Page 1

MICROLIFE® CARDIO+
Blood Pressure Monitor
For accurate blood pressure measurement
Instruction Manual
Page 2

Dear Customer,
We thank you for purchasing the Microlife® Cardio+, the blood pressure
monitor that allows you to easily and accurately measure your blood
pressure.
The Microlife Cardio+ Blood Pressure Monitor is a reliable medical
device designed for use with your iPhone, iPad, or iPod touch. This
simple-to-use, accurate monitor comes highly recommended for
blood pressure monitoring at home. Developed in collaboration with
physicians, the Microlife Cardio+ has been clinically tested to ensure
the highest accuracy of its measurements.*
Read through these instructions carefully to understand this product’s
functions and safety information. We want you to be happy with your
Microlife product.
1
If you have any inquiries, contact Microlife’s customer support center.
Your retailer or pharmacy can provide you with the contact information
of the Microlife dealer in your country. Or visit www.microlife.com for
more information about our products.
Stay healthy!
Microlife AG
* This device uses the same measuring technology as the award winning
«BP 3BTO-A» model tested according to the British Hypertension Society
(BHS) protocol.
Page 3

Table of Contents
Product Overview
The Microlife Cardio+ Blood Pressure Monitor and Application .......... 3
What is included ................................................................................ 4
Before Using the Microlife Cardio+ for the First Time
Charging the Microlife Cardio+ Blood Pressure Monitor .................... 5
Downloading the Microlife Cardio+ App from the App Store ............... 5
Creating a User Prole ...................................................................... 5
Setting a Blood Pressure Target ........................................................ 6
Taking Measurements with the Microlife Cardio+
Before Taking a Measurement ........................................................... 6
Taking a Measurement ...................................................................... 7
During Measurement ........................................................................ 7
Completed Measurement.................................................................. 8
Interpreting the Measurement Results
WHO Indicator .................................................................................. 8
Pulse Arrhythmia Detection (PAD) Indicator ...................................... 8
Managing the Measurement Data
Saving, Deleting, Viewing, or Sharing Data ..................................... 10
Managing a User Prole
Creating, Selecting, or Deleting a User Prole ..................................11
Appendix
Battery Power Indicators ..................................................................11
Error Messages............................................................................... 12
Facts about Blood Pressure and Self-Measurement........................ 13
Safety and Protection ...................................................................... 13
Device care/Cleaning of the Cuff ..................................................... 14
Accuracy Test/Guarantee ................................................................ 14
Technical Specications .................................................................. 15
Guarantee Card ............................................................................. 20
EN
2
Page 4

Product Overview
Microlife Cardio+ Blood Pressure Monitor and
Application
2
1) Connection Slot on iPhone/iPad/iPod touch
2) Microlife Cardio+ Dock
3) On/Off Button
Cancel Button (during measurement)
4) Microlife Cardio+ Plug
5) Preformed Cuff
6) Air Hose
7) Micro USB Port
8) Air Hose Socket
1 5 7 8
3
3 4 6
Page 5

