Page 1

METTLER TOLEDO 33360 + 210260 1
Operating instructions
Bedienungsanleitung
Mode d'emploi
Instrucciones de manejo
Istruzioni per l'uso
METTLER TOLEDO
33360
210260
Density determination kit Page 3
to determine the density of solids (33360) and liquids (33360/210260) with
top-loading electronic METTLER TOLEDO balances with pan diameters of 80 and 130 mm
Dichtebestimmungszusatz Seite 9
für die Dichtebestimmung von Festkörpern (33360) und Flüssigkeiten (33360/210260)
auf oberschaligen elektronischen METTLER TOLEDO-Waagen mit Schalendurchmesser 80 und 130 mm
Accessoires pour la détermination de la masse volumique Page 15
des solides (33360) et des liquides (33360/210260) à l'aide de balances
électroniques METTLER TOLEDO à plateau supérieur ayant un diamètre de 80 et 130 mm
Conjunto de determinación de densidades Página 21
para la determinación de densidades de sólidos (33360) y de líquidos (33360/210260) sobre
balanzas electrónicas METTLER TOLEDO de platillo elevado con diámetros de platillo de hasta 80 y 130 mm
Kit per la determinazione della densità Pagina 27
di solidi (33360) e liquidi (33360/210260) con l'ausilio di bilance
elettroniche METTLER TOLEDO con piatto di pesata superiore del diametro di 80 e 130 mm
Page 2

2 METTLER TOLEDO 33360 + 210260
LEERSEITE
Page 3

33360
210260
Density Determination of Liquids
Dichtebestimmung von Flüssigkeiten
Détermination de la masse volumique de liquides
Determinación de densidades de liquidos
Determinazione della densità dei liquidi
Page 4
33360
Density Determination of Solids
Dichtebestimmung von Festkörpern
Détermination de la masse volumique de solides
Determinación de densidades de sólidos
Determinazione della densità dei solidi
Page 5

METTLER TOLEDO 33360 + 210260 3
Density determination kit
to determine the density of solids and liquids with top-loading electronic METTLER TOLEDO balances with pan
diameters of 80 and 130 mm.
- for the density determination of solids, density determination kit 33360.
- for the density determination of liquids, density determination kit 33360
+ displacement body 210260.
1. Definition of density
- to determine the density of solids and liquids with top-loading electronic METTLER TOLEDO balances with
pan diameters of 80 and 130 mm.
The density ρ of an homogeneous body is the relationship of its mass (m) to its volume (V) or its mass per unit
of volume.
m [g] ρ = Density
ρ = m = Mass
V [cm3] V = Volume
According to DIN (German Engineering Standards) 1305, the term "Weight" can be used instead of "Mass" (m).
2. Principle of density determination
According to the Archimedean Principle, a solid body immersed in a liquid apparently loses as much of its own
weight as the weight of the liquid it has displaced. This makes it possible to determine the unknown value.
Depending on whether the body in question is liquid or solid, a slightly different procedure is involved.
The density of a liquid is determined by using a sinker of known volume. To this end, the sinker is first weighed
while in air, and then while immersed. From these two weighings (made in grams), density ρ1 of the liquid is
calculated as follows:
As - B
s
ρ1 =+ Lsρ1= Density of liquid to be tested at a given
V
s
temperature T
As= Weight of sinker in air
Bs= Weight of sinker when immersed in liquid
or simply Vs= Volume of sinker
Ls= Air buoyancy per ml of sinker (correction of As or
Ps): about + 0,001 g/ ml; see also Section 3.2,
P
s
Air Buoyancy
ρ1 =+ LsPs= Buoyancy of sinker in liquid
V
s
(instead of As - Bs): can be read directly on
METTLER TOLEDO electronic balances; see
Section 4.1
The density of a solid body is determined by using a liquid of known density. To this end, the solid body is first
weighed in air and then immersed. From these two weighings (made in grams or in carats), density ρ2 is calculated
as follows:
A
ρ2 =• ρ
o
ρ2= Density of solid body
A - B A = Weight of solid body in air
B = Weight of solid body when immersed in test liquid
or simply ρo= Density of test liquid at a given temperature T
P = Buoyancy of solid body in test liquid (instead of
A A - B): can be read directly on METTLER TOLEDO
ρ2 =• ρ
o
electronic balances; see Section 4.2
P
The air buoyancy of a solid body is not always taken into account. If necessary, result ρ2 can be corrected by about
+ 0.001 g/ cm3 similar to what was done in the first case; see also Section 3.2, Air Buoyancy.
Page 6
4 METTLER TOLEDO 33360 + 210260
3. Accuracy of density determination
The required accuracy of the result determines whether any factors will be taken into account. The following listing
should make it possible to quantitatively evaluate the influence of these factors.
3.1 Temperature
In the case of solid bodies, the change in density caused by a change in temperature is generally so small that
the temperature of a solid body can be disregarded as far as density determination is concerned (this also applies
to sinkers).
In the case of liquids, however, the density may change on the order of 0.1 … 1 %o pro °C, and this may already
appear at the third decimal place.
Example: Distilled water: Density change about 1 %o per 5 °C
Hydrocarbons + alcohols: Density change about 1 %o per 1 °C
It should be noted that such a change in the density of a liquid is directly taken into account when the density of
solid bodies is being determined because of the test liquid in which the solid body has to be immersed. For this
reason, the temperature of the liquid must always be taken into consideration when the density is to be
determined with better than 1 % accuracy.
Density table for distilled water:
(according to "Handbook of Chemistry and Physics" 66th Ed. 1985-1986, F4-F5)
Temperature (°C) Density (g/ ml) Temperature (°C) Density (g/ ml)
15.0 0.9991 24.0 0.9973
15.5 0.9990 24.5 0.9972
16.0 0.9990 25.0 0.9970
16.5 0.9989 25.5 0.9969
17.0 0.9988 26.0 0.9968
17.5 0.9987 26.5 0.9966
18.0 0.9986 27.0 0.9965
18.5 0.9985 27.5 0.9964
19.0 0.9984 28.0 0.9962
19.5 0.9983 28.5 0.9961
20.0 0.9982 29.0 0.9959
20.5 0.9981 29.5 0.9958
21.0 0.9980 30.0 0.9956
21.5 0.9979 30.5 0.9955
22.0 0.9978 31.0 0.9953
22.5 0.9977 31.5 0.9952
23.0 0.9975 32.0 0.9950
23.5 0.9974
For all other liquids, the density at temperature T must be taken from a book of tables.
Page 7

METTLER TOLEDO 33360 + 210260 5
3.2 Air Buoyancy
Depending on its physical conditions, each cubic centimeter (cm3 ) of air weighs 1 … 1.2 mg. Thus, any object
that is being weighed in air is subject to this kind of buoyancy for each cm3 of its volume. This means that – with
a density of 1 g/ cm3 – an error of approximately 0.1 % would occur if the air buoyancy is not taken into
consideration. If a result with 3 or 4 decimal places is required, the result will have to be corrected for air buoyancy.
The true density is about 0.001 g/ cm3 more than the calculated density.
When the density of liquids is determined, the operating procedure (division by 10 ml, corresponding to the
volume of the sinker), provides a fourth-place result, even if the balance display shows only a three-place
indication. It is therefore advisable – in this case – to make a general buoyancy correction.
3.3 Volume tolerance of the float
German Weights and Measures Regulation EO 13-4 paragraph 9.21
The volume of the float, together with the lower portion of the suspension wire, must be adjusted so that a 30 g
float arrangement does not produce a measuring error exceeding ± 0.00059 g/ cm3 when determining the water
density at a temperature of 20 °C.
3.4 Immersion depth of the sinker or of the gem holder
The sinker (immersion body) is suspended from a platinum wire which has a diameter of 0.2 mm. In the water,
the wire thus experiences a buoyancy of 0.3 mg when 10 mm of the wire are immersed.
If the fluid level stands 10 mm above the eylet of the sinker, about 20 mm of the wire are immersed. With a density
(of liquid) of about 1, this would result in a buoyancy of 0.6 mg. But since this would again have to be divided by
10 ml, this influence can be disregarded.
The submersible part of the gem holder is made of a wire that has a diameter of 0.8 mm. With a liquid density
of approx. 1, this results in a buoyancy of about 5 mg for each 10 mm that is submerged. But since the gem holder
remains submerged while the solid body is weighed in air, and since – with electronic balances – the immersion
depth does not change from one weighing to the next (in spite of the difference in weight), the buoyancy of the
gem holder remains constant and can thus be disregarded. Condition: Do not change the liquid level. (The change
of the liquid level caused by submerging the solid body can – in most cases – be disregarded. A solid body with
a volume of 1 cm3 causes the liquid to rise by about 0.5 mm. This is equivalent to a buoyancy of about 0.15 mg,
i.e. a density error of 0.15 mg/ cm3).
3.5 Surface tension of liquid
Since the liquid adheres to the suspension wire, an apparent weight increase occurs. In the case of the sinker
(wire diameter 0.2 mm), and when water is used as the liquid, this force amounts to about 1 mg. By using wetting
agents or organic liquids, this force can be reduced to 0.3 mg. However, this value will also be divided by 10 ml
so that the resulting density error amounts to no more than 0.0001 g/ ml.
Because of the larger diameter of the wire submerged in the water, a force of up to 3 mg acts upon the gem holder.
Here, too, similar to what is described in Section 3.4, any influence on the result will be virtually eliminated by
having the gem basket submerged during both weighings (A and B). For very high accuracy requirements,
reducing the surface tension would constitute an additional precautionary measure (see also Section 3.6).
Page 8

