 secondes avant de s’arrêter.
Bouton de
remplissage du
gobelet
Bouton de
rinçage de
la cuvette
Bouton de
programme
Pour programmer une nouvelle durée de
remplissage du gobelet...
A) Appuyez brièvement sur le bouton de programme.
Vous entendrez un bip confirmant l’activation du mode
programme.
B) Maintenez appuyé le bouton de remplissage du
gobelet pendant la durée de remplissage du gobelet
souhaitée.
C) Deux bips retentissent pour confirmer le programme.
Remplissage du gobelet...
Appuyez sur le bouton de remplissage du
gobelet pour activer la fonction correspondante. Maintenez appuyé le bouton après
le remplissage du gobelet pour atteindre le
niveau maximal.
Remarque : la fonction de remplissage du gobelet par
défaut permet à l’eau de s’écouler pendant
5 secondes avant de s’arrêter.
Remarque : la fonction de remplissage jusqu’au niveau
maximal ne reste activée que pendant
60 secondes après la fonction de remplissage
du gobelet.
Pour programmer une nouvelle durée de rinçage
de la cuvette...
A) Appuyez brièvement sur le bouton de programme.
Vous entendrez un bip confirmant l’activation du mode
programme.
B) Maintenez appuyé le bouton de rinçage de la cuvette
pendant la durée de rinçage de la cuvette souhaitée.
C) Deux bips retentissent pour confirmer le programme.
Verrouillage du crachoir...
Appuyez trois fois sur le bouton de programme
pour activer la fonction de verrouillage du
crachoir. Un bip retentit pour confirmer que la
fonction de verrouillage est activée.
Appuyez trois fois sur le bouton de programme
pour désactiver la fonction de verrouillage du
crachoir. Deux bips retentissent pour confirmer
que la fonction de verrouillage est désactivée.
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Français - 4
Utilisation et réglages
Page 21

Nettoyage et maintenance
Protections
Midmark recommande d’utiliser des protections jetables sur toutes les commandes cliniques qui peuvent entrer en contact avec les mains et les doigts
des cliniciens lors des procédures dentaires. L’utilisation de protections réduit
considérablement le besoin d’utiliser des nettoyants chimiques, prolongeant
ainsi la durée de vie de l’équipement.
N’utilisez que des matériaux de protection destinés à être utilisés avec du
matériel dentaire. Midmark recommande d’utiliser des protections agréées par
la FDA, comme Pinnacle Cover-All™. Suivez les instructions du fabricant de
protections pour utiliser ces produits correctement.
Nettoyage et désinfection
Outre l’utilisation de protections, Midmark recommande d’utiliser un nettoyant/
désinfectant enregistré auprès de l’EPA et agréé par la FDA, comme Cavicide™, sur toutes les commandes ou surfaces cliniques qui peuvent entrer
en contact avec des instruments dentaires
pendant les procédures dentaires.
Suivez les instructions du fabricant du nettoyant/désinfectant pour utiliser ces produits
correctement. Faites attention à ne pas
mélanger ni appliquer de façon excessive
des liquides.
Accessoires de pièce à main
N’utilisez que des accessoires de pièce à main dentaire agréés par la FDA avec le
système de distribution et consultez les instructions du fabricant pour un nettoyage
et une désinfection corrects. Il est possible d’utiliser soit un embout de seringue
stérilisable à l’autoclave soit un embout de seringue jetable à usage unique.
Avertissement relatif à l’équipement
STÉRILISATION DES EMBOUTS DE SERINGUE STÉRILISABLES À L’AUTOCLAVE
Les embouts de seringue stérilisables à l’autoclave fournis avec le système de
distribution doivent être stérilisés avant leur utilisation sur chaque patient, y compris pour
la première utilisation. Veillez à bien rincer et nettoyer les embouts de seringue avant la
stérilisation, tout débris peut réduire l’efficacité de la stérilisation. Le processus de stérilisation
recommandé est l’autoclave à vapeur. Les paramètres recommandés sont une température de
125 °C (250 °F) et une pression de 106 kPa (15 PSI) pendant 40 minutes.
Nettoyage général
Pour eectuer un nettoyage général, utilisez des nettoyants adaptés à la
situation, tels que de l’eau chaude et des détergents doux, ou une solution
d’eau de Javel à 10 % avec de l’eau.
Contrôle visuel
Après le nettoyage, contrôlez visuellement le produit pour vérier que les
capuchons et blocs tactiles ne présentent aucune détérioration. N’utilisez pas le système de distribution en cas de décoloration excessive, de
ssures ou autres signes d’usure visibles (reportez-vous aux instructions
sous Service après-vente).
Aide au nettoyage et à la désinfection
Pour obtenir de l’aide concernant les instructions de nettoyage et de
désinfection, contactez le Service technique Midmark au 1-937-526-3662, il
peut être utile de fournir les numéros de modèle et de série du système de
distribution lorsque vous sollicitez de l’aide.