What is included:
9 10 11 12
9) Measurement
10) Report
11) Settings
12) Users
• Microlife Cardio+ Dock x 1
• Preformed Cuff, Size M–L (22–42 cm) x 1
• USB Cable for Charging Battery x 1
• Carrying Case x 1
• Instruction Manual x 1
• Quick Start Guide x 1
Read the instructions carefully before using this device.
Type BF applied part.
EN
4
Page 6
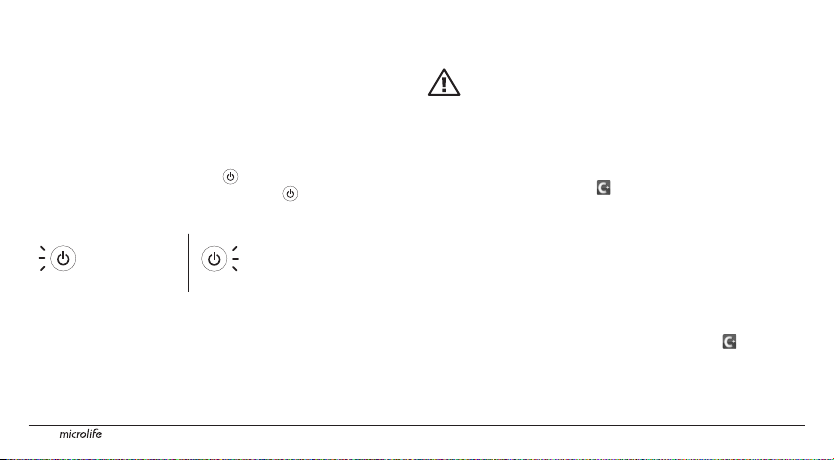
Before Using the Microlife Cardio+ for the First Time
Charging the Microlife Cardio+ Blood Pressure
Monitor
• Connect the dock to an iOS-device USB power adapter using the
supplied USB cable. Connect to a power outlet and charge the dock
for 2 hours.
• During the charging process, the « » button lights up in yellow.
When the battery is completely charged, the « » button lights up
briey in blue, and then the unit automatically switches off.
• The battery status of the blood pressure monitor is displayed at the
• While charging the Microlife Cardio+ Blood Pressure Monitor, you
5
While Charging
Start button lights up
in yellow
bottom of the Microlife Cardio+ app screen.
can also charge your iOS device.
When Charged
Start button lights up briey in
blue and the unit switches off
Charging the Microlife Cardio+ Blood Pressure Monitor directly
through a computer USB port is not recommended.
Downloading the Microlife Cardio+ App from the
App Store
Go to the App Store and search for «Microlife Cardio+». Once this app
has been downloaded, the icon will appear on your iOS device.
☞ To download the app, you need an Apple ID with an email address
and password, as well as an active Internet connection. While the
Microlife Cardio+ app is free to download, you may be subject to
charges from your Internet provider for using the Internet to do so.
Creating a User Prole (optional)
If more than one person will use the monitor, create a user prole to
ensure that each user’s data is stored separately. Open , and go to
«User» to create a prole.
Page 7
Setting a Blood Pressure Target (optional)
If targeted measurement values have been recommended by a
doctor, set a blood pressure target to give a realistic reading based
on these recommendations. Open , and go to «Settings». Set and
save the «Systolic» and «Diastolic» targets.
After completing these steps, you are ready to take a blood pressure
measurement.
Note: Conrm that the time and date on your iOS device are correct.
Taking Measurements with the
Microlife Cardio+
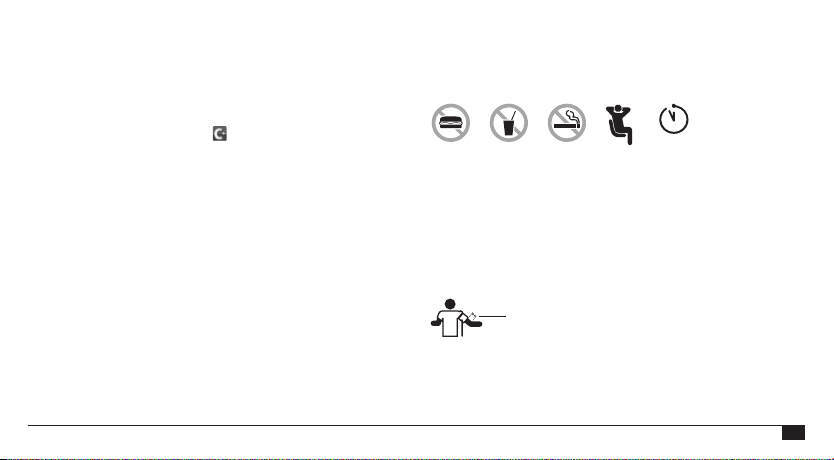
Before Taking a Measurement
• Avoid any activity, eating, or smoking immediately before taking a
measurement.
• Sit down and relax for at least 5 minutes before taking a
measurement.
Relax 5 mins
• Always take measurements on the same arm (normally the left).
• Remove close-tting clothing from the upper arm. To avoid
constriction, your shirt sleeve should not be rolled up; it will not
interfere with the cuff if it is laid at.
• Fit the cuff closely, but not too tightly. Make sure that the cuff is
2 cm (0.75 inches) above your elbow, with the tube on the inside of
your arm.
• Support your arm so that it is relaxed. Ensure that the cuff is at the
same height as your heart.
2 cm (0.75 inches)
EN
6
Page 8