6 METTLER TOLEDO 33360 + 210260
3.6 Air bubbles
With poorly wetting liquids such as water without wetting agents, for example, it is possible that air bubbles will
adhere to the submerged solid body, sinker or wire basket of gem holder. Because of their buoyancy, these
bubbles could affect the result. Thus, a bubble 1 mm in diameter would cause a buoyancy of 0.5 mg, while a bubble
with a diameter of 2 mm would result in a buoyancy of as much as 4 mg.
Precautionary measures:
- Degrease solids that are resistant to solvents.
- Periodically clean gem holder and sinker; do not touch immersible parts with your hands.
- Gently shake gem holder when first immersing it in liquid, before suspending it from hook, so as to loosen air
bubbles that might stick to it.
- Use wetting agents or organic solvents as auxiliary liquid (e.g. Kodak Photo-Flo, Pervitro 75% 72409).
(The change of density caused by the addition of a wetting agent to distilled water is negligible. If, for
example, 0.1 ml wetting agent with a density of 1.2 is added to 250 ml of water, the overall density changes
by 0.001 g/ml.)
3.7 Porous bodies
Since the submersion of porous bodies does not generally cause a one hundred percent displacement of air within
the pores by the liquid, errors will occur as a consequence. Thus the density of the body can only be approximated.
Page 9
METTLER TOLEDO 33360 + 210260 7
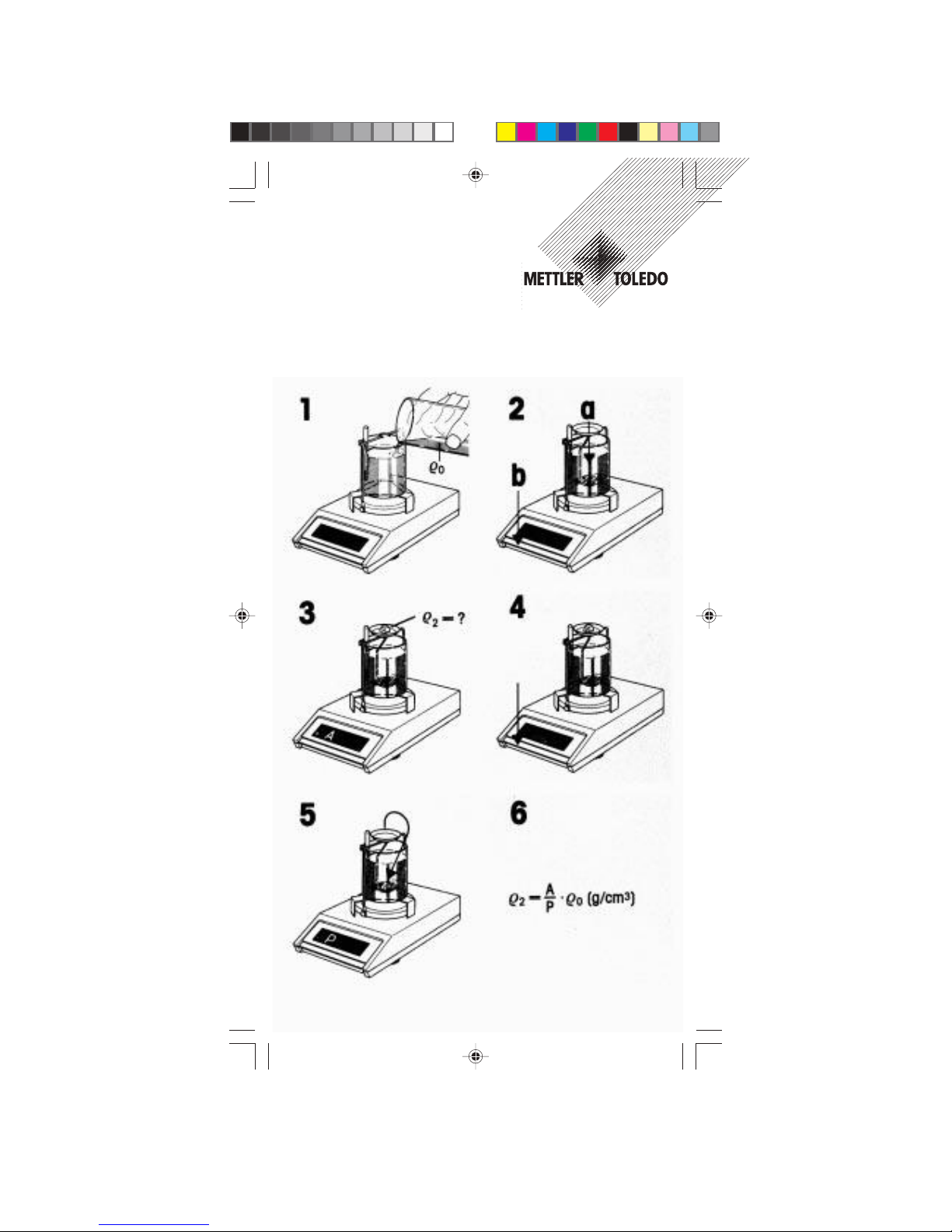
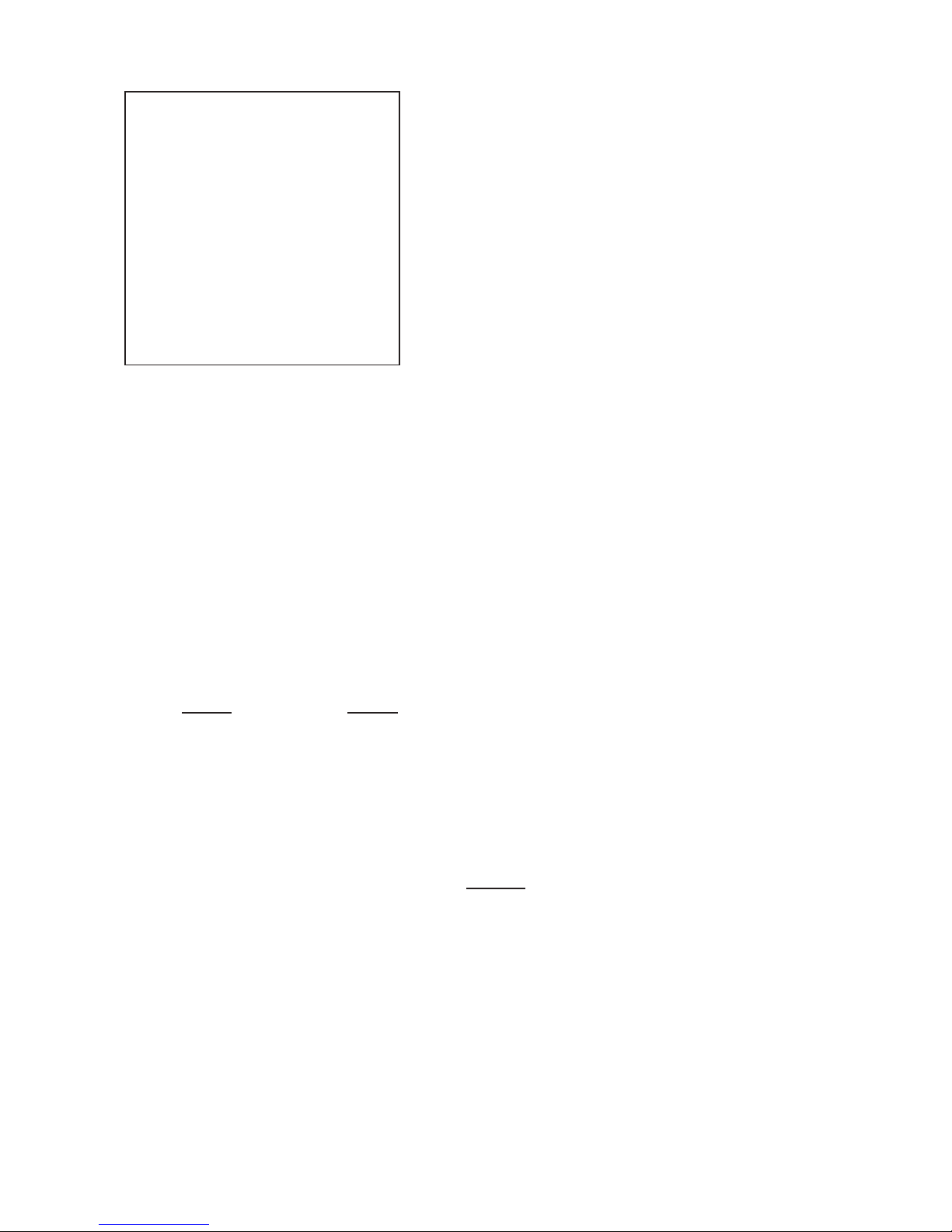
4. How to perform density determinations
Arrangement
1 Weighing pan of METTLER TOLEDO balance
2 Bracket attached to weighing pan …
3 … clamping screws hold bracket in place
4 Bridge placed on balance housing across weighing pan.
Warning: Weighing pan 1 and bracket 2 must not brush
against bridge:
5 250 ml beaker placed on bridge
6 Sinker or gem holder …
7 … suspension; the sinker or gem holder should hang
freely in center of beaker without touching.
Please note: The first weighing is dry!
8 Thermometer
The listing of the various parts is repeated on page 33 (Parts
List)
Reminders:
- It is recommended to check the calibration.
- Along with the text below, please check the illustrated instructions which are enclosed with this description.
4.1 Determining the density of liquids
- Use displacement body 210260 + density determination kit for liquids 33360.
- Place empty beaker on bridge 4, centered below suspension 7; insert thermometer 8.
- Attach sinker 6. It must not touch the beaker or the thermometer.
- Tare balance to show exactly zero.
- Pour liquid to be tested into beaker up to about 10 mm above the eyelet of the sinker. No air bubbles should
adhere to the sinker. If necessary, brush off bubbles with a fine brush or the like.
- The balance displays (with minus sign in front) buoyancy Ps of the sinker. Value Ps displayed by the balance
must now be divided by 10. After that, add 0.001 g/ ml.
The result is density ρ1 (g/ ml) at a given temperature T (to be read).
P
s
Ps [g]
ρ1 =+ L
s
= + 0.001 g/ ml at T °C (Ps = As - Bs)
V
s
10 ml
If the direct buoyancy indication is to be dispensed with, Ps can be obtained from two separate weighings: As =
sinker in air; Bs = sinker in liquid. Procedure is the same as above, but the balance is tared before the sinker is
attached. With the sinker attached, the balance then indicates value As. After the liquid is filled in, the balance
indicates (without additional taring!) value Bs.
Example with buoyancy indication: Determining the density of acetone.
7.898 g
Measured Ps= 7.898 g ρ
1
+ 0.001 g/ ml = 0.7908 g/ ml at 20 °C
values: T = 20 °C 10 ml
The density of the acetone tested amounts to 0.7908 g/ ml at 20 °C.
Page 10

8 METTLER TOLEDO 33360 + 210260
4.2 Determining the density of solids
- Use density determination kit for solids (= gem holder 6) 33360.
- Pour enough auxiliary liquid into beaker so that the solid body after being placed in wire basket of gem holder
will be covered with at least 10 mm of liquid. Insert thermometer. Place beaker on bridge 4 centrally located
below the suspension 7.
- Attach gem holder (watch for air bubbles, especially at the wire basket).
- Tare balance to read exactly zero.
- Place dry solid in upper cup of gem holder. Write down weight displayed by the balance (in grams or carats).
This is weight A of the solid when weighed in air.
- Again tare balance (with solid body in cup).
Balance display indicates exactly zero.
- Remove solid body from cup and place it in lower wire basket (in liquid).
Balance now displays buoyancy P of solid body (with minus sign in front).
Divide first indication A by second indication P, then multiply this intermediate result by the density ρ0 of the
auxiliary liquid (at the given temperature T).
The final result is density ρ2 of the solid body. The unit of measurement is the same as that used for density
ρo of the auxiliary liquid, e.g. g/ cm3 (identical to g/ ml).
A A [g] Since the units of A an P cancel each other, the
ρ2 =• ρo=• ρo [g/ cm3] weighing can be performed just as easily in carats
P P [g] as in grams. Result ρ2 always has the same unit of
measurement as result ρo.
If the direct buoyancy reading is to be dispensed with, P can also be obtained from A - B. The balance will display
B instead of P if it has not been tared after result A was displayed or if it was tared with the solid body removed.
Example with buoyancy indication: Determining the density of a coin with the help of distilled water.
Measurement A = 3.011 g 3.011
values: P = 0.336 g ρ2 = • 0.997 g/ cm3 = 8.93 g/ cm
3
T = 25.5 °C →ρo = 0.997 g/ cm
3
0.336
The coin has a density of 8.93 g/ cm3.
Page 11