Des informations complémentaires sont disponibles auprès des organismes indiqués ci-dessous :
• Organization for Safety & Asepsis Procedures (Organisation
pour les procédures de sécurité et d’asepsie) :
http://www.osap.org
• American Dental Association (Association dentaire américaine) :
http://www.ada.org
• Ministère américain de la santé et des services aux personnes
Centres pour le contrôle et la prévention des maladies (CDC) :
http://www.cdc.gov
• European Dental Association (Association dentaire européenne) :
http://www.eda-eu.org
Lisez
attentivement
les étiquettes !
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Français - 5
Page 22

Renseignements sur la garantie
Garantie limitée Midmark - Produits dentaires
PORTÉE DE LA GARANTIE
Midmark Corporation (« Midmark ») s’engage auprès de l’acquéreur initial à réparer ou à remplacer, à sa
discrétion, les composants des produits dentaires fabriqués par Midmark (hormis les composants non
garantis en vertu des « Exclusions ») qui sont défectueux au niveau du matériel ou de la qualité
d’exécution dans des conditions normales d’utilisation et de service. L’obligation de Midmark au titre de
cette garantie est limitée à la réparation ou au remplacement des composants concernés. La présente
garantie limitée ne s’applique qu’aux défauts signalés à Midmark durant la période de garantie applicable
et qui sont avérés après examen de Midmark. La présente garantie s’étend au seul acquéreur initial d’un
produit et n’est ni transférable, ni cessible. Des composants ou produits de remplacement peuvent être
utilisés, ou encore des composants ou produits rénovés, à condition qu’ils présentent une qualité et des
spécifications identiques à celles des composants ou produits neufs.
PÉRIODE DE GARANTIE APPLICABLE
La période de garantie applicable, qui court à compter de la date de livraison à l’acquéreur initial, est
la suivante : À partir du 1
er
mars 2018, ces périodes de garantie applicables, qui courent à compter
de la date de facturation à l’acquéreur initial, sont les suivantes :
1. PRODUITS OPÉRATOIRES
a. Cinq (5) ans pour tous les produits [excepté les articles signalés aux points b) à e)].
b. Deux (2) ans pour les garnitures (chaises et tabourets).
c. Le module « VANNE BIDIRECTIONNELLE » est garanti pendant dix (10) ans.
d. L’ampoule d’origine sur une nouvelle lampe est garantie pendant un (1) an.
e. Les accessoires non fabriqués par Midmark sont exclus de la garantie, y compris, entre
autres, les systèmes de pièce à main Bien Air, le détartreur Dentsply Cavitron, le
détartreur et la lampe à polymériser Satelec et les caméras Sopro.
2. Les PRODUITS DE CHIRURGIE BUCCALE sont garantis pendant une période d’un (1) an.
3. Les PRODUITS DE STÉRILISATION sont garantis pendant une période d’un (1) an.
4. Les PRODUITS DE NETTOYAGE PAR ULTRASONS sont garantis pendant une période de
deux (2) ans.
5. PRODUITS AIR ET VIDE
a. Compresseurs sans huile PowerAir
®
– Cinq (5) ans ou 3 500 heures d’utilisation, selon la
première occurrence.
b. Aspirateurs secs PowerVac
®
et PowerVac®G – Cinq (5) ans ou 10 000 heures d’utilisation,
selon la première occurrence [à l’exception de la pompe à vide qui est garantie dix (10)
ans ou 20 000 heures d’utilisation, selon la première occurrence].
c. Aspirateurs à anneau humide Classic Series
®
– Cinq (5) ans ou 10 000 heures
d’utilisation, selon la première occurrence.
d. Aspiration chirurgicale PowerMax – Deux (2) ans.
e. Séparateur d’amalgame série Hg5 - Un (1) an. f) Accessoires fabriqués par Midmark - Un
(1) an.
6. MEUBLE POUR CABINET DENTAIRE SYNTHESIS™ ET PRODUIT ARTIZAN
®
EXPRESSIONS
a. Cinq (5) ans pour tous les produits et composants, y compris les faces de portes et de
tiroirs, les roulettes et coulisseaux, sauf les articles figurant dans b), c) et d).
b. Trois (3) ans pour les composants électriques tels que les lampes de travail/lampes LED,
les câbles, les commandes et les accessoires.
c. Deux (2) ans pour le support du moniteur à rail coulissant et les composants et garnitures.
d) Un (1) an pour les plans de travail et résines, y compris les accessoires.
7. Les PRODUITS D’IMAGERIE sont garantis pendant une période de deux (2) ans, à l’exception
du lecteur CR ClearVision, garanti pendant une période d’un (1) an.