Taking a Measurement
• Open the «Microlife Cardio+» app automatically by plugging the iOS
device to the monitor dock. Alternatively, click on the app displayed
on the iOS device screen.
• Select the measurement mode: ONE measurement (single), or MAM
measurement (triple).
• Press «Start» to begin.
• Press «Stop» to cancel anytime during the measurement (e.g., if
you feel uncomfortable because of the pressure from cuff). You can
cancel the measurement through the Apple device interface or the
blood pressure monitor itself.
☞ What Is MAM Measurement?
MAM stands for «Microlife Average Mode». It is a Microlife proprietary
patented technology that takes and analyzes your blood pressure in
3 successive measurements, automatically. Since blood pressure
constantly uctuates, a result determined with MAM is more reliable
than a result obtained from a single measurement. This type of
7
measurement is also recommended by doctors in most of the medical
guidelines for hypertension management.
During Measurement
• The cuff will pump up automatically. Do not move or talk, and do not
tense your arm muscles. Breathe normally.
• When the correct pressure is reached, the pumping stops and the
pressure decreases gradually.
• The heart symbol ashes in the display every time a heartbeat is
detected.
• If you choose MAM measurement, 3 measurements are taken in
succession, with 15-second intervals between each one. A fourth
measurement will be taken if any of the results are unclear.
☞
During the measurement process, if you receive a phone call or
detach the iOS device from the dock, the cuff will automatically deate
and stop the whole measurement process.
Page 9

Completed Measurement
• After the results are shown, the measurement is complete.
• Your systolic and diastolic blood pressure and your pulse are
displayed in the results.
• Remove the cuff and save the measurement results.
Interpreting the Measurement
Results
WHO Indicator
The color of the «WHO» icon indicates the results of your measurement.
To check the evaluation of the measurement, press the «WHO» icon.
The higher value is used in this evaluation, even if the other value
is normal.
Example: 150/85 mmHg indicates «Blood pressure too high».
120/98 mmHg indicates «Blood pressure too high».
Pulse Arrhythmia Detection (PAD) Indicator
PAD
The «
» symbol indicates that certain pulse irregularities were
detected during the measurement:
• If the result deviates from your normal blood pressure, repeat the
measurement.
• If this symbol appears on a regular basis (e.g., several times a week
when measurements are taken daily), we advise you to inform your
doctor and show the following explanation.
☞ Early Detection of Pulse Arrhythmia
This device is an oscillometric blood pressure monitor that also
analyzes pulse rate during measurement. Although it does not replace
a cardiac examination, this device has been clinically tested and can
detect pulse irregularities at an early stage.
PAD
The
detected. If this symbol appears frequently, we recommend that the
patient seek medical advice.
symbol is displayed if pulse irregularities have been
«
»
EN
8
Page 10

Classication of Adult’s Blood Pressure Values in Accordance with the World Health Organization (WHO) in 2003.
Range Systolic (mmHg) Diastolic (mmHg) Recommendation
Blood pressure too low
1
Blood pressure optimum
2
Blood pressure normal
3
Blood pressure slightly high
4
Blood pressure too high
5
Blood pressure far too high
6
Blood pressure dangerously high
9
100 60 Consult your doctor
100–120 60–80 Self-check
120–130 80–85 Self-check
130–140 85–90 Consult your doctor
140–160 90–100 Seek medical advice
160–180 100–110 Seek medical advice
180 110 Urgently seek medical advice
Page 11

Managing the Measurement Data
Saving Data
• Press «Save» to store the measurement results, including
measurement mode, date, and time. If you do not wish to keep a
measurement, press «Cancel».
• To store measurement results from other blood pressure devices, go
to «Manual Input».
☞
The amount of data saved depends on the memory capacity of your
iOS device. A warning message will appear when the memory is full.
Delete old data to save new data.
Deleting Data
• Delete Individual Data: Go to «Report» and press «Edit».
• Delete All Data: Go to «Settings» and press «Reset Data».
Viewing Data
Go to «Report» to view data.
• Viewing Individual Data: Swipe the screen vertically to see data
taken in the same month. To view data taken in different months,
swipe the screen horizontally.
• Viewing Data in a Line Graph: Turn the iOS device horizontally. To
view data in a specic timeline, choose the intended period of view.
• Viewing Data in a Bar Chart*: Turn the iOS device and swipe the
screen horizontally. To view data in a specic timeline, choose the
intended period of view.
1D – One day
1W – One week
1M – One month
3M – Three months
1Y – One year
All – All measurements stored
Sharing Data
Go to «Report» and press « » to share data*.
*Data is shared via email in CSV format. To use this function, an email
account must be set up in your iOS device and an Internet connection
is needed.
10
EN
Page 12