METTLER TOLEDO 33360 + 210260 9
Dichtebestimmungszusatz
für die Dichtebestimmung von Festkörpern und Flüssigkeiten auf oberschaligen elektronischen
METTLER TOLEDO-Waagen mit Schalendurchmessern 80 und 130 mm.
- für die Dichtebestimmung von Festkörpern Dichtebestimmungs-Kit 33360.
- für die Dichtebestimmung von Flüssigkeiten Dichtebestimmungs-Kit 33360
+ Verdrängungskörper 210260.
1. Definition der Dichte
- für die Dichtebestimmung von Festkörpern und Flüssigkeiten auf oberschaligen elektronischen
METTLER TOLEDO-Waagen mit Schalendurchmessern 80 und 130 mm.
Die Dichte ρ eines homogenen Körpers ist das Verhältnis seiner Masse (m) zu seinem Volumen (V) oder,
gleichbedeutend, die Masse einer Volumeneinheit:
m [g] ρ = Dichte
ρ = m = Masse
V [cm3] V = Volumen
Anstelle der Masse (m) kann gemäss DIN 1305 der Begriff “Gewicht” verwendet werden.
2. Prinzip der Dichtebestimmung
Gemäss dem Archimedischen Prinzip verliert ein in eine Flüssigkeit getauchter Körper soviel an Eigengewicht,
wie das Gewicht der von ihm verdrängten Flüssigkeit beträgt. Damit lässt sich die gesuchte Grösse bestimmen.
Das Vorgehen bei Flüssigkeiten und Festkörpern ist etwas unterschiedlich.
Die Dichte einer Flüssigkeit wird mit Hilfe eines Senkkörpers bestimmt, dessen Volumen bekannt ist. Dazu
wiegt man diesen Senkkörper einmal in Luft, sodann in eingetauchtem Zustand. Aus den beiden Wägungen (in
Gramm) errechnet sich die Flüssigkeitsdichte ρ1 wie folgt:
As - B
s
ρ1 =+ Lsρ1= Dichte der zu prüfenden Flüssigkeit bei der
V
s
gegebenen Temperatur T
As= Gewicht des Senkkörpers in Luft
Bs= Gewicht des Senkkörpers in die Flüssigkeit
getaucht
oder einfacher Vs= Volumen des Senkkörpers
Ls= Luftauftrieb pro ml des Senkkörpers
(Korrektur von As bzw. Ps):
P
s
ca. + 0,001 g/ ml; siehe auch 3.2, Luftauftrieb
ρ1 =+ LsPs= Auftrieb des Senkkörpers in der Flüssigkeit
V
s
(anstelle von As - Bs): auf elektronischen
METTLER TOLEDO-Waagen direkt ablesbar,
siehe 4.1
Die Dichte eines Festkörpers wird mit Hilfe einer Flüssigkeit bestimmt, deren Dichte bekannt ist. Dazu wiegt
man den Festkörper einmal in Luft, sodann in eingetauchtem Zustand. Aus den beiden Wägungen (in Gramm
oder Karat) errechnet sich die Dichte ρ2 wie folgt:
A
ρ2 =• ρ
o
ρ2= Dichte des Festkörpers
A - B A = Gewicht des Festkörpers in Luft
B = Gewicht des Festkörpers in die
oder einfacher Hilfsflüssigkeit getaucht
ρo= Dichte der Hilfsflüssigkeit bei der gegebenen
A Temperatur T
ρ2 =• ρ
o
P = Auftrieb des Festkörpers in der Hilfsflüssigkeit
P (anstelle von A - B): auf elektronischen
METTLER TOLEDO-Waagen direkt ablesbar,
siehe 4.2
Der Luftauftrieb wird bei Festkörpern nicht durchgehend berücksichtigt. Bei Bedarf kann, wie im ersten Fall, am
Resultat ρ2 die Korrektur von ca. + 0,001 g/ cm3 angebracht werden; siehe auch 3.2, Luftauftrieb.
Page 12
10 METTLER TOLEDO 33360 + 210260
3. Genauigkeit der Dichtebestimmung
Die allfällige Berücksichtigung von Faktoren, welche das Resultat beeinflussen können, richtet sich stets nach
der geforderten Resultatgenauigkeit. Die folgende Aufstellung im einzelnen soll eine quantitative Wertung dieser
Einflüsse erlauben.
3.1 Temperatur
Bei Festkörpern ist die Veränderung der Dichte durch Temperaturänderung im allgemeinen so gering, dass ihre
Temperatur für die Dichtebestimmung belanglos ist (dies gilt auch für den Senkkörper).
Bei Flüssigkeiten hingegen liegt die Dichteänderung in der Grössenordung von 0,1 … 1 %o pro °C, kann also
schon bei der dritten Nachkommastelle in Erscheinung treten.
Beispiele: destilliertes Wasser Dichteänderung ca. 1 %o auf 5 °C
Kohlenwasserstoffe und Alkohole Dichteänderung ca. 1 %o auf 1 °C
Es ist zu beachten, dass diese Dichteänderung von Flüssigkeiten auch bei Dichtebestimmungen von Festkör-
pern direkt ins Resultat eingeht wegen der Volumenbestimmung des Festkörpers mittels einer Hilfsflüssigkeit,
in die er getaucht wird. Bei Dichtebestimmungen von besser als 1 % Genauigkeit ist daher die Temperatur der
Flüssigkeit stets zu berücksichtigen!
Dichtetabelle für destilliertes Wasser:
(gemäss "Handbook of Chemistry and Physics" 66th Ed. 1985-1986, F4-F5)
Temperatur (C°) Dichte (g/ml) Temperatur (C°) Dichte (g/ml)
15,0 0,9991 24,0 0,9973
15,5 0,9990 24,5 0,9972
16,0 0,9990 25,0 0,9970
16,5 0,9989 25,5 0,9969
17,0 0,9988 26,0 0,9968
17,5 0,9987 26,5 0,9966
18,0 0,9986 27,0 0,9965
18,5 0,9985 27,5 0,9964
19,0 0,9984 28,0 0,9962
19,5 0,9983 28,5 0,9961
20,0 0,9982 29,0 0,9959
20,5 0,9981 29,5 0,9958
21,0 0,9980 30,0 0,9956
21,5 0,9979 30,5 0,9955
22,0 0,9978 31,0 0,9953
22,5 0,9977 31,5 0,9952
23,0 0,9975 32,0 0,9950
23,5 0,9974
Für andere Flüssigkeiten muss die Dichte bei Temperatur T einem Tabellenbuch entnommen werden.
Page 13

METTLER TOLEDO 33360 + 210260 11
3.2 Luftauftrieb
1 cm3 Luft wiegt – je nach Zustand – ca. 1 … 1,2 mg. Ein Körper erfährt also, wenn er in Luft gewogen wird, pro
cm3 seines Volumens einen Auftrieb von dieser Grösse. Dies bedeutet, dass bei einer Dichte um 1 (1 g/ cm3)
durch Nichtberücksichtigung des Luftauftriebs ein Fehler von ca. 1 %o entsteht. Wird ein Resultat von 3 (oder 4)
Nachkommastellen verlangt, muss daher das Resultat um den Luftauftrieb korrigiert werden:
Die wahre Dichte ist um ca. 0,001 g/ cm3 grösser als die errechnete.
Bei der Dichtebestimmung von Flüssigkeiten liefert das Verfahren (Division durch 10 ml, entsprechend dem
Volumen des Senkkörpers) schon bei dreistelliger Gewichtsanzeige ein vierstelliges Resultat. Es ist daher
sinnvoll, in diesem Falle die Luftauftriebskorrektur allgemein vorzunehmen.
3.3 Volumentoleranz des Senkkörpers
Deutsche Eichordnung EO 13-4 Abs. 9.21
Das Volumen des Senkkörpers einschliesslich der unteren Hälfte des Aufhängedrahtes muss so abgeglichen
sein, dass bei der Bestimmung der Dichte des Wassers von 20 °C höchstens ein Fehler von ± 0,0005 g/ cm3 bei
der Senkkörpereinrichtung von 30 g hervorgerufen wird.
3.4 Eintauchtiefe des Senkkörpers bzw. Steinträgers
Der Senkkörper hängt an einem Platindraht von 0,2 mm Durchmesser. Im Wasser erfährt somit der Draht einen
Auftrieb von ca. 0,3 mg auf 10 mm Eintauchlänge.
Steht die Flüssigkeit 10 mm über der Öse des Senkkörpers, sind ca. 40 mm Draht eingetaucht. Bei Dichten um
1 entsteht daraus ein Auftrieb von 1,2 mg. Da aber noch durch 10 ml dividiert wird, fällt dieser Einfluss ausser
Betracht.
Der Steinträger besteht im einzutauchenden Teil aus Draht von 0,8 mm Durchmesser, was bei einer
Flüssigkeitsdichte um 1 einen Auftrieb von ca. 5 mg pro 10 mm Eintauchlänge ergibt. Da jedoch bei der Wägung
des Festkörpers in Luft der Steinträger ebenfalls eingetaucht ist und sich zwischen beiden Wägungen, trotz der
Gewichtsdifferenz, bei elektronischen Waagen die Eintauchtiefe nicht ändert, bleibt die Auftriebskraft am
Steinträger konstant und fällt somit ausser Betracht. Bedingung: Flüssigkeitsstand nicht ändern (Die Änderung
des Flüssigkeitsstandes durch das Eintauchen des Festkörpers ist meist unerheblich: Ein Festkörpervolumen
von 1 cm3 lässt die Flüssigkeit um 0,5 mm ansteigen, was einen Auftrieb von ca. 0,15 mg, d. h. einen Dichtefehler
von ca. 0,15 mg/ cm3 verursacht).
3.5 Oberflächenspannung der Flüssigkeit
Durch Adhäsion der Flüssigkeit am Aufhängedraht entsteht eine scheinbare Gewichtszunahme. Im Falle des
Senkkörpers (Drahtdurchmesser 0,2 mm) und in Wasser beträgt diese Kraft ca. 1 mg; sie kann durch
Verwendung von Netzmitteln oder organischen Flüssigkeiten auf ca. 0,3 mg reduziert werden. Es gilt aber auch
hier, dass anschliessend noch durch 10 ml dividiert wird, sodass der resultierende Dichtefehler höchstens 0,0001
g/ ml beträgt.
Am Steinträger entsteht aufgrund des grösseren Drahtdurchmessers in Wasser eine Kraft von bis zu 3 mg. Auch
in diesem Falle, ähnlich wie unter 3.4, wird aber ein Einfluss auf das Resultat praktisch beseitigt, indem der
Steinträger bei beiden Wägungen (A und B) eingetaucht ist. Für höchste Genauigkeitsansprüche wäre die
Herabsetzung der Oberflächenspannung dennoch eine Vorsichtsmassnahme (siehe auch 3.6).
Page 14

12 METTLER TOLEDO 33360 + 210260
3.6 Luftblasen
Insbesondere bei schlecht benetzenden Flüssigkeiten, wie z. B. netzmittelfreiem Wasser, besteht die Möglichkeit, dass am eingetauchten Festkörper, Senkkörper oder Steinträger Luftblasen hängen bleiben, welche durch
Auftriebserzeugung das Resultat beeinflussen. Eine Blase von 1 mm Ø erzeugt einen Auftrieb von 0,5 mg, eine
Blase von 2 mm Ø aber bereits einen solchen von 4 mg.
Vorsichtsmassnahmen:
- Lösungsmittelbeständige Festkörper entfetten
- Steinträger und Senkkörper periodisch reinigen, eintauchenden Teil nicht mit Händen berühren
- Den Steinträger jeweils beim ersten Eintauchen (Benetzen) in der Flüssigkeit etwas rütteln, bevor er an den
Bügel gehängt wird, um allfällig anhaftende Luftblasen zu lösen.
- Netzmittel oder organische Lösungsmittel als Hilfsflüssigkeit verwenden (z. B. Foto-Flo von Kodak, Pervitro
75% 72409). Die Dichteänderung von destilliertem Wasser durch das Netzmittel ist unerheblich; wird auf 250
ml Wasser z. B. 0,1 ml Netzmittel von der Dichte 1,2 zugefügt, ändert sich die Gesamtdichte um 0,0001 g/
ml.
3.7 Poröse Körper
Da beim Eintauchen von porösen Körpern in der Regel nicht die gesamte Luft in den Poren durch die Flüssigkeit
verdrängt wird, treten Fehler auf. Die Dichte des Körpers kann nur annähernd bestimmt werden.
Page 15