8. Les pièces et accessoires de rechange MIDMARK sont garantis pendant une période de quatrevingt-dix (90) jours.
EXCLUSIONS
Midmark ne peut être tenu pour responsable des cas suivants, qui ne sont pas couverts par la
garantie :
1. malfaçons, dommages ou autres conditions provoqués, en tout ou partie, par une utilisation
abusive ou incorrecte, une négligence, une modification, un accident, des dommages subis
pendant le transport, une négligence dans l’entreposage, une altération ou une demande
de réparation ou de remplacement hors délai ;
2. produits qui ne sont pas installés, utilisés, nettoyés et entretenus correctement tel qu’exigé
ou recommandé dans les guides « Installation » et/ou « Installation/Fonctionnement »
de Midmark pour le produit concerné, y compris les conditions environnementales
structurelles et opérationnelles et les exigences électriques indiquées ;
3. produits considérés comme étant de nature consommable ou stérile ;
4. accessoires ou pièces qui ne sont pas fabriqués par Midmark ;
5. frais appliqués par quiconque pour des réglages, des réparations, des pièces de
remplacement, l’installation ou toute autre tâche accomplie sur ou en rapport avec lesdits
produits, qui ne sont pas expressément autorisés au préalable et par écrit par Midmark ;
6. frais d’entretien et de nettoyage ordinaires ;
7. représentations et garanties données pour toute autre personne ou entité que Midmark ;
8. correspondance de couleur, de grain ou de texture, hormis selon les normes commerciales
acceptables ;
9. changements de couleur causés par la lumière naturelle ou artificielle ;
10. produits de fabrication sur mesure ;
11. altérations ou modifications apportées au produit par toute personne ou entité autre que
Midmark ; et
12. produits qui seraient autrement couverts par les sections 1 et 2 de cette garantie limitée,
mais qui ont été acquis : i) auprès d’une personne ou d’un organisme autre
que Midmark ou l’un de ses revendeurs agréés ; ou ii) auprès d’un revendeur Midmark non
autorisé à vendre le produit en cause sur le territoire géographique où se trouve l’acheteur,
ou non autorisé à vendre le produit en cause sur le marché médical, de la santé animale ou
dentaire, selon le cas, dans lequel l’acheteur a l’intention d’utiliser le produit.
RECOURS EXCLUSIF ; AVIS DE NON-RESPONSABILITÉ POUR LES DOMMAGES INDIRECTS
L’UNIQUE OBLIGATION DE MIDMARK DANS LE CADRE DE LA PRÉSENTE GARANTIE LIMITÉE EST
LA RÉPARATION OU LE REMPLACEMENT DES PIÈCES DÉFECTUEUSES. MIDMARK NE PEUT
ÊTRE TENU POUR RESPONSABLE ET DÉCLINE PAR LA PRÉSENTE TOUTE RESPONSABILITÉ
POUR TOUT RETARD OU DOMMAGE DIRECT, PARTICULIER, INDIRECT, ACCIDENTEL,
EXEMPLAIRE OU CONSÉCUTIF, Y COMPRIS, SANS S’Y LIMITER, LES DOMMAGES RELATIFS À
UNE PERTE DE BÉNÉFICE OU DE REVENU, UNE PERTE D’USAGE, UN TEMPS D’INDISPONIBILITÉ,
UNE COUVERTURE ET LES SALAIRES, PAIEMENTS ET AVANTAGES SOCIAUX D’EMPLOYÉS OU
D’ENTREPRENEURS INDÉPENDANTS.
EXCLUSION DE GARANTIE
LA PRÉSENTE GARANTIE LIMITÉE EST LA SEULE GARANTIE DE MIDMARK ET REMPLACE TOUTE
AUTRE GARANTIE EXPLICITE OU IMPLICITE. MIDMARK N’ÉMET AUCUNE GARANTIE, EXPLICITE
OU IMPLICITE, DE QUELQUE NATURE QUE CE SOIT, Y COMPRIS TOUTE GARANTIE IMPLICITE DE
QUALITÉ MARCHANDE OU D’ADÉQUATION À UNE FIN PARTICULIÈRE. CETTE GARANTIE EST
LIMITÉE À LA RÉPARATION OU AU REMPLACEMENT DE PIÈCES DÉFECTUEUSES.
Règles de prescription
Aucune action ne peut être portée contre Midmark pour violation de la présente garantie limitée,
d’une garantie implicite, le cas échéant, ou pour toute autre revendication découlant de ou relative
aux produits, plus de quatre-vingt-dix (90) jours après expiration de la période de garantie limitée.
© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Français - 6
Page 23

© Midmark Corporation 2016
003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Français - 7
Page 24

003-2302-99
TP200 20-42-FO-00012 Rev A1 C2169
Midmark Corporation
60 Vista Drive
Versailles, OH 45380 États-Unis
1-800-643-6275
1-937-526-3662
www.midmark.com
 Loading...
Loading...