Managing a User Prole Appendix
Creating a User Prole
Creating a prole for an individual user ensures that each individual's
data is stored separately. Go to «User» and choose «+» to create a
new user. Up to 10 proles can be created.
Selecting a User Prole
Go to «User», select the user listed, and proceed to take a
measurement.
Deleting a User Prole
Go to «User», click «Edit», and select the user to be deleted.
11
Battery Power Indicators
The « » button on the blood pressure monitor may show the
following:
• No Light: The battery is completely charged.
• Blue Light: The battery is over 20% charged.
• Yellow Light: The battery is less than 20% charged. Charge the battery.
• Blue and Yellow Lights Flashing Alternately: The battery is defective.
Do not let the battery run down completely, as this shortens the
battery’s service life.
Page 13

Error Messages
If an error occurs during the measurement, the measurement is
interrupted and an error message, e.g., «ERR 3», is displayed.
Error Description Potential cause and remedy
«ERR 1» Signal too weak The pulse signals on the cuff are
«ERR 2» Arm movement During the measurement, error
«ERR 3» No pressure in
the cuff
too weak. Re-position the cuff and
repeat the measurement.*
signals were detected by the cuff,
caused, for instance, by movement
or muscle tension. Repeat the
measurement, keeping your arm still.
Adequate pressure cannot be
generated in the cuff. A leak may
have occurred. Check that the
cuff is correctly connected and
not too loose. Recharge the
batteries if necessary. Repeat the
measurement.
«ERR 5» Abnormal result The measuring signals are
«ERR 6» MAM mode error There were errors during the
Low battery The battery power in the Microlife
* Consult your doctor, if this or any other problem occurs repeatedly.
inaccurate and no result, therefore,
can be displayed. Read through
the checklist for performing reliable
measurements and then repeat the
measurement.*
measurement in MAM mode,
making it impossible to obtain
a nal result. Read through the
checklist for performing reliable
measurements and then repeat the
measurement.*
Cardio+ Blood Pressure Monitor
Dock is too low. Charge the dock
before taking any measurements.
EN
12
Page 14

Facts about Blood Pressure and SelfMeasurement
• Blood pressure is the pressure of the blood owing in the arteries,
generated by the pumping of the heart.
• Two values, the systolic (upper) value and the diastolic (lower) value,
are always measured. This device also indicates pulse rate (the
number of times the heart beats in a minute).
• Permanently high blood pressure can damage your health and must
be treated by your doctor.
• Always discuss your results with your doctor if you have noticed
anything unusual or feel unsure about the results. Never rely on a
single blood pressure reading.
• Under no circumstances should you alter the dosages of any drugs
prescribed by your doctor.
• Depending on physical exertion and other conditions, blood pressure
is subject to wide uctuations, especially as the day progresses.
You should, therefore, take your measurements under the same
conditions, when you are relaxed.
13
• Take at least two measurements per day - one in the morning and
one in the evening.
• It is quite normal for two measurements taken in quick succession to
produce signicantly different results.
• Differences in measurements taken by your doctor or at the
pharmacy and those taken at home are quite normal, as these
environments are very different.
Safety and Protection
• This device and cuff have sensitive components and must be
treated with care.
• Protect this device from:
- water and moisture.
- extreme temperatures.
- damage from impact or drops.
- contamination and dust.
- direct sunlight.
- heat and cold.
Page 15