METTLER TOLEDO 33360 + 210260 13
4. Durchführung von Dichtebestimmungen
Anordnung
1 Waagschale der METTLER TOLEDO-Waagen
2 Bügel, an Waagschale gesteckt und mittels …
3 … Klemmschrauben an ihr festgeklemmt;
4 Brücke, quer über die Waagschale auf das Waagen-
gehäuse gestellt. Achtung: Waagschale 1 und Bügel 2
dürfen nicht an der Brücke streifen!
5 Becherglas 250 ml, auf Brücke gestellt;
6 Senkkörper oder Steinträger, bei …
7 … Aufhängung am Bügel 2 aufgehängt. Der Senkkörper
oder Steinträger soll berührungsfrei mitten im Becherglas hängen. Achtung: Die erste Wägung erfolgt trokken!
8 Thermometer
Die einzelnen Teile sind auf Seite 33 (Ersatzteilliste) noch-
mals aufgeführt.
Hinweise:
- Es empfiehlt sich, die Kalibrierung zu prüfen.
- Beachten Sie, zusammen mit dem nachstehenden Text, die praktische Kurzanleitung in Bildern, welche
dieser Beschreibung beigelegt ist.
4.1 Bestimmen der Dichte von Flüssigkeiten
- Verdrängungskörper 210260 + Dichtebestimmungs-Kit 33360 verwenden.
- Leeres Becherglas auf die Brücke 4 stellen, zentrisch unter die Aufhängung 7; Thermometer einsetzen.
- Senkkörper 6 anhängen. Er darf Becherglas und Thermometer nicht berühren.
- Waage tarieren. Anzeige: genau Null.
- Zu prüfende Flüssigkeit ins Becherglas einfüllen bis ca. 10 mm über die Aufhängeöse des Senkkörpers. Am
Senkkörper dürfen keine Luftblasen hängen bleiben; nötigenfalls Blasen mit feinem Pinsel etc. abstreifen.
- Auf der Waagenanzeige erscheint (mit negativem Vorzeichen) der Auftrieb Ps des Senkkörpers. Diese
Waagenanzeige Ps durch 10 dividieren, dann 0,001 g/ ml hinzuaddieren.
Das Resultat ist die gesuchte Flüssigkeitsdichte ρ1 [g/ ml] bei gegebener Temperatur T (ablesen).
P
s
Ps [g]
ρ1 =+ L
s
= + 0,001 g/ ml bei T °C (Ps = As - Bs)
V
s
10 ml
Soll auf die direkte Auftriebsanzeige verzichtet werden, wird Ps aus zwei getrennten Wägungen gewonnen:
As = Senkkörper in Luft; Bs = Senkkörper in Flüssigkeit. Vorgehen: wie oben, jedoch wird tariert vor dem
Anhängen des Senkkörpers. Mit angehängtem Senkkörper zeigt die Waage dann As, nach dem Einfüllen der
Flüssigkeit (ohne nochmaliges Tarieren!) aber Bs.
Beispiel mit Auftriebsanzeige: Bestimmen der Dichte von Aceton
7,898 g
Messwerte: Ps= 7,898 g ρ
1
+ 0,001 g/ ml = 0,7908 g/ ml bei 20 °C
T = 20 °C 10 ml
Die Dichte des geprüften Acetons ist 0,7908 g/ ml bei 20 °C.
Page 16

14 METTLER TOLEDO 33360 + 210260
4.2 Bestimmen der Dichte von Festkörpern
- Dichtebestimmungs-Kit für Festkörper (= Steinträger 6) 33360 verwenden.
- Soviel Hilfsflüssigkeit ins Becherglas einfüllen, dass der Festkörper, wenn er hernach ins Drahtkörbchen des
Steinträgers gelegt wird, noch mindestens 1 cm hoch mit Flüssigkeit bedeckt ist. Thermometer einsetzen.
Becherglas auf Brücke 4 stellen, zentrisch unter die Aufhängung 7.
- Steinträger anhängen (auf Luftblasen achten, besonders am Drahtkörbchen).
- Waage tarieren. Anzeige: genau Null.
- Trockenen Festkörper ins obere Schälchen des Steinträgers legen, Gewichtsanzeige (in Gramm oder Karat)
notieren. Es ist dies das Gewicht A des Festkörpers in Luft.
- Waage nochmals tarieren (mit Festkörper im Schälchen). Anzeige: genau Null.
- Festkörper aus dem Schälchen nehmen und ins untere Drahtkörbchen (in die Flüssigkeit) legen. Auf der
Waagenanzeige erscheint (mit negativem Vorzeichen) der Auftrieb P des Festkörpers.
Die erste Anzeige A durch die zweite Anzeige P dividieren, dann dieses Zwischenresultat mit der Dichte ρ
o
der Hilfsflüssigkeit (bei der gegebenen Temperatur T) multiplizieren.
Dieses Schlussresultat ist die gesuchte Dichte ρ2 des Festkörpers. Die Einheit entspricht der verwendeten
Einheit für die Dichte ρo der Hilfsflüssigkeit, z. B. g/ cm3 (entspricht g/ ml).
A A [g] Da die Einheiten von A und P sich aufheben, kann
ρ2 =• ρo=• ρo [g/ cm3] ebensogut in Karat wie in Gramm gewogen werden:
P P [g] Das Resultat ρ2 hat stets die Einheit von ρo.
Soll auf die direkte Auftriebsanzeige verzichtet werden, wird auch hier P aus A - B gewonnen: B erscheint anstelle
von P auf der Waagenanzeige, wenn nach der Gewichtsanzeige A entweder gar nicht oder dann mit
abgehobenem Festkörper tariert wird.
Beispiel mit Auftriebsanzeige: Bestimmen der Dichte eines Geldstückes mit Hilfe von destilliertem Wasser.
Messwerte: A = 3,011 g 3,011
P = 0,336 g ρ2 = • 0,997 g/ cm3 = 8,93 g/ cm
3
T = 25,5 °C →ρo = 0,997 g/ cm
3
0,336
Das Geldstück hat eine Dichte von 8,93 g/ cm3.
Page 17

METTLER TOLEDO 33360 + 210260 15
Accessoires pour la détermination de la masse volumique
des solides et des liquides à l'aide de balances électroniques METTLER TOLEDO
à plateau supérieur ayant un diamètre de 80 et 130 mm.
- de solides. Accessoires 33360.
- de liquides. Accessoires 33360 + corps de déplacement 210260.
1. Définition de la masse volumique
- des solides et des liquides à l'aide de balances électroniques METTLER TOLEDO à plateau supérieur ayant
un diamètre de 80 et 130 mm.
La masse volumique ρ d'un corps homogène est le rapport entre sa masse (m) et son volume (V) ou encore la
masse d'une unité de volume de ce corps:
m [g] ρ = masse volumique
ρ = m = masse
V [cm3] V = volume
Suivant la norme allemande DIN 1305, la notion de "poids" peut être utilisée à la place de la masse (m).
2. Principe de la détermination de la masse volumique
Suivant le principe d'Archimède, tout corps plongé dans un liquide en équilibre subit de la part de ce liquide une
poussée verticale orientée de bas en haut égale au poids du liquide déplacé. La grandeur recherchée peut être
déterminée à l'aide de ce principe. Le procédé diffère un peu suivant qu'il s'agit de liquides ou de solides:
La masse volumique d'un liquide est déterminée à l'aide d'un plongeur de volume connu. Pour cela, il faut
peser ce plongeur d'abord dans l'air, puis plongé dans le liquide. A partir de ces deux pesées (en grammes), la
masse volumique de liquide ρ1 se calcule de la manière suivante:
As - B
s
ρ1 =+ Lsρ1= masse volumique du liquide à examiner
V
s
à la température T donnée
As= poids du plongeur dans l'air
Bs= poids du plongeur dans le liquide
ou simplement Vs= volume du plongeur
Ls= poussée de l'air par ml du plongeur (correction de
As ou Ps): environ + 0,001 g/ ml; voir aussi sous 3.2,
P
s
Poussée de l'air
ρ1 =+ LsPs= poussée du liquide sur le plongeur
V
s
(au lieu de As - Bs): donnée directement par les
balances électroniques METTLER TOLEDO, voir
sous 4.1
La masse volumique d'un solide est déterminée à l'aide d'un liquide (liquide auxiliaire) de masse volumique
connue. Pour cela, il faut peser le solide d'abord dans l'air, puis plongé dans ce liquide. A partir de ces deux
pesées (en grammes ou en carats), la masse volumique de solide ρ2 se calcule de la manière suivante:
A
ρ2 =• ρ
o
ρ2= masse volumique du solide
A - B A = poids du solide dans l'air
B = poids du solide plongé dans le
ou simplement liquide auxiliaire
ρo= masse volumique du liquide auxiliaire
A à la température T donnée
ρ2 =• ρ
o
P = poussée du liquide auxiliaire sur le solide
P (au lieu de A - B): donnée directement par les
balances électroniques METTLER TOLEDO, voir
sous 4.2
Pour les solides, la poussée de l'air n'est généralement pas prise en considération. Si nécessaire, comme dans
le premier cas, une correction d'environ + 0,001 g/ cm3 peut être apportée au résultat ρ2; voir aussi sous 3.2,
Poussée de l'air.
Page 18

16 METTLER TOLEDO 33360 + 210260
3. Précision de la détermination de la masse volumique
La prise en considération de facteurs susceptibles d'influencer le résultat se fait toujours en fonction de la
précision recherchée. La présentation détaillée suivante doit permettre une évaluation quantitative de ces
facteurs.
3.1 Température
La variation de la masse volumique des solides due à la variation de la température est généralement si faible
que pour la détermination de la masse volumique leur température est sans importance (ceci est aussi valable
pour le plongeur).
Par contre, la variation de la masse volumique des liquides est de l'ordre de grandeur de 0,1 … 1 %o par °C,
et peut donc déjà apparaître dans le troisième chiffre après la virgule.
Examples de variation Eau distillée: environ 1 %o par 5 °C
de la masse volumique: Carbures d'hydrogène et alcools: environ 1 %o par 1 °C
Il faut noter que cette variation de la masse volumique des liquides se répercute aussi sur le résultat de la
détermination de la masse volumique des solides, parce que le volume de ces derniers est déterminé à l'aide
d'un liquide auxiliaire dans lequel ils sont plongés. Aussi faut-il toujours tenir compte de la température du liquide
si une précision supérieure à 1 % est désirée!
Table de la masse volumique de l'eau distillée:
(d'après "Handbook of Chemistry and Physics", 66th Ed. 1985-1986, F4-F5)
La masse volumique d'autres liquides à la temperature T est à rechercher dans les tables correspondantes.
Température (°C) Masse volumique Température (°C) Masse volumique
(g/ ml) (g/ ml)
15,0 0,9991 24,0 0,9973
15,5 0,9990 24,5 0,9972
16,0 0,9990 25,0 0,9970
16,5 0,9989 25,5 0,9969
17,0 0,9988 26,0 0,9968
17,5 0,9987 26,5 0,9966
18,0 0,9986 27,0 0,9965
18,5 0,9985 27,5 0,9964
19,0 0,9984 28,0 0,9962
19,5 0,9983 28,5 0,9961
20,0 0,9982 29,0 0,9959
20,5 0,9981 29,5 0,9958
21,0 0,9980 30,0 0,9956
21,5 0,9979 30,5 0,9955
22,0 0,9978 31,0 0,9953
22,5 0,9977 31,5 0,9952
23,0 0,9975 32,0 0,9950
23,5 0,9974
Page 19

METTLER TOLEDO 33360 + 210260 17
3.2 Poussée de l'air
1 cm3 d'air pèse, suivant son état, environ 1 … 1,2 mg. Un corps, quand il est pesé dans l'air, subit donc par cm
3
de son volume une poussée de cette valeur. Cela signifie que pour une masse volumique autour de 1 (1 g/ cm3),
une erreur d'environ 1 %o est faite en ne tenant pas compte de la poussée de l'air. C'est pourquoi il faut en tenir
compte si le résultat est demandé avec une précision de 3 (ou 4) chiffres après la virgule:
La masse volumique exacte est environ 0,001 g/ cm3 plus grande que celle calculée.
La façon de calculer la masse volumique des liquides (division par 10 ml, correspondants au volume du plongeur)
fait que, déjà pour un poids donné en trois décimales, le résultat soit donné en quatre décimales. C'est pourquoi
il est judicieux dans ce cas d'effectuer systématiquement la correction due à la poussée de l'air.
3.3 Tolérance du volume du plongeur
Législation allemande relative aux instruments de mesure: EO 13-4 paragraphe 9.21
Le volume du plongeur, y compris la moitié inférieure du fil de suspension, doit être ajusté de façon que, lors de
la détermination de la masse volumique de l'eau à 20 °C, il puisse se produire, au maximum, une erreur de ±
0,0005 g/ cm3 pour un dispositif plongeur de 30 g.
3.4 Profondeur d'immersion du plongeur ou du porte-pierre
Le plongeur est attaché à un fil de platine de diamètre 0,2 mm. Ce fil subit dans l'eau une poussée d'environ 0,3
mg par 10 mm de longueur immergée.
Si le liquide se trouve 10 mm au-dessus de l'anneau du plongeur, environ 20 mm de fil sont immergés. Pour une
masse volumique proche de 1, il résulte de ce fait une poussée d'environ 0,6 mg. Mais comme on divise encore
par 10 ml, cette source d'erreur devient parfaitement négligeable.
Le porte-pierre se compose dans sa partie immersible d'un fil de diamètre 0,8 mm qui subit, pour une masse
volumique du liquide proche de 1, une poussée d'environ 5 mg par 10 mm de longueur immergée. Comme le
porte-pierre est également immergé lors de la pesée dans l'air du solide et que sa profondeur d'immersion malgré
la différence de poids, ne change pas sur les balances électroniques, la poussée du liquide sur le porte-pierre
reste constante et n'entre donc pas en considération. Condition: Ne pas changer le niveau du liquide. (Le
changement du niveau du liquide dû à l'immersion du solide est le plus souvent négligeable: un volume de solide
d'1 cm3 fait monter le niveau du liquide de 0,5 mm, ce qui provoque une poussée d'environ 0,15 mg, c'est à dire
une erreur de la masse volumique d'environ 0,15 mg/ cm3).
3.5 Tension superficielle du liquide
L'adhésion du liquide sur le fil de suspension crée une augmentation de poids fictive. Dans le cas du plongeur
(diamètre du fil 0,2 mm) et de l'eau, cette force est environ 1 mg; en utilisant des agents mouillants ou des liquides
organiques, elle peut être réduite à 0,3 mg. Mais dans ce cas aussi, il faut encore diviser par 10 ml, de telle manière
que l'erreur de la masse volumique résultante atteint au maximum 0,0001 g/ ml.
Le diamètre du fil étant plus grand, il se crée sur le porte-pierre dans l'eau une force pouvant atteindre 3 mg. Dans
ce cas aussi, comme dans le paragraphe 3.4, une influence sur le résultat est pratiquement exclue, car le portepierre est immergé lors des deux pesées (A et B). Si une grande précision est désirée, il serait néanmoins
préférable, par précaution, de réduire la tension superficielle (voir aussi sous 3.6).
Page 20

18 METTLER TOLEDO 33360 + 210260
3.6 Bulles d'air
Particulièrement dans un liquide peu mouillant, comme par exemple de l'eau pure, il est possible qu'il reste
accroché au solide, plongeur ou porte-pierre immergé des bulles d'air qui exercent une poussée pouvant modifier
le résultat. Une bulle d'1 mm de diamètre exerce une poussée de 0,5 mg, mais une bulle de 2 mm de diamètre
en exerce une atteignant déjà 4 mg.
Mesures de précaution:
- Dégraisser au solvant les solides y résistant.
- Nettoyer régulièrement le porte-pierre et le plongeur, ne pas les toucher avec les mains.
- Secouer un peu le porte-pierre lors de sa première immersion dans le liquide, avant de l'accrocher à l'étrier,
pour éliminer les bulles d'air.
- Utiliser des agents mouillants ou des solvants organiques comme liquide auxiliaire, (par ex. Photo-Flo de
Kodak, Pervitro 75% 72409). La variation de la masse volumique de l'eau distillée, due à l'apport d'agent
mouillant, est insignifiante; si par exemple à 150 ml d'eau est ajouté 0,1 ml d'agent mouillant de masse
volumique 1,2 g/ ml, la masse volumique globale change de 0,0001 g/ ml.
3.7 Corps poreux
En règle générale, lors de l'immersion d'un corps poreux, l'air n'est pas chassé en totalité par le liquide; de ce
fait, il y a une source d'erreur. La masse volumique d'un corps poreux ne peut donc être définie que d'une manière
approximative.
Page 21

METTLER TOLEDO 33360 + 210260 19
4. Détermination des masses volumiques
Disposition:
1 Plateau de la balance METTLER TOLEDO
2 Etrier, placé sur le plateau
3 Vis de fixation de l'étrier sur le plateau
4 Pont, placé au travers du plateau et reposant sur le bâti
de la balance. Attention: Le plateau 1 et l'étrier 2 ne
doivent pas toucher le pont!
5 Bécher de 250 ml, posé sur le pont
6 Plongeur ou porte-pierre
7 Coupelle de suspension à l'étrier 2
Le plongeur ou le porte-pierre doit pendre librement au
centre du bécher. Attention: La première pesée s'effectue à sec!
8 Thermomètre
Les différentes pièces sont à nouveau décrites à la page 33
(liste des pièces de rechange).
Indications:
- Il est recommandé de vérifier le calibrage.
- Consultez, avec le texte ci-après, les instructions abrégées en images qui sont jointes à cette description.
4.1 Détermination de la masse volumique des liquides
- Utiliser le corps de déplacement 210260 + les accessoires de détermination de la masse volumique de
liquides 33360.
- Poser le bécher vide sur le pont 4, centré sous la suspension 7; placer le thermomètre dans le bécher.
- Suspendre le plongeur 6 sans qu'il touche le bécher ou le thermomètre.
- Tarer la balance. Affichage: exactement zéro.
- Verser le liquide à examiner dans le bécher jusqu'à 10 mm au-dessus de l'anneau de fixation du plongeur.
Aucune bulle d'air ne doit rester accrochée au plongeur; si nécessaire, les enlever avec un petit pinceau.
- Sur l'affichage de la balance apparaît (avec un signe négatif) la poussée Ps sur le plongeur. Diviser cette
donnée de la balance par 10, puis y ajouter 0,001 g/ ml. Le résultat est la masse volumique p1 [g/ ml]
recherchée du liquide à la température T (à relever).
P
s
Ps [g]
ρ1 =+ L
s
= + 0,001 g/ ml à T °C (Ps = As - Bs)
V
s
10 ml
S'il faut renoncer à la donnée directe de la poussée, Ps sera déterminé par deux pesées distinctes: As = plongeur
dans l'air; Bs = plongeur dans le liquide. Marche à suivre: comme ci-dessus, mais il faut tarer avant de suspendre
le plongeur. Le plongeur étant suspendu, la balance donne As et, après avoir versé le liquide (sans tarage
supplémentaire!), Bs.
Exemple avec affichage de la poussée: Détermination de la masse volumique de l'acétone.
7,898 g
Valeurs mesurées: Ps= 7,898 g ρ
1
+ 0,001 g/ ml = 0,7908 g/ ml à 20 °C
T = 20 °C 10 ml
La masse volumique de l'acétone vérifiée est de 0,7908 g/ ml à 20 °C.
Page 22

20 METTLER TOLEDO 33360 + 210260
4.2 Détermination de la masse volumique des solides
- Utiliser les accessoires de détermination de la masse volumique de solides (= porte-pierre 6) 33360.
- Verser dans le bécher assez de liquide auxiliaire pour que le solide, une fois déposé dans le panier, soit
recouvert par au moins 1 cm de liquide. Placer le thermomètre dans le bécher. Poser le bécher sur le pont
4, centré sous la suspension 7.
- Suspendre le porte-pierre (faire attention aux bulles d'air, surtout sur le panier).
- Tarer. Affichage: exactement zéro.
- Poser le solide sec dans la coupelle supérieure du porte-pierre, noter le poids indiqué (en grammes ou carats).
C'est le poids A du solide dans l'air.
- Tarer à nouveau (le solide étant dans la coupelle). Affichage: exactement zéro.
- Sortir le solide de la coupelle et le déposer plus bas dans le panier (dans le liquide). Sur l'affichage de la
balance apparaît (avec un signe négatif) la poussée P sur le solide.
Diviser la première valeur affichée A par la deuxième P, puis multiplier ce résultat intermédiaire par la masse
volumique ρo du liquide auxiliaire (à la température T).
Ce résultat final est la masse volumique ρo recherchée du solide. Les unités correspondent à celles utilisées
pour la masse volumique ρo du liquide auxiliaire, par exemple g/ cm3 (identique à g/ ml).
A A [g] Comme les unités de A et P s'annulent, les pesées
ρ2 =• ρo=• ρo [g/ cm3] peuvent se faire tout aussi bien en carats qu'en
P P [g] grammes: le résultat ρ2 a toujours les unités de ρo.
S'il faut renoncer à la donnée directe de la poussée, P sera déterminé par A - B: B sera indiqué à la place de P
sur l'affichage de la balance, si après l'affichage du poids A, le tarage n'est pas effectué du tout ou alors effectué
en ayant préalablement enlevé le solide.
Example avec affichage de la poussée: Détermination de la masse volumique d'une monnaie à l'aide d'eau
distillée.
Valeurs mesurées: A = 3,011 g 3,011
P = 0,336 g ρ2 = • 0,997 g/ cm3 = 8,93 g/ cm
3
T = 25,5 °C →ρo = 0,997 g/ cm
3
0,336
La monnaie a une masse volumique de 8,93 g/ cm3.
Page 23

METTLER TOLEDO 33360 + 210260 21
Conjunto de determinar densidades
para la determinación de densidades de sólidos y de líquidos sobre balanzas electrónicas METTLER TOLEDO
de platillo elevado con diámetros de platillo de hasta 80 y 130 mm.
- para sólidos, conjunto de determinar densidades 33360.
- para líquidos, conjunto de determinar densidades 33360 + cuerpo de desplazamiento 210260.
1. Definición de la densidad
- para la determinación de densidades de sólidos y de líquidos sobre balanzas electrónicas METTLER
TOLEDO de platillo elevado con diámetros de platillo de hasta 80 y 130 mm.
La densidad ρ de un cuerpo homogéneo es la relación de su masa (m) a su volumen (V) o, lo que es lo mismo,
la masa de una unidad de volumen:
m [g] ρ = densidad
ρ = m = masa
V [cm3] V = volumen
En lugar de la masa (m) puede utilizarse el término "peso" de acuerdo con DIN 1305.
2. Principio de la determinación de densidades
Según el principio de Arquímedes un cuerpo sumergido en un líquido pierde de su peso propio una cantidad igual
al peso del líquido por él desalojado. De esta forma puede determinarse la magnitud buscada. El procedimiento
es algo diferente para los líquidos que para los sólidos:
La densidad de un líquido se determina con ayuda de un cuerpo sumergible o hundidor cuyo volumen es
conocido. Para tal fin este cuerpo se pesa una vez en el aire y acto seguido en estado sumergido. A partir de
ambas pesadas (en gramos) se calcula la densidad del líquido ρ1 de la forma siguiente:
As - B
s
ρ1 =+ Lsρ1= densidad del líquido ensayado a la temperatura
V
s
dada T
As= peso del cuerpo en el aire
Bs= peso del cuerpo sumergido en el líquido
o más sencillo Vs= volumen del cuerpo
Ls= empuje del aire por ml del cuerpo (corrección de
Asó Ps): + 0,001 g/ ml aprox.; véase también 3.2,
P
s
Empuje del aire.
ρ1 =+ LsPs= empuje del cuerpo en el líquido
V
s
(en lugar de As - Bs): directamente leíble en balan-
zas METTLER TOLEDO electrónicas, véase 4.1
La densidad de un sólido se determina con ayuda de un líquido (líquido auxiliar) cuya densidad sea conocida.
Para ello se pesa el sólido una vez en el aire y a continuación en estado sumergido. A partir de ambas pesadas
(en gramos o en quilates) se calcula la densidad ρ2 de la forma siguiente:
A
ρ2 =• ρ
o
ρ2= densidad del sólido
A - B A = peso del sólido en el aire
B = peso del sólido sumergido en el líquido auxiliar
o más sencillo ρo= densidad del líquido auxiliar a la temperatura
dada T
P = empuje del sólido en el líquido auxiliar (en lugar de
A A - B): directamente leíble en balanzas METTLER
TOLEDO electrónicas véase 4.2
ρ2 =• ρ
o
P
En el caso de los sólidos no se tiene en cuenta constantemente el empuje del aire. Si fuera necesario, puede
aplicarse en el resultado ρ2, al igual que en el primer caso, la corrección aproximada de + 0,001 g/ cm3; véase
también 3.2, Empuje del aire.
Page 24