• Do not use the device if you think it is damaged or if you notice
anything unusual about it.
• Never open the device.
• Use only the cuff provided.
Ensure that children do not use the device unsupervised;
some parts are small enough to be swallowed.
Accuracy Test
We recommend that this device is tested for accuracy every 2 years or
after any mechanical impact (e.g., being dropped). Contact Microlife’s
service center to arrange a test.
Batteries and electronic devices must be disposed of in accordance
with the locally applicable regulations, not with domestic waste.
Device care
Clean the device with a soft, dry cloth. Do not use gas, thinners or
similar solvents.
Cleaning the Cuff
Spots on the cuff can be removed carefully with a damp cloth and
soapsuds.
The cuff with bladder must not be washed in a dishwasher,
clothes washer, or submerged in water.
Guarantee
This device is covered by a 2-year guarantee from the date of
purchase. The guarantee is valid only on presentation of the guarantee
card completed by the dealer (see back) conrming date of purchase
or the receipt.
• Batteries, cuff, and normal wear and tear are not included.
• Opening or altering the device invalidates the guarantee.
• The guarantee does not cover damage caused by improper
handling, discharged batteries, accidents, or non-compliance with
the operating instructions.
Contact Microlife’s customer service center for further details.
14
EN
Page 16

Technical Specications
Product name: Microlife® Cardio+ Blood Pressure Monitor
Runs on Apple iOS
devices:
BP4GAPO-2M
iPod touch (4th generation), iPod touch (3rd
generation), iPhone 4S, iPhone 4, iPhone
3GS, iPad (3rd generation), iPad 2, iPad.
Operating system: Apple iOS 5.0 or higher
Operating temperature: +10 °C to +40 °C, 15 - 90% humidity
Storage temperature: -20 °C to +55 °C, 15 - 90% humidity
Weight: Approx. 213 g
Dimensions (ø x H): Approx. 120 mm x 93.5 mm
Cuff size: 22 - 42 cm adult cuff; supports size M to L
Measuring procedure: Oscillometric; single or MAM readings
15
Measurement range: 30 - 280 mmHg: blood pressure
Cuff pressure display
range:
40 - 200 beats per minute: pulse
0 - 299 mmHg
Resolution: 1 mmHg
Static accuracy: Pressure within ±3 mmHg or
2% of reading > 200 mmHg
Pulse accuracy: ± 5% of the readout value
Voltage source: Via USB (5 V , 500 mAh) or integrated
Reference to standards: ANSI/AAMI SP10, IEC 60601-1,
This device complies with the requirements of the Medical Device
Directive 93/42/EEC. Technical alterations reserved.
battery (3.7 V , 500 mAh)
IEC 60601-1-2, EN 1060 -1/-3/-4
Page 17

Guidance and manufacturer's declaration - electromagnetic emissions
The BP4GAPO-2M is intended for use in the electromagnetic environment specied below. The customer or the user of the BP4GAPO-2M should assure that
it is used in such an environment.
Emission test Compliance Electromagnetic environment-guidance
RF emissions CISPR 11 Group 1 The BP4GAPO-2M uses RF energy only for its internal function. Therefore, its
RF emissions CISPR 11 Group B The BP4GAPO-2M is suitable for use in all establishments, including domestic
Harmonic emissions IEC 61000-3-2 Not applicable
Voltage uctuations / icker emissions
IEC 61000-3-3
Not applicable
RF emissions are very low and are not likely to cause any interference in nearby
electronic equipment.
establishments and those directly connected to the public low-voltage power
supply network that suppies buildings used for domestic purposes.
16
EN
Page 18

Guidance and manufacturer's declaration - electromagnetic immunity
The BP4GAPO-2M is intended for use in the electromagnetic environment specied below. The customer or the user of the BP4GAPO-2M should assure that
it is used in such an environment.
Immunity test IEC 60601 test level Compliance
Electrostatic discharge
(ESD)
Electrical fast transient/burst
IEC 61000-4-4
Surge IEC 61000-4-5 + 1kV line(s) to line(s)
Voltage Dips, short
interruption and voltage
variations on power supply
input lines IEC 61000-4-11
Power frequency (50/60 Hz)
magnetic eld
IEC 61000-4-8
Note: UT is the a.c. mains voltage prior to application of the test level.
17
+ 6 kV contact
+ 8 kV air
+ 2kV for power supply lines
+ 1kV for input/output lines
+ 2kV line(s) to earth
<5% UT (>95% dip in UT) for 0,5 cycle
40% UT (60% dip in UT) for 5 cycles
70% UT (30% dip in UT) for 25 cycles
<5% UT (>95% dip in UT) for 5 s
3 A/m 3 A/m The BP4GAPO-2M power frequency magnetic elds should be at
level
+ 6 kV contact
+ 8 kV air
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Electromagnetic environment-guidance
Floors should be wood, concrete or ceramic tile. If oors are covered
with synthetic material, the relative humidity should be at least 30 %
Mains power quality should be that of a typical commerical or
hospital environment
Mains power quality should be that of a typical commerical or
hospital environment
Mains power quality should be that of a typical commerical or
hospital environment. If the user of the BP4GAPO-2M requires
continued operation during power mains interruptions, it is
recommended that the BP4GAPO-2M be powered from an
uninterruptible power supply or a battery.
levels characteristic of a typical location in a typical commercial or
hospital environment.
Page 19