22 METTLER TOLEDO 33360 + 210260
3. Exactitud de la determinación de densidades
La posible consideración de factores que pueden afectar al resultado se acomoda siempre a la exactitud
requerida en el mismo. La exposición siguiente permitirá una valoración cuantitativa de cada uno de estos
factores.
3.1 Temperatura
En el caso de los sólidos, la variación de la densidad por el cambio de temperatura es, en general, tan pequeña
que su temperatura carece de importancia para la determinación de densidad (lo mismo puede decirse del
cuerpo sumergible).
Por el contrario, en el caso de los líquidos, la variación de densidad se sitúa dentro del orden de magnitud de
0,1 … 1 %o por cada °C y , por tanto, puede manifestarse ya en la tercera posición detrás de la coma.
Ejemplos: Agua destilada: variación de densidad de 1 %o aprox. a 5 °C
Hidrocarburos y alcoholes: variación de densidad de 1 %o aprox. a 1 °C
Hay que tener en cuenta que esta variación de densidad de los líquidos interviene también directamente en el
resultado de las determinaciones de densidad de los sólidos, debido a que el volumen del sólido se determina
mediante un líquido auxiliar en el que se sumerge. De ahí que para determinar la densidad con una exactitud
superior al 1 % haya que tener siempre en cuenta la temperatura del líquido.
Tabla de densidad para agua destilada:
(según "Handbook of Chemistry and Physics" 66th Ed. 1985-1986, F4-F5)
Para otros líquidos hay que tomar la densidad a la temperatura T de un libro de tablas.
Temperatura (°C) Densidad (g/ ml) Temperatura (°C) Densidad (g/ ml)
15,0 0,9991 24,0 0,9973
15,5 0,9990 24,5 0,9972
16,0 0,9990 25,0 0,9970
16,5 0,9989 25,5 0,9969
17,0 0,9988 26,0 0,9968
17,5 0,9987 26,5 0,9966
18,0 0,9986 27,0 0,9965
18,5 0,9985 27,5 0,9964
19,0 0,9984 28,0 0,9962
19,5 0,9983 28,5 0,9961
20,0 0,9982 29,0 0,9959
20,5 0,9981 29,5 0,9958
21,0 0,9980 30,0 0,9956
21,5 0,9979 30,5 0,9955
22,0 0,9978 31,0 0,9953
22,5 0,9977 31,5 0,9952
23,0 0,9975 32,0 0,9950
23,5 0,9974
Page 25

METTLER TOLEDO 33360 + 210260 23
3.2 Empuje del aire
1 cm3 de aire pesa - según las condiciones - entre 1 y 1,2 mg. Así, pues, un cuerpo que se pese en el aire
experimenta un empuje de esta magnitud por cada cm3 de su volumen. Esto quiere decir que para una densidad
en torno a 1 (1 g/ cm3) se produce un error de cerca del 1 %o si no se tiene en cuenta el empuje del aire. De ahí
que si se requiere un resultado de 3 (ó de 4) decimales sea necesario corregir el empuje del aire en el resultado:
La densidad verdadera es aproximadamente 0,001 g/ cm3 mayor que la calculada.
En la determinación de densidades de líquidos, el procedimiento (división por 10 ml, de acuerdo con el volumen
del cuerpo sumergible) facilita ya un resultado de cuatro cifras con una indicación del peso de tres cifras. Por
tanto es conveniente, por regla general, efectuar en este caso la corrección por empuje del aire.
3.3 Tolerancia de volumen del cuerpo sumergible
Norma de contraste alemana EO 13-4, párrafo 0.21
El volumen del cuerpo sumergible, incluyendo la mitad inferior del hilo de suspensión, debe ser ajustado de forma
que, al determinar la densidad del agua a 20 °C, se pueda producir como máximo un error de ± 0,0005 g/ cm
3
con un dispositivo sumergible de 30 g.
3.4. Profundidad de inmersión del cuerpo sumergible o portapiedras
El cuerpo sumergible pende de un alambre de platino de 0,2 mm de diámetro. Por consiguiente, el alambre
experimienta en el agua un empuje de unos 0,3 mg sobre 10 mm de longitud sumergida.
Si el líquido se encuentra 10 mm por encima del ojete del cuerpo sumergible, hay sumergidos unos 20 mm de
alambre, por lo que con densidades próximas a 1 se origina un empuje de 0,6 mg. Pero como todavía se divide
por 10 ml, este efecto no entra en consideración.
El portapiedras está formado, en la parte que se sumerge, por alambre de 0,8 mm de diámetro, lo que en el
caso de una densidad de líquido próxima a 1 produce un empuje de unos 5 mg por cada 10 mm de longitud
sumergida. Pero como al pesar el sólido en el aire el portapiedras está igualmente sumergido, y entre ambas
pesadas no varía la profundidad de inmersión en las balanzas electrónicas, a pesar de la diferencia de peso, la
fuerza ascensional en el portapiedras permanece constante y, por lo tanto, no interviene. Condición: El
portapiedras debe estar suspendido del estribo de igual forma las dos veces y el estado del líquido no variar. (La
variación del estado del líquido producida por la inmersión del sólido es generalmente despreciable: un volumen
del sólido de 1 cm3 hace subir el líquido unos 0,5 mm, lo que da lugar a un empuje de unos 0,15 mg, es decir,
un error de densidad de 0,15 mg/ cm3 aproximadamente).
3.5. Tensión superficial del líquido
Debido a la adhesión del líquido al alambre de suspensión, se produce un aumento aparente del peso. En el caso
del cuerpo sumergible (diámetro del alambre 0,2 mm) y en agua, esta fuerza es de cerca de 1 mg y puede ser
reducida a cerca de 0,3 mg empleando humectantes o líquidos orgánicos. Pero como también a continuación
se divide por 10 ml, el error de densidad resultante se eleva a 0.0001 g/ ml, como máximo.
Debido al mayor diámetro del alambre en el agua, se origina en el portapiedras una fuerza de hasta 3 mg. Pero
también en este caso, análogamente a lo que ocurre en 3.4, se elimina en la práctica un efecto sobre el resultado
sumergiendo el portapiedras en ambas pesadas (A y B). Sin embargo, para las máximas exigencias de exactitud
la reducción de la tensión superficial sería una medida de precaución (véase también 3.6).
Page 26

24 METTLER TOLEDO 33360 + 210260
3.6 Burbujas de aire
Particularmente en el caso de líquidos poco mojadores, por ejemplo agua exenta de humectantes, existe la
posibilidad de que se adhieran burbujas de aire al sólido sumergido, cuerpo sumergible o portapiedras, que
influyan sobre el resultado por producir empuje. Una burbuja de 1 mm de ø produce un empuje de 0,5 mg,
mientras que otra de 2 mm produce un empuje de 4 mg.
Medidas de precaución
- Desengrase los sólidos que resistan a los disolventes.
- Limpie periódicamente portapiedras y cuerpo sumergible, sin tocar con las manos la parte que se sumerge.
- Sacuda siempre algo el portapiedras en la primera inmersión (mojadura) en el líquido, antes de suspenderlo
del estribo, para desprender las posibles burbujas de aire adheridas.
- Utilice humectantes o disolventes orgánicos como líquido auxiliar (p. ej. Foto-Flo de Kodak, Pervitro 75%
72409). Es despreciable el cambio de densidad del agua destilada producido por el humectante; si se añade
a 250 ml de agua por ejemplo 0,1 ml de humectante de la densidad 1.2, la densidad total varía en 0,0001 g/
ml.
3.7 Cuerpos porosos
Puesto que al sumergir cuerpos porosos generalmente no se desaloja todo el aire de los poros a través del
líquido, aparecen errores. La densidad del cuerpo sólo puede ser determinada de forma aproximada.
Page 27

METTLER TOLEDO 33360 + 210260 25
4. Ejecución de las determinaciones de densidad
Disposición:
1 platillo de la balanza METTLER TOLEDO
2 estribo, encajado y fijado en el platillo mediante …
3 … tornillos de apriete;
4 puente, colocado transversalmente encima del platillo
sobre la caja de la balanza. Atención: platillo 1 y estribo
2 no deben rozar el puente!
5 vaso de 250 ml, colocado sobre el puente;
6 cuerpo sumergible o portapiedras, suspendidos del
estribo 2 en el …
7 … colgante. Deben estar suspendidos en el centro del
vaso sin tocarlo. Atención: La primera pesada se hace
en seco!
8 termómetro
Las distintas partes se vuelven a presentar en la página 33
(lista de repuestos).
Notas:
- Se recomienda comprobar la calibración.
- Tenga en cuenta, además del texto siguiente, las breves instrucciones prácticas de las ilustraciones que
acompañan a esta descripción.
4.1 Determinación de la densidad de líquidos
- Utilice el cuerpo de desplazamiento 210260 + el conjunto de determinar densidades para líquidos
33360.
- Ponga el vaso vació sobre el puente 4, concéntricamente debajo del colgante 7; introduzca el termómetro.
- Suspenda el cuerpo sumergible 6. No debe rozar el vaso ni el termómetro.
- Tare la balanza. Indicación: cero exacto.
- Eche el líquido a ensayar en el vaso hasta unos 10 mm por encima del ojete de suspensión del cuerpo
sumergible. No debe quedar adherida burbuja alguna al mismo; en caso necesario quite las burbujas con un
pincel fino, etc.
- En el indicador de la balanza aparece (con signo negativo) el empuje Ps del cuerpo sumergible.
Divida esta indicación Ps de la balanza por 10 y luego añada 0.001 g/ ml.
El resultado es la densidad de líquido buscada ρ1 [g/ ml] a la temperatura T (lea).
P
s
Ps [g]
ρ1 =+ L
s
= + 0,001 g/ ml a T °C (Ps = As - Bs)
V
s
10 ml
En el caso de que se omita la lectura directa del empuje, se obtiene Ps a partir de dos pesadas separadas: A
s
= cuerpo sumergible en el aire; Bs = cuerpo sumergible en el líquido. Procedimiento: igual que antes, pero tarando
antes de suspender el cuerpo sumergible. Con éste suspendido, la balanza señala así As y, después de agregar
el líquido, Bs (sin nuevo tarado!).
Ejemplo con indicación de empuje: Determinación de la densidad de la acetona.
7,898 g
Medidas: Ps= 7,898 g ρ
1
+ 0,001 g/ ml = 0,7908 g/ ml a 20 °C
T = 20 °C 10 ml
La densidad de la acetona verificada es 0,7908 g/ ml a 20 °C.
Page 28

26 METTLER TOLEDO 33360 + 210260
4.2 Determinación de la densidad de sólidos
- Utilice el conjunto de determinar densidades para sólidos (= portapiedras 6), 33360.
- Eche líquido auxiliar en el vaso hasta que el sólido, una vez colocado en la cestita de alambre del portapiedras,
esté cubierto como mínimo con 1 cm de líquido. Meta el termómetro. Coloque el vaso en el puente 4,
concéntricamente debajo del colgante 7.
- Suspenda el portapiedras (atención a las burbujas, sobre todo en la cestita).
- Tare la balanza. Indicación: cero exacto.
- Ponga el sólido seco en la capsulita superior del portapiedras, anote la indicación del peso (en gramos o en
quilates). Este es el peso A del sólido en el aire.
- Tare de nuevo la balanza (con sólido en la capsulita). Indicación: cero exacto.
- Saque el sólido de la capsulita y póngalo en la cestita de alambre inferior (dentro del líquido).
En el indicador de la balanza aparece (con signo negativo) el empuje p del sólido.
Divida la primera indicación A por la segunda P y luego multiplique este resultado intermedio por la densidad
ρo del líquido auxiliar (a la temperatura dada T).
Este resultado final es la densidad buscada ρ2 del sólido. La unidad corresponde a la unidad utilizada para
la densidad ρo del líquido auxiliar, por ejemplo g/ cm3 (igual a g/ ml).
A A [g] Puesto que las unidades de A y de P se anulan,
ρ2 =• ρo=• ρo [g/ cm3] puede pesarse, por ejemplo, tanto en quilates como en
P P [g] gramos: el resultado ρ2 tiene siempre la unidad de ρo.
En el caso de que se omita la indicación directa, también aqui P se obtiene a partir de A - B: B aparece en lugar
de P en el indicador de la balanza, cuando después de la indicación de peso A no se tara de ninguna forma o
se hace con el sólido levantado.
Ejemplo con indicación de empuje: Determinación de la densidad de una moneda con ayuda de agua
destilada.
Medidas: A = 3,011 g 3,011
P = 0,336 g ρ2 = • 0,997 g/ cm3 = 8,93 g/ cm
3
T = 25,5 °C →ρo = 0,997 g/ cm
3
0,336
La moneda tiene una densidad de 8,93 g/ cm3.
Page 29

METTLER TOLEDO 33360 + 210260 27
Kit per la determinazione della densità
di solidi e liquidi con l'ausilio di bilance elettroniche METTLER TOLEDO con piatto di pesata superiore avente
un diametro di 80 e 130 mm.
- per la determinazione della densità di solidi: kit di determinazione della densità 33360.
- per la determinazione della densità di liquidi: kit di determinazione della densità 33360
+ corpo da immergere 210260.
1. Definizione della densità
- di solidi e liquidi con l'ausilio di bilance elettroniche METTLER TOLEDO con piatto di pesata superiore avente
un diametro di 80 e 130 mm.
La densità ρ di un corpo omogeneo è il rapporto tra la sua massa (m) ed il suo volume (V) oppure, in altre parole,
è la massa di un'unità di volume di questo corpo:
m [g] ρ = densità
ρ = m = massa
V [cm3] V = volume
In luogo di massa (m) si può porre, conformemente alla DIN 1305, il termine "peso".
2. Principio della determinazione della densità
Conformemente al principio di Archimede, un corpo immerso in un liquido subisce da parte di questo liquido una
spinta verticale dal basso verso l'alto pari al peso del liquido spostato. In base a questo principio si può
determinare la grandezza cercata. Il procedimento varia alquanto a seconda che si tratti di liquidi oppure di corpi
solidi.
La densità di un liquido viene determinata con l'ausilio di un corpo da immergere, del quale si conosce il volume.
Allo scopo, questo corpo viene pesato dapprima liberamente in aria e poi immerso nel liquido. In base alle due
pesate (in grammi) si calcola da densità ρ1 del liquido come segue:
As - B
s
ρ1 =+ Lsρ1= densità del liquido in esame ad una
V
s
data temperatura T
As= peso del corpo in aria
Bs= peso del corpo immerso nel liquido
oppure più semplicemente Vs= volume del corpo
Ls= spinta dell'aria ogni ml del corpo
(correzione di As o Ps): circa + 0,001 g/ ml;
P
s
vedere anche 3.2, Spinta dell'aria
ρ1 =+ LsPs= spinta del liquido sul corpo
V
s
(in luogo di As - Bs): leggibile direttamente
sulle bilance elettroniche METTLER TOLEDO,
vedere 4.1
La densità di un solido viene determinata con l'ausilio di un liquido (liquido ausilario) del quale si conosce la
densità. Allo scopo, il corpo solido viene pesato prima in aria e poi immerso in detto liquido. In base alle due
pesate (in grammi oppure in carati) si calcola la densità ρ2 come segue:
A
ρ2 =• ρ
o
ρ2= densità del corpo solido
A - B A = peso del solido in aria
B = peso del solido immerso nel liquido ausiliario
oppure più semplicemente ρo= densità del liquido ausiliario ad una data
temperatura T
A P = spinta del liquido ausiliario sul solido
ρ2 =• ρ
o
(in luogo di A - B): leggibile direttamente
P sulle bilance elettroniche METTLER TOLEDO,
vedere 4.2
La spinta dell'aria non viene generalmente presa in considerazione per i corpi solidi. Se necessario, come nel
primo caso, si può apportare al risultato ρ2 la correzione di circa + 0,001 g/ cm3; vedere anche 3.2, Spinta dell'aria.
Page 30

28 METTLER TOLEDO 33360 + 210260
3. Precisione della determinazione della densità
I fattori suscettibili di influire sul risultato vengono presi in considerazione a seconda della precisione richiesta
per il risultato. I punti che seguono hanno lo scopo di consentire in dettaglio una valutazione quantitativa di detti
fattori.
3.1 Temperatura
Per i corpi solidi, la variazione della densità dovuta a variazioni della temperatura è in generale così minima che
la loro temperatura non ha importanza ai fini della determinazione della densità (ciò vale anche per il corpo da
immergere).
Per i liquidi invece, la variazione della densità ha un ordine di grandezza di 0,1 … 1 %o ogni °C, per cui può già
apparire nella terza cifra dopo la virgola.
Esempi: Acqua distillata: variazione della densità circa 1 %o ogni 5 °C
Idrocarburi ed alcooli: variazione della densità circa 1 %o ogni 1 °C
Si deve tenere presente che questa variazione della densità dei liquidi si ripercuote direttamente sul risultato delle
determinazioni della densità di corpi solidi, in quanto il volume del corpo solido viene determinato per mezzo
di un liquido ausiliario, nel quale il corpo viene immerso. Se nelle determinazioni della densità si desidera una
precisione maggiore dell' 1 %, si deve sempre tener conto della temperatura del liquido!
Tabella delle densità dell'acqua distillata:
Tratto da: "Handbook of Chemistry and Physics" 66th Ed. 1985-1986, F4-F5)
Per altri liquidi, la densità alla temperatura T dovrà essere tratta da un tabellario.
Temperatura (°C) Densità (g/ ml) Temperatura (°C) Densità (g/ ml)
15,0 0,9991 24,0 0,9973
15,5 0,9990 24,5 0,9972
16,0 0,9990 25,0 0,9970
16,5 0,9989 25,5 0,9969
17,0 0,9988 26,0 0,9968
17,5 0,9987 26,5 0,9966
18,0 0,9986 27,0 0,9965
18,5 0,9985 27,5 0,9964
19,0 0,9984 28,0 0,9962
19,5 0,9983 28,5 0,9961
20,0 0,9982 29,0 0,9959
20,5 0,9981 29,5 0,9958
21,0 0,9980 30,0 0,9956
21,5 0,9979 30,5 0,9955
22,0 0,9978 31,0 0,9953
22,5 0,9977 31,5 0,9952
23,0 0,9975 32,0 0,9950
23,5 0,9974
Page 31

METTLER TOLEDO 33360 + 210260 29
3.2 Spinta dell'aria
1 cm3 di aria pesa – a seconda della sua condizione – circa 1 … 1,2 mg. Pertanto, un corpo pesato in aria subisce
una spinta di questa grandezza per ogni cm3 del suo volume. Ciò significa che, data una densità attorno ad
1 (1 g/ cm3), si verifica, non tenendo conto della spinta dell'aria, un errore di circa 1 %o. Se è richiesto un risultato
con 3 (oppure 4) cifre dopo la virgola, sarà quindi necessario correggere il risultato in base alla spinta dell'aria:
La vera densità è di circa 0,001 g/ cm3 maggiore di quella calcolata.
Il metodo di caldolo della densità di liquidi (divisione per 10 ml, conformemente al volume del corpo immerso)
fa si che già per un peso indicato con tre decimali si ottiene un risultato con quattro decimali. E' pertanto opportuno
in questo caso eseguire sistematicamente la correzione dovuta alla spinta dell'aria.
3.3 Tolleranza sul volume del corpo immerso
Legislazione tedesca di metrologia EO 13-4, paragrafo 9.21
Il volume del corpo immerso, comprensivo della metà inferiore del filo di sospensione, deve essere regolato
in modo tale che la determinazione della densità dell'acqua a 20 °C sia affetta da un errore massimo di
± 0,0005 g/ cm3 con un corpo immerso del peso di 30 g.
3.4 Profondità d'immersione del corpo da immergere o del portapietra
Il corpo da immergere è appeso ad un filo di platino di 0,2 mm di diametro. Quindi il filo subisce nell'acqua una
spinta di circa 0,3 mg ogni 10 mm di lunghezza immersa.
Se il liquido si trova 10 mm al disopra dell'occhiello del corpo, sono immersi circa 20 mm di filo. Per densità
prossime ad 1 ne risulta una spinta di 0,6 mg. Dato però che si divide ancora per 10 ml, questa fonte d'errore
diventa del tutto trascurabile.
Il portapiertra è costituito, nella sua parte da immergere, da un filo di 0,8 mm di diametro, il che, data una densità
del liquido attorno ad 1, comporta una spinta di circa 5 mg ogni 10 mm di lunghezza immersa. Dato però che il
portapietra nella pesata del corpo solido in aria è analogamente immerso nel liquido e dato inoltre che la
profondità d'immersione, nonostante la differenza di peso, non varia sulle bilance elettroniche, la spinta del
liquido sul portapietra rimane costante e non viene quindi di considerata. Condizione: non variare il livello del
liquido (La variazione del livello del liquido a seguito dell'immersione del corpo solido è per lo più trascurabile:
un volume del solido di 1 cm3 fa salire il livello del liquido di 0,5 mm, il che provoca una spinta di circa 0,15 mg,
cioè un errore nella determinazione della densità di circa 0,15 mg/ cm3).
3.5 Tensione superficiale del liquido
A seguito dell'adesione del liquido al filo di sospensione si verifica un aumento fittizio del peso. Nel caso del corpo
immerso (diametro del filo 0,2 mm) ed in acqua, questa forza ammonta a circa 1 mg; essa può essere ridotta a
circa 0,3 mg con l'impiego di agenti tensioattivi o di liquidi organici. Però anche in questo caso si deve poi dividere
per 10 ml, per cui l'errore di densità risultante ammonta al massimo a 0,0001 g/ml.
Poiché il diametro del filo è più grande, sul portapietra immerso in acqua agisce una forza che può arrivare fino
a 3 mg. Anche in questo caso, analogamente a quanto detto nel punto 3.4, una ripercussione sul risultato viene
eliminata praticamente dal fatto che il portapietra è immerso durante entrambe le pesate (A e B). Se è richiesta
la massima precisione, sarà nondimeno preferibile, per precauzione, ridurre la tensione superficiale (vedere
anche 3.6).
Page 32