Guidance and manufacturer's declaration - electromagnetic immunity
The BP4GAPO-2M is intended for use in the electromagnetic environment specied below. The customer or the user of the BP4GAPO-2M should assure that
it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment-guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from structures, objects and people.
a
Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio
broad-cast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to xed RF transmitters, an electromagnetic site
survey should be considered. If the measured eld strength in the location in which the BP4GAPO-2M is used exceeds the applicable RF compliance level
above, the BP4GAPO-2M should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such
as re-orienting or relocating the BP4GAPO-2M.
b
Over the frequency range 150 kHz to 80 MHz, eld strenghts should be less than 3 V/m.
3 Vrms
150 KHz to 80 MHz
3 V/m
80MHz to 2,5 GHz
Not applicable Portable and mobile RF communications equipment should be used no closer to any part of the
3 V/m Recommended separation distance:
BP4GAPO-2M including cables, than the recommended separation distance calculated from
the equation applicable to the frequency of the transmitter.
d = 1,2 √P d = 1,2 √P 80MHz to 800 MHz d = 2,3 √P 800MHz to 2,5 GHz
Where P is the maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer and d is the recommended separation distance in meters (m)
Field strengths from xed RF transmitters, as determined by an electromagnetic site
survey, a should be less than the compliance level in each frequency range.
Interference may occur in the vicinity of equipment marked with the following symbol:
b
18
EN
Page 20

Recommended separation distance between portable and mobile RF communication equipment and
the BP4GAPO-2M
The BP4GAPO-2M is intended for use in a electromagnetic environment in which radiated RF disturbance are controlled. The customer or the user of
the BP4GAPO-2M can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications
equipment (transmitters) and the BP4GAPO-2M as recommended below, according to the maximum output power of the communications equipment.
Rated maximum output power of
transmitter
W
0,01 N/A 0,12 0,23
0,1
1
10
100
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the
equation applicable to the frequency of the transmitter, wher P is the maximum output power rating of the transmitter in watts (W) according to the transmitter
manufacturer.
Note 1: At 80 MHz and 800 MHz the separation distance for the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from structures,
objects and people.
19
150 kHz to 80 MHz
d = 1,2√P
Separation distance according to frequency of transmitter
N/A 0,38
N/A 1,2
N/A 3,8
N/A 12
m
80 MHz to 800 MHz
d = 1,2√P
800 MHz to 2,5 GHz
d = 2,3√P
0,73
2,3
7,3
23
Page 21

Guarantee Card
Name of Purchaser Serial Number
Page 22

Date of Purchase Specialist Dealer
Page 23

Europe / Middle-East / Africa
Microlife AG
Espenstrasse 139
9443 Widnau / Switzerland
Tel. +41 / 71 727 70 30
Fax +41 / 71 727 70 39
Email admin@microlife.ch
www.microlife.com
Asia
Microlife Corporation
9F, 431, RuiGang Road, NeiHu
Taipei, 11492, Taiwan, R.O.C.
Tel. 886 2 8797-1288
Fax.886 2 8797-1283
Email service@microlife.com.tw
www.microlife.com
North / Central / South America
Microlife USA, Inc.
1617 Gulf to Bay Blvd., 2nd Floor Ste A
Clearwater, FL 33755 / USA
Tel. +1 727 442 5353
Fax +1 727 442 5377
Email msa@microlifeusa.com
www.microlife.com
Designed for use with iPhone, iPad, or iPod touch.
IB CardioPlus EN 4912
 Loading...
Loading...