30 METTLER TOLEDO 33360 + 210260
3.6 Bollicine d'aria
Particolarmente nel caso di liquidi scarsamente bagnanti, come è p.es. il caso dell'acqua priva di prodotti
tensioattivi, sussiste la possibilità che sul corpo solido, sul corpo immerso o sul portapietra rimangano attaccate
delle bollicine d'aria che alterano il risultato per effetto della spinta verso l'alto da esse esercitata. Una bollicina
di 1 mm Ø genera una spinta verso l'alto di 0,5 mg, una bollicina di 2 mm Ø però già una spinta di 4 mg.
Accorgimenti precauzionali:
- Sgrassare con solventi il corpo solido ad essi resistente.
- Pulire a regolari intervalli di tempo il portapietra ed il corpo da immergere e non toccare con le mani la parte
che va immersa.
- Scuotere alquanto il portapietra nella sua prima immersione (bagnatura) nel liquido, prima ancora di
agganciarlo alla staffa, affinchè si distacchino le eventuali bollicine d'aria.
- Impiegare quale liquido ausiliario agenti tensioattivi oppure solventi organici (p.es. Foto-Flo della Kodak,
Pervitro 75% 72409). La variazione della densità dell'acqua distillata dovuta all'apporto del prodotto
tensioattivo è irrilevante; se, p.es., si immette in 250 ml d'acqua 0,1 ml di prodotto tensioattivo avente la
densità di 1.2, la densità globale varia di 0,0001 g/ ml.
3.7 Corpi porosi
Dato che immergendo corpi porosi, di regola, non tutta l'aria contenuta nei pori viene espulsa dal liquido, si
verificano degli errori. La densità del corpo può essere determinata soltanto approssimativamente.
Page 33

METTLER TOLEDO 33360 + 210260 31
4. Esecuzione delle determinazioni della densità
Kit montato
1 Piatto di pesata della bilancia METTLER TOLEDO
2 Staffa, sistemata sul piatto di pesata
3 Viti fissaggio staffa sul piatto di pesata
4 Ponte, disposto trasversalmente al disopra del piatto di
pesata e poggiante sullo chassis della bilancia.
Attenzione: il piatto 1 e la staffa 2 non devono strisciare
contro il ponte!
5 Bicchiere da 250 ml, posato sul ponte;
6 Corpo da immergere o portapietra
7 Piattello di sospensione sulla staffa 2.
Il corpo da immergere ovvero il portapietra devono
pendere liberamente al centro del bicchiere senza toccarlo. Attenzione: la prima pesata avviene a secco!
8 Termometro
I singoli componenti sono nuovamente elencati a pag. 33
(distinta delle parti di ricambio).
Avvertenze:
- Si raccomanda di verificare la calibrazione.
- Consultare, unitamente al testo che segue, le brevi istruzioni pratiche illustrate allegate alla presente
descrizione.
4.1 Determinazione della densità di liquidi
- Utilizzare il corpo da immergere 210260 + il kit di determinazione della densità 33360.
- Porre sul ponte 4 il bicchiere vuoto, centrandolo al disotto della sospensione 7; sistemare il termometro entro
il bicchiere.
- Sospendere il corpo da immergere 6. Esso non deve toccare né il bicchiere né il termometro.
- Tarare la bilancia. Indicazione: esattamente zero.
- Introdurre nel bicchiere il liquido da esaminare, fino a circa 10 mm al disopra dell'occhiello di sospensione del
corpo immerso. Sul corpo non deve aderire alcuna bollicina d'aria; staccare eventualmente le bollicine con
un pennellino o simili.
- Sull'indicazione della bilancia compare (con segno negativo) la spinta Ps sul corpo immerso. Dividere per 10
questa indicazione Ps della bilancia, aggiungere poi 0,001 g/ ml.
Il risultato rappresenta la densità cercata del liquido p1 [g/ ml] alla temperatura T (da leggere).
P
s
Ps [g]
ρ1 =+ L
s
= + 0,001 g/ ml a T °C (Ps = As - Bs)
V
s
10 ml
Se si deve rinunciare all'indicazione diretta della spinta, si determinerà Ps mediante due distinte pesate: As = corpo
da immergere in aria; Bs = corpo immerso nel liquido. Procedura: come sopra, però si tara prima della
sospensione del corpo da immergere. A corpo agganciato la bilancia indica poi As, e dopo l'introduzione del
liquido (senza ripetere la taratura!) invece Bs.
Esempio con indicazione della spinta: Determinazione della densità di acetone.
7,898 g
Valori misurati: Ps= 7,898 g ρ
1
+ 0,001 g/ ml = 0,7908 g/ ml a 20 °C
T = 20 °C 10 ml
La densità dell'acetone impiegato nella prova è di 0,7908 g/ ml a 20 °C.
Page 34

32 METTLER TOLEDO 33360 + 210260
4.2 Determinazione della densità di corpi solidi
- Utilizzare il kit di determinazione della densità di solidi 33360 (= portapietra 6).
- Versare nel bicchiere una quantità di liquido ausiliario tale che il corpo solido, quando verrà successivamente
introdotto nel cestello in filo metallico del portapietra, risulti ricoperto dal liquido per almeno
1 cm. Inserire il termometro. Posare il bicchiere sul ponte 4, centrandolo al disotto della sospensione 7.
- Agganciare il portapietra (attenzione alle bollicine d'aria, specialmente sul cestello).
- Tarare la bilancia. Indicazione: esattamente zero.
- Porre il corpo solido asciutto nel piattello superiore del portapietra, prendere nota del peso indicato (in grammi
o carati). Si tratta del peso A del corpo solido in aria.
- Tarare nuovamente la bilancia (con il corpo solido nel piattello). Indicazione: esattamente zero.
- Prelevare il corpo solido dal piattello e porlo nel cestello (immerso nel liquido). Nell'indicazione della bilancia
compare (con segno negativo) la spinta P sul corpo solido.
Dividere la prima indicazione A per la seconda indicazione P poi moltiplicare questo risultato parziale per la
densità ρo del liquido ausiliario (alla temperatura T).
Questo risultato finale rappresenta le densità cercata ρ2 del corpo solido. L'unità corrisponde a quella adottata
per la densità ρo del liquido ausiliario, p.es. g/ cm3 (il che corrisponde g/ ml).
A A [g] Pioché le unità di A e P si elidono, le pesate possono
ρ2 =• ρo=• ρo [g/ cm3] essere eseguite tanto in carati quanto in grammi: il
P P [g] risultato ρ2 ha sempre l'unità di ρo.
Se si deve rinunciare all'indicazione diretta della spinta, P sarà determinato con A - B: B comparirà nell'indicazione della bilancia in luogo di P se dopo l'indicazione del peso A non si esegue per nulla la tara, oppure se si
tara dopo aver tolto il corpo solido.
Esempio con indicazione della spinta: Determinazione della densità di una moneta con l'ausilio di acqua
distillata.
Valori A = 3,011 g 3,011
misurati: P = 0,336 g ρ2 = • 0,997 g/ cm3 = 8,93 g/ cm
3
T = 25,5 °C →ρo = 0,997 g/ cm
3
0,336
La moneta ha una densità di 8,93 g/ cm3.
Page 35

METTLER TOLEDO 33360 + 210260 33
Spare parts Order No.
Ersatzteile Bestell-Nr.
Pièces de rechange No de commande
Repuestos No de pedido
Parti di ricambio N. ordinazione
Glass beaker 250 ml 71578
Becherglas
Bécher
Vaso
Bicchiere
Bracket 80 mm 33341
Bügel
Etrier
Estribo
Staffa
Bracket 130 mm 33342
Bügel
Etrier
Estribo
Staffa
Float packaged 210260
Senkkörper verpackt
Plongeur sous emballage
Cuerpo sumergible embalado
Corpo da immergere imballato
Gem holder 33343
Steinträger
Porte-pierre
Portapiedras
Portapietra
Thermometer 238767
Thermometer
Thermomètre
Termómetro
Termometro
Clamping screw 40288
Klemmschraube
Vis de fixation
Tornillo de apriete
Vite di fissaggio
Bridge 33347
Brücke
Pont
Puente
Ponte
Pervitro 75% 72409
Page 36

34 METTLER TOLEDO 33360 + 210260
For fast, reliable and efficient determination of density of solid, porous or fluid substances we also recommend
the METTLER TOLEDO LabWare "Density".
Für schnelle, einfache und zuverlässige Dichtebestimmung von festen, porösen und flüssigen Stoffen verwenden
Sie auch die METTLER TOLEDO LabWare "Dichte".
Pour une détermination rapide, simple et fiable de la masse volumique de solides poreux ou de liquides, utilisez
également le METTLER TOLEDO LabWare "Masse volumique".
Para una determinación rápida, sencilla y fiable de densidades de sustancias sólidas, porosas y líquidas, utilice
también el LabWare METTLER TOLEDO "Densidad".
Per una determinazione rapida, semplice ed affidabile della densità di sostanze solide, porose e di liquidi
impiegate anche il METTLER TOLEDO LabWare "Densità".
Page 37

METTLER TOLEDO 33360 + 210260 35
LEERSEITE
Page 38

36 METTLER TOLEDO 33360 + 210260
To protect your METTLER TOLEDO product’s future:
METTLER TOLEDO Service assures the quality, measuring accuracy and preservation of value of all METTLER TOLEDO products for years to come.
Please send for full details about our attractive terms of service.
Thank you.
Für eine gute Zukunft Ihres METTLER TOLEDO Produktes:
METTLER TOLEDO Service sichert Ihnen auf Jahre Qualität, Messgenauigkeit und
Werterhaltung der METTLER TOLEDO Produkte.
Verlangen Sie bitte genaue Unterlagen über unser attraktives Service-Angebot.
Vielen Dank.
Pour assurer l´avenir de vos produits METTLER TOLEDO:
Le service après-vente METTLER TOLEDO vous garantit pendant des années leur
qualité, leur précision de mesure et le maintien de leur valeur.
Demandez-nous notre documentation sur les excellentes prestations proposées
par le service après-vente METTLER TOLEDO.
Merci.
Para un mejor futuro de sus productos METTLER TOLEDO:
El servicio postventa de METTLER TOLEDO garantiza durante años su calidad, su
precisión metrológica y la conservación de su valor.
Pida nuestra documentación sobre las excelentes prestaciones que le ofrece el
servicio postventa de METTLER TOLEDO.
Gracias.
Per un buon futuro dei Vostri prodotti METTLER TOLEDO:
Il servizio assistenza tecnica METTLER TOLEDO Vi garantisce nel corso degli anni
la loro qualità, la loro precisione di misura e la conservazione del loro valore.
Richiedeteci subito la documentazione illustrativa del servizio altamente professionale che Vi offriamo.
Grazie.
*P702578*
© Mettler-Toledo GmbH 1998 702578A Printed in Switzerland 9811/2.45
Mettler-Toledo GmbH, Laboratory & Weighing Technologies, CH-8606 Greifensee, Switzerland
Phone +41-1-944 22 11, Fax +41-1-944 30 60, Internet: http://www.mt.com
Subject to technical changes and to the availability
of the accessories supplied with the instruments.
Technische Änderungen und Änderungen im
Lieferumfang des Zubehörs vorbehalten.
Sous réserve de modifications techniques
et de disponibilité des accessoires.
Reservadas las modificaciones técnicas
y la disponibilidad de los accesorios.
Con riserva di apportare modifiche tecniche
e di disponibilità degli accessori.
 Loading...
Loading...